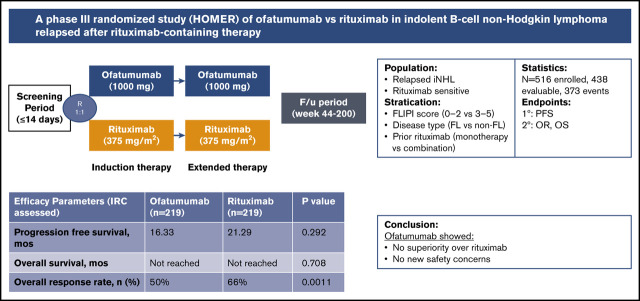
Key Points
Ofatumumab showed no superiority over rituximab in patients with FL who had relapsed after a rituximab-containing therapy.
Ofatumumab showed no new safety concerns in the current study and the adverse events were well managed.
Abstract
Because of high relapse rates with rituximab combinations, there is an unmet need for new therapeutic agents for treatment of indolent B-cell non-Hodgkin lymphoma (iNHL) or follicular lymphoma (FL). In previous trials, ofatumumab in combination with chemotherapy showed good results in relapsed/refractory FL pretreated with rituximab. This phase 3 trial evaluated the efficacy and safety of single-agent ofatumumab vs single-agent rituximab in rituximab-sensitive relapsed FL that relapsed at least 6 months after completing the last prior treatment with single-agent rituximab or a rituximab-containing regimen. Patients were randomized 1:1 to receive either ofatumumab (1000 mg) or rituximab (375 mg/m2) every week for 4 weeks for the induction phase, followed by once every 2 months for 4 additional doses. The primary endpoint, progression-free survival (PFS) and secondary endpoints, overall response rate (ORR) and overall survival (OS), were evaluated. Overall, 438 patients were assigned to receive ofatumumab (n = 219) and rituximab (n = 219). Baseline characteristics were similar in both arms. The independent review committee assessed whether median PFS was shorter in the ofatumumab arm than in the rituximab arm (16.33 vs 21.29 months), with no significant difference (hazard ratio, 1.15; 95% confidence interval, 0.89-1.49; P = .29) and also showed a lower ORR (50%) compared with the rituximab arm (66%). At the time of analysis, data were not matured for OS results. The number of grade >3 adverse events was higher in the ofatumumab arm (37%) than the rituximab arm (28%). Ofatumumab showed no superiority over rituximab in patients with FL who had relapsed after a rituximab-containing therapy. This study was registered at www.clinicaltrials.gov as #NCT01200589.
Visual Abstract
Introduction
Non-Hodgkin lymphomas (NHLs) represent 4% of all malignancies with an incidences of 72 240 in United States, 2017.1 Indolent NHLs (iNHLs) are a heterogeneous group of low-grade lymphomas which classically include follicular lymphoma (FL), marginal zone lymphoma, a subset of mantle cell lymphoma, and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma. FL is the most common iNHL, which accounts for 20% to 30% of all newly diagnosed cases of B-cell NHL,2 and has an annual incidence of 1.6 to 3.1/100 000 cases in western countries.3,4 High expression of CD20 in FL makes this population a specific target for treatment with anti-CD20 antibodies.5 Development of the anti-CD20 antibody rituximab has had a remarkable impact on the management of FL. Rituximab is prescribed either as a single agent or as combination therapy and has become the standard of care for both FL and other types of iNHL patients.6 Rituximab in combination with chemotherapy has significantly improved the outcomes in FL, including response rates, progression-free survival (PFS), and overall survival (OS).7-10 However, there remains an unmet need for novel therapeutic agents in the relapsed iNHL because patients have an ongoing risk of a relapse after treatment, despite high initial response rates.
A range of novel therapeutic agents for the treatment of FL are currently available or are in development, including phosphoinositide 3-kinase inhibitors, immunomodulatory agents, inhibitors of Bruton tyrosine kinase, antibody-drug conjugates, novel anti-CD20 monoclonal antibodies, chimeric antigen receptor T-cell therapy, immune checkpoint inhibitors, inhibitors of nuclear export proteins, and others.11-16 Recently, obinutuzumab, an anti-CD20 antibody, in combination with the alkylating chemotherapeutic agent, bendamustine, was approved in patients who failed to respond to an rituximab-containing regimen.17 Even with the approval of obinutuzumab, treatment options for these patients are limited and there is an unmet need for the FL patients who have relapsed after rituximab-containing therapy.
