Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and clinically challenging hematologic malignancy with dismal outcomes. With a median age of ∼70 years, the majority of patients with BPDCN have experienced historically suboptimal responses with intensive chemotherapy regimens. The major scientific breakthrough in this field was the recognition of overexpression of a surface receptor, CD123/interleukin 3 (IL-3) receptor α, in all patients. Importantly, a novel therapeutic agent consisting of a truncated diphtheria toxin (DT) payload fused to recombinant human IL-3 was being developed, one that targeted CD123, initially known as DT-IL-3 (later known as SL401; tagraxofusp; tagraxofusp-erzs [Elzonris]). The identification of this agent, and subsequent clinical trials specifically dedicated to patients with BPDCN (including a pilot study, followed by a larger phase 1/2 multicenter study [90% overall response rate [ORR] in frontline and 67% ORR in relapsed/refractory setting]), in part led to approval of tagraxofusp-erzs on 21 December 2018. Tagraxofusp-erzs was the first agent approved for BPDCN (for patients ages 2 years and older), and importantly, established this drug as the first CD123-targeted agent ever approved. The most notable toxicity of tagraxofusp-erzs is occurrence of the capillary leak syndrome, which occurs frequently at all grades, and has also been observed to be life-threatening, appropriately leading to a US Food and Drug Administration “black box” warning in the package insert. The preclinical and clinical aspects of drug development of tagraxofusp-erzs as monotherapy leading to drug approval are reviewed herein, with discussion of future directions of this novel agent, including consideration for rational combinations in BPDCN and beyond.
Background
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy noted for its unique clinical presentation (skin, bone marrow, lymph nodes) and historically poor outcomes.1 Its true incidence is unknown, owing to its many nomenclature changes over time and historical difficulty in clinicopathologic recognition.2,3 Accounting for <1% of all hematologic malignancies and <1% of all cutaneous lymphoma/lymphoma-like entities, a recent Surveillance, Epidemiology, and End Results (SEER) US database inquiry noted 0.04 cases per 100 000, definitely marking BPDCN as an orphan/ultra-rare disease.4,5 One unique aspect of BPDCN is that it has been found to be a male-predominant cancer, with some series reporting 2:1, others higher, 5:1, male-to-female incidence patterns, which may, in part, be explained by dosage effect from X-chromosome linkage to ZRSR2.6,7 Because of its historical rarity, data are sparse in the medical literature, with median overall survival (OS) for BPDCN of <2 years reported in most series prior to the targeted era.1,8 Although there is no definitive recurrent chromosomal aberration yet identified in all cases, it has been noted that patients with BPDCN, with or without bone marrow involvement, exhibit recurring mutations commonly found in myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML), and acute myeloid leukemia (AML), for example, TET2, ASXL1, RAS, TP53.7,9-11 The pathologic hallmark of BPDCN has been revealed by the identification of a consistent and reproducible immunophenotypic profile.12 The triad of positivity for CD123, CD4, and CD56 (or, as we have put forward as a helpful memory device, “Think 123456”)13 has yielded a high level of sensitivity for this elusive diagnosis.14,15 Furthermore, additional markers, including TCL-1, CD303, and TCF4, have all added specificity in securing a diagnosis of BPDCN, particularly in the context of differentiating from mimicking diseases such as AML with leukemia cutis.16,17 Indeed, we have found it to be of highest importance to approach a new diagnosis of BPDCN with close collaboration with a multidisciplinary team, including a pathology team, once a diagnosis of BPDCN is suspected. Based on the understanding that BPDCN has a separate and unique clinical course, the World Health Organization (WHO) in 2008 crystallized the term “blastic plasmacytoid dendritic cell neoplasm” under AML and its related family of neoplasms, and then in 2016, appropriately appointed BPDCN as its own separate category under myeloid neoplasms.18,19
