Abstract
Background
Postoperative hypoxemia (POH) is common and primarily treated with temporary oxygen supplementation. Because the clinical impact of postoperative hypoxemia is sometimes presumed as minor, efforts to better understand and minimize it have been limited. Here, we hypothesized that, after adjusting for opioids received perioperatively and other confounders, the frequency of POH events reported within the first 3 postoperative days (POD) is associated with increased postoperative 1-year mortality.
Methods
With prior IRB approval, the Epic Clarity database was queried for all adult inpatient anesthesia encounters performed at our health system (1 academic and 2 community hospitals) from 1/1/2012 to 3/31/2016. Patients with multiple hospitalizations or subsequent surgeries within the same hospitalization were excluded. We classified patients based on the presence (POH) or not (No-POH) of ≥1 documented peripheral saturation of oxyhemoglobin (SpO2) ≤85% event of any duration occurring between the discharge from the post-anesthesia care unit until POD 3. Demographics, comorbidities, surgery duration, morphine milligram equivalents administered perioperatively, respiratory therapies, intensive care unit (ICU) admission and hospital length of stay were also collected. Logistic regression was used to characterize the association between POH and 1-year postoperative mortality after adjusting for perioperatively administered opioids and other confounding factors.
Results
A total of 43,011 patients met study criteria. At least one POH event was reported in 10,727 (24.9%) patients. Of these, 7,179 (66.9%) had ≥1 hypoxemic event on POD1, 5,340 (49.8%) on POD2 and 3,455 (32.3%) on POD3. Patients with ≥1 POH event, compared to No-POH patients, were older, had more respiratory and other comorbidities, underwent longer surgeries, received greater opioid doses on the day of surgery and POD1 and received more continuous pulse oximetry monitoring. POH patients required more frequent postoperative oxygen therapy, noninvasive ventilation, reintubation, and ICU admission. One-year postoperative mortality occurred in 4.4% of patients with ≥1 POH and 3.0% of No-POH patients (p<0.001). After adjusting for confounding factors, for every 10% increase in the frequency of SpO2≤85% readings, the odds of postoperative 1- year mortality were 1.20 (95%CI: 1.11–1.29, p<0.001). Perioperative opioids were not independently associated with increased 1-year mortality.
Conclusions
After adjusting for perioperative opioids and other confounders, moderate/severe POH within the first 3 postoperative days was independently associated with increased 1-year postoperative mortality. Increased efforts should be directed to understand if efforts to detect and reduce postoperative hypoxemia lead to improved patient outcomes.
Introduction
Periods of postoperative hypoxemia (POH) are common after surgery, with a frequency ranging from 5% to 65.5%1–6 depending on the surgical population involved, definition of hypoxemia (severity and duration) and monitoring methods (intermittent or manual vs. continuous or automated pulse oximetry). Accurate characterization of the frequency, duration and severity of POH requires continuous pulse oximetry7, but routine postoperative care relies primarily on intermittent visual assessments of ventilation and manual checks of peripheral saturation of oxyhemoglobin (SpO2). Due to practical challenges in accurate characterization and reporting, most POH events are underdiagnosed in frequency but also severity and duration2,8. In a study with simultaneous monitoring methods, sporadic SpO2 manual assessments missed 90% of episodes of SpO2 <90% for >1h detected with continuous pulse oximetry2. The clinical impact of POH may also be underappreciated. Although it has been associated with increased risk of postoperative cardiac ischemia9 and cognitive dysfunction10, most POH episodes are resolved with temporary oxygen supplementation. However, the use of prolonged (>1 day) postoperative oxygen therapy has been associated with prolonged hospital stay, admission to the intensive care unit (ICU) and increased need of resources during and after hospital discharge1,11,12. The relationship of POH to 1-year postoperative mortality is unknown, but this information would reinforce the need to better characterize the etiology and reduce POH.
Episodes of POH are multifactorial, but are often related to perioperatively administered opioids6,13,14. Interestingly, a recent study found no significant increase in opioid-induced hypoxemia in patients using long-acting opioids versus short-acting opioids for patient-controlled-analgesia14. Nonetheless, there are ongoing efforts to limit opioid administration perioperatively, also with the goal of reducing chronification of opioid use15. Enhanced-Recovery After Surgery (ERAS) protocols, usually build on opioid-minimizing strategies aimed at reducing adverse opioid-related side effects16. However, the relationship between perioperative opioid use, POH events, and their relationship with postoperative mortality remains poorly understood.17
Here, we hypothesized that independently of opioids received perioperatively and other confounding factors, moderate/severe POH (SpO2≤85%) documented after discharge from the PACU and within the first 3 postoperative days (POD 1–3) would be associated with increased 1-year postoperative mortality. Such an association would justify increased efforts to understand, prevent and reduce POH events that currently are often viewed as having little relevance. We tested our hypothesis in a retrospective analysis of our health system clinical data warehouse, which included one academic and two community hospitals.
Methods
We performed a retrospective database study after approval by the Colorado Multiple Institutional Review Board (COMIRB #09–0674). The requirement for informed consent was waived by the IRB.
