Abstract
The HIV pandemic disproportionately impacts sub-Saharan Africa where in 2017, 71% of people living with HIV resided, 65% of new infections and 75% of deaths were reported. Prevention, screening and treatment strategies have led to progress in addressing this disease. HIV diagnostics have been crucial for prevention and treatment but more progress is required to reduce HIV infection. The Center for Innovation in Point-of-Care Technologies for HIV/AIDS at Northwestern University (C-THAN) is a vital partner in the National Institute of Biomedical Imaging and Bioengineering Point-of-Care Technologies Research Network. C-THAN’s mission is to develop and commercialize a pipeline of point-of-care technologies critical for improved prevention and management of HIV in low- and middle-income countries with specific emphasis on sub-Saharan Africa.
Graphical Abstract
1. Introduction
In 2017, 36.9 million people were living with HIV/AIDS (PLWHA) and 940,000 deaths were attributed to HIV with the heaviest burden born by low and middle income countries (LMICs). Sub-Saharan Africa (SSA) alone accounted for 71% of PLWHA, 75% of HIV-related deaths and 65% of new infections [2, 3].
Despite the high number of HIV-related deaths, significant improvements have been made compared to 10 years ago. In 2005, 1.9 million deaths were attributed to HIV/AIDS [4]. The 49% mortality decrease was caused by a massive increase in antiretroviral therapy (ART) access. Likewise, new infections decreased by more than 50% each year from 2000 to 2010 caused by decreases in risky behavior and ART reducing HIV transmission [5].
In 2014, the United Nations’ Program on HIV and AIDS (UNAIDS) set the ambitious 90-90-90 goals to curb the AIDS epidemic: by 2020, achieve detection of 90% of HIV cases, treatment for 90% of those cases, and viral suppression for 90% of those treated [6]. The 90-90-90 goals are likely to be reached in southern and eastern Africa, but western and central Africa lag behind [7]. This disparity in HIV/AIDS prevention and treatment may intensify as the largest-ever generation of young people age into adolescence and adulthood potentially causing a rebound of the pandemic [7]. Gaps in HIV prevention, diagnosis and treatment must be identified and strategies to bridge these gaps developed.
The HIV care cascade outlines HIV care from initial diagnosis to viral suppression (Figure 1) and can be used to identify treatment gaps and intervention opportunities. Each step requires diagnostic tests to provide the necessary information to move on to the following step [8]. Increased HIV testing, earlier diagnosis, linkage and retention to care and earlier ART initiation are key requirements for achieving 90-90-90 targets [8]. The HIV care cascade and its concomitant diagnostic pathway are the focus of this review.
Figure 1.
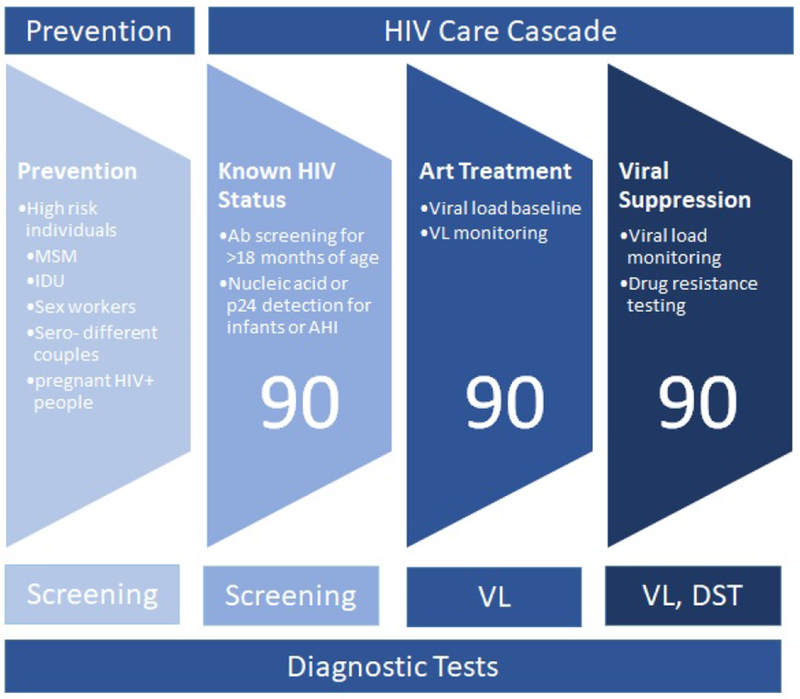
HIV prevention and care cascade. HIV+, HIV positive; IDU, injection drug use; MSM, men who have sex with men; AHI, acute HIV infection; ART, antiretroviral therapy; VL, viral load; DST, drug sensitivity testing. The three steps of cascade associated with UNAIDs 90:90:90 are labeled.