Ofatumumab targets a novel CD20 epitope on B cells and is released slowly from the target molecule compared with rituximab. Data from preclinical trials have shown that ofatumumab has the potential to treat B-cell malignancies with low CD20 and high CD55 and CD59 expressions, such as B-CLL and rituximab-relapsed/refractory (r/r) FL.18-21 In previous phase 1/2 studies, ofatumumab showed an overall response rate (ORR) of 43% along with a durable median duration of response of 29.9 months in rituximab-r/r FL patients.22 This phase 3 randomized trial was designed to evaluate the efficacy and safety of single-agent ofatumumab vs single-agent rituximab in patients with iNHL who had relapsed after prior rituximab-containing therapy.
Patients and methods
Study design and patient population
Single Agent Ofatumumab vs. Single Agent Rituximab in Indolent B-Cell Non Hodgkin Lymphoma Relapsed After Rituximab-Containing Therapy (HOMER) was a phase 3 randomized open-label study of single-agent ofatumumab compared with single-agent rituximab in iNHL relapsed after rituximab monotherapy or a rituximab-containing regimen. The patient population consisted of patients who were at least 18 years old and carried a diagnosis of FL (grades 1-3A), small lymphocytic lymphoma, marginal zone lymphoma, and lymphoplasmacytic lymphoma that was rituximab-sensitive. Rituximab sensitivity was defined as partial response (PR) or complete response (CR) to their last prior treatment with rituximab or a rituximab-containing regimen lasting at least 6 months following completion of the rituximab treatment. Patients must have had 1 or more of the following indications for treatment: (1) cytopenias; (2) at least 1 lymphoma-related symptom; (3) progressive or massive lymphadenopathy; or (4) progressive or massive organomegaly. Patients had an Eastern Cooperative Oncology Group Performance Status of 0-2. Key exclusion criteria included: prior treatment with ofatumumab; prior exposure to anti-CD20 radioimmunotherapy or non-rituximab anti-CD20 therapy within 6 months before randomization; autologous stem cell transplant within 6 months before randomization; prior allogeneic stem cell transplant; systemic therapy for lymphoma within 3 months before randomization; chronic or current active infectious disease; clinically significant cardiac, cerebrovascular, liver, or biliary disease; and/or screening laboratory abnormalities (neutrophils <1.5 × 109/L, platelets <50 × 109/L, alkaline phosphatase >1.5 times upper limit of normal [ULN], total bilirubin >1.5 times ULN, and/or transaminases >3 times ULN).
All patients were required to provide written informed consent before any screening procedures. The study protocol was approved by the institutional review board or ethics committee at each participating center, and the study was conducted according to the ethical principles of the Declaration of Helsinki. Patients were stratified according to the type of last prior rituximab therapy (monotherapy vs combination therapy) and disease type (FL vs non-FL). Those with FL were further stratified by FL international prognostic index-1 score.23 Patients were randomized 1:1 to receive either ofatumumab (1000 mg) or rituximab (375 mg/m2) every week for 4 weeks for the induction phase, followed by once every 2 months for 4 additional doses. Dose reductions were not allowed, but investigators could use medical discretion to delay treatment due to infection or other adverse events (AEs).
Assessment of efficacy and safety
The response assessments of CR, PR, stable disease, or progressive disease were based on criteria defined in the modified 2007 Revised Response Criteria for Malignant Lymphoma.24 Response assessments were determined based on history and physical examination; complete blood count; and computed tomography scans of the neck, chest, abdomen and pelvis with contrast (unless contraindicated). To confirm CR, bone marrow aspirate and biopsy was also required. Response assessments were performed 2 months (week 12) after the fourth dose of antibody to determine response to induction and repeated at 2 months after dose 6 (week 28) and 2 months after dose 8 of antibody (week 44). Follow-up response assessments were performed every 26 weeks until week 200. The primary endpoint of the study was PFS defined as time from randomization to disease progression or death due to any cause as determined by an independent review committee (IRC). Because of the study’s early closure for futility, only the primary endpoint, PFS, and selected secondary endpoints, ORR, CR, and OS, were analyzed. Other secondary efficacy objectives and exploratory objectives (duration of response, time to next treatment, and pharmacokinetics) were considered out of scope in the present study.