Study design
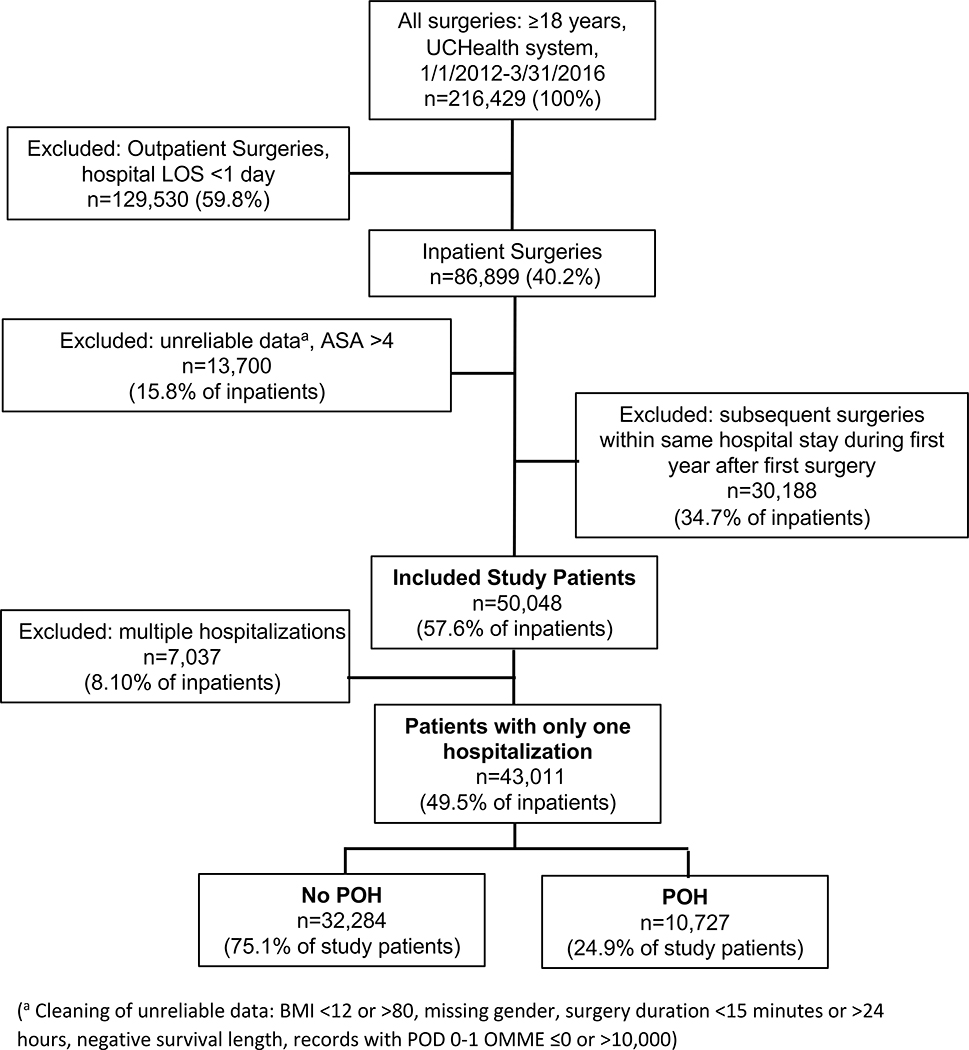
This study was designed following the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guideline. The primary and secondary outcomes were defined and established a priori at initiation of the study design. We queried the Epic Clarity electronic medical record (EMR) database at the University of Colorado Health (UCHealth) system for all adults undergoing inpatient anesthesia care in one of its hospitals between 1/1/2012 to 3/31/2016. During this period, UCHealth was composed of a large academic tertiary medical center and two community hospitals. Patients were included if they fulfilled the following criteria: ≥18 years and receiving anesthesia care for any surgical procedure. Exclusion criteria included: subsequent procedures for each patient within a year from the first date of surgery, outpatient procedures, organ donations, missing procedure type, procedure duration <15 minutes or >24 hours, patients with Body Mass Index (BMI) <12 or >80, American Society of Anesthesiologists (ASA) physical status 5–6 or missing. Only patients with one hospitalization over the study period were included to avoid the confounding effect of patients with worse systemic health or staged procedures. Figure 1 shows the study schematic flowchart.
Figure 1.
Study schematic flowchart.
Data collected
We extracted demographic information, comorbid conditions, surgery duration, oral morphine milligram equivalents (OMME) administered on the date of surgery and daily on postoperative days 1–3 (POD1–3). The OMME dose on the date of surgery was chosen as a covariate as it likely also reflects the degree of procedure complexity and surgical trauma inflicted. We noted the documentation of any POH event, defined as any episode of SpO2≤85% of any duration and cause obtained with continuous or intermittent pulse oximetry from discharge from the post-anesthesia care unit (PACU) until POD 3. All postoperative SpO2 values in our electronic medical record system are either automatically transferred to the medical chart (continuous pulse oximetry) or entered and validated by the patient’s nursing staff (for intermittent pulse oximetry). The specific method of SpO2 monitoring is not recorded in the EMR. Therefore, we assumed pulse oximetry was intermittent if SpO2 measurements documented in the EMR daily were ≤8 versus continuous if >8. We expressed the frequency of POH events (POH%) as follows:
POH% = (Number of SpO2≤85% measurements POD 1–3) / (Total number of SpO2 measurements POD 1–3)
We also collected the presence postoperatively of the following respiratory interventions: supplemental oxygen therapy, noninvasive ventilation (NIV, including auto-titrated, bi-level or continuous positive airway pressure) and reintubation with mechanical ventilation. Postoperative transfer to the ICU, either planned (if occurring immediately after surgery) or unplanned (if happening ≥12h after discharge from PACU) and the hospital length of stay were also recorded. Mortality was extracted from the EMR.