With its varied clinical presentation and challenging management features, the treatment approach to BPDCN has historically centered around multiagent chemotherapy regimens repurposed from acute leukemias and lymphomas, with notably suboptimal rates, including high early death rates and complete remissions (CRs) of ∼40% to 60% in most larger series1,20-22 with these intensive approaches. In the chemotherapy era, clinical responses have been induced with a variety of multiagent chemotherapy approaches, with administration of acute lymphoblastic leukemia–based approaches (including the hyperfractionated cyclophosphamide, vincristine, Adriamycin, dexamethasone alternating with methotrexate and ARA-C [HCVAD] regimen in patients who are fit/able to receive intensive treatment) and AML-based approaches being the 2 most commonly used.22,23 In BPDCN, both autologous and allogeneic stem cell transplants (SCTs) have been used successfully for selected patients with more data needed in the field to determine ideal scenarios for type of transplant. Of course, as would be expected, in terms of timing of transplant, in the fit/younger patient with BPDCN, outcomes have been noted to be most optimal with intensive chemotherapy followed by allogeneic SCT in first remission.24-26 However, unfortunately, this approach will not be feasible for the majority of patients, as most patients with BPDCN present with older age and/or are unfit for intensive chemotherapy in the setting of comorbidities; more prospective data with regards to type and timing of SCT will be of great importance to the field.27 Given the continued overall poor outcomes in patients with BPDCN, despite various therapeutic strategies in the past, there was an urgent unmet medical need for novel therapeutic approaches and personalized targeted regimens.28
Preclinical: basic and translational science and drug development: target→CD123
The modern, emerging field of targeting CD123/interleukin 3 (IL-3) receptor α (IL-3Rα) in hematologic malignancies may well be able to trace its origins to the seminal paper by Jordan and colleagues in the year 2000.29 Through analysis of flow cytometry studies and in a series of advanced in vitro and in vivo experiments in AML-based model systems, Jordan et al demonstrated that CD123 was strongly expressed on the surface of primitive leukemia stem cells (LSCs) and that CD123 may represent a novel target for therapy in acute leukemia.29 These results were later confirmed and expanded by Konopleva’s group with single-cell mass cytometry/cytometry by time of flight and other approaches highlighting the importance of CD123 expression as part of LSC populations and identifying CD123 as an attractive possible target in myeloid malignancies.30 Because these primitive or blast acute LSCs had previously been shown to be resistant in many cases to standard cytotoxic chemotherapy, groups around the world were investigating novel, targeted approaches for therapy for relapsed/refractory (R/R) hematologic malignancies.31
A second parallel story, involving the development of diphtheria toxin (DT)–based approaches, was being pioneered in a series of elegant investigations by Thorpe, Ross, and colleagues, and sought to establish the antileukemic potency of DT in the field of leukemia. Their work showed specifically that DT appeared to be directly toxic to human lymphoblastoid cell–based systems, both on its own and in combination with antithymocyte globulin.32,33
Putting it all together, also in 2000, Frankel and colleagues published the first data set outlining a novel, engineered construct targeting CD123: a truncated DT protein fused to human IL-3, with a linker (G4S)2. This new agent, termed DT-IL-3, demonstrated ≥10-fold reduction of colony-forming cells in 36% of patient samples with chronic myeloid leukemia and in 33% of patients samples with AML.31,34 It was hypothesized that this novel construct (known at that time as DT-IL-3 or DT(388)-IL-3) might be able to target LSC populations and that further work was warranted to attempt to develop it for clinical investigation particularly in patients with chronic and acute leukemias.35,36 Further studies revealed that there was a significant correlation in log cell kill for DT(388)-IL-3 vis-à-vis a high-blast-affinity IL-3R density. Moreover, these data demonstrated that 1 of the most critical components to AML patient sample blast kill with this agent was a specific, high-affinity IL-3–binding profile.37,38