All AEs and serious AEs (SAEs), regardless of the relationship to study drug, were collected from the first dose of treatment until 60 days after the last treatment. SAE collection continued to be reported from 61 days after the last dose until the end of follow-up period or until subsequent antilymphoma therapy initiated.
Statistical analysis
The primary analysis of PFS was to be performed when 373 PFS events as assessed by the IRC had been reached. A planned interim analysis for efficacy and safety was performed when 182 IRC PFS events had been reached, and an independent data monitoring committee had recommended the study be terminated because of futility. There were no safety concerns noted at that time.
Because of the early closure of this study for futility, the final primary endpoint analysis was conducted based on all available study data at that time. The intent-to-treat (ITT) population was used for efficacy analyses. The safety population comprised all patients who received at least 1 dose of study drug. The statistical analysis for this study was performed using currently supported version of SAS (version 9.3). All data up to the time of study completion/withdrawal from study were included in the analysis regardless of treatment duration. All confidence intervals (CIs) were 2-sided and used a 95% confidence level. All P values reported in this document are nominal P values, not adjusted for multiplicity.
Results
Patients and treatment
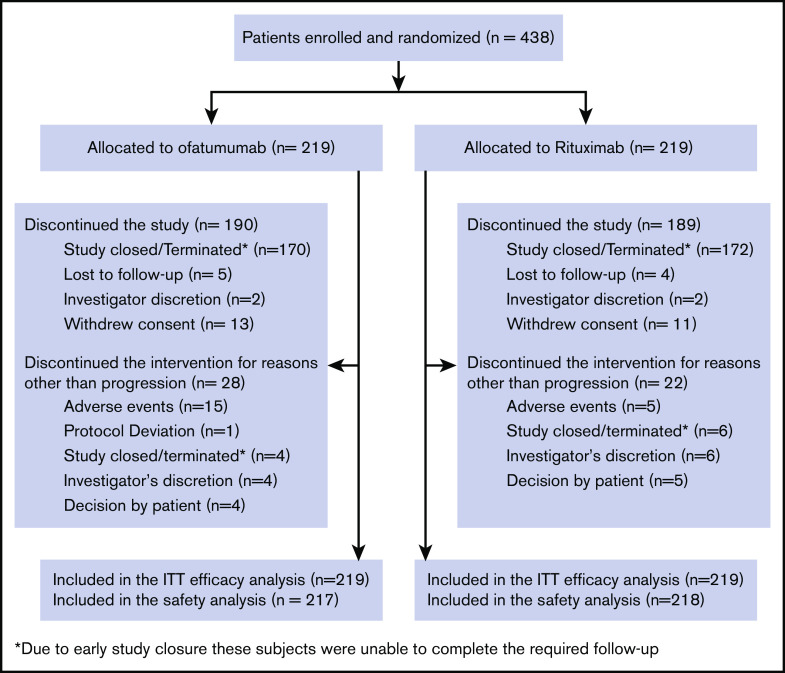
Between October 2010 and May 2016, a total of 438 patients were enrolled and randomized from 99 sites across 16 countries (219 in each arm; Figure 1). Following the interim analysis, the independent data monitoring committee recommended for early closure of the study because of futility (no superiority of ofatumumab over rituximab in efficacy).
Figure 1.
Consort diagram.
The baseline demographic and disease characteristics were well balanced between the 2 treatment arms (Table 1), with the exception of median time from initial diagnosis, which was shorter for the ofatumumab arm (4.7 years) vs rituximab (5.3 years). The median time from disease progression was similar for the 2 arms. Chemotherapy (90%) and biologic therapy (79%) were the most common prior anticancer therapies. All patients in both the treatment arms received rituximab containing regimen before the start of study; overall, 98% patients and 99% patients had FL in ofatumumab and rituximab arms, respectively. ORR for the most recent prior anticancer therapy was 96% and 95% in the ofatumumab and rituximab arms, respectively.
Table 1.
