Abstract
Purpose:
Patterns of resistance to first-line osimertinib are not well-established and have primarily been evaluated using plasma assays which cannot detect histologic transformation and have differential sensitivity for copy number changes and chromosomal rearrangements.
Experimental Design:
To characterize mechanisms of resistance to osimertinib, patients with metastatic EGFR-mutant lung cancers who received osimertinib at Memorial Sloan Kettering and had next-generation sequencing performed on tumor tissue before osimertinib initiation and after progression were identified.
Results:
Among 62 patients who met eligibility critieria, histologic transformation, primarily squamous transformation, was identified in 15% of first-line osimertinib cases and 14% of later-line cases. Nineteen percent (5/27) of patients treated with first-line osimertinib had off-target genetic resistance (2 MET amplification, 1 KRAS mutation, 1 RET fusion, and 1 BRAF fusion) whereas 4% (1/27) had an acquired EGFR mutation (EGFR G724S). Patients with squamous transformation exhibited considerable genomic complexity; acquired PIK3CA mutation, chromosome 3q amplification and FGF amplification were all seen. Patients with transformation had shorter time on osimertinib and shorter survival compared to patients with on-target resistance. Initial EGFR sensitizing mutation, time on osimertinib treatment and line of therapy also influenced resistance mechanism that emerged. The compound mutation EGFR S768 + V769L and the mutation MET H1094Y were identified and validated as resistance mechanisms with potential treatment options.
Conclusion:
Histologic transformation and other off-target molecular alterations are frequent early emerging resistance mechanisms to osimertinib and are associated with poor clinical outcomes.
Keywords: EGFR, osimertinib, TKI, acquired resistance
Introduction:
The identification of EGFR T790M as the dominant mechanism of resistance to first- and second-generation EGFR-TKIs resulted in the development of osimertinib, a third-generation EGFR-TKI.(1,2) Osimertinib’s initial approval was in the setting of progression on initial EGFR-TKI in patients with tumors harboring EGFR T790M.(3) More recently, osimertinib exhibited superior progression-free survival (PFS) compared to erlotinib or gefitinib as initial treatment in patients with EGFR-mutant non-small cell lung cancer, positioning osimertinib as the preferred first-line treatment where available.(4) Nevertheless, acquired resistance to osimertinib invariably develops with a median PFS of 19 months.(4)
Similar to earlier EGFR inhibitors, characterization of the landscape of resistance enables the development of subsequent therapies. Our knowledge about mechanisms of resistance to osimertinib is primarily derived from patients who received osimertinib after other EGFR-TKIs; these patients have pre-existing EGFR T790M which may induce fundamentally distinct resistance mechanisms compared with patients receiving first-line osimertinib.(5–9) In the later-line osimertinib setting, the most commonly reported acquired on-target EGFR mutation is EGFR C797S, with other EGFR mutations such as G724, L792 and L718/G719 also reported.(5,6,8,10–12) MET amplification is the most frequently identified off-target resistance mechanism, though alterations in RET, ALK, BRAF, and FGFR also occur.(5–8,10–15) Small cell and squamous cell histologic transformation have been reported as infrequent mechanisms of resistance.(5,8,16–20)
Analysis of circulating tumor (ct)DNA has been the predominant method for investigating resistance, but cannot detect histologic transformation and has differential sensitivity for copy number changes and chromosomal rearrangements compared to tissue analysis.(5,6,11,12,21–25) Further, published studies have lacked paired tumor samples pre- and post-osimertinib, which makes determination of acquired alterations and putative resistance mechanisms challenging. Therefore, we sought to use paired tumor tissue to detect molecular and histologic mechanisms of resistance to osimertinib and identify potential associations with clinical outcomes.
Patients and Methods
In accordance with the Belmont report and following Institutional Review Board/Privacy Board at Memorial Sloan Kettering (MSK) for retrospective review of records and waiver of consent, we retrospectively identified all patients with EGFR-mutant metastatic lung cancers who received osimertinib and had pretreatment and post-progression tumor samples (acquired resistance defined by Jackman criteria)(26) where targeted hybrid capture, next-generation sequencing (NGS) of tumor DNA had been performed. The primary NGS platform was MSK-IMPACT,(27) but other NGS platforms, such as MSK-Ampliseq (supplementary methods) and Foundation Medicine NGS,(28) were occasionally utilized. For MSK-IMPACT, patients were consented to MSK IRB protocol 12–245. Patients were divided into two cohorts: 1) patients who received osimertinib without prior EGFR-TKI exposure (“first-line” osimertinib), and 2) patients who received osimertinib after prior EGFR-TKIs (“later-line” osimertinib).
Patient records were reviewed to extract demographic information, clinical outcomes, and molecular and histologic data. Time-to-treatment discontinuation (TTD) was defined as time from start of EGFR-TKI to last administered dose prior to a treatment change.(29) Overall survival (OS) was defined as date of osimertinib initiation to date of death or last follow-up as of May 1, 2019. Fisher’s exact and log-rank tests were used to identify associations between clinical, molecular, and histologic features, and Kaplan-Meier methodology was used for TTD and OS. In all cases of transformation, the original pathology samples were re-reviewed to confirm the absence of pre-existing neuroendocrine, squamous/adenosquamous, or small cell histology using immunohistochemistry performed for p40, TTF1, RB1, and p53.
MSK-Fusion Solid, a custom RNAseq panel, was used to detect fusions in cases where no resistance mechanism was identified by NGS and sufficient tissue was available.(30) Single nucleotide variants (SNVs) and copy number variants (CNVs) identified by MSK IMPACT were analyzed on the cBioPortal.(31) Specific mutations were assessed for enrichment with the McNemar test using paired samples and Fisher’s exact test in unpaired analyses. Additional methods are provided in the supplement.
Results
Clinical characteristics
Sixty-two patients were identified with acquired resistance to osimertinib and paired tumor tissue available for analyses. Twenty-seven patients received first-line osimertinib and 35 patients received later-line osimertinib (after prior EGFR-TKI). The clinical characteristics of the two groups are presented in Table 1. The cohorts had similar clinical characteristics with the exception of shorter follow-up on the first-line patients, which reflects the recent approval of osimertinib as a first-line treatment (April 2018). By the data cutoff date (May 1st, 2019), over two thirds of patients treated with first-line osimertinib at MSKCC were still on therapy (32% discontinued first-line osimertinib) whereas the majority of patients treated with later-line osimertinib at MSKCC had discontinued treatment (64% discontinued later-line osimertinib). Median follow-up on for patients who received first-line osimertinib was 17.2 mos (95% CI 12.0 – 24.9 mos) and later-line osimertinib 28.5 mos (95% CI 19.7 – 32.5 mos). Among the patients reported with resistance to first-line osimertinib, the median TTD on osimertinib was 13.6 months (95% CI: 12.4 – 25.5 mo) with 19 (70%) patients alive at data cutoff. The median TTD on osimertinib for the later-line cohort was 15.2 months (95% CI: 13.0 – 19.9 mos) with 25 (71%) patients alive at data cutoff.
Table 1.
