Abstract
Background
Niemann-Pick disease, type C1 is a neurodegenerative condition that arises from mutations of NPC1 and is often diagnosed in children. Recently, several drug trials have been implemented to minimize neurodegeneration, including a trial of 2-hydoxypropyl-β-cyclodextrins (VTS-270).
Objectives
The current study extends findings from a previous report of 18 months of disease severity data by describing neuropsychological outcomes over the course of 36 months post-baseline.
Design
An open-label, dose-escalation phase 1/2a study of VTS-270 was performed in participants with NPC1 aged 4 to 23 years.
Methods
Fourteen participants were sequentially assigned to receive monthly initial intrathecal VTS-270 at doses of 50, 200, 300, or 400 mg per month. After initial dosing, participants were dose-escalated (to 600 or 1200 mg) as tolerated. Participants were evaluated at 6-month intervals using a standardized neuropsychological battery, including tests of cognition, language, and adaptive behavior. A random effects model with restricted maximum likelihood estimation was constructed for each outcome, and the slope was the parameter of interest.
Results
Findings based on IQ scores and both standard scores and age equivalents of adaptive functioning indicate that there were not meaningful declines in these areas during the study period. The average annualized change in FSIQ was negative: B = −1.28, SE = 0.70, t(34.2) = −1.83, p = .076. The Vineland-II ABC standard score decreased by 1.76 points per year [SE = 0.67, t(59.1) = −2.62, p = .011], but annualized slopes for each of the domain age equivalents were positive: Communication [B = 0.71, SE = 3.12, t(60.7) = 0.23, p = .82], Socialization [B = 2.99, SE = 2.92, t(60.4) = 1.03, p = .30], Daily Living Skills [B = 2.76, SE = 2.76, t(60.3) = 1.18, p = .24], and Motor Skills [B = 1.42, SE = 0.94, t(50.5) = 1.51, p = .14], indicating not worsening but slower-than-average acquisition of skills.
Conclusion
In conjunction with previous findings, these results provide support for the slowing of disease progress up to 36 months post-initiation of intrathecal VTS-270.
Registration
ClinicalTrials.Gov, Hydroxypropyl Beta Cyclodextrin for Niemann-Pick type C1 Disease, NCT01747135
1. Introduction
Niemann-Pick disease, type C1 (NPC1) is a lysosomal storage disorder characterized by progressive neurodegeneration. The few natural history studies of the neuropsychological and language functioning of NPC1 confirm a progressive cognitive decline in both children and adults [1, 2]. Neurological disease progression can be tracked using the NPC Neurological Severity Score [3]. The age of onset of neurological disease is variable, but steadily progressive with increasing impairment of both motor and cognitive function [3–5].
The potential therapeutic efficacy of 2-hydoxypropyl-β-cyclodextrins (HPβCD) is supported by studies in both NPC1 mouse [6–9] and feline [10] models demonstrating delayed progression of neurological signs and death. A phase 1/2a study HPβCD (VTS-270) was initiated at the National Institutes of Health (NIH), and 18-month outcomes in several domains have been reported [11]. Findings from this study indicated that despite mid-frequency to high-frequency hearing loss in all participants, communication was not significantly impacted and the NPC Neurological Severity Score showed decreased progression in the domains of ambulation, cognition, and speech when compared to archival natural history data from 21 NPC1 patients. The objective of this current analysis was to evaluate the change in neurodevelopmental outcomes at up to 36 months post-baseline in this Phase 1/2a trial of escalating doses of lumbar intrathecal HPβCD in NPC1.
2. Methods
2.1. Study Design and Procedures
This was an open-label dose-escalation study of the safety and efficacy of intrathecal HPβCD (NCT02124083) (see [11] for a full description of methods and results).
Patients received a monthly infusion of HPβCD. During each visit to the NIH Clinical Center in Bethesda, MD, USA, assessments of adverse events and clinical efficacy were performed. A standardized neuropsychological battery, the focus of this report, was administered every 6 months. This analysis includes outcomes collected out to 36 months post-baseline.
Study monitoring was provided by a study safety committee and an independent Data Safety Monitoring Board. Independent auditing was provided by Amarex Clinical Research (Gaithersburg, MD). An investigator IND supported the study (IND 113273). This IND was subsequently transferred to Vtesse, Inc. (Gaithersburg, MD) to support further drug development.