Patient characteristics
| Parameter | Ofatumumab (n = 219) | Rituximab (n = 219) |
|---|---|---|
| Age, n (%), y | ||
| ≤65 | 148 (68) | 142 (65) |
| >65 | 71 (32) | 77 (35) |
| Age, y | ||
| Median (min, max) | 61.0 (27, 90) | 62 (26, 85) |
| Sex, n (%) | ||
| Female | 115 (53) | 109 (50) |
| Male | 104 (47) | 110 (50) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 15 (7) | 21 (10) |
| Not Hispanic or Latino | 204 (93) | 198 (90) |
| Race, n (%) | ||
| White | 122 (56) | 131 (60) |
| Asian | 89 (41) | 79 (36) |
| Other | 7 (3) | 6 (3) |
| Time since diagnosis, y | ||
| Median (min, max) | 4.7 (1, 24) | 5.3 (1, 20) |
| Time since disease progression, y | ||
| Median (min, max) | 0.1 (0, 13) | 0.2 (0, 13) |
| Number of prior therapies | ||
| Median (min, max) | 2 (1, 10) | 2 (1, 9) |
| Number of prior rituximab therapies | ||
| Median (min, max) | 1 (1, 6) | 1 (1, 8) |
| Rituximab-based therapy, n (%) | ||
| Any therapy | 219 (100) | 219 (100) |
| Monotherapy | 78 (36) | 84 (38) |
| Combination therapy | 199 (91) | 202 (92) |
| Collected FL international prognostic index-1 score, n (%) | ||
| 0-3 | 173 (79) | 165 (75) |
| 4 and 5 | 19 (9) | 15 (7) |
| Bulky lymphadenopathy at screening, n (%) | ||
| Yes | 13 (6) | 17 (8) |
| iNHL subtype, n (%) | ||
| FL | 214 (98) | 214 (98) |
| Non-FL | 5 (2) | 5 (2) |
| Prior anticancer therapy n (%) | ||
| Biologic therapy | 177 (81) | 169 (77) |
| Chemotherapy | 196 (89) | 199 (91) |
| Immunotherapy | 28 (13) | 43 (20) |
| Small molecule targeted therapy | 2 (<1) | 6 (3) |
| Other | 5 (2) | 4 (2) |
| Best response, n (%) | ||
| Complete response | 136 (62) | 140 (64) |
| Partial response | 74 (34) | 69 (32) |
| Stable disease | 4 (2) | 3 (1) |
| Progressive disease | 2 (<1) | 1 (<1) |
| Ann Arbor staging at screening (A + B), n (%) | ||
| I | 22 (10) | 18 (8.2) |
| II | 49 (22.4) | 41 (18.7) |
| III | 74 (33.8) | 80 (36.5) |
| IV A | 74 (33.8) | 80 (36.5) |
min, minimum; max, maximum.
Only 13% of patients in the ofatumumab arm and 14% in the rituximab arm completed the study. The majority of the patients discontinued the study early primarily as a result of study termination (78% in the ofatumumab arm and 79% in the rituximab arm). Despite the early study closure, a total of 292 patients completed the study treatment per protocol: 142 patients (65%) in the ofatumumab arm and 150 patients (68%) in the rituximab arm. Disease progression was the most common reason for discontinuation of the study treatment (ofatumumab 22% vs rituximab 21%). The median duration of exposure was the same in the 2 treatment groups (8.082 months each). A slightly lower proportion of patients in the ofatumumab arm received 8 cycles of study treatment compared with those in the rituximab arm (66% vs 72%).
Efficacy
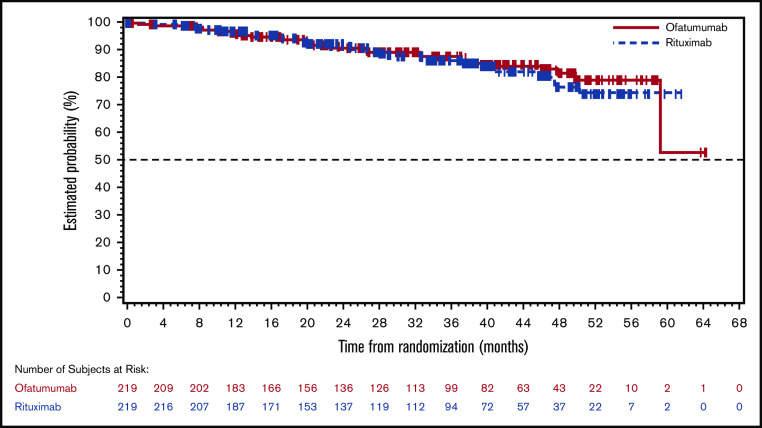
At the time of the final analysis, 231 PFS events (ofatumumab, 114; rituximab, 117) had been assessed by the IRC. The median IRC-assessed PFS based on these events was shorter in the ofatumumab arm (16.33 months) than in the rituximab arm (21.29 months), with no significant difference observed between the 2 arms (hazard ratio, 1.15; 95% CI, 0.89-1.49; P = .292; Figure 2A). Investigator-assessed PFS demonstrated similar results (16.16 months in ofatumumab arm vs 19.22 months in rituximab arm) (Figure 2B). No significant benefit in PFS was seen with ofatumumab compared with the rituximab arm in all analyzed subgroups, including baseline demographic characteristics. Based on the data available at the data cutoff, the estimates of OS were similar in the 2 treatment arms; however, data were not mature for