Clinical characteristics of patients with acquired resistance to osimertinib by line of therapy
| Clinical characteristics | First-line Osimertinib (%) | Later-line Osimertinib (%) | Total N (%) |
|||
|---|---|---|---|---|---|---|
| Total | 27 | 35 | 62 | |||
| Age | ||||||
| Median (range) | 58 (44–75) | 59 (40–75) | 58 (40–75) | |||
| Sex | ||||||
| Male | 12 (44) | 11 (31) | 23 (37) | |||
| Female | 15 (56) | 24 (69) | 39 (63) | |||
| Smoking | ||||||
| Never-smoker | 17 (63) | 22 (63) | 39 (63) | |||
| Former smoker | 10 (37) | 13 (37) | 23 (37) | |||
| Histology | Before Osi | After Osi | Before Osi | After Osi | Before Osi | After Osi |
| Adenocarcinoma | 24 (89) | 20 (74) | 35 (100) | 28 (80) | 59 (95) | 49 (79) |
| Squamous | 2 (7) | 4 (15) | 0 (0) | 3 (9) | 2 (3) | 6 (10) |
| Neuroendocrine | 0 (0) | 1 (4) | 0 (0) | 2 (6) | 0 (0) | 3 (5) |
| Other | 1 (4) | 2 (7) | 0 (0) | 2 (6) | 1 (2) | 4 (7) |
| EGFR mutation | ||||||
| Exon 19 deletion | 14 (52) | 28 (80) | 42 (68) | |||
| L858R | 11 (41) | 6 (17) | 17 (27) | |||
| T790M | 1 (4) | 32 (91) | 33 (53) | |||
| Other | 2 (7) | 1 (3) | 3 (5) | |||
The demographics, histology, and sensitizing EGFR mutation for the two cohorts and the overall population is shown. Abbreviations: Osi, osimertinib; EGFR, epidermal growth factor receptor.
Concurrent genomic alterations seen with first-line osimertinib
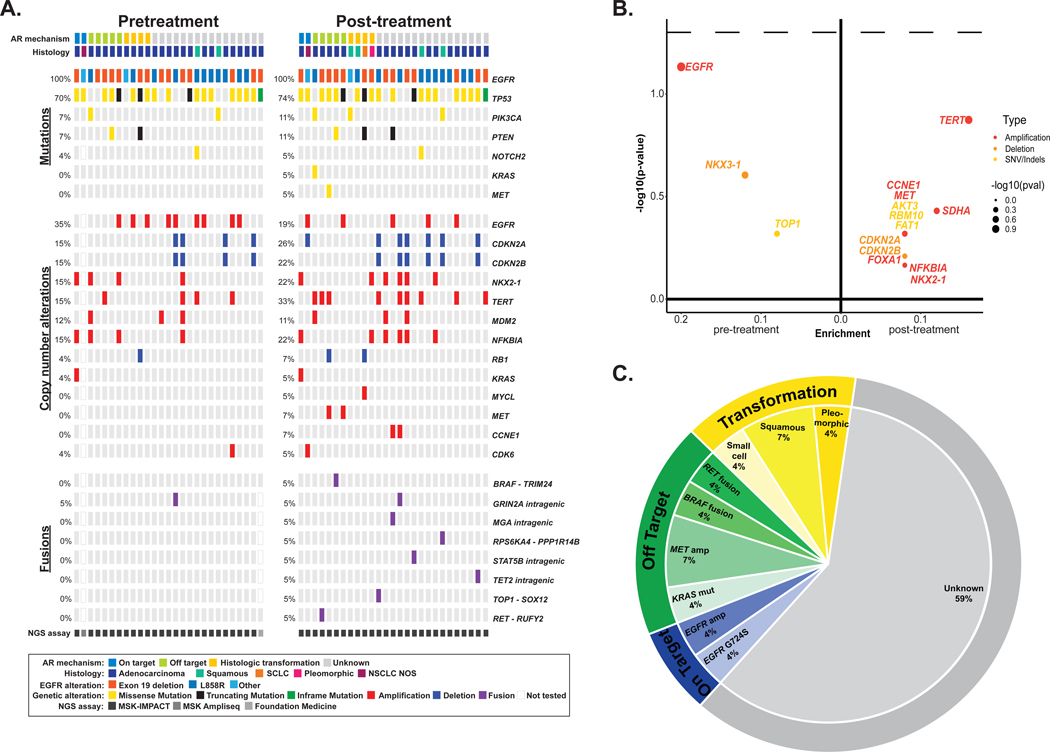
The molecular landscape of concurrent alterations identified before and after treatment with first-line osimertinib is depicted in Figure 1A. The most frequent co-occurring pretreatment mutation was TP53 (70%, n=19). Common co-occuring amplifications/deletions were EGFR amplifications (33%, n=9) and CDKN2A/B deletions (15%, n=4 each). There was no significant enrichment of specific alterations in either the pre-treatment or post-treatment setting (Figure 1B). Known mechanisms of acquired resistance to EGFR-TKIs were identified in 41% (n=11) of cases (Figure 1C). In the first-line setting, EGFR G724S was the only on-target acquired mutation identified. Off-target resistance mechanisms included MET amplifications (Supplemental Table 1), MET H1094Y mutation, KRAS G12A mutation, TRIM24-BRAF fusion, and RUFY2-RET fusion.
Figure 1. Genomic alterations identified with first-line osimertinib.
A, Frequency of alterations pre- and post-osimertinib in patients treated with first-line osimertinib. The in-figure legend specifies details on acquired resistance mechanism, histology, initial EGFR mutation, alteration type, and next-generation sequencing (NGS) assay. B, Enrichment of individual altered genes pre-osimertinib (left) and post-osimertinib (right). Alteration type is indicated in the in-figure legend. The dashed line represents a P-value of 0.05. The frequency difference between the two sample sets is plotted on the x-axis and its significance [-log10(p-value)] on the y-axis. C, The distribution of established mechanisms of resistance by type of alteration in patients treated with first-line osimertinib. SCLC, small-cell lung cancer; NSCLC, non-small cell lung cancer; AR, acquired resistance.
Concurrent genomic alterations seen with later-line osimertinib
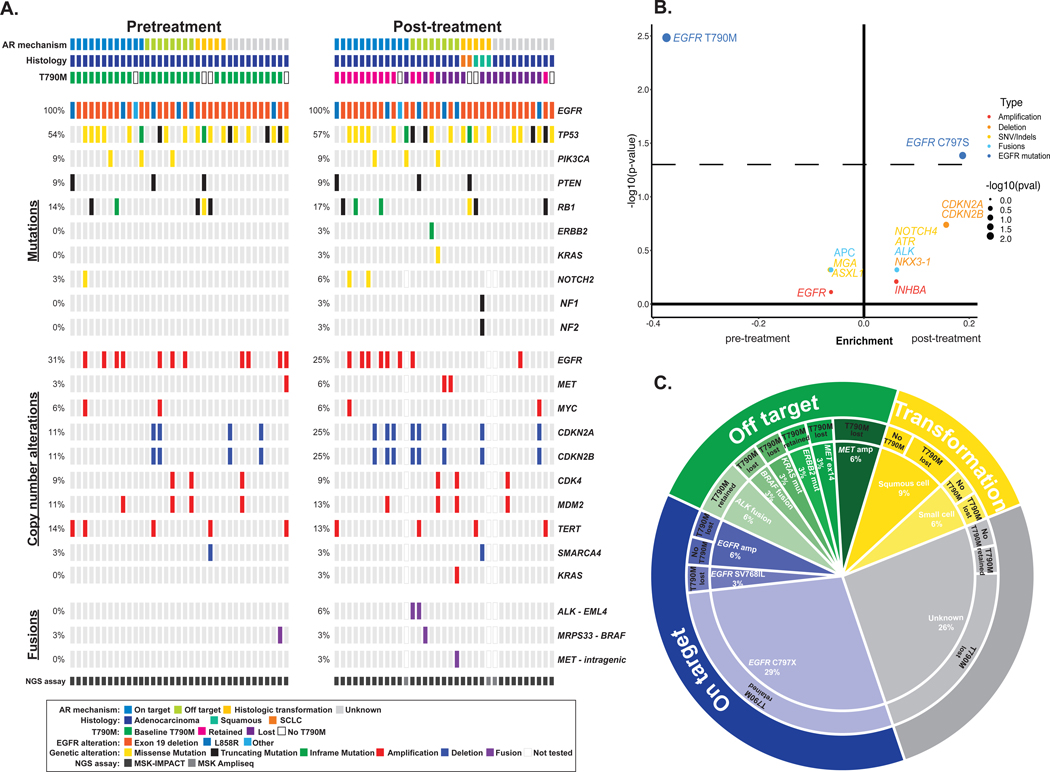
In patients who received later-line osimertinib, the most frequent co-occurring mutations pre-osimertinib were TP53 (54%, n=19), and RB1 (14%, n=5); the most common co-occurring amplification was EGFR (31%, n=11) (Figure 2A). Among the patients that received later-line osimertinib, EGFR T790M (P = 0.003) was enriched in the pre-osimertinib samples and acquisition of EGFR C797S (P = 0.04) was enriched in the post-progression samples (Figure 2B). Established mechanisms of resistance were identified in 71% (25 patients) of cases (Figure 2C). EGFR C797 mutations (C797S = 9 or C797G = 1) were common, occurring in 29% of cases. Off-target resistance mechanisms, including a AGK-BRAF fusion, MET exon 14 alteration, and KRAS G12D mutation, occurred in cells lacking EGFR T790M, while EGFR T790M was retained with ALK fusions and an ERBB2 Y772_A775dup.