Patient classification
Patients were classified into 2 groups as presenting ≥1 POH event, defined as the presence in the patient’s chart of SpO2≤85% of any duration after discharge from the PACU until POD 3 (POH) or not presenting any POH (No-POH). This classification occurred independently of cause, any concurrent oxygen supplementation or other respiratory therapies.
Endpoints
The primary outcome was mortality within 1 year after the date of surgery.
Statistical analysis
Data were inspected for outliers, missing data, and data entry errors using graphical and univariate analyses. Missing data and outlier data values were individually revised for completion, correction or left as missing data points. Cases with insufficient data for Body Mass Index (BMI) calculation, BMI values <12 or >80, and surgery durations >24h or <15min were excluded. Values of SpO2<50% were assumed unreliable and considered missing and values >100% were coded as 100%. The analyses were restricted to patients with only one record, excluding those with multiple records due to convergence issues with statistical models that account for correlated observations (e.g., mixed effects models and generalized estimates equations).
To examine the association between POH and the primary (1-year postoperative mortality) and secondary outcomes, two-sample t-tests or Chi square tests were used to estimate the unadjusted association for continuous or categorical outcomes, respectively. Multiple logistic regression was used to evaluate the adjusted association of POH and 1-year postoperative mortality, adjusting for OMME received and other variables selected a priori based on clinical relevance.
All analyses were performed using R v3.5.018. Due to the large sample size a conservative level of significance of p<0.010 was used for all statistical tests.
Results
Characteristics
A total of 43,011 patients met study criteria (see Consort study diagram in Figure 1). At least one POH event was observed in 10,727 (24.9%) patients and 32,284 (75.1%) of patients had no POH events reported (No-POH). Table 1 shows that patients in the POH group were older than No-POH patients and had a greater systemic comorbidity load with the exception of similar smoking history and neurological disease, underwent longer surgeries performed more likely at the academic center, required higher OMMEs on date of surgery and POD1, and received more often continuous SpO2 monitoring. Opioids received on POD2 and POD3 should be interpreted cautiously due to the high presence of missing data and variability in daily doses on those days. Several of these differences (e.g. age, BMI, asthma) were statistically significant despite a small difference between the groups due to the large sample size.
Table 1.
Characteristics of patients presenting at least one or none moderate/severe postoperative hypoxemic (POH) events. Values are shown as mean (SD) or N (% of respective group) unless otherwise indicated.
| Total | No POH | POH | P value | |
|---|---|---|---|---|
| (N=43,011) | (N=32,284) | (N=10,727) | ||
| Demographics | ||||
| Age, years | 58.0 (17.0) | 57.2 (17.3) | 60.5 (15.7) | <0.001 |
| Male Gender | 20,321 (47.2%) | 15,531 (48.1%) | 4,790 (44.7%) | <0.001 |
| BMI, kg/m2 | 28.6 (7.1) | 28.3 (6.9) | 29.5 (7.7) | <0.001 |
| ASA Physical Status: | <0.001 | |||
| 1 | 1,778 (4.1%) | 1,482 (4.6%) | 296 (2.8%) | |
| 2 | 17,243 (40.1%) | 13,930 (43.1%) | 3,313 (30.9%) | |
| 3 | 18,820 (43.8%) | 13,399 (41.5%) | 5,421 (50.5%) | |
| 4 | 5,170 (12.0%) | 3,473 (10.8%) | 1,697 (15.8%) | |
| Type of medical center | ||||