OS analysis at the time of the final analysis (hazard ratio, 0.91; 95% CI, 0.54-1.52], P = .708) (Figure 3). Per the IRC assessment, ORR (CR + PR) was lower in the ofatumumab arm than the rituximab arm (50% vs 66%; P = .0011). CR was observed in 36 patients (16%) in the ofatumumab arm and 44 patients (20%) in the rituximab arm. PR was the best response in 74 patients (34%) and 100 patients (46%) in the ofatumumab and rituximab arms, respectively (Table 2). Similar results were seen in investigator-assessed ORR (ofatumumab 46% vs rituximab 61%). Investigator-assessed rates of CR and PR in the ofatumumab vs rituximab arms were 16% and 30% vs 21% and 40%, respectively. In the ofatumumab arm, 117 (53%) patients received subsequent anticancer therapy, as compared with 105 (48%) in the rituximab arm.
Figure 2.
Kaplan-Meier PFS. (A) Independent reviewer-assessed Kaplan-Meier PFS (ITT population). (B) Investigator-assessed Kaplan-Meier PFS (ITT population).
Figure 3.
Kaplan-Meier OS.
Table 2.
IRC-assessed best response
| Ofatumumab (n = 219) | Rituximab (n = 219) | |
|---|---|---|
| Best response, n (%) | ||
| CR | 36 (16) | 44 (20) |
| PR | 74 (34) | 100 (46) |
| Stable disease | 71 (32) | 50 (23) |
| Progressive disease | 22 (10) | 23 (11) |
| Not evaluable | 16 (7) | 1 (< 1) |
| Responder | ||
| Yes (CR + PR), n (%) | 110 (50) | 144 (66) |
| No, n (%) | 109 (50) | 75 (34) |
| 95% CI, % | 43, 57 | 59, 72 |
| P* | .0011 | |
| Test for homogeneity of odds ratios across strata for responder | ||
| P† | .77 |
CMH, Cochran-Mantel-Haenszel.
CMH test adjusted for stratum.
Test of homogeneity of odds ratios across planned stratum (Breslow-Day test).
Safety
The majority of the patients in either treatment arm experienced at least 1 AE during the course of the study. The incidence of AEs was higher in the ofatumumab arm compared with the rituximab arm (93% vs 84%). In the ofatumumab arm, 37% of patients experienced a grade ≥3 AEs as compared with 28% in the rituximab arm, of which 27% and 15%, respectively, were suspected to be related to study drug. The most commonly reported grade ≥3 AEs (≥5% incidence in either arm) were urticaria (ofatumumab 7% vs rituximab 0%), infusion-related reactions (ofatumumab 5% vs rituximab 0%), and neutropenia (ofatumumab 2% vs rituximab 6%) (Table 3). The incidence of SAEs during the study was similar in the 2 arms (ofatumumab 18% vs rituximab 17%). The most frequent SAEs (≥1% incidence in either groups) in both the arms were infusion related reactions (ofatumumab 2% vs rituximab 0%), pneumonia (ofatumumab <1% vs rituximab 2%) and sepsis (ofatumumab <1% vs rituximab 1%) (Table 3). The incidence of AEs of special interest was higher in the ofatumumab arm compared with the rituximab arm, including infusion-related AEs (ofatumumab 82% vs rituximab 51%), infection-related AEs (ofatumumab 32% vs rituximab 37%), cardiac events (ofatumumab 7% vs rituximab 4%), neoplasms (ofatumumab 6% vs rituximab 3%), and mucocutaneous reactions (ofatumumab 58% vs rituximab 22%) (Table 3). AEs leading to study drug discontinuation were reported in 6% of patients in the ofatumumab arm including urticaria (1%) and hypotension (< %), and in 3% of patients in the rituximab arm. The incidence of AEs leading to dose interruption/delay was higher in the ofatumumab arm compared with the rituximab arm (72% vs 26%). The most frequent AEs leading to drug interruption/delay (≥5% in either arm) were infusion-related reactions (ofatumumab 17% vs rituximab 5%), urticaria (ofatumumab 17% vs rituximab <1%), rash (ofatumumab 15% vs rituximab 2%), and pruritus (ofatumumab 7% vs rituximab 2%).