Figure 2. Genomic alterations identified with later-line osimertinib.
A, Frequency of alterations pre- and post-osimertinib in patients treated with later-line osimertinib. The in-figure legend specifies details on acquired resistance mechanism, histology, initial EGFR mutation, alteration type, and next-generation sequencing (NGS) assay. B, Enrichment of individual altered genes pre-osimertinib (left) and post-osimertinib (right). The dashed line represents a P-value of 0.05. the frequency difference between the two sample sets is plotted on the x-axis and its significance [-log10(P-value)] on the y-axis. C, The distribution of established mechanisms of resistance by type of alteration in patients treated with later-line osimertinib. SCLC, small-cell lung cancer; NSCLC, non-small cell lung cancer; AR, acquired resistance
Comparisons between first-line and later-line osimertinib
Overall, EGFR-mediated acquired resistance was associated with a longer time on osimertinib and improved OS compared to other resistance mechanisms (median TTD 18.0 mo, 95% CI: 13.3–33.2; vs 13.2 mos, 95% CI: 10.6–16.3, P = 0.04; median OS not reached vs 29 mos 95% CI: 24.6 – not reached, P < 0.001). Notably, the proportion of off-target resistance in the first-line setting was higher than the later-line cohort (P = 0.01) suggesting that off-target resistance emerges earlier and/or treatment with first-line osimertinib may enrich for off-target resistance.
Associations with sensitizing EGFR mutation
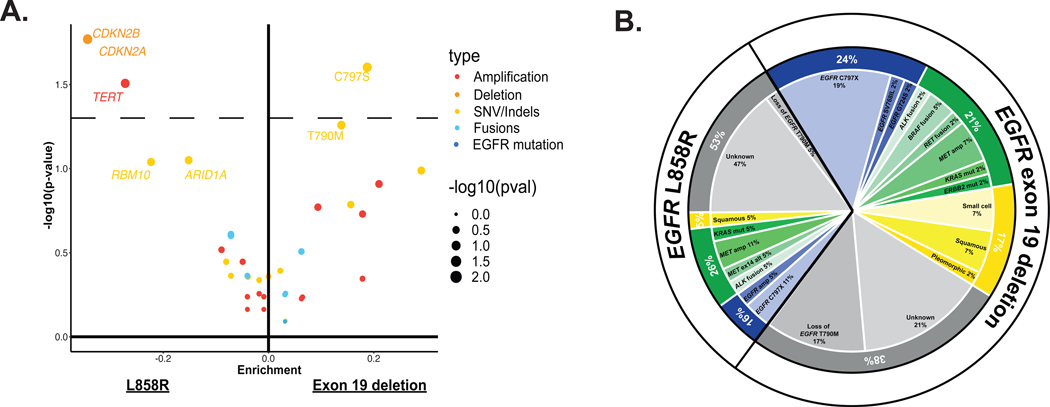
Acquired alterations were analyzed by initial sensitizing EGFR mutation (Exon 19 deletion versus L858R mutation, Figure 3a and b). EGFR C797S was more frequently seen with EGFR exon 19 deletion (Figure 3a and b, P = 0.03). Post-treatment CDKN2A/B deletions and TERT amplifications were more commonly seen with EGFR L858R mutations (P = 0.02, P = 0.03, respectively). However, these associations were not significant when accounting for multiple comparisons.
Figure 3. Molecular alterations by sensitizing EGFR mutation.
A, Enrichment of genomic alterations by sensitizing EGFR mutation. The dotted line represents a P-value of 0.05. Enrichment of mutations in patients with EGFR exon 19 deletions (right) versus EGFR L858R (left). For each mutation, the frequency difference between the two cohorts is plotted on the x-axis and its significance -log10(P-value) on the y-axis. B, The distribution of mechanisms of resistance organized by sensitizing EGFR mutation
Histologic transformation
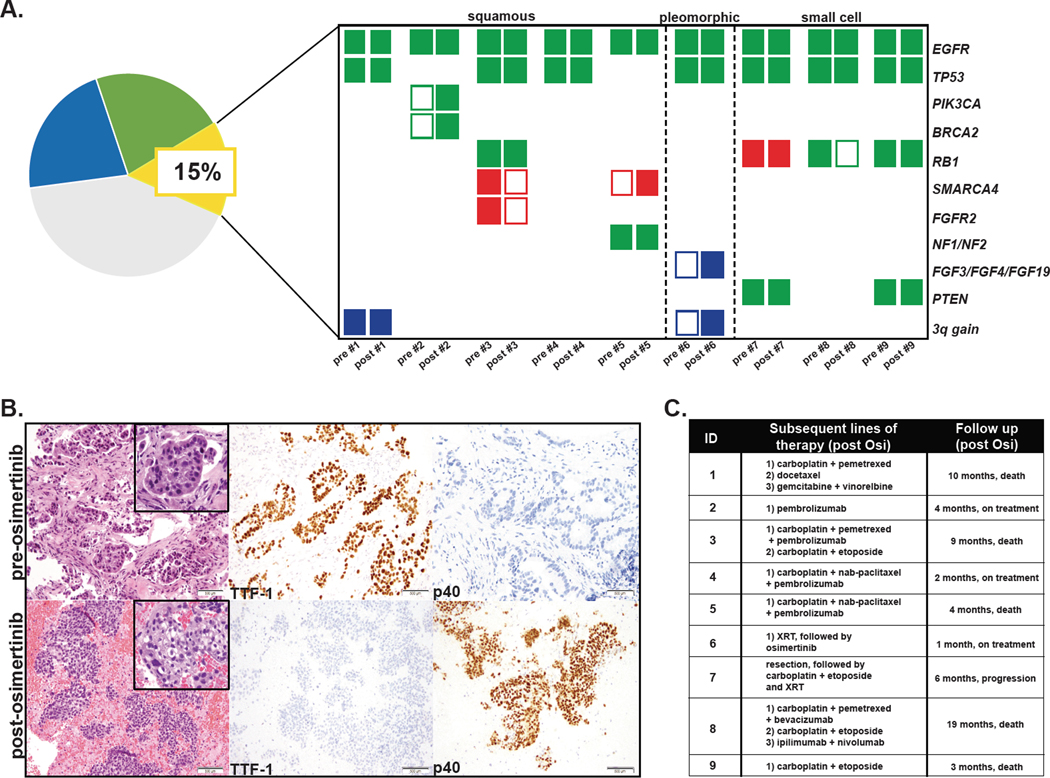
Histologic transformation was identified in 15% of cases (9 patients), 15% in the first-line setting and 14% in the later-line setting (Figure 4A). We identified 5 cases of squamous cell transformation (Figure 4B), 1 case of pleomorphic transformation with squamous, sarcomatoid, and small cell features and 3 cases of small cell transformation. There was no overarching genomic correlate associated with squamous transformation. One patient acquired a PIK3CA E726K mutation; no pre-existing or acquired SOX2 amplifications were identified, but one patient had low-level chromosome 3q gain and one had low level PIK3CA copy number gain in both the pre-treatment and post-treatment samples (Figure 4A). The patient with pleomorphic transformation acquired a very high level chromosome 3q amplification (49-fold) and FGF3/FGF4/FGF19 amplification (15-fold) (Figure 4A). Consistent with prior literature, the small cell lung cancer tumors (SCLC) all had pre-existing alterations of RB1 and TP53 identified in their pre-treatment samples (Figure 4A).