| Academic Center | 20,228 (47.0%) | 13,771 (42.7%) | 6,457 (60.2%) | <0.001 |
| Community Centers | 22,783 (53.0%) | 18,513 (57.3%) | 4,270 (39.8%) | |
| Preoperative Comorbidities | ||||
| Asthma | 5,004 (11.6%) | 3,619 (11.2%) | 1,385 (12.9%) | <0.001 |
| COPD | 3,852 (9.0%) | 2,556 (7.9%) | 1,296 (12.1%) | <0.001 |
| OSA | 6,707 (15.6%) | 4,602 (14.3%) | 2,105 (19.6%) | <0.001 |
| Other Respiratory Diseases | 5,473 (12.7%) | 3,665 (11.4%) | 1,808 (16.9%) | <0.001 |
| Smoker | 5,187 (12.1%) | 3,937 (12.2%) | 1,250 (11.7%) | 0.140 |
| Hypertension | 20,715 (48.2%) | 14,819 (45.9%) | 5,896 (55.0%) | <0.001 |
| Cardiac Disease (excl. hypertension) | 9,725 (22.6%) | 6,991 (21.7%) | 2,734 (25.5%) | <0.001 |
| Neurological Disease | 9,856 (22.9%) | 7,457 (23.1%) | 2,399 (22.4%) | 0.120 |
| Renal Disease | 6,259 (14.6%) | 4,432 (13.7%) | 1,827 (17.0%) | <0.001 |
| Liver Disease | 2,862 (6.7%) | 2,024 (6.3%) | 838 (7.8%) | <0.001 |
| Obesity (BMI >30 kg/m2) | 14,990 (34.9%) | 10,700 (33.1%) | 4,290 (40.0%) | <0.001 |
| Diabetes | 7,463 (17.4%) | 5,209 (16.1%) | 2,254 (21.0%) | <0.001 |
| Other Endocrine Diseases | 10,688 (24.8%) | 7,515 (23.3%) | 3,173 (29.6%) | <0.001 |
| Surgery Duration, minutes | 181 (124.4) | 168 (118.6) | 220 (132.9) | <0.001 |
| Postoperative continuous SpO2 monitoring | 22,066 (51.3%) | 12,589 (39.0%) | 9,477 (88.3%) | <0.001 |
| Opioid requirements, OMME * | ||||
| Day of Surgery | 181 (242.2) | 173 (238.3) | 203 (252.3) | <0.001 |
| POD 1 | 47.7 (69.0) | 45.0 (67.9) | 55.8 (71.8) | <0.001 |
| POD 2 | 41.7 (66.9)10,712 | 39.2 (65.2)10,161 | 47.2 (70.1)551 | <0.001 |
| POD 3 | 38.4 (66.6)20.837 | 36.0 (65.7)18,266 | 42.7 (68.1)2,571 | <0.001 |
(Opioid requirement on Day of Surgery used as surrogate for surgery complexity and related-trauma; Opioid Requirement on Postoperative Days 2 and 3 are presented but were excluded from the analysis due to high proportion of null data and variability between patients; Superscripts indicate the number of missing values for POD 2 or POD 3. These missing values are related, in part, to patients being discharged from the hospital)
(ASA=American Society of Anesthesiologists Physical Status classification; BMI=Body Mass Index; COPD=Chronic Obstructive Pulmonary Disease; CPAP=Continuous Positive Airway Pressure; DOS=Date of Service; OMME=Oral Morphine Milligram Equivalents; OSA=Obstructive Sleep Apnea, including formally diagnosed (based on existing diagnosis and/or positive airway pressure, PAP, prescription in patient’s chart, independently of therapy compliance) and suspected (presence of ≥3 positive STOP-BANG risk factors on the anesthesia preoperative interview on the DOS); POD=Postoperative Day; POH= Postoperative Hypoxemia; SpO2=peripheral saturation of oxyhemoglobin)
Postoperative hypoxemia, 1-year postoperative mortality and other clinical outcomes, unadjusted
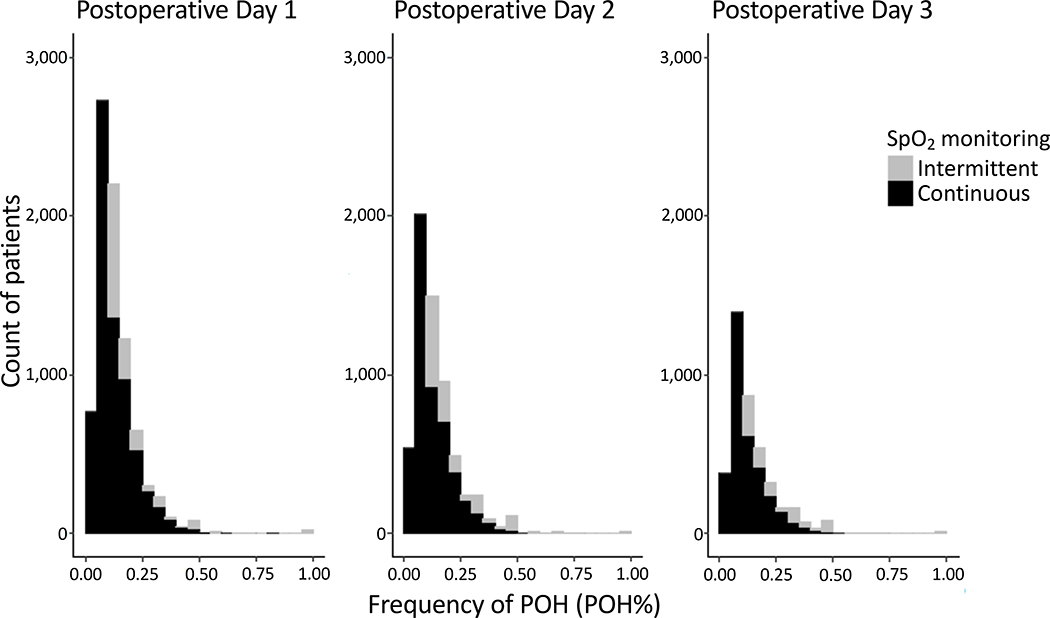
The POH event(s) occurred primarily on POD1 (in 7,179 (66.9%) of POH patients), progressively decreasing on POD2 (5,340 (49.8%) of POH patients) and POD3 (3,455 (32.2%) of POH patients) (Table 2). As expected, patients with ≥1 POH event on POD1–3 required significantly more respiratory interventions, including oxygen supplementation, NIV, reintubation, as well as greater admission to ICU and longer hospital length of stay (Table 1). Mortality within 1 year of surgery was greater in patients with ≥1 POH event compared to No-POH patients (4.4% vs. 3.0%, respectively) (Table 2). Figure 2 describes the distribution of POH% at each POD 1–3 for both SpO2 monitoring methods.
Table 2.