2.2. Participants
Eligible participants were aged 2 – 25 years and neurological manifestations, although participants were excluded if these were severe. All participants had neurological symptoms by 13 years of age thus would be categorized as childhood/adolescent onset. Disease progression is similar for both childhood and adolescent onset [3]. Fourteen participants were enrolled at the NIH between September 2013 and January 2015 (see Table 1).
Table 1.
Participant information (N = 14)
| Mean ± Standard Deviation | Range | |
|---|---|---|
| Age at diagnosis (years) | 9.07 ± 5.65 | 2 − 20 |
| Age at baseline (years) | 15.07 ± 5.53 | 4 − 23 |
| Niemann-Pick Type C1 Total Severity | 19.29 ± 7.46 | 5 − 32 |
| Score at Baseline | ||
| n (%) | ||
| Female | 7 (50%) | |
| White | 14 (100%) | |
| Non-Latino/Non-Hispanic | 13 (93%) | |
| Hearing loss at baseline | 11 (79%) | |
| Seizures | 5 (36%) | |
| Miglustat | 11 (79%) | |
2.3. HPβCD and Drug Administration
HPβCD (VTS-270) was formulated and provided by Johnson & Johnson (Janssen Research and Development). HPβCD was administered monthly by intrathecal injection in a total volume of 10 ml over 2–3 minutes. Patients were sequentially assigned to starting doses of 50 mg (CDA101–103), 200 mg (CDA104–106), 300 mg (CDA107–109), and 400 mg (CDA110–112). Two participants (CDA113–114) were initially dosed at 900 mg. After initial dosing, participants were dose-escalated (to 600 or 1200 mg) as tolerated.
2.4. Outcomes
The primary neurocognitive outcomes in this study were IQ and adaptive behavior. The appropriate cognitive measure was selected from the Wechsler Abbreviated Scales of Intelligence, Wechsler Preschool and Primary Scale of Intelligence, 3rd edition, Wechsler Intelligence Scales for Children-Fourth Edition [12–14], and the Mullen Scales of Early Learning [15], based on developmental level and chronological age of the participant. If the participant’s developmental level required an out-of-age-range Mullen, a developmental quotient was calculated using the age equivalents generated by the test. Verbal (VIQ), Nonverbal/Perceptual Reasoning (NVIQ), and Full-Scale (FSIQ) IQ were reported; these scores have a population mean of 100 and a standard deviation of 15.
Adaptive behavior was measured with the Vineland Adaptive Behavior Scales, Second Edition [16]. The Vineland is a semi-structured caregiver interview that assesses adaptive functioning in Communication, Daily Living Skills, Socialization, and Motor Skills (standard scores available only for children under the age of 7 years). The overall level of adaptive behavior is reflected in the Adaptive Behavior Composite, which is a standard score with a population mean of 100 and a standard deviation of 15. In addition to standard scores, age equivalents for each of the domains were calculated.
Due to its clinical relevance, a measure of memory was included as a secondary outcome. The Wide Range Assessment of Memory and Learning – Second Edition [WRAML-2;17] is an individually administered test battery to assess verbal, visual, and more global memory deficits. Here, we obtained the Story Memory, Story Recall, and Story Recognition scaled scores (population mean = 10, standard deviation = 3).
Other tests were administered, but high rates of missing data were driven by (a) priority, as cognitive and adaptive tests were collected first, and (b) ability, as some patients were incapable of completing some tests, even at baseline. These tests included the Hooper Visual Organization Test [18], which is a neurological screening measure designed to assess visual perceptual functioning; the Beery-Buktenica Developmental Test of Visual-Motor Integration [19], a test of integration of visual and motor abilities (eye-hand coordination); and the Purdue Pegboard Test [20], which is a test of manual dexterity and bimanual coordination. Descriptive results of these tests are included in Supplementary Figure 1.
2.5. Statistical Analysis
No comparison group was available, so the goal of this set of analyses was to describe the course of the neuropsychological outcomes during the trial. We accomplished this using a random effects model with restricted maximum likelihood estimation for each outcome. Continuous time from baseline (in years) was entered as a fixed effect, and a random intercept was used with an unstructured covariance structure. The small sample size precluded the use of a random slope; the mean slope was calculated and is presented as annualized change. Influence diagnostics were requested, and observations with Cook’s d values exceeding 0.2 were excluded and the model re-assessed. Distributional assumptions were assessed via visual inspection of plots. All models were run with and without the following covariates: age at baseline (centered at 15 years) and time since initial onset of neurological symptoms. Results with these covariates are presented in Supplementary Table 1.
