Abstract
Our previous study found that hydrogen gas (H2) could efficiently inhibit lung cancer progression; however, the underlying mechanisms still remains to be elucidated. The present study aimed to explore the roles of H2 in lung cancer cell autophagy, and reveal the effects of autophagy on H2-mediated lung cancer cell apoptosis and the underlying mechanisms. The expression levels of proteins associated with cell apoptosis and autophagy were detected using western blot analysis. Cell autophagy was inhibited by 3-methyladenine treatment or Beclin1 downregulation, while rapamycin was used to induce autophagy. Cell growth and apoptosis were detected using the Cell Counting Kit-8 and flow cytometry assays, respectively. The results demonstrated that cell apoptosis and autophagy were significantly enhanced in the A549 and H1975 lung cancer cell lines treated with H2. However, autophagy enhancement weakened H2 roles in promoting cell apoptosis and vice versa. In addition, it was found that H2 treatment induced marked decreases in the protein expression levels of phosphorylated STAT3 and Bcl2, and overexpression of STAT3 abolished H2 roles in promoting cell apoptosis and autophagy. Overall, the present study revealed that H2 can promote lung cancer cell apoptosis and autophagy via inhibiting the activation of STAT3/Bcl2 signaling and suppression of autophagy can enhance H2 roles in promoting lung cancer cell apoptosis.
Keywords: hydrogen gas, cell apoptosis, cell autophagy, STAT/Bcl2 signaling
Introduction
Hydrogen gas (H2), as a type of endogenous gas, has been demonstrated to not only serve as a crucial energy source, but also to exert crucial roles in physiological regulation (1). Hydrogen molecules can enter tissues and exert anti-inflammatory, antioxidant and anti-apoptotic roles (2). Notably, H2 shows high safety and efficacy to patients in a clinical setting (3). Intravenous administration of 500 ml H2 significantly improved the erythema and associated symptoms in 4 patients with acute erythematous skin diseases, with no changes in the physiological parameters, such as body temperature, blood pressure and pulse rate, or deterioration of liver and kidney function. In addition, two volunteers (one for intravenous H2 administration and the other for H2 inhalation) demonstrated no discomfort (3). Recently, H2 has been also applied in a clinical setting to improve the hearing loss induced by radiotherapy in patients with nasopharyngeal cancer (ClinicalTrials.gov identifier, NCT03818347) (4). In that study, H2 (67% hydrogen and 33% oxygen) was generated using the hydrogen-oxygen nebulizer through the electrolysis of water and the results demonstrated that hydrogen-oxygen therapy could markedly improve binaural hearing (4). Another Phase 1 trial (ClinicalTrials.gov identifier, NCT04046211) aims to explore the safety of H2 on healthy adult volunteers. In the study, 8 volunteers were included, 2 of which were exposed to 2.4% H2 in medical air for 24 h, 2 who were exposed to the same gas for 48 h and the other 4 patients exposed to the same gas for 72 h (https://clinicaltrials.gov/ct2/show/NCT04046211). In addition, Dole et al (5) proposed in 1975, for the first time, that H2 has anti-cancer potential. Similarly, our research group previously found that H2 administration significantly represses cell growth, migration and invasion capacities and induced cell apoptosis in lung cancer A549 and H1975 cells (6). However, the molecular mechanisms remain largely unknown.
Autophagy and apoptosis are indispensable biological processes and serve crucial roles in individual development (7). Autophagy, also known as macroautophagy, is a ‘self-eating’ process, which engulfs cytoplasmic proteins, complexes or organelles into the autophagosome (a cytoplasmic double membrane structure), leading to degradation and recycling (8). Autophagy is modulated by autophagy-related genes (ATGs) through two evolutionarily conserved ubiquitin-like conjugation systems, known as the ATG12-ATG5 and the ATG8 light chain 3 (LC3)-phosphatidylethanolamine (PE) systems. Microtubule-associated protein 1A/1B LC3BI is conjugated with PE to become LC3BII, which associates with the outer and inner membranes of the autophagosome (9). It is known that autophagy maintains cellular homeostasis and protects against various diseases, including cancer (10–12). However, its role in carcinogenesis is controversial. Accumulating evidence has found that autophagy to be a ‘double-edge sword’ in the progression of cancer (13,14).
Signal transducer and activator of transcription (STAT) 3 is a latent transcription factor which modulates extracellular signals by interacting with polypeptide receptors (15). STAT3 protein becomes transcriptionally activated primarily through tyrosine phosphorylation, which then dimerizes, translocates to the nucleus and binds to sequence-specific DNA elements, leading to the transcription of target genes (16). STAT3 has been found to serve as an oncogene and frequently hyper-activated in a number of types of cancer, including lung cancer, contributing to cancer cell survival, proliferation, metastasis and angiogenesis (17,18). In addition, STAT3 serves a vital role in the process of autophagy (19). For example, Guo et al (20) reported that repression of STAT3 via its inhibitor, isocryptotanshinone enhances autophagy in the A549 lung cancer cell line.