Figure 4. The genomic profile and clinical outcomes of patient with histologic transformation.
A, Genomic patterns pre- and post-osimertinib by histologic transformation subtype. Green shaded rectangle represents mutation. Blue shaded rectangle represents copy number gain. Red shaded rectangle represents deletion. B, Pre-treatment biopsy shows adenocarcinoma, which is positive for TTF-1 and negative for p40 by immunohistochemistry. Post-treatment sample show squamous cell carcinoma, which is negative for TTF-1 and positive for p40 by immunohistochemistry. C, Treatment regimens received by patients with histologic transformation and overall survival after osimertinib.
Clinical course after treatment with osimertinib
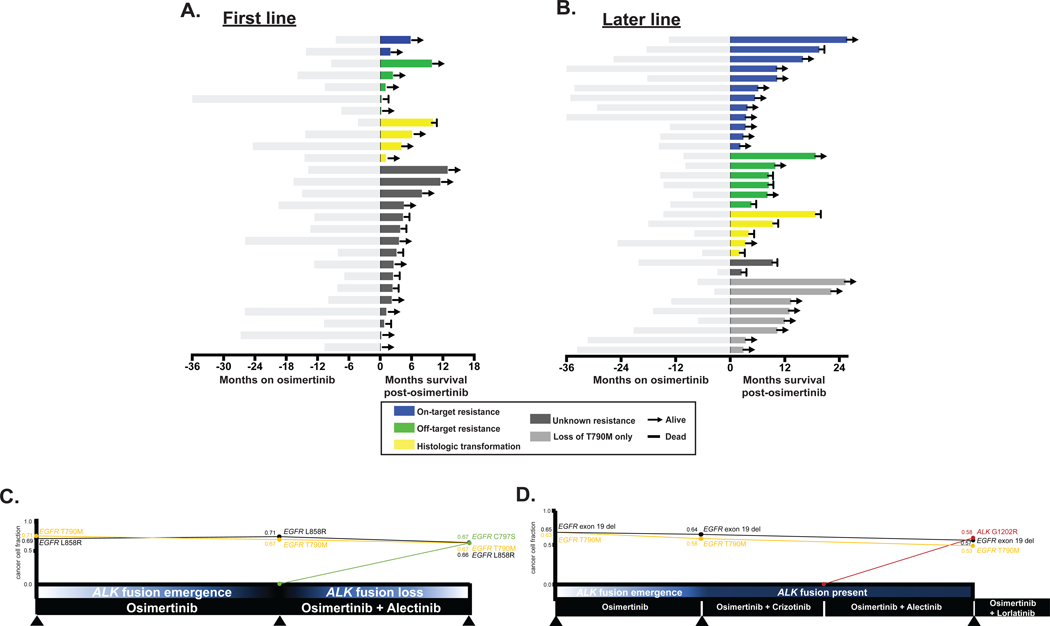
Patient outcomes post-osimertinib are summarized by resistance mechanism and line of therapy (Figure 5). Five of the 9 patients with histologic transformation have died (4 within 10 months post-osimertinib progression). The patients with small cell transformation all received platinum with etoposide as a part of their subsequent therapy (Figure 4C). Among the 5 patients with squamous transformation, varying treatment strategies were employed and outcomes were mixed with limited follow-up (Figure 4C). In several instances, patients with acquired off-target alterations were treated with targeted therapies. For instance, 2 patients with tumors that acquired ALK fusions were treated with osimertinib and ALK TKIs (crizotinib, alectinib, lorlatinib) with durable responses (Figure 5C and D). The clonal evolution of identified alterations is illustrated over interval biopsy samples for 2 patients (Figure 5C and D).
Figure 5. Clinical outcomes pre/post osimertinib progression by resistance mechanism.
A,B Time on osimertinib (months) is depicted to the left (light grey) and overall survival after osimertinib (months) is depicted on the right for patients who A, received first-line osimertinib. B, received later-line osimertinib. C,D Longitudinal analysis of 2 patients who received later-line osimertinib. C, One patient acquired an EGFR C797S mutation and lost an ALK fusion after treatment with osimertinib and alectinib. D, The other patient acquired an ALK G1202R mutation after treatment with osimertinib and alectinib. Treatment summaries from osimertinib onwards are noted in black bars with time (months) along the x-axis. Summary of molecular alterations (MSK-IMPACT) prior to starting osimertinib and at 2 resistance timepoints are shown for each patient (colored lines). Cancer cell fractions (CCF) of driver and resistance alterations based on FACETS analysis (see supplementary methods) at each biopsy timepoint.
Functional studies of MET H1094 and EGFR SV768IL, as novel resistance mechanisms to osimertinib
We hypothesized that acquired alterations in MET H1094, identified in the first-line setting and EGFR S768I+V769L (EGFR SV768IL) mutations found in the later-line setting could represent putative resistance mechanisms and explored these alterations in pre-clinical models.