Identification and confirmation of BPDCN as CD123-high–expressing tumor as potential for targeting and evolution of drug nomenclature (DT-IL-3; SL401; tagraxofusp; tagraxofusp-erzs [Elzonris])
IL-3R along with IL-5 and granulocyte-macrophage colony-stimulating factor receptors all share the common signaling subunit βc. Each of these 3 receptors is a heterodimer. IL-3Rα (CD123) is found on pluripotent progenitor cells and is critical for the induction of tyrosine phosphorylation, promotion of cell proliferation, and differentiation within hematopoietic cell lines.39,40 IL-3 binding to IL-3R CD123 leads to rapid internalization of the IL-3/IL-3R complex through receptor-mediated endocytosis. CD123 is expressed in a host of normal tissues throughout the body. Interestingly, there is low/absent expression of CD123 on normal hematopoietic progenitors; moderate to low expression in monocytes, mast cells, eosinophils, neutrophils, myeloid dendritic cells, and osteoblasts; notable expression on endothelial cells; and high expression on basophils and plasmacytoid dendritic cells.40,41 In terms of malignant tissues/states, a number of different myeloid and lymphoid malignancies have been shown to express/overexpress CD123 including: AML, chronic myeloid leukemia, CMML, hairy cell leukemia, MDS, systemic mastocytosis, primary eosinophilic disorders, multiple myeloma (MM), and acute lymphoblastic leukemia.40 Even more so than in AML, it became apparent that CD123 overexpression was more pronounced in BPDCN and, therefore, BPDCN might represent an important area for developing the novel DT-IL-3 agent.42 As investigation with this novel agent continued, its later name became SL-40143,44 (tagraxofusp [Elzonris]; Stemline Therapeutics, New York, NY), and then ultimately tagraxofusp-erzs.44 SL-401 binds, with high affinity, to IL-3R. The drug was ultimately confirmed to become internalized by receptor-mediated endocytosis. Additionally, the catalytic domain of DT is then cleaved and it translocates into the cytosol from the endosome, at which point it leads to the inactivation of elongation factor 2 (EF2). Finally, EF2 inactivation leads to disruption of protein synthesis and ultimately to promotion of apoptotic cell death.41,45
By 2013, studies of SL-401 testing specific to BPDCN were being initiated. One of the major preclinical breakthroughs in the modern era of BPDCN has been the creation and propagation of 2 independent cell lines specific to CD123+ BPDCN, thought to be among the first ever created: CAL-1 (Japan)46 and GEN 2.2 (France).42 In terms of in vitro investigation, Angelot-Delettre et al demonstrated that SL-401 had high potency against BPDCN cell lines (both CAL-1 and GEN 2.2) and primary patient samples, with the 50% inhibitory concentration noted in femtomolar range.42 In further experiments, Angelot-Delettre and team conducted a series of in vivo experiments. In these, immunodeficient NSG mice were irradiated and given IV the GEN 2.2 cell line. The mice were then treated 1 week later with 5 daily injections of SL-401 or phosphate-buffered saline control; with 1 cycle of SL-401 therapy, there was a significant improvement in median OS in the SL-401–treated group vs control (58 days vs 17 days, respectively). Additionally, circulating BPDCN cells were found to be undetectable for up to ∼2 weeks in the treatment group, whereas in control group, the amount of BPDCN cells gradually increased until time of death.42 SL-401 underwent further testing in patient-derived xenografts generated from BPDCN primary cells. In work by Christie et al, immunodeficient NSG mice were injected with patient BPDCN blasts. Once the average disease burden (peripheral blood reached >0.2%, the mice were then randomized for therapy with either vehicle or 5 daily injections of SL-401. Once they experienced relapsed BPDCN, the mice were then randomly assigned to retreatment with SL-401 or vehicle. SL-401-retreated mice were able to achieve second peripheral blood remission in this study. Overall, the mice treated with SL-401 demonstrated reduced peripheral blood disease BPDCN burden and decreased spleen sizes. Treatment with SL-401 resulted in improved OS in BPDCN patient-derived xenograft mouse models (71 days for treated mice vs 35 days in control).47,48
Clinical development of SL-401