The present study aimed to investigate the effects of H2 on autophagy and apoptosis in lung cancer cells, and the role of STAT3 in this process. The results demonstrated that H2 could promote lung cancer cell apoptosis and autophagy by inhibiting the activation of the STAT3/Bcl2 signaling pathway and suppression of autophagy could enhance H2 roles in promoting lung cancer cell apoptosis.
Materials and methods
Cell lines and culture
The human A549 and H1975 lung cancer cell lines, were purchased from American Type Culture Collection. The A549 cell line was cultured in F-12K medium, supplemented with 10% fetal bovine serum (FBS), while the H1975 cell line was cultured in RPMI-1640 medium containing 10% FBS (all from Gibco; Thermo Fisher Scientific, Inc.). The two cell lines were maintained in an incubator at 37°C with 5% CO2.
Cell transfection and treatments
Three short interfering (si)RNAs used to silence Beclin1 (si-Beclin1-1/2-3; cat. no. SR322490) in the A549 and H1975 cells, and the negative control vector (si-NC, cat. no. SR322490) were purchased from OriGene Technologies, Inc.. The overexpressing plasmid STAT3 (OE-STAT3, cat. no. SC124165) and OE-NC (cat. no. SC124165) were also obtained from OriGene Technologies, Inc.. Cell transfection was performed using the transfection reagent Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols with 2 µg OE-STAT3/OE-NC or 0.3 µg si-Beclin1/si-NC for each well of a 6-well plate. Following 48 h of transfection, cells were harvested for analysis. The siRNA sequences were as follow: si-NC, Forward: 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse: 5′-ACGUGACACGUUCGGAGAATT-3′; si-Beclin1-1, forward: 5′-CUGGACACGAGUUUCAAGATT-3′ and reverse: 5′-UCUUGAAACUCGUGUCCAGTT-3′; si-Beclin1-2, forward: 5′-GUGGAAUGGAAUGAGAUUATT-3′ and reverse: 5′-UAAUCUCAUUCCAUUCCACTT-3′; si-Beclin1-3, forward: 5′-GCUGCCGUUAUACUGUUCUTT-3′ and reverse: 5′-AGAACAGUAUAACGGCAGCTT-3′.
For H2 treatment, A549 and H1975 cells were cultured in different concentrations of H2 (20, 40 and 60%) with the assistance of Shanghai Nanobubble Technology Co., Ltd. (http://www.nanobubble.cn/p-about.html?app=mb) for different time points (12, 24, 36, 48 or 72 h), and 5% CO2 served as the negative control.
A549 and H1975 cells were treated with 2 mM 3-methyladenine (3-MA) or 100 µM rapamycin (RAPA) for 48 h to repress and induce autophagy, respectively.
Western blot analysis
Total protein was extracted from the cells using the RIPA lysis buffer (Sangon Biotech Co., Ltd.), containing protease inhibitor (Beyotime Institute of Biotechnology) and according to the manufacturer's instructions. After quantification with a bicinchoninic acid protein kit (Bio-Rad Laboratories, Inc.), 30 µg protein from each sample was loaded per lane and separated using 10% SDS-PAGE. Then, the proteins were transferred onto polyvinylidene difluoride membranes (EMD Millipore), and blocked with 5% skimmed milk at room temperature for 1 h, following which the membranes were incubated with the following primary antibodies, overnight at 4°C: Cleaved-caspase 3 (1:1,000 dilution; cat. no. ab2302; Abcam), cleaved poly ADP-ribose polymerase (PARP; 1:1,000 dilution; cat. no. ab32064; Abcam), Beclin1 (1:2,000 dilution; cat. no. ab207612; Abcam), p62 (1:1,000 dilution; cat. no. ab56416; Abcam), LC3B (1:3,000 dilution; cat. no. ab51520; Abcam), Bcl2 (1:1,000 dilution; cat. no. 15071; Cell Signaling Technology, Inc.), STAT3 (1:1,000 dilution; cat. no. 9139; Cell Signaling Technology, Inc.), phosphorylated (p)-STAT3 (1:1,000 dilution; cat. no. 9145; Cell Signaling Technology, Inc.) and β-actin (1:4,000 dilution; cat. no. 3700; Cell Signaling Technology, Inc.). After the incubation with the corresponding secondary antibodies (Santa Cruz Biotechnology, Inc.), the protein expression levels were detected using a western blot imaging and quantitative system (Bio-Rad Laboratories, Inc.). Protein quantification was performed using the ImageJ software (version 1.48; National Institutes of Health) after background subtraction, with β-actin as an internal reference.