Clinical outcomes of patients presenting moderate/severe postoperative hypoxemic (POH) events. Values are shown as mean (SD) or N (% of group) unless otherwise indicated.
| Total | No POH | POH | P value | |
|---|---|---|---|---|
| (N=43,011) | (N=32,284) | (N=10,727) | ||
| Postoperative In-hospital Course | ||||
| Patients With At Least One Hypoxemic Event (SpO2 ≤85%, any duration): | ||||
| PACU (not considered POH) | 1,585 (3.7%) | 1,018 (3.2%) | 567 (5.3%) | <0.001 |
| POD 1 | 7,179 (16.7%) | 0 (0.0%) | 7,179 (66.9%) | <0.001 |
| POD 2 | 5,340 (12.4%) | 0 (0.0%) | 5,340 (49.8%) | <0.001 |
| POD 3 | 3,455 (8.0%) | 0 (0.0%) | 3,455 (32.2%) | <0.001 |
| Any POH event on POD 1, 2, or 3 | 10,727 (24.9%) | 0 (0.0%) | 10,727 (100.0%) | |
| % of Hypoxemic Events (POH%) * | ||||
| POD 1 | 2.4 (6.7) | 0 (0.0) | 9.5 (10.6) | <0.001 |
| POD 2 | 1.9 (6.3) | 0 (0.0) | 7.5 (10.8) | <0.001 |
| POD 3 | 1.2 (5.3) | 0 (0.0) | 4.9 (9.8) | <0.001 |
| Postoperative O2 Therapy Duration, days | 3.7 (5.4) | 3.1 (5.3) | 5.3 (5.5) | <0.001 |
| Postoperative NIV (CPAP, BiPAP, APAP) | 5,905 (13.7%) | 3,783 (11.7%) | 2,122 (19.8%) | <0.001 |
| Postoperative Reintubation | 1,535 (3.6%) | 1,025 (3.2%) | 510 (4.8%) | <0.001 |
| ICU Admission: | ||||
| Planned (direct transfer after surgery) | 4,447 (10.3%) | 2,849 (8.8%) | 1,598 (14.9%) | <0.001 |
| Unplanned (>12h after PACU discharge) | 926 (2.2%) | 500 (1.5%) | 426 (4.0%) | <0.001 |
| Hospital Length of Stay, days | 4.9 (5.8) | 4.5 (5.7) | 6.1 (5.9) | <0.001 |
| Death within 1 year from DOS | 1,426 (3.3%) | 958 (3.0%) | 468 (4.4%) | <0.001 |
The frequency of POH events is expressed as percentage of hypoxemia events out of the total SpO2 measurements per day for POD 1–3.
(APAP=Auto-titrating Positive Airway Pressure; BiPAP= Bi-level Positive Airway Pressure; CPAP=Continuous Positive Airway Pressure; DOS=Date of Service; ICU=Intensive Care Unit; NIV=Non-Invasive Ventilation, including CPAP, APAP or BiPAP; PACU=Post-Anesthesia Care Unit; POD=Postoperative Day; POH= Postoperative Hypoxemia, SpO2=Peripheral Saturation of Oxyhemoglobin by pulse oximetry)
Figure 2. Histograms of frequency of postoperative hypoxemic (POH) events (POH%) for postoperative days 1–3 for both monitoring methods of peripheral saturation of oxyhemoglobin (SpO2).
Only patients with POH included. Stacked histograms show the non-overlapping number of patients with POH events in 5% intervals as detected by continuous (black bars) and intermittent (gray bars) SpO2 monitoring. Note, for intermittently (≤8 measurements/day) monitored patients, one hypoxemic event in 24h would correspond to POH% 12.5%, and hence not reflected in the first two columns (intervals 0.00–0.10).
Factors associated with 1-year postoperative mortality, logistic regression results
Mortality within 1 year from date of surgery was significantly associated with the frequency of POH events (POH%) after adjusting for confounders (Table 3). After adjusting for other confounding factors, for every 10% increase in the frequency of SpO2≤85% measurements, the odds of 1-year postoperative mortality were 1.20 (95%CI: 1.11–1.29) (p<0.001). The variables with the strongest association with 1-year postoperative mortality were the ASA physical status 4 and 3, and reintubation (Table 3). Other variables independently associated with mortality were, in decreasing order of their OR: unplanned ICU admission, postoperative continuous SpO2 monitoring, liver disease, renal disease, other respiratory diseases, diabetes, POH%, male gender, and older age (Table 3). Pre-existing hypertension and the BMI were inversely associated with 1-year postoperative mortality. Interestingly, OMME on date of surgery or POD1, cardiac disease or hospital length of stay were not significantly associated with postoperative mortality. Results of alternative logistic regression models using different definitions of POH (including mild hypoxemia with POH defined as SpO2<90%, and severe hypoxemia only with POH defined as SpO2<80%), as well the effect of accumulated POD on which POH events occurred are shown in the Supplement.
Table 3.
Logistic regression model results including the frequency of postoperative hypoxemic (POH) events obtained during POD 1–3 and death within 1 year from day of surgery as the outcome.