SL-401 was initially investigated clinically in several myeloid malignancy populations. The novel agent was originally investigated by Frankel and colleagues in patients with MDS and AML.49 Given that BPDCN overexpresses CD123 in almost all cases, and that SL-401 exhibited femtomolar level potency specifically against BPDCN cells,42 a pilot phase 1 study was initiated in patients with BPDCN.43 In this important study, 11 patients (all male) with a median age of 70 years were enrolled, with 9 of 11 receiving at least 1 of a planned 5 doses of SL-401. Among these 9 patients, 7 of 9 (78%) achieved a major response (which included 5 patients with CR and 2 additional patients with partial remission). Remarkably, these results were achieved with only 1 cycle of active drug available for most patients. Encouragingly, there was evidence for reduction of both skin and bone marrow compartments of disease. The most notable toxicity noted was that of the vascular leak syndrome, or as it is now termed, capillary leak syndrome (CLS), observed in most of the patients. This phenomenon was hypothesized to occur in relation to the drug’s diphtheria-containing domain or due to the CD123-targeted aspect (endothelial cells, other cells which express CD123) or both.43 Despite the signal for CLS, the drug was found to be quite safe and manageable, and was felt to be a possible breakthrough for patients with the rare blood cancer of BPDCN, and was recommended for further larger clinical trial investigations.50
Building on these promising results, Pemmaraju et al then conducted a multicenter phase 1/2 clinical trial, STML-401-0114 (NCT02113982), featuring multiple cycles of SL-401. This study was designed in 4 stages, including the pivotal stage 3 cohort. In stage 1, a lead-in, dose escalation (“3+3” design) was completed. This cohort enrolled patients with BPDCN with both untreated and R/R disease, in doses of SL-401 ranging from 7, 9, or 12 μg/kg IV daily (days 1-5, every 21 days). No maximum tolerated dose was found, but based on evaluation of toxicity, efficacy, and assessment of the overall experience, by investigator consensus, the target dose of 12 μg/kg IV daily was chosen to move forward to stage 2. In stage 2, the expansion phase, the key objective was to further define safety from stage 1 and to determine efficacy at the selected target dose. Again, both untreated and R/R patients with BPDCN were enrolled. In stage 3, the pivotal, confirmatory cohort, only untreated patients with BPDCN were enrolled and treated at the 12 μg/kg IV dosing from stage 2, with prespecified statistical focus on rate of CR, to serve as confirmation of efficacy for possible study drug registration consideration. A stage 4 was opened to continue enrolling patients with BPDCN, but these results have not yet been published. The inclusion criteria for the clinical trial included ages 18 years and older, Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, and importantly, albumin of 3.2 g/dL or higher, at baseline. Notably, the initial albumin cutoff at trial start was 3.0 g/dL; this was amended for a higher albumin after serious CLS was noted in cohort 1 (grade 5 CLS/death in a patient >80 years of age) and this new albumin 3.2 g/dL or higher was carried out for the remainder of the study. Exclusion criteria included receipt of chemotherapy or other investigational agent within prior 14 days of study start, or clinically significant cardiopulmonary disease. A novel set of response criteria was successfully put forward for prospective use on this study which included evaluating disease response in the 3 major compartments involved (skin based on the modified severity weighted assessment tool [mSWAT]51; bone marrow evaluation based on AML criteria52; lymph node assessment based on Cheson criteria53).44 A subtype of CR, known as clinical CR, or CRc, was denoted as no detectable/gross evidence for disease in the 3 organ compartments, but with evidence of residual microscopic skin disease on biopsy (see Table 1 for modified presentation of response criteria used). A total of 47 patients were treated in all lines of therapy, with a median age of 70 years (range, 22-84 years) and 83% male. Among the n = 29 frontline patients treated with tagraxofusp-erzs at 12 μg/kg, 21 of 29 achieved a major response for a 90% overall response rate (ORR; 72% CR/CRc), with OS at 24 months observed to be 52%. Remarkably, 45% of these frontline-treated patients were able to be bridged successfully to SCT. Among the 15 patients in R/R setting, the ORR was 67% with a median OS reported as 8.5 months.44
Table 1.