Cell counting Kit-8 assay (CCK-8)
A CCK-8 assay was used to determine cell proliferation ability according to the manufacturer's instructions. In brief, A549 and H1975 cells were cultured in 96-well plates at a density of 2×103 cells/well, overnight at 37°C. Then, the cells were treated with different concentrations of H2 for 12, 24, 36, 48 or 72 h. Following which, the cell culture medium was replaced with 10 µl CCK-8 reagent (Beyotime Institute of Biotechnology) and 90 µl fresh medium, and incubated at 37°C for another 4 h. The absorbance at 450 nm was determined using a plate reader (model 680; Bio-Rad Laboratories, Inc.).
Flow cytometry assay
Cell apoptosis was determined using flow cytometry and an Annexin V-FITC/propidium iodide (PI) kit (Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's protocols. Briefly, A549 and H1975 cells were harvested through centrifugation at 110 × g for 5 min at 4°C and washed once with PBS following 48 h of H2 treatment and/or cell transfection. Then, the cells were incubated with Annexin V-FITC and PI solution for 15 min in the dark, at room temperature. The fluorescent signals were evaluated using flow cytometry (BD Biosciences) within 1 h of staining using Flowjo version 7.6 software (FlowJo LLC). Cells in the FITC−/PI− quadrant represented live cells, while FITC+/PI− represented early apoptotic cells and FITC+/PI+ represented late apoptotic cells.
Statistical analysis
Each experiment was performed in triplicate. Data are presented the mean ± standard deviation. Statistically significant comparisons between two groups and multiple groups were performed using unpaired Student's t-test and one-way ANOVA, followed by Dunnett's or Tukey's post hoc test. Data analysis was performed using the SPSS software (version 23.0, IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
H2 treatment induces significant increases in cell apoptosis and autophagy
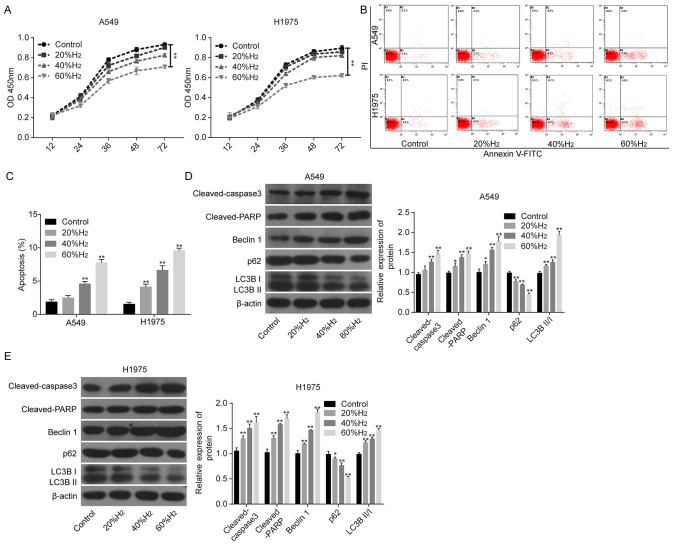
The present study first investigated the effects of H2 treatment on the apoptosis and autophagy of lung cancer cells. Compared with that in the control group, cell viability was reduced following treatment with H2, in a time-dependent manner, in both A549 and H1975 cells (Fig. 1A), along with increased apoptosis rates (Fig. 1B and C). The expression levels of pro-apoptotic proteins, including cleaved-caspase 3 and cleaved-PARP were significantly increased followed H2 treatment compared with that in the control group (Fig. 1D and E), in a dose-dependent manner, in both cell lines. In addition, Beclin1 expression and the ratio of LC3BII/I were also significantly increased following H2 treatment, while the expression of p62 was significantly reduced (Fig. 1D and E), also in a dose-dependent manner. These findings demonstrated that H2 could induce cell apoptosis and autophagy in lung cancer cell lines.
Figure 1.
H2 treatment enhances lung cancer cell apoptosis and autophagy. After A549 and H1975 cells were treated with 20, 40 or 60% H2, the following assays were performed. (A) Cell Counting Kit-8 assay was used to investigate cell growth. (B and C) Flow cytometry assay was used to detect cell apoptosis. (D and E) The protein levels of cleaved-caspase 3, cleaved-PARP, p62, Beclin1, LC3BII and LC3BI were detected using western blot analysis. *P<0.05 and **P<0.01 vs. control. PARP, poly ADP-ribose polymerase; LC3, light chain 3; H2, hydrogen gas.