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| % of POH (in 10% increments) * | 1.20 | (1.11,1.29) | <0.001 |
| Age, years | 1.02 | (1.02,1.03) | <0.001 |
| Male Gender | 1.20 | (1.07,1.35) | 0.002 |
| BMI | 0.96 | (0.95,0.97) | <0.001 |
| ASA 2 (vs. 1) | 3.14 | (1.00,9.91) | 0.051 |
| ASA 3 (vs. 1) | 12.51 | (4.00,39.17) | <0.001 |
| ASA 4 (vs. 1) | 24.59 | (7.81,77.43) | <0.001 |
| Academic Center (vs. Community Centers) | 1.21 | (1.07,1.38) | 0.003 |
| Asthma | 0.77 | (0.63,0.95) | 0.016 |
| COPD | 0.97 | (0.82,1.15) | 0.722 |
| OSA | 0.78 | (0.64,0.95) | 0.014 |
| Other Respiratory Diseases | 1.48 | (1.28,1.71) | <0.001 |
| Hypertension | 0.75 | (0.66,0.86) | <0.001 |
| Cardiac Disease (excl. hypertension) | 1.05 | (0.92,1.19) | 0.455 |
| Renal Disease | 1.71 | (1.51,1.95) | <0.001 |
| Liver Disease | 1.86 | (1.57,2.19) | <0.001 |
| Diabetes | 1.31 | (1.14,1.49) | <0.001 |
| Other Endocrine Disease | 0.95 | (0.82,1.10) | 0.518 |
| Surgery Duration, mins | 0.998 | (0.997,0.998) | <0.001 |
| DOS OMME | 1.00 | (1.00,1.00) | 0.731 |
| POD 1 OMME | 0.999 | (0.9975,0.99996) | 0.006 |
| Postoperative continuous SpO2 monitoring | 1.33 | (1.16,1.53) | <0.001 |
| Postoperative NIV (CPAP, BiPAP, APAP) | 0.92 | (0.77,1.09) | 0.328 |
| Postoperative Reintubation | 3.62 | (2.96,4.41) | <0.001 |
| Planned ICU admission | 0.91 | (0.77,1.08) | 0.297 |
| Unplanned ICU admission | 2.75 | (2.22,3.41) | <0.001 |
| Hospital Length of Stay, days | 1.01 | (1.01,1.02) | <0.001 |
Interpretation: For each 10% increase in POH events out of the total measured, the OR for 1-year mortality is 1.2 (increases by 20%)
(APAP=Auto-titrating Positive Airway Pressure; ASA=American Society of Anesthesiologists Physical Status classification; BiPAP= Bi-level Positive Airway Pressure; BMI=Body Mass Index; COPD=Chronic Obstructive Pulmonary Disease; CPAP=Continuous Positive Airway Pressure; DOS=Date of Service; ICU=Intensive Care Unit; NIV=Non-Invasive Ventilation, including CPAP, APAP or BiPAP; OMME=Oral Morphine Milligram Equivalents; OSA=Obstructive Sleep Apnea; PACU=Post-Anesthesia Care Unit; POD=Postoperative Day; POH= Postoperative Hypoxemia; SpO2=Peripheral Saturation of Oxyhemoglobin by pulse oximetry)
Discussion
This system-wide retrospective study evaluated if moderate/severe POH is associated with increased 1-year postoperative mortality independently of perioperatively-administered opioids and other confounders. After inpatient surgeries performed at one large academic and two community centers, the frequency of SpO2≤85% measurements of any duration and cause observed within the first 3 postoperative days was associated with increased 1-year postoperative mortality. Although perioperative opioid equivalents were higher in patients with ≥1 POH event, they were not independently associated with 1-year postoperative mortality.
We observed ≥1 POH event in approximately 25% of patients. The POH frequency described in the literature varies widely1–6: from 5% with intermittent SpO2 measurements after non-cardiac surgery2 to 65.5% when arterial blood gas analyses were used after cardiac surgery5. Within our health system and including both intermittent and continuous pulse oximetry, we have previously reported that 27–40% of all surgical (cardiac and non-cardiac) inpatients presenting ≥1 SpO2≤85% event after discharge from PACU4. Patients with multiple hospitalizations were included in that study and POH events throughout the whole hospital stay were included, which may explain the greater POH incidence observed. To minimize potential selection bias of patients with more complex health conditions or surgical interventions associated with a greater risk for mortality, we excluded patients with multiple hospitalizations in this study. We used routine postoperative SpO2 monitoring and a threshold of moderate/severe hypoxemia (SpO2≤85%). The incidence of POH at the academic center was greater than the observed at community centers, which could be related, in part, to the greater complexity of surgeries performed as reflected by longer duration and higher opioid doses administered on the date of surgery (data not shown).
Hypoxemia of different degrees and at diverse time points after surgery has been associated with increased risk of cardiopulmonary complications, confusion, prolonged hospital LOS, and discharge to a nursing facility and healthcare costs1,9,10,12. In our study, for every 10% increase in the frequency of SpO2≤85% measurements of any duration and cause observed within the first 3 postoperative days, the odds of 1-year postoperative mortality increased by 20%. To our knowledge, the association between POH and 1-year postoperative mortality has not been evaluated. The retrospective study design precludes any causal conclusions on the nature of this association. However, several pathophysiologic mechanisms triggered by intermittent hypoxia could contribute: activation of the sympathetic system, oxidative stress and inflammation, impaired immune responses, altered glucose and lipid metabolism19–21 and other biological processes22–25.