Proposed response criteria for patient evaluation in BPDCN
| BPDCN compartment of disease* | Evaluation method | Response criteria disease area | Year/reference | Notes |
|---|---|---|---|---|
| Cutaneous (skin) | mSWAT | CTCL | 2011/51 | mSWAT = modified severity weighted assessment tool |
| Blood/bone marrow | CBC; bone marrow aspiration/biopsy | AML | 2003/52 | BPDCN can transform to AML, so always confirm diagnosis with expert pathology team |
| Lymph node | PET/CT; CT | NHL | 2014/53 | Can also image other visceral/extramedullary sites |
BPDCN response criteria based on Pemmaraju et al.44 CR equals no detectable disease in all 3 compartments.
CBC, complete blood count; CT, computed tomography; CTCL, cutaneous T-cell lymphoma; NHL, non-Hodgkin lymphoma; PET, positron emission tomography.
CRc equals no detectable disease noted in nonskin organs, with gross decrease in cutaneous lesions; evidence of microscopic residual skin disease.
CLS in the bacterial toxin targeted-therapy era
A vital aspect of novel drug discovery in modern targeted therapy must include recognition and management of novel toxicities. The occurrence of the vascular leak syndrome, now more commonly known as the CLS, is the most notable toxicity directly related to tagraxofusp-erzs41,44 and should be appreciated by all administering this new agent.
As detailed on the package insert, among a total of 94 patients with hematologic malignancies evaluated for safety in tagraxofusp-erzs clinical trials, CLS occurred with incidence of 55% (52 of 94 patients) at all grades. This included grade 4 in 1 patient (1%) and grade 5 (death) in 2 patients (2 of 94, or 2%). The occurrence of the CLS generally occurred in association with: edema, weight gain, hypotension, and, most notably, hypoalbuminemia.
When approaching CLS, the clinician should remember that there are various disease states themselves and additionally, various agents, including cytotoxic drugs, SCT, and other targeted agents that have been linked to its occurrence all throughout different oncologic settings over the past several decades.54 With regards to recent bacterial toxin–based targeted-therapy examples, denileukin diftitox and moxetumomab pasudotox-tdfk were both associated with CLS. Denileukin diftitox is an IL2R-directed/CD25 agent with a DT-based platform. It was approved for recurrent/persistent cutaneous lymphoma (CTCL) and also carries a “black box” warning for CLS. In clinical studies, this agent resulted in 32.5% of patients (76 of 234) with CLS; of 76 patients, 1 of 3 required inpatient hospitalization or intervention to prevent a hospitalization. Moxetumomab pasudotox-tdfk is a CD22-directed cytotoxin that is based on the fragment of Pseudomonas exotoxin A as its mechanistic platform. This agent represents a first-in-class drug that was approved for R/R hairy cell leukemia in 2018. Both CLS and hemolytic uremic syndrome were noted during approval studies. CLS of grade 3 or 4 occurred in 1.6% and 2% of patients, respectively.55-58