2. Prevention of HIV Transmission
In addition to the HIV care cascade, effective prevention strategies are critical for reducing HIV transmission (Figure 1). Condom use and medical male circumcision prevent transmission. Additionally, there are two pharmacologic approaches: 1) Treatment (ART) as prevention: durable suppression of viremia to an undetectable level such that transmission to uninfected individuals is prevented (Undetectable = Untransmittable or U=U) [9], and 2) pre-exposure prophylaxis (PrEP) with antiviral therapies for individuals without HIV who are at high risk for infection.
2.1. ART as Prevention
It has long been recognized that ART reduces risk of HIV transmission [5]. Two large studies, PARTNER and PARTNER2, followed sero-discordant couples (one HIV infected, the other HIV uninfected) and demonstrated that when the HIV-infected partner was successfully treated with ART, the reported risk of transmission was zero [10, 11].
2.1.1. Diagnostics that support ART as prevention
Viral load monitoring is performed at treatment initiation, then again after three to six months of ART, and at least once annually when VL in “undetectable”. Monitoring VL detects treatment failure. Any detectable VL may result in HIV transmission.
2.2. Pre-Exposure Prophylaxis
Expanding ART has substantial prevention benefits but other strategic prevention interventions will aid in curbing the HIV epidemic [7]. PrEP is recommended for people who are at high risk for acquiring HIV. It works by blocking HIV infection using a combination of two antiviral medications taken daily [13]. If taken as prescribed, daily PrEP reduces the risk of HIV acquisition from sex by more than 90%, but it is much less effective when adherence is suboptimal. [14].
2.2.1. Diagnostic tests required to support PrEP use
Diagnostic support of PrEP requires frequent HIV testing to detect acute HIV infection (AHI) thus minimizing risk of drug resistance selection [15]. Before initiating PrEP, all patients must have a negative HIV test (4th generation antibody/antigen preferred). In patients with symptoms consistent with AHI or who report unprotected sex with an HIV-infected partner, the patient must have both a negative HIV antibody test and a negative HIV RNA test. It would be desirable to confirm that a patient is HIV negative with an HIV RNA test on the day that PrEP is initially prescribed; however, this is not routinely done in clinical care [16]. The patient is monitored every 3 months with a 4th generation HIV antibody/antigen test to detect potential AHI.
2.3. Prevention of Mother to Child Transmission (PMTCT)
HIV transmission from mother to infant can take place during pregnancy, childbirth or through breast feeding. [17]. Without intervention, 15-30% of infants born to HIV-infected mothers will acquire HIV [18]; however, transmission can be prevented. A recent study reported that the risk of HIV transmission to infants from mothers who had suppressed VL was zero [20]. Unfortunately, there is often poor retention of new mothers in care. WHO infant feeding guidelines recommend that women with HIV in settings that lack clean water to reconstitute infant formula should breastfeed infants exclusively for 6 months and continue for 12 months supplementing with appropriate complementary foods, and the mother should remain on treatment and receive adherence support [21].
2.3.1. Diagnostics that support PMTCT
Diagnostic tests required for PMTCT include HIV antibody screening before or during pregnancy with subsequent ART to prevent HIV transmission [22].
3. HIV Care Cascade
Appropriate diagnostic test at different steps of the care cascade depends upon: molecular target, when target is detectable after infection, concentration of target in specimen, volume of specimen tested and limit of detection of test [23].
3.1. Determining HIV Status
The HIV status of most people can be effectively determined by using an antibody test. For outreach testing, point-of-care (POC) testing is an obvious choice; however, longer window periods and lower sensitivities with POC assays compared to lab tests are important trade-offs.
Certain outreach testing populations may be more likely to have recent HIV infections so 4th generation antibody/antigen tests should be prioritized. For PrEP testing, those who become infected in the context of poor adherence may have delayed seroconversion and nucleic acid testing should augment 4th generation antibody/antigen testing [23].
3.2. ART Treatment
VL testing is an important tool for monitoring treatment efficacy. WHO’s “treat all” policy recommends treating all infected patients regardless of CD4+ count. A recent study of six African nations demonstrated that national adoption of the “treat all” policy was associated with large increases in rapid ART initiation [12].