We pre-selected covariates for the logistic regression that are known to contribute directly or indirectly to POH and/or postoperative mortality, including the perioperatively-administered opioid doses. Perioperative opioids have received attention for their contribution to POH6,12,13. However, the relationship between the type and dose of perioperative opioids on POH is still not completely understood14,26. In this study, POH patients received greater opioid daily doses than No-POH patients (Table 1). The incidence of POH was greater on POD1 (∼67% of POH patients, Table 2), which is similar to the timing observed in other studies. In a case-control study in adults after major inpatient surgery, approximately 77.4% of opioid-induced respiratory depression episodes requiring reversal with naloxone occurred during the first 24h after surgery27. Nonetheless, opioid doses on the date of surgery or POD1 were not associated with increased 1-year postoperative mortality (Table 3). Interestingly, attempts to limit perioperative opioids have reduced respiratory failure yet failed to decrease mortality28.
Our logistic regression results highlight other variables that are associated with increased 1-year postoperative mortality (Table 3). However, most are non-modifiable variables: older age, male gender, respiratory comorbidities and/or overall systemic disease burden. Continuous SpO2 monitoring, more often implemented in patients at high risk for POH, was the only modifiable variable independently associated with 1-year postoperative mortality. As expected, reintubation and unplanned ICU admission were associated with increased mortality. POH will often precede reintubation and unplanned ICU admission. However, our logistic model suggests that POH is associated with 1-year mortality even after adjusting for the presence of continuous SpO2 monitoring, reintubation and/or unplanned ICU admission.
This study presents several limitations to the generalizability of our results, primarily related to its database retrospective nature and the challenge of the accurate assessment of hypoxemia. First and foremost, the accurate characterization of POH is limited in a retrospective study. Continuous pulse oximetry or blood gas analyses would be required to provide details of duration and severity of POH. Even POH events from patients receiving intermittent SpO2 measurements were possibly underestimated. A study in 16 ICU patients at high risk for hypoxemia found that the reported manual SpO2 values were an average 6.5% (95% CI, 4.0–9.0%) greater than simultaneously automated ones8. Similarly, our postoperative mortality was restricted to deaths reported to the EMR and may also be underestimated29. To increase the likelihood of detecting reported deaths after discharge, we obtained the mortality data from the data warehouse >3 years after the last surgical procedure. Other limitations to the generalizability of our results may arise from our exclusion criteria: We excluded events of mild hypoxemia (SpO2=86–89%), with less likely negative impact, particularly if brief. In fact, when mild POH events were included in the analyses, POH was no longer associated with 1-year mortality (Supplement). In addition, we excluded patients with multiple surgical procedures and/or hospitalizations, which likely constitute patients with more severe systemic disease and/or more complex procedures, and thus may be at increased risk of both POH events and postoperative mortality. Previous studies have suggested risk factors for POH (e.g. age >50 years, BMI >30 kg/m2, respiratory comorbidities, SpO2≤96% before or immediately after surgery, but not the type or duration of surgery)26. The purpose of this study was to determine if POH, once occurred, is independently associated with postoperative mortality without establishing any cause-effect relationships.
In conclusion, in this large database study we found that moderate/severe POH of any duration within the first 3 postoperative days is independently associated with increased 1-year postoperative mortality. Perioperative opioid doses were not independently associated with 1-year postoperative mortality. Increased efforts should be directed to understand the etiology and ultimately reduce episodes of postoperative hypoxemia.
Supplementary Material
Key Points Summary.
Question
Is the frequency of moderate/severe postoperative hypoxemic events (SpO2≤85%) within the first 3 postoperative days associated with increased 1-year postoperative mortality after adjusting for opioids received perioperatively and other confounding factors?
Findings
After adjusting for confounders, the OR for 1-year mortality is 1.2 (increases by 20%) with each 10% increase in the frequency of POH events.
Meaning
Future research should aim to characterize postoperative hypoxemia and to assess if interventions to minimize it can improve patient safety and reduce major morbidity and mortality after surgery.
Acknowledgments
Disclosure of Funding: This study was supported by a Seed Grant by the University of Colorado School of Medicine, Department of Anesthesiology (AFB) and the National Institutes of Health / National Institute on Drug Abuse, Award Number K23DA040923 (KB). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary of Terms
- ASA physical status
American Society of Anesthesiologists physical status
- BMI
Body Mass Index
- EMR
electronic medical record
- ERAS
Enhanced-Recovery After Surgery
- ICU
intensive care unit
- LOS
length of stay
- NIV
noninvasive ventilation
- OR
odds ratio
- OMME
oral morphine milligram equivalents
- PACU
post-anesthesia care unit
- POD
postoperative day
- POH
postoperative hypoxemia
- SpO2
peripheral saturation of oxyhemoglobin
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- UCHealth
University of Colorado Health
Footnotes
Conflicts of Interest: None
Clinical trial number: Not applicable.
Contributor Information
Karsten Bartels, Departments of Anesthesiology, Medicine, and Surgery, University of Colorado School of Medicine.
Alexander Kaizer, Department of Biostatistics, University of Colorado School of Medicine.
Leslie Jameson, Department of Anesthesiology, University of Colorado School of Medicine.
Kenneth Bullard, Department of Anesthesiology, University of Colorado School of Medicine.
Colleen Dingman, Department of Anesthesiology, University of Colorado School of Medicine.
Ana Fernandez-Bustamante, Department of Anesthesiology, University of Colorado School of Medicine.