Of note, it is important to highlight to the reader that there is no absolute definition or definite treatment of CLS, and, thus, for the tagraxofusp-erzs clinical trials, investigators designed and agreed by consensus upon prevention and treatment schemas. CLS management with tagraxofusp-erzs consists of an individualized patient-by-patient approach. In general, given the authors considerable experience in treating patients with tagraxofusp-erzs, we recommend a 3-pronged strategy with regards to management of CLS: (1) first and foremost is recognition of this life-threatening phenomenon and extensive counseling, education, and discussion with the patient, caregiver, nursing and inpatient teams, chemotherapy unit team, and all others involved in administration of this novel agent. This cannot be overstated as prevention and early recognition by all stakeholders involved can be potentially life-saving. Furthermore, it is imperative to perform a thorough cardiopulmonary assessment of each patient prior to administration of tagraxofusp-erzs, with special attention to volume status, cardiac ejection fraction, and history of significant cardiac or pulmonary disease that may affect cardiopulmonary reserve. (2) Closely following the package label with regards to baseline and follow-up of key chemistry values including albumin, creatinine, liver function tests (LFTs), and daily weights. Remarkably, all of the laboratory parameters we instituted and followed ourselves on the clinical trial leading to the drug’s approval have been appropriately carried over onto the package insert. Pay special attention to baseline albumin, the kinetic rate of drop of albumin, and when and how to appropriately replace with IV albumin. As noted on the package insert, do not start a patient on tagraxofusp-erzs if baseline albumin is <3.2 g/dL, as we found patients with lower baseline albumin with rapid drop in the albumin during therapy to be the patients at highest risk for CLS. (3) Once CLS begins, it can be rapidly fatal and must be approached with a clinically aggressive team-based methodology. Early intervention, based on individual patient needs, will be necessary by a multidisciplinary team including but not limited to administration of IV albumin, corticosteroids, fluid diuresis (or fluid replacement, depending on individual patient situation, if concomitant other conditions such as sepsis, etc), and low threshold for transfer to higher level of care such as the intensive care unit and early administration of vasopressors.