Table 3.
AEs, AEs ≥grade 3 and serious AEs, and AEs of special interest
| Preferred term | Ofatumumab (n = 217) | Rituximab (n = 218) |
|---|---|---|
| AEs (≥10% in either arm), n (%) | ||
| Any event | 202 (93) | 183 (84) |
| Infusion-related reaction | 45 (21) | 19 (9) |
| Rash | 42 (19) | 11 (5) |
| Urticaria | 41 (19) | 3 (1) |
| Fatigue | 21 (10) | 29 (13) |
| Diarrhea | 21 (10) | 15 (7) |
| Nasopharyngitis | 14 (6) | 22 (10) |
| White blood cell count decreased | 12 (6) | 23 (11) |
| Pyrexia | 9 (4) | 22 (10) |
| AEs ≥ grade 3 (≥5% of patients), n (%) | ||
| Any event | 81 (37) | 60 (28) |
| Urticaria | 16 (7) | 0 (0) |
| Infusion-related reaction | 11 (5) | 0 (0) |
| Neutropenia | 4 (2) | 12 (6) |
| Serious AEs (≥1% of patients), n (%) | ||
| Any event | 38 (18) | 37 (17) |
| Infusion-related reaction | 5 (2) | 0 (0) |
| Pneumonia | 2 (< 1) | 5 (2) |
| Sepsis | 1 (< 1) | 3 (1) |
| AEs of special interest (≥1% of patients), n (%) | ||
| Infusion-related AEs | 178 (82) | 112 (51) |
| Infection-related AEs | 69 (32) | 81 (37) |
| Cardiac events | 15 (7) | 8 (4) |
| Neoplasms | 13 (6) | 7 (3) |
| Muco-cutaneous reactions | 125 (58) | 49 (22) |
| Tumor lysis syndrome | 3 (1) | 0 (0) |
Death
A total of 28 deaths were reported in the ofatumumab arm and 30 deaths in the rituximab arm. One on-treatment death (time from treatment initiation to stopping the last dose plus 30 days) occurred during the study in the ofatumumab arm from sepsis related to the study drug. The other death reported in ofatumumab arm related to study drug was due to pneumonia. Primary causes of death were disease under study (ofatumumab 8% vs rituximab 10%), SAE possibly related to study treatment (ofatumumab <1% vs rituximab 0%), and other (ofatumumab 4% vs rituximab 4%).
Discussion
The CD20-directed monoclonal antibody rituximab was approved for single-agent maintenance therapy in patients with previously untreated follicular CD20+ B-NHL who achieved a CR or PR to rituximab in combination with first-line chemotherapy. Single-agent rituximab can be used in relapsed FL,22 but more patients are becoming rituximab refractory after receiving the drug with primary therapy and as maintenance.
Compared with rituximab, ofatumumab binds to a novel epitope of the CD20 molecule on B cells and releases only very slowly from the target. Ofatumumab was observed to be superior to rituximab in its ability to lyse different B cell lines (eg, SU-DHL-4, Daudi, Raji) and to destroy B-CLL cells resistant to rituximab in in vitro studies.20,21 In animal studies, ofatumumab has been shown to deplete B cells effectively; depletion of B cells from peripheral blood and lymph nodes of cynomolgus monkeys induced by ofatumumab lasted longer than that induced by rituximab.19 Ofatumumab has also proved therapeutic against Daudi cell growth in an in vivo mouse xenograft model with maximum effect at 0.5 mg/kg intraperitoneally.18
HOMER is the largest phase 3 study comparing the efficacy and safety profile of 2 type I anti-CD20 monoclonal antibodies, ofatumumab and rituximab, in patients with iNHL who were sensitive to and had relapsed after a prior rituximab-containing therapy. The study was stopped because of futility based on the results of the planned interim analysis and consequently did not meet its primary objective to show superiority of the treatment with ofatumumab over rituximab in patients with iNHL relapsed after rituximab-containing therapy. The data presented here are from the final analysis of the study.