3.3. Viral Suppression
Viral load monitoring is performed regularly with the goal of HIV suppression to lower than the detection limit of the most sensitive assays. If HIV is detected, virologic failure and ART resistance are possible. Genotypic ART resistance can be determined by either DNA sequencing or nucleic acid hybridization/melting assay which can direct second line drug therapy.
4. Diagnostic challenges in SSA
There is substantial variation in HIV prevalence between and within countries in SSA. A recent mapping study of sub-national HIV prevalence reported approximately one-third of PLWHA are spatially concentrated in a few areas of greater than 1000 PLWHA per 5 X 5 kilometer grid. A similar number live in areas with fewer than 100 per grid, and 7.2% live in grids with fewer than 10 [24]. Prevention, screening and treatment strategies differ between these regions. Diagnostic tests and treatment performed in central facilities can serve highly concentrated populations, but are not available in less concentrated areas which may require alternatives such as POC diagnostics.
POC tests primarily lateral flow assays that detect HIV antibodies have been in use in LMICs for nearly 20 years. Twenty-seven different commercial in vitro diagnostic tests are WHO prequalified [25] and have contributed to the reduction in mortality from HIV/AIDS by facilitating testing outside laboratory settings [26, 27] which has driven the scale-up of ART in decentralized clinics.
Other POC tests essential for ART initiation, VL monitoring and drug sensitivity testing (Table 1) are not readily available at the POC. To increase the number of people aware of their status, persons in high risk populations need to be screened frequently with AHI tests. Once HIV has been diagnosed, ART should be initiated immediately [28], with treatment monitoring as it is the most reliable biological indicator for treatment adherence and treatment failure [29]. Recent reports reveal POC testing may contribute to achieving the 90-90-90 guidelines by addressing barriers to HIV testing and treatment.
Table 1.
Diagnostic tests required for HIV care cascade. Molecular Test is a test that detects nucleic acids; RDT = rapid diagnostic test that detects antibodies and/or antigens; ART = antiretroviral therapy; VL = viral load.
| Testing Need | Patient Population | Test Biomarker | Test Format |
|---|---|---|---|
| Diagnosis | |||
| General Screening | People of unknown status | Antibodies | Lateral Flow Assay |
| Acute Infection | Exposed infants & High Risk Groups | Viral Marker: RNA, proviral DNA, p24 | Molecular Test or Lateral Flow Assay, 4th Generation RDT |
| Monitoring | |||
| Treatment Monitoring | Patients on ART | Viral RNA | Molecular Test |
| Drug Resistance | VL>1000cp/ml; Treatment Failure | Viral RNA | Molecular; Sequencing |
In 2017, 1.4 million pregnant women were living with HIV and 180,000 children were infected during pregnancy, child birth, or breastfeeding [30]. Without treatment, 50% of infants with HIV will die before their second birthday with peak mortality at 8-10 weeks. Early and accurate diagnosis could not be more critical. In SSA, early infant diagnosis (EID) typically involves collection of dried blood spot specimens at local health facilities which are transported to central laboratories for nucleic acid testing. The results are returned to the caregiver in 30-90 days which delays treatment initiation and frequently results in failure to initiate treatment [31–34].
4.2. Benefits of point-of-care testing in SSA
Two POC EID assays, m-PIMA HIV-1/2 Detect (Abbott Laboratories; Lake Forest, IL, USA) and Xpert HIV-1 Qual (Cepheid; Sunnyvale, CA, USA) received WHO prequalification in 2016 [35, 36]. These automated diagnostic devices use capillary blood, require little training and have a time to result of 52–90 minutes with similar sensitivity and specificity to the gold standard, Roche CAPP/CTM assay.
In an observational study, Bianchi et al [37] reported that POC EID testing introduced in eight African countries showed dramatic improvements in a number of outcomes over conventional testing including: substantially more test results were returned to caregivers within 30 days [98⋅3% vs 18⋅7] and the median time from sample collection to ART initiation was markedly decreased [0 vs 49 days] at a significantly lower cost [37].
Drain, et al [38] recently reported a positive effect of POC VL testing on viral suppression and retention in care for PLWHA in a randomized controlled trial. For 12 months, the participants were randomized to receive either POC VL testing (Xpert® HIV-1 VL, Cepheid) and same day counseling or standard-of-care (SOC) laboratory VL testing. After 12 months, a 13.9% increase was observed in the number of patients retained with suppressed VL who received POC testing compared to the SOC. The disaggregated results demonstrated that POC VL testing increased VL suppression by 10.3% and increased retention by 7.7%.