No a priori power calculation was performed for these analyses. There are no agreed-upon sample size requirements for valid implementation of random effects models, and characteristics of the data such as the intraclass correlation and the number of observations per person moderate the effect of small sample size on the bias of model estimates [21]. The sample size in this study is just commensurate with the minimum suggested for valid interpretation of fixed effects and variance (n ≈ 15), but is below that suggested for the standard errors of the fixed effect (n ≈ 30) [21]. Simulation studies suggest that when the sample size is small, standard error estimates are biased downward. For this reason, we used the Kenward-Roger adjustment to denominator degrees of freedom and the covariance matrix, which improves underestimation and results in valid inference [22]. Participants with any available data were included in analysis; list-wise deletion was not used. However, where missing data resulted in a sample size <10 at more than one major assessment point, parametric analysis was not performed. Following the current recommendations of the American Statistical Association [23], we do not specify a threshold for “statistical significance,” and instead report parameter estimates (B) with their associated standard errors (SE), and specific p-values. Statistical analyses were performed in SAS/STAT 9.4.
3. Results
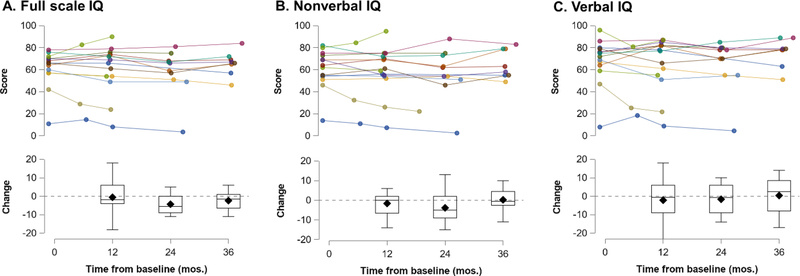
Individual IQ data are plotted in Figure 1. The average annualized change in FSIQ was negative: B = −1.28, SE = 0.70, t(34.2) = −1.83, p =0 .076. The slopes in each of the IQ subdomains were weaker: NVIQ, B = −0.65, SE = 0.82, t(35.3) = −0.79, p = 0.43; VIQ, B = −0.80, SE = 1.02, t(34.3) = −0.79, p = 0.44.
Fig. 1.
IQ scores by individual (top) and by study visit (bottom). Change refers to the change from baseline score per individual. Mos = months.
On average, the Vineland-II ABC standard score decreased by 1.76 points per year [SE = 0.67, t(59.1) = −2.62, p = 0.011]. Declines of similarly small magnitude were observed in each of domain standard scores: Communication [B = −1.88, SE = 1.05, t(60.3) = −1.79, p = 0.08], Socialization [B = −2.13, SE = 1.00, t(60.3) = −2.12, p = 0.04], and Daily Living Skills [B = −1.32, SE = 0.83, t(59.2) = −1.60, p = 0.11] (see Supplementary Figure 2). However, examination of the average age equivalent scores (in months) for these domains (shown in Figure 2) indicated that the small decreases in standard scores were not due to worsening, but rather to slower-than-typical (or no) acquisition of skills. Annualized slopes for each of the domain age equivalents were positive, although the 95% CI included zero: Communication [B = 0.71, SE = 3.12, t(60.7) = 0.23, p = 0.82], Socialization [B = 2.99, SE = 2.92, t(60.4) = 1.03, p = 0.30], Daily Living Skills [B = 2.76, SE = 2.76, t(60.3) = 1.18, p = 0.24], and Motor Skills [B = 1.42, SE = 0.94, t(50.5) = 1.51, p = 0.14].
Fig. 2.
Adaptive behavior age equivalents (AE) by individual (top) and by study visit (bottom). Change refers to the change from baseline score per individual. Mos = months.