MET H1094 mutations confer resistance to osimertinib
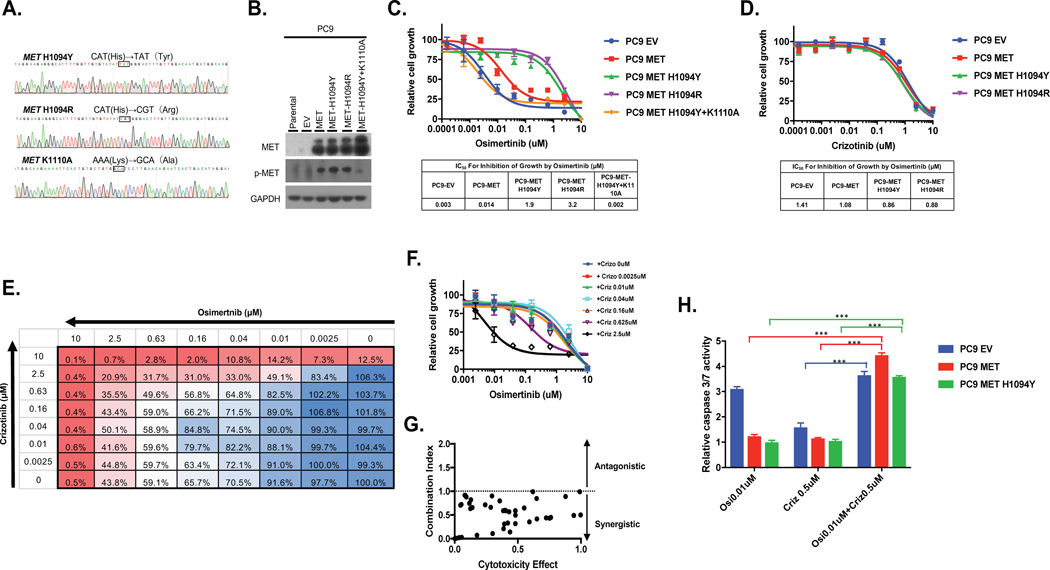
We expressed wildtype MET, MET H1094R, MET H1094Y and a kinase dead MET H1094Y (K1110A mutant) in the PC9, EGFR exon 19 deleted lung adenocarcinoma cell line. Mutations were introduced into MET using site-directed mutagenesis and mutations confirmed by DNA sequencing (Figure 6A). Western blotting of whole-cell extracts confirmed overexpression of MET and the corresponding mutants (Figure 6B). The two MET mutants demonstrated similar levels of MET phosphorylation versus wildtype MET. The kinase dead MET H1094Y mutant showed a low level of MET phosphorylation. Sensitivity to growth inhibition by osimertinib was reduced in PC9 cells expressing either MET H1094 mutant compared to PC9 cells expressing an empty vector (EV)plasmid (PC9-EV) or PC9 cells overexpressing wildtype MET (PC9-MET) (Figure 6C). The MET H1094R mutant induced greater resistance to osimertinib compared to the MET H1094Y mutant (959 vs 136-fold increase in IC50 value for growth inhibition, compared to PC9-EV cells). PC9 cells expressing the two MET H1094 mutants were slightly more sensitive to crizotinib than the PC9-EV or PC9-MET cells (Figure 6D).
Figure 6. Combined inhibition of MET and EGFR overcomes MET-H1094-mediated osimertinib resistance.
A, MET-H1094 mutations were introduced by site-directed mutagenesis and confirmed by Sanger sequencing. B, Plasmids containing the MET mutants were stably introduced into PC9 cells via lentiviral transduction and then expression of MET confirmed by Western blotting. C and D, Isogenic PC9 cells were treated with osimertinib, C, or crizotinib, D, for 96 h and then growth determined using alamarBlue viability dye (top panel). IC50 values were determined by non-linear regression analysis using GraphPad Prism (bottom panel). E-G, Cells were treated with the indicated combined concentrations of osimertinib and crizotinib for 96 h and then growth determined. E and F, The percent inhibition of growth at each drug combination. G, The Chou-Talalay method was used to examine whether crizotinib and osimertinib inhibited growth in a synergistic manner. The dot plot shows the combination index (CI) as a function of the fraction affected. CI<1 indicates synergy between the two inhibitors. H, Caspase 3/7 activity was determined in cells treated for 48 h with the indicated inhibitors for 48 h and then caspase 3/7 activity determined. Results represent the fold change in caspase 3/7 enzymatic activity above the corresponding untreated cells. All experiments were repeated 3 times and included triplicate determinations of each condition. EV: empty vector. The K110A mutation results in an inactive kinase. Results represent the mean ± SD. *p<0.05 **p<0.01, ***p<0.001.
Combined inhibition of MET and EGFR overcomes MET H1094-mediated osimertinib resistance
To determine if combination crizotinib and osimertinib would be an effective therapeutic strategy for EGFR-mutant cancers expressing MET-H1094, we examined if these two drugs act synergistically to inhibit growth. We used the method of Chou-Talalay,(32) where cells are treated with drug combinations and then a parameter called the combination index (CI) is determined. CI<1 indicates synergy, CI>1 indicates antagonism between the two drugs and CI=1 indicates an additive effect. Growth was inhibited in PC9-MET-H1094Y cells when the two drugs were combined, compared to either drug alone (Figure 6E and F). The CI values were <1 for all drug concentrations tested (Figure 6G). We next examined the effect of the MET H1094Y mutation on osimertinib induced caspase 3/7 activity as a measure of apoptosis. Treatment of PC9-EV cells with osimertinib induced activation of capase 3/7 as expected but did not stimulate caspase 3/7 activity in PC9-MET or PC9-MET-H1049Y cells (Figure 6H). In contrast, treatment of the PC9 cell lines with crizotinib alone did not stimulate caspase 3/7 activity in any of the cell lines (Figure 6H). However, a combination of crizotinib and osimertinib resulted in a significant increase in caspase 3/7 activity in PC9-MET and PC9-MET-H1094Y (Figure 6G). Taken together, these results endorse MET H1094Y to be a novel mechanism of resistance to osimertinib that can be overcome by combined inhibition of MET and EGFR.
Compound EGFR S768I+V769L (EGFR SV768IL) mutations confer resistance to osimertinib
To determine if the observed mutations in EGFR exon 20 confer resistance to osimertinib, we generated cell lines with the S768I and V769L mutations (EGFR-SV768IL) by site directed mutagenesis (Supplemental Figure 1A). Wildtype EGFR or EGFR-SV768IL was expressed in PC9 and HCC827 cell lines and expression confirmed by Western blotting (Supplemental Figure 1B). EGFR-SV768IL was more heavily phosphorylated than wildtype EGFR in both cell lines, indicating higher level of activity (Supplemental Figure 1B) of the SV768IL variant. Similarly, expression of EGFR-SV768IL resulted in phosphorylation of ERK1/2 and AKT to a higher extent than that observed with expression of wildtype EGFR. Growth of PC9 (Supplemental Figure 1C) and HCC827 (Supplemental Figure 1C) expressing EGFR-SV768IL was resistant to the inhibitory effects of osimertinib, compared to cells expressing either an empty vector (EV) or wildtype EGFR. The IC50 value of osimertinib for PC9-EGFR-SV768IL cells was 166-fold higher than that for PC9-EV cells (Supplemental Figure 1C). Similarly, the IC50 value of osimertinib in HCC827-EGFR SV768IL cells was 244-fold higher than that of HCC827-EV cells (Supplemental Figure 1C). We also examined the effect of EGFR-SV768IL on osimertinib-induced caspase 3/7 activity in PC9 cells. Whereas osimertinb caused a significant increase in caspase 3/7 activity in PC9-EV and PC9-EGFR cells, no increase was observed in PC9-EGFR-SV768IL cells (Supplemental Figure 1D).
Discussion
We identified an array of acquired resistance mechanisms to osimertinib using paired pre- and post-treatment tissue samples. In our cohort of first-line patients with limited follow-up and shorter time on osimertinib, EGFR-mediated resistance was uncommon, whereas off-target resistance, including histologic transformation, was seen frequently. Notably, there appears to be a time-dependent pattern of resistance with off-target resistance emerging earlier resulting in less durable responses to osimertinib. This mirrors our earlier finding that on-target resistance mutations( i.e. EGFR T790M) are associated with more indolent disease and arise as after a longer time on EGFR-TKI and with better post-progression survival.(33) Development of off-target resistance after a short period may result from pre-existing subclones that emerge quickly on treatment. To ascertain whether resistance mechanisms to first- and later-line osimertinib truly differ will require lengthier follow-up in patients on first-line osimertinib.