A similar study, the GAUSS: A Study of Obinutuzumab (RO5072759) in Patients With Indolent Non-Hodgkin’s Lymphoma (GAUSS) study,16 was a randomized, phase 2 trial conducted in 175 patients with relapsed CD20+ iNHL to evaluate the efficacy of obinutuzumab, a type II anti-CD20 mAb, as compared with rituximab. The results showed a significant improvement in the ORR (CR + PR) with obinutuzumab vs rituximab (44.6% vs 33.3%, P = .08).16 This is contrary to the results of HOMER, which showed a significantly lower ORR with ofatumumab compared with rituximab. Of note, the ORR of both treatments arms was higher in the HOMER study (ofatumumab, 50%; rituximab, 66%; P = .0011) than in the GAUSS study (obinutuzumab, 44.6%; rituximab, 33.3%; P = .08).16 Although our study showed better ORR in the 2 arms than the GAUSS study,16 the difference between the ORR for ofatumumab and for rituximab was not observed to be significantly different. Despite the GAUSS trial demonstrating an improvement in ORR with obinutuzumab, it failed to translate to a prolonged PFS (17.6 months in the obinutuzumab arm vs 25.4 months in the rituximab arm, P = .74).16 This finding is consistent with the results of our study where no statistically significant difference in the PFS between the 2 treatment arms.
The safety profile of ofatumumab observed in the study was consistent with prior experience with ofatumumab as a single agent in the oncology setting,22,25 with no major safety concerns. Grade ≥3 AEs observed in the ofatumumab arm (37%) were slightly higher compared with the rituximab arm (28%). Infections occurred in 32% of patients in the ofatumumab arm, similar to 33% observed in the previous phase 1/2 trial of ofatumumab in r/r FL.22 Overall, 21% of patients experienced infusion-related AEs in the ofatumumab arm, of whom only 3% of patients discontinued the treatment. No fatal infusion-related AEs were reported. All the AEs reported during the study were well managed. A limitation of this study is that around 2/3 of the patients completed 8 cycles of both the study drugs.
In conclusion, ofatumumab showed no superiority over rituximab in patients with iNHL who had relapsed after a rituximab-containing therapy. In rituximab-sensitive patients with iNHL, use of ofatumumab over rituximab should be evaluated with caution. Ofatumumab showed no new safety concerns in the current study and the AEs were well managed.
Acknowledgments
The authors thank patients and their families, investigators, and staff from all the participating sites and Saurabh Agarwal of Novartis Healthcare Pvt. Ltd. for providing medical editorial assistance with this manuscript.
This work was supported by research funding from Novartis Pharma AG, Basel, Switzerland.
Footnotes
Data sharing statement: Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Authorship
Contribution: D.G.M., J.L., N.F., M.O., M.I., and K.T. contributed to collection and assembly of data; D.G.M. and M.O. contributed to conception and design; D.M., J.D., M.O., M.I., and K.T. contributed to data analysis and interpretation; D.G.M., N.F., J.D., J.L., M.O., M.I., and K.T. contributed to manuscript writing; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: D.G.M. reports stock options from AZ Biotherapeutics; honoraria from Bioline RX, Kite Pharma, Gilead, Novartis, Juno Therapeutics, Celgene, AZ Biotherapeutics, and Pharmacyclics; research funding from Kite Pharma, Juno Therapeutics, and Celgene; and intellectual property interest from Juno Therapeutics; N.F. reports honoraria from Chugai Pharma, Eisai, Kirin Pharmaceuticals, Janssen, Mochida, Mundi, Nippon Shinyaku, Ono, Takeda, and Zenyaku; a consulting role with Celgene, Chugai, and Ono; and research funding from Celgene, Eisai, AbbVie, Bayer, Gilead, Solasia Pharma, and Takeda; J.D., J.L., and M.I. report they are employees of Novartis and have stock ownership to disclose from Novartis. M.O. reports honoraria from Celgene and MeijiSeika Pharma and a consulting role with Celltrion, Mundi Pharma, Symbio, Verastem, Eisai, Teva Takeda, and Denevo Biopharma; HB is an employee of Novartis; K.T. reports honoraria from Zenyaku Kogyo, Eisai, Takeda, Mundipharma, HUYA Bioscience, Kyowa Kirin, Celgene, Chugai Pharma, Ono Pharmaceutical, Yakult, Daiichi Sankyo, Bristol-Meyers Squibb, Meiji Seika Kaisha, Solasia Pharma, and Verastem; consulting roles with Zenyaku Kogyo, Takeda, Mundipharma, HUYA Bioscience, Celgene, Ono Pharmaceutical, and Daiichi Sankyo; and research funding from Eisai, Takeda, Mundipharma, Kyowa Kirin, Celgene, Chugai Pharma, Ono Pharmaceutical, AbbVie, and Janssen.