FDA approval, package insert, labeling indication
Tagraxofusp-erzs is administered as an IV infusion given over 15 minutes with ample premedication including corticosteroids, H2-histamine blocking agent, H1-histamine blocking agent, and acetaminophen. It is given at dose of 12 μg/kg on days 1 through 5 on a 21-day cycle with a 10-day window in which to receive the 5 doses. The 10-day window in which to administer a cycle of the drug is important to note, as we observed that dose interruptions are frequently necessary with administration of tagraxofusp-erzs in order to manage fluctuations in albumin, daily weight, creatinine, and/or LFTs and in monitoring for CLS. Patients have been noted to achieve remission with BPDCN treated with this agent even if all 5 doses are not able to be administered, so it is of highest importance to follow closely prescribing instructions and hold parameters for this novel drug. Very importantly, the first cycle must be given in the inpatient hospital setting, in order to adequately monitor for CLS and other toxicities; remaining cycles may be considered for inpatient vs outpatient setting based on provider team discretion.59
In terms of safety, we reviewed CLS in detail. In addition to the “black box” warning for CLS, the other 2 notable warnings/precautions are: (1) drug infusion hypersensitivity reaction in 46% and (2) hepatotoxicity in 88%. The hepatotoxicity is notable in that daily LFT is expected to be measured and if either aspartate aminotransferase or alanine aminotransferase increases to >5 times the upper limit of normal, the drug should be held.60 Additionally, other common toxicities of tagraxofusp-erzs that occurred at 30% incidence or higher included: fever, nausea, edema, fatigue, and weight gain. Commonly noted abnormalities of laboratory values that occurred 50% or higher included: thrombocytopenia (notably mostly in first cycle), hypoalbuminemia, anemia, and LFT abnormalities. It was noted that 85% of patients developed antidrug/neutralizing antibodies. Ultimately, based on the pivotal group (stage 3, STML-401-0114, n = 13), among frontline-treated patients, a CR/CRc rate was noted in 54%; median duration of CR was not reached at median follow-up of ∼1 year.61
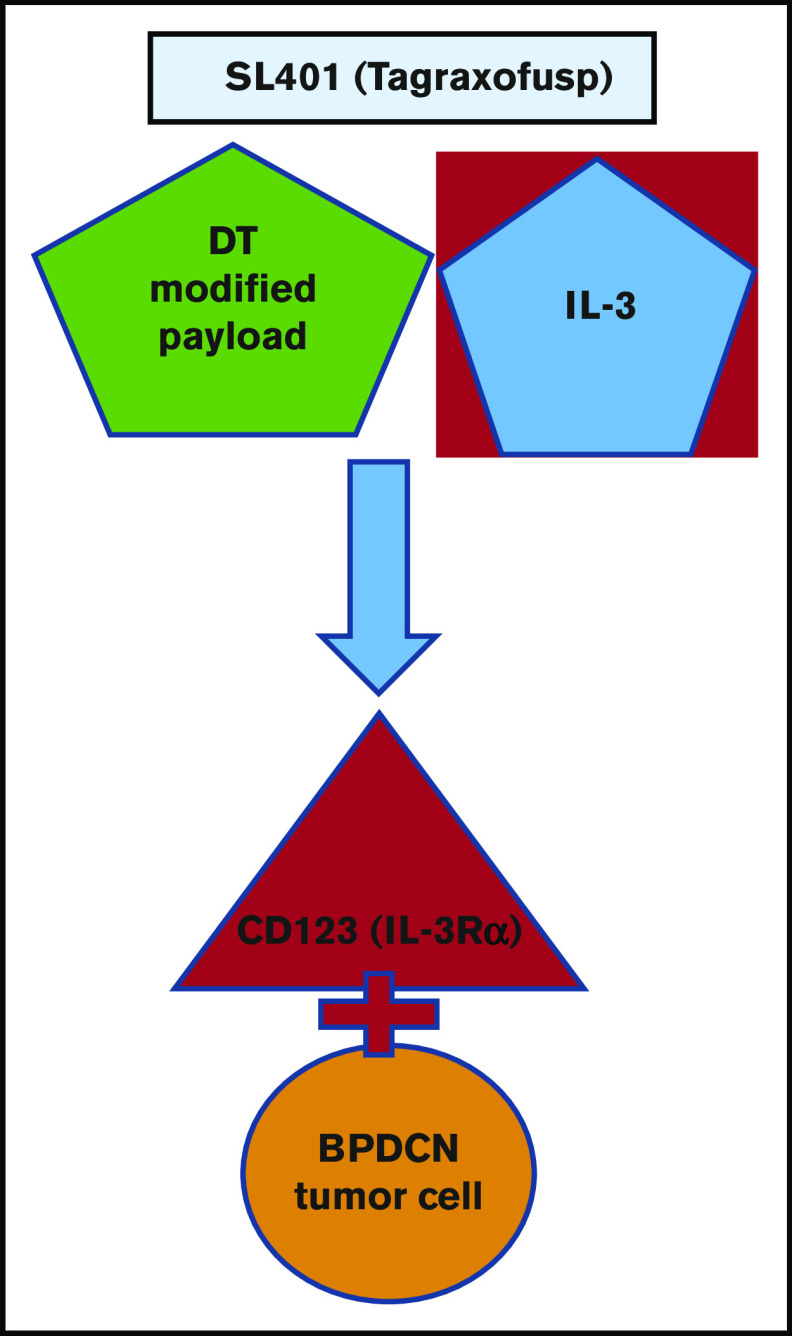
Tagraxofusp was officially approved as tagraxofusp-erzs (Elzonris) by the US Food and Drug Administration (FDA) on 21 December 2018, for patients with BPDCN ages 2 years and older, making it the first approved agent specifically for patients with BPDCN, now widely available for usage in both newly diagnosed and R/R settings, and notably the first CD123-directed targeted agent approved in the field of hematology/oncology (Figure 1).61
Figure 1.
Tagraxofusp, a novel CD123-targeted agent.