Autophagy weakens the role of H2 in promoting cell apoptosis in lung cancer cells
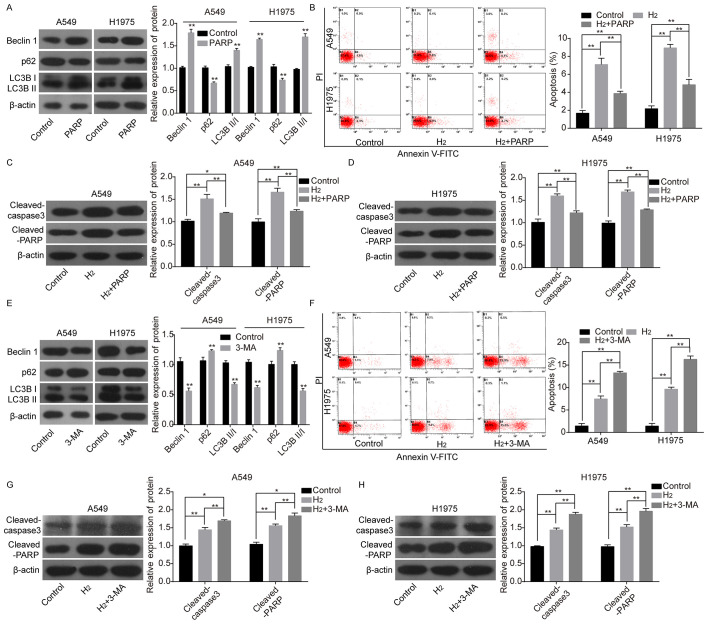
The autophagy roles in H2-induced cell apoptosis in lung cancer cells were subsequently investigated. The protein expression levels of Beclin1 and LC3BII/I were significantly increased, while that of p62 was reduced when A549 and H1975 cells were treated with RAPA, an autophagy inducer (Fig. 2A). As 60% H2 resulted in significant changes in cell viability and apoptosis, 60% H2 was selected for the subsequent experiments. Following RAPA treatment, cell apoptosis rates (Fig. 2B) and the protein expression levels of cleaved-caspase 3 and cleaved-PARP (Fig. 2C and D) were significantly decreased compared with that in the H2 group. By contrast, the protein expression levels of Beclin1 and LC3BII/I were significantly decreased and p62 expression was increased following cell treatment with 3-MA, an autophagy repressor (Fig. 2E). In addition, 3-MA treatment enhanced H2-mediated increases in cell apoptosis rates (Fig. 2F) and the protein expression levels of cleaved-caspase 3 and cleaved-PARP as compared with the H2 group (Fig. 2G and H). These results revealed that autophagy weakened H2 roles in inducing cell apoptosis in lung cancer cells.
Figure 2.
Evaluation of autophagy effects on H2-induced lung cancer cell apoptosis. (A) The protein expression levels of p62, Beclin1, LC3BII and LC3BI, following treatment of A549 and H1975 cells with RAPA were detected using western blot analysis. (B) Cell apoptosis was investigated using a flow cytometry assay. Western blot analysis of the expression of cleaved-caspase 3 and cleaved-PARP in (C) A549 and (D) H1975 cells treated with H2 or H2+RAPA. (E) The protein expression levels of p62, Beclin1, LC3BII and LC3BI were detected using western blot analysis following treatment of A549 and H1975 cells with 3-MA. (F) Cell apoptosis was investigated using flow cytometry. Western blot analysis of the protein expression levels of cleaved-caspase 3 and cleaved-PARP following treatment of (G) A549 and (H) H1975 cells with H2 or H2+3-MA. *P<0.05. **P<0.01. LC3, light chain 3; RAPA, rapamycin; PARP, poly ADP-ribose polymerase; PI, propidium iodide; 3-MA, 3-methyladenine; H2, hydrogen gas.
Knockdown of Beclin1 enhances the roles of H2 in promoting cell apoptosis in lung cancer cell lines
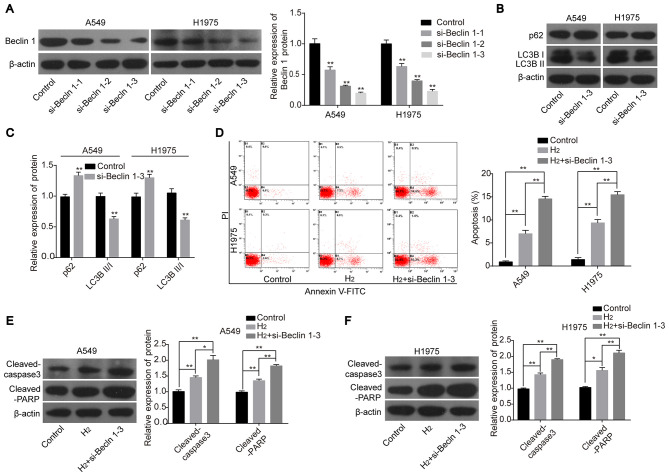
To further clarify the role of autophagy in H2-mediated lung cancer cell apoptosis, knockdown experiments were performed. The expression of Beclin1 was significantly decreased in A549 and H1975 cells transfected with the siRNAs targeting the human Beclin1 gene. As si-Beclin1-3 significantly reduced the protein expression level of Beclin1 among the 3 siRNAs (Fig. 3A), this was chosen for the subsequent experiments. Following cell transfection with si-Beclin1-3, the protein expression level of p62 was increased and the expression ratio of LC3BII:LC3BI was decreased (Fig. 3B and C). Notably, cell apoptosis induced by H2 treatment was significantly increased when Beclin1 was silenced in both A549 and H1975 cells as compared with the H2 group (Fig. 3D). In addition, knockdown of Beclin1 increased the protein expression levels of cleaved-caspase 3 and cleaved-PARP in A549 and H1975 cells following H2 treatment compared with cells treated with H2 only (Fig. 3E and F). These findings further confirmed that autophagy inhibition could enhance the effects of H2 on inducing cell apoptosis in lung cancer cell lines.