The studies described above highlight the potential for POC testing to make significant impact in LMICs by providing a timely and actionable result. Technology designed specifically for low resource settings is required to expand this benefit to more remote locations by eliminating the need for trained and computer-literate operators, stable supply of electricity or an uninterrupted power device, air conditioning to moderate operating and reagent storage temperatures, refrigeration, land-line communication and central water supply [39].
5. Sponsorship of POC Technology Development
In 2018, as part of National Institute of Biomedical Imaging and Bioengineering’s (NIBIB) Point of Care Technology Research Network, the Center for Innovation in Point-of-Care Technologies for HIV/AIDS at Northwestern University (C-THAN) was founded with funds from NIBIB, Fogarty International Center, and Office of AIDS Research to develop POC technologies critical for improved management of HIV/AIDS and facilitate technology commercialization in LMICs with specific emphasis on SSA (Figure 2).
Figure 2.
C-THAN Logo, mission statement and website.
C-THAN has seven clinical sites in SSA: Universities of Lagos, Ibadan and Jos (Nigeria), Cape Town and Stellenbosch (South Africa), University of Sciences, Techniques and Technologies Bamako (Mali) and Muhimbili University of Health and Allied Sciences (Tanzania) and three biomedical engineering sites: Universities of Lagos, Ibadan and Cape Town.
Four collaborative cores coordinate product development: Administrative, Technology Development and Refinement, Clinical Translation and Validation and Technology Training and Dissemination (Figure 3). Clinical and user needs are incorporated into the development process while expertise and resources to address barriers to commercialization and implementation are provided.
Figure 3.
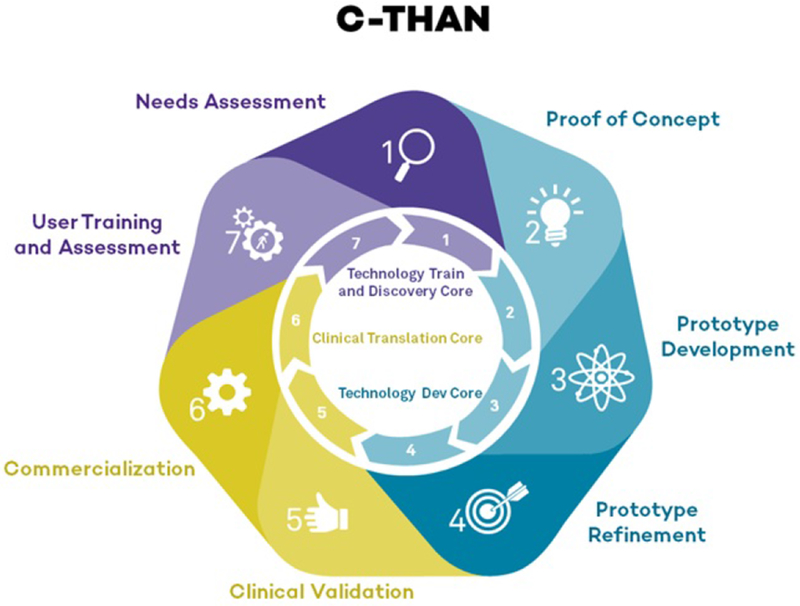
C-THAN product pipeline/ product development cycle. Each step of the product development cycle was assigned a number from 1-7 to assign a level of technology readiness so that different projects can be evaluated via the same metrics. The turquoise section of the wheel is managed by the Technology Development and Refinement Core, the gold section is managed by the Clinical Translation and Validation Core and the lavender section is managed by the Technology Training and Dissemination Core.
Annual solicitations are published in January on the C-THAN website (Figure 2). The $100,000 awards address high priority HIV/AIDS research topics, are designed for low resource settings, have commercialization potential and are intended to stimulate development of funding proposals and/or attract venture capital. Applicants from LMICs, either independently or in collaboration with developers in high resource countries, are encouraged to apply. Consultations with C-THAN staff is encouraged.
6. Conclusions
Meeting UNAID’s HIV/AIDS 90-90-90 goals requires healthcare transformation for PLWHA. C-THAN supports collaborations across disciplines, institutions and geographies to drive innovation from concept to patient care and seeks technology development, clinical implementation, manufacturing and regulatory collaborators.
Highlights.
UNAIDS 90-90-90 treatment target to be reached by 2020 will help end AIDS epidemic.
Point-of-care technologies designed for HIV care cascade will help meet this target.
C-THAN supports development and facilitates commercialization of POC technology.