References
- 1.Ramachandran SK, Thompson A, Pandit JJ, Devine S, Shanks AM. Retrospective observational evaluation of postoperative oxygen saturation levels and associated postoperative respiratory complications and hospital resource utilization. PloS one. 2017;12(5):e0175408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Z, Sessler DI, Dalton JE, et al. Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth Analg. 2015;121(3):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge H, Jiang Y, Jin Q, Wan L, Qian X, Zhang Z. Nomogram for the prediction of postoperative hypoxemia in patients with acute aortic dissection. BMC Anesthesiology. 2018;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Bustamante A, Bartels K, Clavijo C, et al. Preoperatively Screened Obstructive Sleep Apnea Is Associated With Worse Postoperative Outcomes Than Previously Diagnosed Obstructive Sleep Apnea. Anesth Analg. 2017;125(2):593–602. [DOI] [PubMed] [Google Scholar]
- 5.Sutton AD, Bailey M, Bellomo R, Eastwood GM, Pilcher DV. The association between early arterial oxygenation in the ICU and mortality following cardiac surgery. Anaesth Intensive Care. 2014;42(6):730–735. [DOI] [PubMed] [Google Scholar]
- 6.Siriussawakul A, Mandee S, Thonsontia J, Vitayaburananont P, Areewatana S, Laonarinthawoot J. Obesity, epidural analgesia, and subcostal incision are risk factors for postoperative desaturation. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2010;57(5):415–422. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen T, Nicholson A, Hovhannisyan K, Moller AM, Smith AF, Lewis SR. Pulse oximetry for perioperative monitoring. The Cochrane database of systematic reviews. 2014(3):CD002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taenzer AH, Pyke J, Herrick MD, Dodds TM, McGrath SP. A comparison of oxygen saturation data in inpatients with low oxygen saturation using automated continuous monitoring and intermittent manual data charting. Anesth Analg. 2014;118(2):326–331. [DOI] [PubMed] [Google Scholar]
- 9.Gill NP, Wright B, Reilly CS. Relationship between hypoxaemic and cardiac ischaemic events in the perioperative period. British journal of anaesthesia. 1992;68:471–473. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg J, Kehlet H. Postoperative mental confusion--association with postoperative hypoxemia. Surgery. 1993;114(1):76–81. [PubMed] [Google Scholar]
- 11.Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostin P, Teja BJ, Friedrich S, et al. The association of early postoperative desaturation in the operating theatre with hospital discharge to a skilled nursing or long-term care facility. Anaesthesia. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg J, Oturai P, Erichsen CJ, Pedersen MH, Kehlet H. Effect of general anesthesia and major versus minor surgery on late postoperative episodic and constant hypoxemia. J Clin Anesth. 1994;6(3):212–216. [DOI] [PubMed] [Google Scholar]
- 14.Belcher AW, Khanna AK, Leung S, et al. Long-Acting Patient-Controlled Opioids Are Not Associated With More Postoperative Hypoxemia Than Short-Acting Patient-Controlled Opioids After Noncardiac Surgery: A Cohort Analysis. Anesth Analg. 2016;123(6):1471–1479. [DOI] [PubMed] [Google Scholar]
- 15.Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery - A retrospective cohort study. Drug Alcohol Depend. 2018;187:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott MJ, McEvoy MD, Gordon DB, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) Joint Consensus Statement on Optimal Analgesia within an Enhanced Recovery Pathway for Colorectal Surgery: Part 2-From PACU to the Transition Home. Perioper Med (Lond). 2017;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopf HW. Preventing Opioid-Induced Postoperative Hypoxemia: No Simple Answer? Anesth Analg. 2016;123(6):1356–1358. [DOI] [PubMed] [Google Scholar]
- 18.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. Published 2018. Accessed. [Google Scholar]
- 19.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147(1):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snow JB, Kitzis V, Norton CE, et al. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. Journal of applied physiology. 2008;104(1):110–118. [DOI] [PubMed] [Google Scholar]
- 21.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best practice & research Clinical endocrinology & metabolism. 2010;24(5):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almendros I, Gozal D. Intermittent hypoxia and cancer: Undesirable bed partners? Respir Physiol Neurobiol. 2018;256:79–86. [DOI] [PubMed] [Google Scholar]
- 23.Nanduri J, Semenza GL, Prabhakar NR. Epigenetic changes by DNA methylation in chronic and intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2017;313(6):L1096–L1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiernan EA, Smith SM, Mitchell GS, Watters JJ. Mechanisms of microglial activation in models of inflammation and hypoxia: Implications for chronic intermittent hypoxia. J Physiol. 2016;594(6):1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang LP, Chen NH, Lin Y, Ko WS, Pang JS. Increased MCP-1 gene expression in monocytes of severe OSA patients and under intermittent hypoxia. Sleep Breath. 2015. [DOI] [PubMed] [Google Scholar]
- 26.Kaushal A, Goyal P, Dhiraaj S, Agarwal A, Singh PK. Identification of Various Perioperative Risk Factors Responsible for Development of Postoperative Hypoxaemia. Turk J Anaesthesiol Reanim. 2018;46(6):416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190(5):752–756. [DOI] [PubMed] [Google Scholar]
- 28.Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276–1282. [DOI] [PubMed] [Google Scholar]
- 29.Jones B, Vawdrey DK. Measuring mortality information in clinical data warehouses. AMIA Jt Summits Transl Sci Proc. 2015;2015:450–455. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.