Figure 3.
Knockdown of Beclin1 enhances the role of H2 in promoting lung cancer cell apoptosis. (A) The knockdown efficiency of si-Beclin1 in A549 and H1975 cells was detected using western blot analysis. (B) Western blot analysis was used to detect the protein expression levels of p62, LC3BII and LC3BI in A549 and H1975 cells and the results were subsequently (C) quantified. (D) Flow cytometry assay was used to investigate cell apoptosis. The protein expression levels of cleaved-caspase 3 and cleaved-PARP were determined using western blot analysis in (E) A549 and (F) H1975 cells. *P<0.05 and **P<0.01. si, short interfering; LC3, light chain 3; PARP, poly ADP-ribose polymerase; PI, propidium iodide; H2, hydrogen gas.
H2 treatment induces lung cancer cell apoptosis and autophagy by repressing the STAT3/Bcl2 signaling pathway
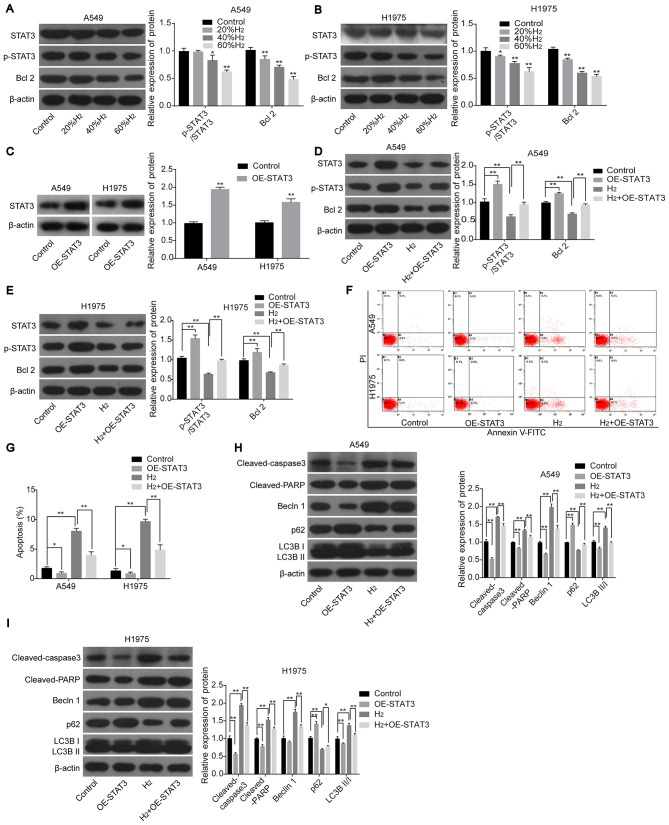
Next, the roles of the STAT3/Bcl2 signaling pathway in H2-mediated cell apoptosis and autophagy in lung cancer cell lines were investigated. The protein expression levels of p-STAT3 and Bcl2 were significantly decreased following H2 treatment in H1975 and A549 cells in a dose-dependent manner (Fig. 4A and B), whereas STAT3-overexpression abolished this effect (Fig. 4C-E). In addition, overexpression of STAT3 in H2-treated cells inhibited lung cancer cell apoptosis and neutralized H2-mediated increases in apoptosis (Fig. 4F and G), along with the decreased protein expression levels of cleaved-caspase 3, cleaved-PARP, Beclin1, LC3BII/I and the increased expression of p62 (all vs. H2 group; Fig. 4H and I). The aforementioned findings suggested that H2 treatment promoted lung cancer cell apoptosis and autophagy by repressing the activation of the STAT3/Bcl2 signaling pathway.
Figure 4.