The current affiliation for M.O. is Department of Hematology and Oncology, Kasugai Municipal Hospital, Kasugai, Japan.
Correspondence: David G. Maloney, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109-1024; e-mail dmaloney@fredhutch.org.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392. [PubMed] [Google Scholar]
- 3.Shirley MH, Sayeed S, Barnes I, Finlayson A, Ali R. Incidence of haematological malignancies by ethnic group in England, 2001-7. Br J Haematol. 2013;163(4):465-477. [DOI] [PubMed] [Google Scholar]
- 4.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevodnik VK, Lavrenčak J, Horvat M, Novakovič BJ. The predictive significance of CD20 expression in B-cell lymphomas. Diagn Pathol. 2011;6(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825-2833. [DOI] [PubMed] [Google Scholar]
- 7.Bachy E, Houot R, Morschhauser F, et al. ; Groupe d’Etude des Lymphomes de l’Adulte (GELA) . Long-term follow up of the FL2000 study comparing CHVP-interferon to CHVP-interferon plus rituximab in follicular lymphoma. Haematologica. 2013;98(7):1107-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herold M, Dölken G, Fiedler F, et al. Randomized phase III study for the treatment of advanced indolent non-Hodgkin’s lymphomas (NHL) and mantle cell lymphoma: chemotherapy versus chemotherapy plus rituximab. Ann Hematol. 2003;82(2):77-79. [DOI] [PubMed] [Google Scholar]
- 9.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725-3732. [DOI] [PubMed] [Google Scholar]
- 10.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579-4586. [DOI] [PubMed] [Google Scholar]
- 11.Advani RH, Flinn I, Sharman JP, et al. Two doses of polatuzumab vedotin (PoV, anti-CD79b antibody-drug conjugate) in patients (pts) with relapsed/refractory (RR) follicular lymphoma (FL): durable responses at lower dose level. J Clin Oncol. 2015;33(15):8503. [Google Scholar]
- 12.Bartlett NL, LaPlant BR, Qi J, et al. Ibrutinib monotherapy in relapsed/refractory follicular lymphoma (FL): preliminary results of a Phase 2 Consortium (P2C) Trial. Blood. 2014;124(21):800. [Google Scholar]
- 13.Flinn I, Oki Y, Patel M, et al. A Phase 1 evaluation of duvelisib (IPI-145), a PI3K-delta,gamma inhibitor, in patients with relapsed/refractory iNHL. Blood. 2014;124(21):802. [Google Scholar]
- 14.Gerecitano JF, Roberts AW, Seymour JF, et al. A phase 1 study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with relapsed/refractory non-Hodgkin lymphoma [abstract]. Blood. 2015;126(23):254 Abstract 623. [Google Scholar]
- 15.Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33(31):3635-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehn LH, Goy A, Offner FC, et al. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B-cell non-Hodgkin lymphoma: final analysis of the GAUSS Study. J Clin Oncol. 2015;33(30):3467-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081-1093. [DOI] [PubMed] [Google Scholar]
- 18.Bleeker WK, Munk ME, Mackus WJ, et al. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol. 2008;140(3):303-312. [DOI] [PubMed] [Google Scholar]
- 19.Dechant M, Teeling J, Beyer T, et al. Novel fully human CD20 antibodies with different mechanisms of action. Blood. 2003;102(11):103. [Google Scholar]
- 20.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104(6):1793-1800. [DOI] [PubMed] [Google Scholar]
- 21.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362-371. [DOI] [PubMed] [Google Scholar]
- 22.Hagenbeek A, Gadeberg O, Johnson P, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111(12):5486-5495. [DOI] [PubMed] [Google Scholar]
- 23.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258-1265. [DOI] [PubMed] [Google Scholar]
- 24.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 25.van Oers MH, Kuliczkowski K, Smolej L, et al. ; PROLONG study investigators . Ofatumumab maintenance versus observation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label, multicentre, randomised phase 3 study. Lancet Oncol. 2015;16(13):1370-1379. [DOI] [PubMed] [Google Scholar]