C-THAN focuses on HIV High Priority Research Topics within sub-Saharan Africa.
Acknowledgements
Funding: C-THAN is funded by 1U54EB027049-01NIBIB, Fogarty International Center and Office of AIDS Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The view expressed in this article are of the authors and not the official position of the NIH, institution or funder.
Disclosures
The authors declare no conflict of interest.
References
- 1.Lahuerta M, Ue F, Hoffman S, Elul B, Kulkarni SG, Wu Y, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24(1):359–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2017 G. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2017 G. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. HIV/AIDS Data and Statistics 2016. [cited 2018 05 January]. Available from: http://www.who.int/hiv/data/mortality_targets_2016.
- 5.WHO. Progress Report 2016 Prevent HIV, Test and Treat All. 2016. 05 January.
- 6.UNAIDS. UNAIDS. 90-90-90: A Transformative Agenda to Leave No One Behind 2017. [cited 2017 Available from: http://www.unaids.org/en/speeches/2014/20141025_SP_EXD_Vietnam_launch_of_909090_en.pdf.
- 7.Bekker LG, Alleyne G, Baral S, Cepeda J, Daskalakis D, Dowdy D, et al. Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: The International AIDS Society-Lancet Commission. Lancet (London, England). 2018;392(10144):312–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gueler A, Vanobberghen F, Rice B, Egger M, Mugglin C. The HIV Care Cascade from HIV diagnosis to viral suppression in sub-Saharan Africa: a systematic review and meta-regression analysis protocol. Systematic reviews. 2017;6(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS. Successful treatment of HIV eliminates sexual transmission. Lancet (London, England). 2019. [DOI] [PubMed] [Google Scholar]
- 10.*.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Degen O, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet (London, England). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; In the PARTNER2 study, gay sero-discordant couples were followed and if the HIV-positive partner received ART and maintained a plasma HIV-1 RNA level of less than 200/mL, then the risk of HIV transmission to his HIV-negative partner was zero.
- 11.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–81. [DOI] [PubMed] [Google Scholar]
- 12.*.Tymejczyk O, Brazier E, Yiannoutsos CT, Vinikoor M, van Lettow M, Nalugoda F, et al. Changes in rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis. PLoS Med 2019;16(6):e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors provide a rigorous assessment of the real-world impact of WHO’s “treat all” policy on antiretroviral treatment ART uptake in 6 sub-Saharan countries. Strong effects on increasing rates of rapid ART initiation were observed in all six countries but with different trajectories.
- 13.Chou R, Evans C, Hoverman A, Sun C, Dana T, Bougatsos C, et al. Preexposure Prophylaxis for the Prevention of HIV Infection: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2019;321(22):2214–30. [DOI] [PubMed] [Google Scholar]
- 14.CDC. Pre-Exposure Prophylaxis (PrEP) Atlanta: CDC; 2018. [cited 2019 12 June 2019]. Available from: https://www.cdc.gov/hiv/basics/prep.html. [Google Scholar]
- 15.Fransen K, de Baetselier I, Rammutla E, Ahmed K, Owino F, Agingu W, et al. Detection of new HIV infections in a multicentre HIV antiretroviral pre-exposure prophylaxis trial. Journal of Clinical Virology: the official publication of the Pan American Society for Clinical Virology. 2017;93:76–80. [DOI] [PubMed] [Google Scholar]
- 16.Center PAEaT. HIV Pre-Exposure Prophylaxis (PrEP): A brief guide for providers 2016. [Available from: http://paetc.org/wp-content/uploads/2016/01/PAETC-PrEP-primer-1.pdf.
- 17.Arkell C. Pregnancy and infant feeding: Can we say U=U about the risk of passing HIV to an infant? 2018. [Available from: https://www.catie.ca/en/pif/spring-2018/pregnancy-and-infant-feeding-can-we-say-uu-about-risk-passing-hiv-infant.