H2 treatment increases lung cancer cell apoptosis and autophagy by repressing the activation of the STAT3/Bcl2 signaling pathway. The protein expression levels of STAT3, p-STAT3 and Bcl2 were detected using western blot analysis in (A) A549 and (B) H1975 cells following treatment with different concentrations of H2. (C) STAT3 expression was detected using western blot analysis following transfection with OE-STAT3 or OE-NC. The protein expression levels of STAT3, p-STAT3 and Bcl2 were detected using western blot analysis in (D) A549 and (E) H1975 cells following treatment with H2 and/or transfected with OE-STAT3. Cell apoptosis was investigated using (F) flow cytometry and the results were subsequently (G) quantified. The protein expression levels of cleaved-caspase 3, cleaved-PARP, Beclin1, p62, LC3BII and LC3BI in (H) A549 and (I) H1975 cells were detected using western blot analysis following transfection with OE-STAT3 and/or treated with H2. *P<0.05 and **P<0.01. STAT, signal transducer and activator of transcription; p-, phosphorylated; PARP, poly ADP-ribose polymerase; LC3, light chain 3; H2, hydrogen gas; OE, overexpression; NC, negative control
Discussion
As a colorless, odorless, tasteless, non-toxic and highly combustible gas, H2 exerts multiple roles, including anti-reactive oxygen species, anti-inflammation and antitumor (21). Increasing evidence has revealed that H2 not only improved the side effects induced by chemotherapeutics, but also inhibited cancer growth (22–24). In addition, Chen et al (25) reported that H2 treatment reduced the metastases size in the abdominal cavity, improved anemia and hypoalbuminemia and gradually returned the lymphocyte and tumor marker levels, including CA19-9, α fetoprotein and carcinoembryonic antigen to normal. In our previous study, it was found that H2 treatment effectively inhibited lung cancer cell growth and induced cell apoptosis by targeting the structural maintenance of chromosomes 3 gene in a dose-dependent manner, with no side effects on the non-tumor burdened mice (6). The current study demonstrated that H2 treatment repressed cell growth and increased cell apoptosis by inhibiting the STAT3/Bcl2 signaling pathway in A549 and H1975 lung cancer cell lines.
Autophagy and apoptosis, which lead to the degradation of proteins and organelles or cell death upon cellular stress, serve vital roles in the progression of lung cancer (26). Previous studies indicated that autophagy serves as a ‘double-edged sword’ in lung cancer cell apoptosis (26). Typically, autophagy repression decreases the cell's ability to overcome stress and maintain homeostasis (27). However, several studies have reported that autophagy induced cell apoptosis (28–30). Autophagy exerts an oncogenic or a tumor suppressive role in carcinogenesis (31). In lung cancer, inhibition of autophagy enhances the anti-angiogenic property of anlotinib (32), suggesting an angiogenic role of autophagy in lung cancer. Yin et al (33) reported that inhibition of autophagy induced by mucolipin-1 (which is a member of the transient receptor potential cation channel family) downregulation repressed the progression of lung cancer. Li et al (34), demonstrated that autophagy enhancement caused by apurinic endonuclease 1 significantly increased the resistance of lung cancer cells to cisplatin. These findings suggested that autophagy may exert an oncogenic role in lung cancer progression. By contrast, Fan et al (35) reported that Bruceine D, a quassinoid compound, which can be extracted from the seeds of Brucea javanica, inhibits lung cancer progression by inducing cell apoptosis and autophagy, suggesting a suppressive role of autophagy in lung cancer progression. These findings indicate that autophagy serves an important role in lung cancer progression, therefore autophagy plays a role in H2-induced cell apoptosis in lung cancer cell lines.
When autophagy is induced, Beclin1 and LC3 are distributed to the autophagosome membrane to trigger the formation of autophagosome, whereas p62 expression is decreased (36,37). The transformation of LC3I to LC3BII is the most common autophagosome marker, as the amount of LC3BII reflects the number of autophagosomes and autophagy-related structures (38). The present study found that H2 treatment significantly increased the protein expression level of Beclin1, and the conversion of LC3BI to LC3BII, and decreased the expression level of p62, indicating that H2 treatment could trigger lung cancer cell autophagy. Several studies have investigated the role of H2 treatment in autophagy. In detail, Guan et al (39) reported that H2 treatment induced autophagy and protected Sprague-Dawley rats against chronic intermittent hypoxia-induced renal dysfunction. In addition, inhaling 2% H2 resulted in an increase in the expression of LC3BII and attenuated sepsis-induced liver injury (40). However, in a myocardial ischemia reperfusion model of rats, H2 inhalation significantly reduced the ischemic size and serum troponin I level by repressing autophagy (41). The effects might be tissue specific.
In addition, the present study also investigated the role of autophagy in H2-mediated cell apoptosis. The results demonstrated that autophagy enhancement by RAPA stimulation significantly weakened H2-mediated cell apoptosis in both A549 and H1975 lung cancer cell lines, whereas autophagy inhibition by 3-MA treatment or siRNA-Beclin1 transfection significantly enhanced the roles of H2 in promoting lung cancer cell apoptosis, indicating that autophagy inhibition could enhance the antitumor role of H2 in lung cancer.