EGFR C797S, the most common EGFR mutation acquired on later-line osimertinib, was not identified in our first-line cohort of patients. The frequency of EGFR C797S in the first-line FLAURA study was 8%;(15) both first-line cohorts (ours and FLAURA) report lower frequencies of EGFR C797S compared to later-line osimertinib cohorts (15%−32%, Supplemental Figure 2)(6,8,10,34,35) again suggesting first-line and later-line osimertinib may have different resistance spectra. Later-line osimertinib is utilized only in tumors with acquired EGFR T790M; these tumors have demonstrated continued dependence on EGFR signaling and may be predisposed to acquire tertiary EGFR mutations (i.e. EGFR C797S) compared to EGFR-mutant tumors at large resulting in the disparate frequencies of EGFR acquired mutations in the first-line and later-line setting. MET amplification was also identified at a lower rate (7%) than most reports in the later-line setting (10%−26%, Supplemental Figure 2)(6,8,10,34,35) and is on the lower-end of first-line reports (5–15%, Supplemental Figure 2).(15,36) Previous studies lacking pretreatment tissue or plasma may overestimate acquired MET amplifications, which can be seen concurrently with EGFR prior to treatment.(6,35,37,38) In addition, plasma-based platforms typically have lower sensitivity to assess copy number changes.(21–25)
Tissue analysis is critical to characterizing resistance mechanisms. Histologic transformation, which cannot be detected via plasma testing, was a frequent mechanism of resistance in our study. Rates of transformation and other off-target resistance mechanisms may be higher with osimertinib compared to earlier generation TKIs due to better on-target inhibition. Prior to this report, squamous cell transformation was identified infrequently. (5,8,16–20) This phenomenon is surely underrecognized due to the increasing reliance on circulating tumor (ct)DNA for identification of resistance mechanisms. Recognition of histologic transformation is imperative as it has prognostic and therapeutic implications. Patients with squamous cell transformation in our cohort had short post-progression survival (Figure 4 and 5). Similar to small-cell transformation,(1,2,39) patients with squamous cell transformation may require treatments tailored to this cancer type. Primary squamous cell lung cancers, unlike the prerequisite RB1 and TP53 mutations identified in small cell lung cancers, exhibit considerable genomic complexity. Understanding the etiology of squamous cell transformation will require comprehensive investigation, made more challenging by the fact that de novo squamous cell lung cancers do not have a overarching genomic signature. Further study will include understanding the gene expression subtype of the transformed cases and assessing non-genomic processes that may play a role in histologic transdifferentiation such as transcription factor networks and the epigenome.
Recent data suggest that the initial sensitizing EGFR mutation may bias the resistance mechanisms that emerge. To date, EGFR G724S has only been identified with EGFR exon 19 deletions, and structural and in vitro models support EGFR G724S as conferring resistance only when concurrent with an EGFR exon 19 deletion.(40) We similarly demonstrate that EGFR C797S is preferentially coupled with EGFR exon 19 deletions. We also confirm the previous findings that EGFR C797S was only seen in tumors that retained EGFR T790M suggesting continued EGFR dependence in these tumors. Typically, off-target or unknown resistance mechanisms are seen in the absence of EGFR T790M suggesting a loss of EGFR dependence in these tumors.
We and others have described acquired chromosomal rearrangements (ALK, RET, BRAF, ERBB2, MET exon 14) as resistance mechanisms to EGFR-TKIs.(5–7,10–15,41–43) We again identified BRAF, ALK, and RET fusions in this series. The relatively high frequency of these otherwise extremely rare oncogene fusions in the setting of acquired resistance to osimertinib requires further exploration. Eight percent of all RET fusions and 50% of all BRAF fusions identified in lung cancers at MSK by MSK-IMPACT over the time period of this study were found in patients with EGFR-mutant lung cancer and acquired resistance to osimertinib(31,44). This high frequency of acquired fusions supports a predisposition for genomic rearrangements driven by the selective pressure of osimertinib.
We also identified and validated the mutation MET H1094Y and the compound mutation EGFR S768 + V769L as resistance mechanisms with potential associated treatments. Prior work demonstrated increased catalytic activity and cognate autophosphorylation of MET H1094Y as compared to the wildtype MET kinase domain confirming MET H1094Y to be an oncogenic and transformative mutation. From a structural perspective, this mutation resides in close proximity to the ATP binding site, and based on molecular modeling studies, these mutations may activate MET kinase by destabilizing the inhibitory conformation of the activation loop.(45,46) Prior case reports of de novo EGFR S768I and V769L compound mutations have been publishedwith mixed responses to first and second generation EGFR TKIs.(47–49). It is not clear if treating a patient who acquires on-target resistance to osimertinib will respond to early generation TKIs and the appropriate trials are underway (NCT03755102).(9)
Over half of our first-line cohort had unknown mechanisms of resistance. In these cases, resistance may be due to epigenetic modifications, changes in protein expression or novel genomic alterations. Further analyses will need to integrate epigenetic, RNA, and protein expression analyses to uncover the yet undetermined mechanisms of resistance to osimertinib. In addition, clonal evolution and tumor heterogeneity also play a fundamental role in resistance to targeted therapies and should be considered in future analyses. As osimertinib has only recently been integrated as first-line treatment, our first-line cohort was biased towards resistance mechanisms that emerge earlier on treatment and makes directed comparison to the later-line cohort challenging but provides the unique perspective of identifying early emerging mechanisms of resistance. Another limitation of our study is that histologic transformation could also represent outgrowth of a pre-existing clone of tumor cells that were not previously identified. However, multiple sections throughout each sample of pathologic tissue were re-examined to confirm no evidence of pre-existing squamous cell or small cell histology. This will be a overarching limitation for all future studies of lineage plasticity in this patient subset since most metastatic patients only have small core needle biopsies done. Also, molecular data from single-lesion biopsies may not reflect the entirety of genetic alterations due to tumoral heterogeneity Finally, although our sample size was modest, this is the largest analysis to date of osimertinib resistance utilizing paired tumor tissue.
In conclusion, our study establishes that mechanisms of resistance to osimertinib are diverse, with sensitizing EGFR mutation, time on osimertinib therapy, and line of therapy all influencing the resistance spectra identified. Off-target resistance arises early on first-line osimertinib after a shorter duration of osimertinib treatment. Histologic transformation appears common with first-line osimertinib and highlights the continued importance of tissue-based assays to evaluate acquired resistance. With resistance mechanisms dependent on original sensiziting EGFR mutation, further assessment of how pretreatment alterations forecast resistance will be important as the field amends first-line treatments to delay or prevent resistance. Identifying and overcoming these resistance mechanisms will require a multifaceted approach utilizing both plasma and tissue molecular and histopathological analyses.
Supplementary Material
A, The EFGR-SV768IL mutation was introduced by site-directed mutagenesis and confirmed by Sanger sequencing. B, Plasmids containing EGFR or EFGR-SV768IL were stably introduced into PC9 and HCC827 cells via lentiviral transduction and then expression of EGFR confirmed by Western blotting. C, Isogenic PC9 (left panel) and HCC827 (right panel) cells were treated with osimertinib for 96 h and then growth determined using alamarBlue viability dye (top panel). IC50 values were determined by non-linear regression analysis using GraphPad Primsm (bottom panel). Isogenic PC9 cells were treated with osimertinib for 48 h and then caspase 3/7 activity determined. Results represent the fold change in caspase 3/7 enzymatic activity above the corresponding untreated cell line. All experiments were repeated 3 times and included triplicate determinations of each condition. Results represent the mean ± SD. EV: empty vector. **p<0.01, ***p<0.001.