- 18.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. The Cochrane database of systematic reviews. 2011(7):Cd003510. [DOI] [PubMed] [Google Scholar]
- 19.Mandelbrot L, Tubiana R, Le Chenadec J, Dollfus C, Faye A, Pannier E, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis 2015;61(11):1715–25. [DOI] [PubMed] [Google Scholar]
- 20.Luoga E, Vanobberghen F, Bircher R, Nyuri A, Ntamatungiro AJ, Mnzava D, et al. Brief Report: No HIV Transmission from Virally Suppressed Mothers During Breastfeeding in Rural Tanzania. J Acquir Immune Defic Syndr 2018;79(1):e17–e20. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 22.UNAIDS. Fast-Track Ending the AIDS Epidemic by 2030. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- 23.Hurt CB, Nelson JAE, Hightow-Weidman LB, Miller WC. Selecting an HIV Test: A Narrative Review for Clinicians and Researchers. Sexually transmitted diseases. 2017;44(12):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.*.Dwyer-Lindgren L, Cork MA, Sligar A, Steuben KM, Wilson KF, Provost NR, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors performed a mapping study exploring within country variation at a 5 X %-km resolution of sub-Saharan Africa by estimating the prevalence of HIV among adults and the corresponding number of people living with HIV from 2000-2017. These estimates of HIV prevalence across geography and time are an important tool for targeting HIV interventions to specific populations.
- 25.WHO. In vitro diagnostics and laboratory technology Public reports of WHO prequalified IVDs 2018. [Available from: http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-rdts/public_report/en/.
- 26.Pai NP, Wilkinson S, Deli-Houssein R, Vijh R, Vadnais C, Behlim T, et al. Barriers to Implementation of Rapid and Point-of-Care Tests for Human Immunodeficiency Virus Infection: Findings from a Systematic Review (1996-2014). Point Care. 2015;14(3):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid SD, Fidler SJ, Cooke GS. Tracking the progress of HIV: the impact of point-of-care tests on antiretroviral therapy. Clin Epidemiol 2013;5:387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV Geneva, Switzerland: 2015. [Available from: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. [PubMed] [Google Scholar]
- 29.Usdin M, Guillerm M, Calmy A. Patient needs and point-of-care requirements for HIV load testing in resource-limited settings. J Infect Dis 2010;15(201):650384. [DOI] [PubMed] [Google Scholar]
- 30.UNICEF. Elimination of mother-to-child transmission 2018. [Available from: https://data.unicef.org/topic/hivaids/emtct/.
- 31.Mugambi ML, Deo S, Kekitiinwa A, Kiyaga C, Singer ME. Do diagnosis delays impact receipt of test results? Evidence from the HIV early infant diagnosis program in Uganda. PLoS One. 2013;8(11):e78891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutcliffe CG, van Dijk JH, Hamangaba F, Mayani F, Moss WJ. Turnaround time for early infant HIV diagnosis in rural Zambia: a chart review. PLoS One. 2014;9(1):e87028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutcliffe CG, van Dijk JH, Muleka M, Munsanje J, Thuma PE, Moss WJ. Delays in Initiation of Antiretroviral Therapy Among HIV-infected Children in Rural Zambia. The Pediatric infectious disease journal. 2016;35(4):e107–12. [DOI] [PubMed] [Google Scholar]
- 34.Phiri NA, Lee HY, Chilenga L, Mtika C, Sinyiza F, Musopole O, et al. Early infant diagnosis and outcomes in HIV-exposed infants at a central and a district hospital, Northern Malawi. Public health action. 2017;7(2):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. WHO prequalification of in vitro diagnostics public report. Product: Xpert HIV-1 Qual Assay Geneva, Switzerland: WHO; 2016. [Available from: https://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/160613PQPublicReport_0259-0700-00_XpertQualHIV_v2.pdf. [Google Scholar]
- 36.WHO. WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: m-PIMA HIV-1/2 VL Geneva, Switzerland: WHO; 2016. [Available from: https://www.who.int/diagnostics_laboratory/evaluations/pq-list/190408_pqdx_0359_032_00_pqpr_mpima.pdf. [Google Scholar]
- 37.*.Bianchi F, Cohn J, Sacks E, Bailey R, Lemaire JF, Machekano R. Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: an observational study in eight African countries. The lancet HIV. 2019;6(6):e373–e81. [DOI] [PubMed] [Google Scholar]; Authors report on observation study where service deliver and clinical outcomes in eight sub-Saharan countries before and after a point-of-care early infant HIV diagnostic intervention was introduced. Point-of-care testing dramatically improved the speed of return HIV test results and thus enabled prompt ART initiation which could potentially reduce morbidity and mortality in infants with HIV.
- 38.Drain PK, Dorward J, Violette L, Quame-Amaglo J, Thomas K, Samsunder N, et al. Point-Of-Care Viral Load Testing Improves HIV Viral Suppression and Retention in Care. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington 2019. [Google Scholar]
- 39.McNerney R, Cunningham J, Hepple P, Zumla A. New tuberculosis diagnostics and rollout. International Journal of Infectious Diseases. 2015;32:81–6. [DOI] [PubMed] [Google Scholar]