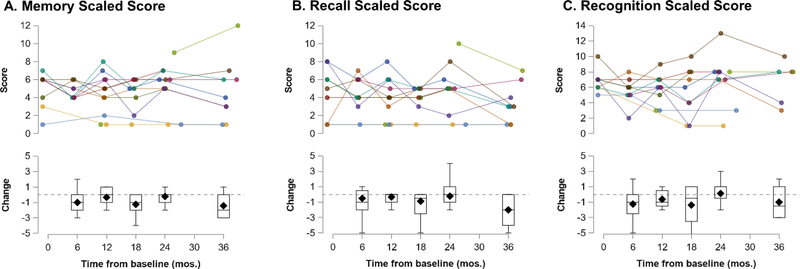
Fewer than 10 WRAML observations were available at most timepoints (including baseline), so these data were not subjected to parametric analysis. Scaled scores are plotted in Figure 3. The median change in scaled score was slightly negative at most time points for Memory, Recall, and Recognition.
Fig. 3.
WRAML scaled scores by individual (top) and by study visit (bottom). Change refers to the change from baseline score per individual. Mos = months.
Eight trial participants had archival natural history data [24]; too few datapoints were available for parametric analysis. For the purposes of visual comparison, these data were plotted next to the trial data (Figure 4).
Fig. 4.
Natural history IQ data (n = 8). Data in the shaded portion of the graphs are from the trial (duplicated from Figure 1).
4. Discussion
A previous report described a slowed neurological disease progression among patients with NPC1 in a phase 1/2a trial of intrathecal HPβCD [11]. In this secondary analysis, we demonstrate that contemporaneously measured cognitive and adaptive skills did not significantly decline during this trial.
Immediate memory, recall and recognition were evaluated with the WRAML. Although small sample size prohibited statistical analysis, participants demonstrated negligible change even until the 36-month time point. Even fewer timepoints were available for other measures, including the Hooper Test of Visual Integration, the Berry Visual Motor Integration test and Purdue Pegboard. Visual inspection of the raw data (see Supplementary Materials) suggests generally stable scores across these measures, for those participants who were able to withstand these tests and receive basal scores at baseline.
Data from up to 6 years of natural history study from a limited number of participants does indicate some decline prior to treatment initiation, but it is difficult to interpret this without a larger sample and a cohort of participants who did not go on to receive treatment. While conclusions regarding changes over time are limited overall, the presentation of data from various measures and types of scores suggest that the adaptive behavior age equivalent scores are the most clinically useful scores for studies of this nature. Most importantly, standard scores are an indicator of relative standing, not actual ability. They are expected to remain stable in the general population and to decrease with progression of a neurodegenerative disease. To observe an increase in standard scores, which is the goal of standard analyses of clinical trials, the participant would need to gain skills at a rate substantially exceeding age-based expectations. Thus, improvements in skill may still present as worsening in standard scores. This was borne out in the Vineland scores in this study; although the mean Adaptive Behavior Composite score decreased during the trial, the age equivalents in each of the domains remained stable. The recently published third edition of the Vineland includes scores specifically designed to measure change (growth scale values), which may dramatically improve the power of clinical trials to detect treatment effects. The limitations of this study are similar to those described elsewhere [11], including the small sample size, the lack of a contemporaneous control group, and that the dose-escalation design limited our ability to establish a dose-response relationship to the neuropsychological outcomes. Additionally, we were not able to include a historical control group. We did plot previously obtained natural history data from a subset of the participants, but this has attendant limitations including increasing time since time of onset. The descriptive nature of this report was necessary because the study was not designed to specifically target clinical efficacy with respect to neuropsychological functioning. Finally, 11 of 14 participants were taking miglustat at baseline and throughout the trial. Due to the small number of participants not taking miglustat, we were unable to ascertain its potential confounding effect.
5. Conclusions
These findings not only provide further evidence for the lack of disease progression associated with intrathecal HPβCD in NPC1, but they also provide proof-of-concept for including cognitive and adaptive function as targets in future treatment studies of neurodegenerative diseases affecting youth, such as NPC-1.
Supplementary Material
Key points.
An open-label phase 1/2a study of 2-hydoxypropyl-β-cyclodextrin (VTS-270) was performed in 14 individuals with Niemann-Pick disease, type C1.
Up to 36-months post-baseline, there were no significant declines in the areas of cognition and adaptive behavior.
This secondary analysis provides support for the slowing of disease progression with intrathecal VTS-270.
Acknowledgements
We thank the study participants and their families.
Funding: This research was supported by the Intramural Research Programs of the National Institute of Mental Health (1ZICMH002961) and the Division of Translational Research of the Eunice Kennedy Shriver National Institute of Child Health and Development (ZIA HD008824).