Summary of mechanisms of resistance reported in recent prospective studies and the largest retrospective series of acquired resistance mechanisms to osimertinib in the first-line and later-line.(5,6,15,34–36)
Translational relevance.
Prior reports of mechanisms of resistance to osimertinib primarily focus on patients who received osimertinib after other EGFR-TKIs and rely heavily upon circulating tumor (ct)DNA. We utilized paired tumor tissue with next-generation sequencing performed before osimertinib and after progression to analyze resistance mechanisms in a cohort of 62 patients with EGFR-mutant lung cancer treated with osimertinib, either as first-line or later-line treatment. We identified lineage plasticity and in particular, squamous histologic transformation, as unexpectedly frequent with first-line osimertinib and associated with considerable genomic complexity, highlighting the importance of tissue-based analyses to evaluated acquired resistance. We also detected a diverse array of off-target genomic resistance mechanisms that may be amenable to targeted therapy. Sensitizing EGFR mutation, time on osimertinib therapy, and line of therapy all may influence the resistance spectra identified. Finally, we validate two novel resistance alterations, EGFR S768 + V769L and MET H1094Y and explore relevant potential treatment options.
Acknowledgement of research support:
This research was supported in part by the National Cancer Institute of the National Institutes of Health (T32 CA009207, P30 CA008748) and the Druckenmiller Center for Lung Cancer Research at MSK.
Disclosures: AJS, JMC, DK, HS, HR, YD, JC, DP, NL have no financial interests to declare. PKP has served on advisory boards (w/ honoraria) for Celgene, Abbvie, Boehringer Ingelheim; IDSMC: Takeda, Travel support: EMD Serono Research funding (to institution): Celgene, EMD Serono. MO has consulted for PharmaMar. MAE is a consultant for AstraZeneca and received support from Astrazeneca, Invivoscribe, and Raindance Technologies. MGK is a consultant for Ariad, AstraZeneca and Genentech Roche and received research funding from Genentech Roche and Puma Biotechnology. RS has received research funding from Helsinn Health Care and Loxo Oncology. ML has received advisory board compensation from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Takeda, and Bayer, and research support from LOXO Oncology and Helsinn Healthcare. GJR has research funding to his institution from Pfizer, Novartis, Takeda, and Roche. HAY has consulted for AstraZeneca and has research funding to her institution from AstraZeneca, Lilly, Pfizer, Novartis, and Daiichi.
References
- 1.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19(8):2240–7 doi 10.1158/1078-0432.ccr-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine 2011;3(75):75ra26 doi 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. The New England journal of medicine 2017;376(7):629–40 doi 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. The New England journal of medicine 2018;378(2):113–25 doi 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 5.Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer discovery 2018;8(12):1529–39 doi 10.1158/2159-8290.Cd-18-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le X, Puri S, Negrao MV, Nilsson M, Robichaux JP, Boyle TA, et al. Landscape of EGFR -dependent and -independent resistance mechanisms to osimertinib and continuation therapy post-progression in EGFR-mutant NSCLC. Clinical cancer research : an official journal of the American Association for Cancer Research 2018. doi 10.1158/1078-0432.Ccr-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michels S, Heydt C, Veggel Bv, Deschler-Baier B, Pardo N, Monkhorst K, et al. Genomic Profiling Identifies Outcome-Relevant Mechanisms of Innate and Acquired Resistance to Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy in Lung Cancer. Jco Precis Oncol 2019;3(3):1–14 doi 10.1200/po.18.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehlman C, Cadranel J, Rousseau-Bussac G, Lacave R, Pujals A, Girard N, et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: A multicentric retrospective French study. Lung Cancer 2019;137:149–56 doi 10.1016/j.lungcan.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Rangachari D, To C, Shpilsky JE, VanderLaan PA, Kobayashi SS, Mushajiang M, et al. EGFR-Mutated Lung Cancers Resistant to Osimertinib through EGFR C797S Respond to First-Generation Reversible EGFR Inhibitors but Eventually Acquire EGFR T790M/C797 S in Preclinical Models and Clinical Samples. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2019;14(11):1995–2002 doi 10.1016/j.jtho.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24(13):3097–107 doi 10.1158/1078-0432.ccr-17-2310. [DOI] [PubMed] [Google Scholar]
- 11.Blakely CM, Watkins TBK, Wu W, Gini B, Chabon JJ, McCoach CE, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nature genetics 2017;49(12):1693–704 doi 10.1038/ng.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nature communications 2016;7:11815 doi 10.1038/ncomms11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piotrowska Z, Thress KS, Mooradian M, Heist RS, Azzoli CG, Temel JS, et al. MET amplification (amp) as a resistance mechanism to osimertinib. Journal of Clinical Oncology 2017;35(15_suppl):9020– doi 10.1200/JCO.2017.35.15_suppl.9020. [DOI] [Google Scholar]
- 14.Iams W YC Acquired Resistance to Osimertinib by CCDC6-RET Fusion in a Patient with EGFR T790M Mutant Metastatic Lung Adenocarcinoma. Journal Thoracic Oncology 2017;12(11S2):2. [Google Scholar]
- 15.Ramalingam S Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. ESMO2018. [Google Scholar]
- 16.Bruno R, Proietti A, Ali G, Puppo G, Ribechini A, Chella A, et al. Squamous cell transformation and EGFR T790M mutation as acquired resistance mechanisms in a patient with lung adenocarcinoma treated with a tyrosine kinase inhibitor: A case report. Oncology letters 2017;14(5):5947–51 doi 10.3892/ol.2017.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izumi H, Yamasaki A, Ueda Y, Sumikawa T, Maeta H, Nakamoto S, et al. Squamous Cell Carcinoma Transformation from EGFR-mutated Lung Adenocarcinoma: A Case Report and Literature Review. Clinical lung cancer 2018;19(1):e63–e6 doi 10.1016/j.cllc.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Lin CC, Shih JY, Yu CJ, Ho CC, Liao WY, Lee JH, et al. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. The Lancet Respiratory medicine 2018;6(2):107–16 doi 10.1016/s2213-2600(17)30480-0. [DOI] [PubMed] [Google Scholar]
- 19.Paik PK, Varghese AM, Sima CS, Moreira AL, Ladanyi M, Kris MG, et al. Response to erlotinib in patients with EGFR mutant advanced non-small cell lung cancers with a squamous or squamous-like component. Molecular cancer therapeutics 2012;11(11):2535–40 doi 10.1158/1535-7163.Mct-12-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara S, Ichiki Y, Fukuichi Y, Honda Y, Kanayama M, Taira A, et al. Squamous cell carcinoma transformation from adenocarcinoma as an acquired resistance after the EGFR TKI therapy in (EGFR-mutated) non-small cell lung cancer. Journal of thoracic disease 2018;10(7):E526–e31 doi 10.21037/jtd.2018.06.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stetson D, Ahmed A, Xu X, Nuttall BRB, Lubinski TJ, Johnson JH, et al. Orthogonal Comparison of Four Plasma NGS Tests With Tumor Suggests Technical Factors are a Major Source of Assay Discordance. Jco Precis Oncol 2019;3(3):1–9 doi 10.1200/PO.18.00191. [DOI] [PubMed] [Google Scholar]
- 22.Paweletz CP, Lau CJ, Oxnard GR. Does Testing Error Underlie Liquid Biopsy Discordance? Jco Precis Oncol 2019(3):1–3 doi 10.1200/PO.18.00408. [DOI] [PubMed] [Google Scholar]