As the STAT3/Bcl2 signaling pathway serves an important role in cell autophagy and apoptosis (42,43), the present study also investigated the effects of the STAT3/Bcl2 signaling pathway on H2-mediated lung cancer cell autophagy and apoptosis. It was found that the phosphorylation protein expression levels of STAT3 and Bcl2 were significantly inhibited when A549 and H1975 cells were treated with H2, suggesting that H2 could repress the activation of the STAT3/Bcl2 signaling pathway. Consistent with this result, Bai et al (44) found that hydrogen-rich saline treatment increased LC3B and Beclin1 protein expression levels and decreased the protein phosphorylation level of STAT3 in hypoxic-ischemic brain damaged mice. To further investigate the roles of STAT3/Bcl2 in H2-mediated cell apoptosis and autophagy in lung cancer, rescue experiments were also performed in the present study. The results demonstrated that overexpression of STAT3 neutralized the H2 roles in increasing cell autophagy and apoptosis, suggesting that H2 induced lung cancer apoptosis and autophagy by inhibiting the STAT3/Bcl2 signaling pathway.
In summary, the present study revealed that H2 could promote lung cancer cell apoptosis and autophagy by inhibiting the STAT3/Bcl2 signaling pathway and that suppression of autophagy could enhance the roles of H2 in promoting lung cancer cell apoptosis. The current study might provide a novel application of H2 in the treatment of lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Excellent Personnel Training Program (Studying of the Molecular Mechanism and Clinical Transformation of Hydrogen in the Treatment of Lung Cancer; grant no. zh2018006).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
GC conceived and designed the project. LL performed the majority of the experiments and wrote the paper. ZY analyzed the data. YW and JM performed parts of the experiments. All authors approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhao P, Jin Z, Chen Q, Yang T, Chen D, Meng J, Lu X, Gu Z, He Q. Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 2018;9:4241. doi: 10.1038/s41467-018-06630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 3.Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Nakazawa J, Kaneko K, Nakao A. Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med Gas Res. 2012;2:14. doi: 10.1186/2045-9912-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Kong X, Mu F, Lu T, Du D, Xu K. Hydrogen-oxygen therapy can alleviate radiotherapy-induced hearing loss in patients with nasopharyngeal cancer. Ann Palliat Med. 2019;8:746–751. doi: 10.21037/apm.2019.11.18. [DOI] [PubMed] [Google Scholar]
- 5.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science. 1975;190:152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 2018;104:788–797. doi: 10.1016/j.biopha.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Gordy C, He YW. The crosstalk between autophagy and apoptosis: Where does this lead? Protein Cell. 2012;3:17–27. doi: 10.1007/s13238-011-1127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotthibundhu A, McDonagh K, von Kriegsheim A, Garcia-Munoz A, Klawiter A, Thompson K, Chauhan KD, Krawczyk J, McInerney V, Dockery P, et al. Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res Ther. 2016;7:166. doi: 10.1186/s13287-016-0425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B. Cell biology: Autophagy and cancer. Nature. 2007;446:745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–880. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasik AM, Grabarek J, Pantovic A, Cieślar-Pobuda A, Asgari HR, Bundgaard-Nielsen C, Rafat M, Dixon IM, Ghavami S, Łos MJ. Reprogramming and carcinogenesis-parallels and distinctions. Int Rev Cell Mol Biol. 2014;308:167–203. doi: 10.1016/B978-0-12-800097-7.00005-1. [DOI] [PubMed] [Google Scholar]
- 14.Sridhar S, Botbol Y, Macian F, Cuervo AM. Autophagy and disease: Always two sides to a problem. J Pathol. 2012;226:255–273. doi: 10.1002/path.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy DE, Darnell JE., Jr Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 17.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 18.Tong M, Wang J, Jiang N, Pan H, Li D. Correlation between p-STAT3 overexpression and prognosis in lung cancer: A systematic review and meta-analysis. PLoS One. 2017;12:e0182282. doi: 10.1371/journal.pone.0182282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You L, Wang Z, Li H, Shou J, Jing Z, Xie J, Sui X, Pan H, Han W. The role of STAT3 in autophagy. Autophagy. 2015;11:729–739. doi: 10.1080/15548627.2015.1017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S, Luo W, Liu L, Pang X, Zhu H, Liu A, Lu J, Ma DL, Leung CH, Wang Y, Chen X. Isocryptotanshinone, a STAT3 inhibitor, induces apoptosis and pro-death autophagy in A549 lung cancer cells. J Drug Target. 2016;24:934–942. doi: 10.3109/1061186X.2016.1157882. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Liao R, Sheng X, Luo X, Zhang X, Wen X, Zhou J, Peng K. Hydrogen gas in cancer treatment. Front Oncol. 2019;9:696. doi: 10.3389/fonc.2019.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li FY, Zhu SX, Wang ZP, Wang H, Zhao Y, Chen GP. Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem Toxicol. 2013;61:248–254. doi: 10.1016/j.fct.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Zhou P, Lin B, Wang P, Pan T, Wang S, Chen W, Cheng S, Liu S. The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J Radiat Res. 2019;60:17–22. doi: 10.1093/jrr/rry074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Yuan M, Song J, Chen X, Yang H. Hydrogen gas from inflammation treatment to cancer therapy. ACS Nano. 2019;13:8505–8511. doi: 10.1021/acsnano.9b05124. [DOI] [PubMed] [Google Scholar]
- 25.Chen JB, Pan ZB, Du DM, Qian W, Ma YY, Mu F, Xu KC. Hydrogen gas therapy induced shrinkage of metastatic gallbladder cancer: A case report. World J Clin Cases. 2019;7:2065–2074. doi: 10.12998/wjcc.v7.i15.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR, Cho WC. Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 2017;18:367. doi: 10.3390/ijms18020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang S, Yu ZM, Zhang CY, Zheng J, Li KL, Wu Y, Qian LL, Yang ZY, Li XR, Zhang Y, Wang RX. Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther. 2015;6:247. doi: 10.1186/s13287-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Su L, Xiao Z, Liu X, Liu X. Methyl jasmonate induces apoptosis and pro-apoptotic autophagy via the ROS pathway in human non-small cell lung cancer. Am J Cancer Res. 2016;6:187–199. [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, Wang L, Xia Y, Qiao Y, Sun W, et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2019;38:71. doi: 10.1186/s13046-019-1093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin C, Zhang H, Liu X, Zhang H, Zhang Y, Bai X, Wang L, Li H, Li X, Zhang S, et al. Downregulated MCOLN1 attenuates the progression of non-small-cell lung cancer by inhibiting lysosome-autophagy. Cancer Manag Res. 2019;11:8607–8617. doi: 10.2147/CMAR.S216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Wang Y, Wu L, Dong Y, Zhang J, Chen F, Xie W, Huang J, Lu N. Apurinic endonuclease 1 promotes the cisplatin resistance of lung cancer cells by inducing Parkin-mediated mitophagy. Oncol Rep. 2019;42:2245–2254. doi: 10.3892/or.2019.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan J, Ren D, Wang J, Liu X, Zhang H, Wu M, Yang G. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 2020;11:126. doi: 10.1038/s41419-020-2317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Khattouti A, Selimovic D, Haikel Y, Hassan M. Crosstalk between apoptosis and autophagy: Molecular mechanisms and therapeutic strategies in cancer. J Cell Death. 2013;6:37–55. doi: 10.4137/JCD.S11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorburn A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis. 2008;13:1–9. doi: 10.1007/s10495-007-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta NA, Kolachala VL, Jiang R, Abramowsky C, Shenoi A, Kosters A, Pavuluri H, Anania F, Kirk AD. Mitigation of autophagy ameliorates hepatocellular damage following ischemia-reperfusion injury in murine steatotic liver. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1088–G1099. doi: 10.1152/ajpgi.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan P, Sun ZM, Luo LF, Zhou J, Yang S, Zhao YS, Yu FY, An JR, Wang N, Ji ES. Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019;225:46–54. doi: 10.1016/j.lfs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Yan M, Yu Y, Mao X, Feng J, Wang Y, Chen H, Xie K, Yu Y. Hydrogen gas inhalation attenuates sepsis-induced liver injury in a FUNDC1-dependent manner. Int Immunopharmacol. 2019;71:61–67. doi: 10.1016/j.intimp.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Gao Y, Yang H, Chi J, Xu Q, Zhao L, Yang W, Liu W, Yang W. Hydrogen gas attenuates myocardial ischemia reperfusion injury independent of postconditioning in rats by attenuating endoplasmic reticulum stress-induced autophagy. Cell Physiol Biochem. 2017;43:1503–1514. doi: 10.1159/000481974. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Gong W, Yang ZY, Zhou XS, Gong C, Zhang TR, Wei X, Ma D, Ye F, Gao QL. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 2017;22:544–557. doi: 10.1007/s10495-016-1334-2. [DOI] [PubMed] [Google Scholar]
- 43.Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, Zheng B, Guo W. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8:e3015. doi: 10.1038/cddis.2017.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai X, Liu S, Yuan L, Xie Y, Li T, Wang L, Wang X, Zhang T, Qin S, Song G, et al. Hydrogen-rich saline mediates neuroprotection through the regulation of endoplasmic reticulum stress and autophagy under hypoxia-ischemia neonatal brain injury in mice. Brain Res. 2016;1646:410–417. doi: 10.1016/j.brainres.2016.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.