Footnotes
Conflicts of Interest:VTS-270 was provided by Janssen Pharmaceuticals, a Johnson & Johnson company. CAF, AT, NF, SB, LAK, and FDP have no other disclosures.
Compliance with Ethical Standards
Ethical Approval: This study was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Informed Consent: Written informed guardian permission or participant consent were obtained. Assent was obtained when possible.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Thurm A, Farmer C, Farhat NY, Wiggs E, Black D, Porter FD. Cohort study of neurocognitive functioning and adaptive behaviour in children and adolescents with Niemann-Pick Disease type C1. Dev Med Child Neurol. 2015. doi: 10.1111/dmcn.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klarner B, Klunemann HH, Lurding R, Aslanidis C, Rupprecht R. Neuropsychological profile of adult patients with Niemann-Pick C1 (NPC1) mutations. J Inherit Metab Dis. 2007;30(1):60–7. doi: 10.1007/s10545-006-0417-6. [DOI] [PubMed] [Google Scholar]
- 3.Yanjanin NM, Velez JI, Gropman A, King K, Bianconi SE, Conley SK et al. Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):132–40. doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iturriaga C, Pineda M, Fernandez-Valero E, Vanier M, Coll M. Niemann–Pick C disease in Spain: clinical spectrum and development of a disability scale. Journal of the neurological sciences. 2006;249(1):1–6. [DOI] [PubMed] [Google Scholar]
- 5.Stampfer M, Theiss S, Amraoui Y, Jiang X, Keller S, Ory DS et al. Niemann-Pick disease type C clinical database: cognitive and coordination deficits are early disease indicators. Orphanet journal of rare diseases. 2013;8(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo F, Erickson RP, Garver WS, Hossain GS, Carbone PN, Heidenreich RA et al. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life sciences. 2001;70(2):131–42. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Li H, Repa JJ, Turley SD, Dietschy JM. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. Journal of lipid research. 2008;49(3):663–9. doi: 10.1194/jlr.M700525-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci U S A. 2009;106(7):2377–82. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K et al. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PloS one. 2009;4(9):e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vite CH, Bagel JH, Swain GP, Prociuk M, Sikora TU, Stein VM et al. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease. Sci Transl Med. 2015;7(276):276ra26. doi: 10.1126/scitranslmed.3010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L et al. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet. 2017;390(10104):1758–68. doi: 10.1016/s0140-6736(17)31465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wechsler D Wechsler Preschool and Primary Scale of Inteligence, Third Edition. San Antonio, TX: Pearson; 2002. [Google Scholar]
- 13.Wechsler D Wechsler Intelligence Scale for Children, Fourth Edition. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 14.Wechsler D Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- 15.Mullen EM, editor. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 16.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales, Second Edition. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- 17.Sheslow D, Adams W. Wide range assessment of memory and learning second edition administration and technical manual. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 18.Hooper EH. Hooper visual organization test (VOT). Western Psychological Services; 1983. [Google Scholar]
- 19.Beery KE, Beery NA. Beery VMI.: The Beery-Buktenica Developmental Test of Visual-motor Integration with Supplemental Developmental Tests of Visual Perception and Motor Coordination: And, Stepping Stones Age Norms from Birth to Age Six. Administration, Scoring, and Teaching Manual. PsychCorp; 2010. [Google Scholar]
- 20.Instrument L Purdue pegboard test: user instructions. Lafayette, IN: Lafayette Instrument; 2002. [Google Scholar]
- 21.McNeish DM, Stapleton LM. The effect of small sample size on two-level model estimates: A review and illustration. Educational Psychology Review. 2016;28(2):295–314. [Google Scholar]
- 22.Kenward MG, Roger JH. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Computational Statistics & Data Analysis. 2009;53(7):2583–95. [Google Scholar]
- 23.Wasserstein RL, Schirm AL, Lazar NA. Moving to a World Beyond “p < 0.05”. The American Statistician. 2019;73(sup1):1–19. doi: 10.1080/00031305.2019.1583913. [DOI] [Google Scholar]
- 24.Thurm A, Farmer C, Farhat NY, Wiggs E, Black D, Porter FD. Cohort study of neurocognitive functioning and adaptive behaviour in children and adolescents with Niemann-Pick Disease type C1. Developmental Medicine & Child Neurology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.