- 23.Guibert N, Hu Y, Feeney N, Kuang Y, Plagnol V, Jones G, et al. Amplicon-based next-generation sequencing of plasma cell-free DNA for detection of driver and resistance mutations in advanced non-small cell lung cancer. Ann Oncol 2018;29(4):1049–55 doi 10.1093/annonc/mdy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabari JK, Offin M, Stephens D, Ni A, Lee A, Pavlakis N, et al. A Prospective Study of Circulating Tumor DNA to Guide Matched Targeted Therapy in Lung Cancers. J Natl Cancer Inst 2018. doi 10.1093/jnci/djy156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Supplee JG, Milan MSD, Lim LP, Potts KT, Sholl LM, Oxnard GR, et al. Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer 2019;134:96–9 doi 10.1016/j.lungcan.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28(2):357–60 doi 10.1200/jco.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD 2015;17(3):251–64 doi 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature Biotechnology 2013;31(11):1023 doi 10.1038/nbt.269610.1038/nbt.2696https://www.nature.com/articles/nbt.2696#supplementary-informationhttps://www.nature.com/articles/nbt.2696#supplementary-information . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra-Kalyani P, Gong Y, Goldberg KB, Kluetz PG, Pazdur R, Khozin S, et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann Oncol 2019;30(5):830–8 doi 10.1093/annonc/mdz060. [DOI] [PubMed] [Google Scholar]
- 30.Benayed R, Offin MD, Mullaney KA, Sukhadia P, Rios KM, Desmeules P, et al. Comprehensive detection of targetable fusions in lung adenocarcinomas by complementary targeted DNAseq and RNAseq assays. Journal of Clinical Oncology 2018;36(15_suppl):12076– doi 10.1200/JCO.2018.36.15_suppl.12076. [DOI] [Google Scholar]
- 31.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling 2013;6(269):pl1 doi 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research 2010;70(2):440–6 doi 10.1158/0008-5472.Can-09-1947. [DOI] [PubMed] [Google Scholar]
- 33.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clinical cancer research : an official journal of the American Association for Cancer Research 2011;17(6):1616–22 doi 10.1158/1078-0432.Ccr-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M–Positive Lung Cancer and Acquired Resistance to OsimertinibResistance to Osimertinib in Patients With EGFR T790M–Positive Lung CancerResistance to Osimertinib in Patients With EGFR T790M–Positive Lung Cancer. JAMA Oncology 2018;4(11):1527–34 doi 10.1001/jamaoncol.2018.2969 %J JAMA Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadimitrakopoulou VA. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. ESMO2018. [Google Scholar]
- 36.Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(9):841–9 doi 10.1200/jco.2017.74.7576. [DOI] [PubMed] [Google Scholar]
- 37.Jakobsen JN, Santoni-Rugiu E, Grauslund M, Melchior L, Sørensen JB. Concomitant driver mutations in advanced EGFR-mutated non-small-cell lung cancer and their impact on erlotinib treatment. Oncotarget 2018;9(40):26195–208 doi 10.18632/oncotarget.25490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schildhaus HU, Schultheis AM, Ruschoff J, Binot E, Merkelbach-Bruse S, Fassunke J, et al. MET amplification status in therapy-naive adeno- and squamous cell carcinomas of the lung. Clinical cancer research : an official journal of the American Association for Cancer Research 2015;21(4):907–15 doi 10.1158/1078-0432.Ccr-14-0450. [DOI] [PubMed] [Google Scholar]
- 39.Marcoux N, Gettinger SN, O’Kane G, Arbour KC, Neal JW, Husain H, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37(4):278–85 doi 10.1200/jco.18.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown BP, Zhang YK, Westover D, Yan Y, Qiao H, Huang V, et al. On-target Resistance to the Mutant-Selective EGFR Inhibitor Osimertinib Can Develop in an Allele-Specific Manner Dependent on the Original EGFR-Activating Mutation. Clinical cancer research : an official journal of the American Association for Cancer Research 2019. doi 10.1158/1078-0432.Ccr-18-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Offin M, Somwar R, Rekhtman N, Benayed R, Chang JC, Plodkowski A, et al. Acquired ALK and RET Gene Fusions as Mechanisms of Resistance to Osimertinib in EGFR-Mutant Lung Cancers. Jco Precis Oncol 2018;2 doi 10.1200/po.18.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzawa K, Offin M, Schoenfeld AJ, Plodkowski AJ, Odintsov I, Lu D, et al. Acquired MET Exon 14 Alteration Drives Secondary Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in EGFR-Mutated Lung Cancer. Jco Precis Oncol 2019;3 doi 10.1200/po.19.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vojnic M, Kubota D, Kurzatkowski C, Offin M, Suzawa K, Benayed R, et al. Acquired BRAF Rearrangements Induce Secondary Resistance to EGFR therapy in EGFR-Mutated Lung Cancers. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2019;14(5):802–15 doi 10.1016/j.jtho.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery 2012;2(5):401–4 doi 10.1158/2159-8290.Cd-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt L, Junker K, Nakaigawa N, Kinjerski T, Weirich G, Miller M, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 1999;18(14):2343–50 doi 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 46.Pal SK, Ali SM, Yakirevich E, Geynisman DM, Karam JA, Elvin JA, et al. Characterization of Clinical Cases of Advanced Papillary Renal Cell Carcinoma via Comprehensive Genomic Profiling. European urology 2018;73(1):71–8 doi 10.1016/j.eururo.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 47.Asahina H, Yamazaki K, Kinoshita I, Yokouchi H, Dosaka-Akita H, Nishimura M. Non-responsiveness to gefitinib in a patient with lung adenocarcinoma having rare EGFR mutations S768I and V769L. Lung Cancer 2006;54(3):419–22 doi 10.1016/j.lungcan.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Niogret J, Coudert B, Boidot R. Primary Resistance to Afatinib in a Patient with Lung Adenocarcinoma Harboring Uncommon EGFR Mutations: S768I and V769L. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2018;13(7):e113 doi 10.1016/j.jtho.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Shao YW, Xia Y. Responsiveness to Full-Dose Afatinib in a Patient With Lung Adenocarcinoma Harboring EGFR S768I and V769L Mutations. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2019;14(2):e25–e7 doi 10.1016/j.jtho.2018.10.165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, The EFGR-SV768IL mutation was introduced by site-directed mutagenesis and confirmed by Sanger sequencing. B, Plasmids containing EGFR or EFGR-SV768IL were stably introduced into PC9 and HCC827 cells via lentiviral transduction and then expression of EGFR confirmed by Western blotting. C, Isogenic PC9 (left panel) and HCC827 (right panel) cells were treated with osimertinib for 96 h and then growth determined using alamarBlue viability dye (top panel). IC50 values were determined by non-linear regression analysis using GraphPad Primsm (bottom panel). Isogenic PC9 cells were treated with osimertinib for 48 h and then caspase 3/7 activity determined. Results represent the fold change in caspase 3/7 enzymatic activity above the corresponding untreated cell line. All experiments were repeated 3 times and included triplicate determinations of each condition. Results represent the mean ± SD. EV: empty vector. **p<0.01, ***p<0.001.
Summary of mechanisms of resistance reported in recent prospective studies and the largest retrospective series of acquired resistance mechanisms to osimertinib in the first-line and later-line.(5,6,15,34–36)