Future directions for tagraxofusp-erzs in BPDCN and beyond
In the BPDCN and AML fields, several novel directions are being developed for use of tagraxofusp-erzs either as a single agent, or of interest, in new combinations. In terms of MDS and AML clinical development, although single-agent activity with tagraxofusp-erzs has been investigated, the field is now moving forward with rational doublet and triplet combinations. Togami and Lane recently demonstrated preclinical mechanistic rationale for possible resistance mechanisms in tagraxofusp-erzs monotherapy (hypermethylation of DPH1 gene important for synthesis of diphthamide) and possibility of being overcome combination with hypomethylating agents (via restoration of DPH1 expression).48,62 Based on this seminal work, we are now able to investigate doublet (and now, triplet) combination with tagraxofusp-erzs and hypomethylator agent (with recently, addition of venetoclax), which is currently being investigated by Lane and Pemmaraju and colleagues in the ongoing phase 1/2 clinical trial (NCT03113643)63,64 for patients with high-risk MDS and AML.62 Based on preclinical and clinical demonstration of activity with venetoclax in BPDCN, Pemmaraju and colleagues recently opened the novel triplet for patients with BPDCN with tagraxofusp-erzs in combination with venetoclax and HCVAD chemotherapy (NCT04216524).65,66 And finally, a single-center post-SCT maintenance study for patients with BPDCN treated with tagraxofusp-erzs has recently been launched by Bashir and team (NCT04317781). Outside of BPDCN and AML, this novel CD123-directed agent is actively being investigated in a number of other fields, including myelofibrosis, CMML, and MM. See Table 2 for clinical trials involving tagraxofusp-erzs.
Table 2.
Active clinical trials with tagraxofusp (SL401)
| Active trial titles | Area of study | Clinicaltrials.gov identifier |
|---|---|---|
| SL-401 in combination with pomalidomide and dexamethasone in R/R MM | MM | NCT02661022 |
| SL-401 in combination with AZA or AZA/VEN in AML or high-risk MDS | MDS, AML | NCT03113643 |
| Tagraxofusp (SL-401) in patients with CMML or MF | MF, CMML | NCT02268253 |
| SL-401, venetoclax, and HCVAD chemotherapy for the treatment of BPDCN | BPDCN | NCT04216524 |
| Tagraxofusp in treating patients with BPDCN after SCT | BPDCN | NCT04317781 |
Source: Clinicaltrials.gov (accessed May 2020).
AZA, azacitidine; MF, myelofibrosis; VEN, venetoclax.
Conclusions
The unique mechanism of action of tagraxofusp-erzs and its recent FDA approval has opened a new era for targeted therapy in patients with BPDCN. In the largest clinical trial experience published, with a median age of 70 years, a 90% ORR with 72% CR/CRc rate was observed among 29 previously untreated patients, with 45% being bridged to SCT. The most important toxicity for clinicians to be aware of is that of CLS, which was generally manageable, found to occur frequently at all grades, and which can be recognized early and mitigated with a multidisciplinary management strategy in most cases. Notably, CLS was fatal in 2/94 (2%) patients available for safety evaluation treated with tagraxofusp-erzs leading to is approval. This novel agent represents the first BPDCN-specific therapy now available in the United States, and is a now a welcome option for consideration for therapy in the frontline or R/R setting in those patients with adequate albumin, renal, hepatic, and cardiopulmonary reserve. Now, 1.5 years after tagraxofusp-erzs as a monotherapy agent has been approved, it will be of high interest to continue investigation of this CD123-directed therapy in rational combinations with other targeted agents, hypomethylating agents, and cytotoxic chemotherapy in patients with BPDCN and other hematologic malignancies that overexpress CD123.
Acknowledgments
This work was supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672 (from the National Cancer Institute of the National Institutes of Health) and the SagerStrong Foundation.
Footnotes
Data-sharing requests may be e-mailed to the corresponding author, Naveen Pemmaraju, at npemmaraju@mdanderson.org.
Authorship
Contribution: N.P. and M.K. conceived of the study and wrote, edited, and approved the final draft of this manuscript.
Conflict-of-interest disclosure: N.P. provided consulting services to and received honoraria from Celgene, Stemline, Incyte, Novartis, MustangBio, Roche Diagnostics, LFB, Pacylex, and AbbVie; and received research funding and clinical trial support from Stemline, Novartis, AbbVie, Samus, Cellectis, Plexxikon, Daiichi-Sankyo, Affymetrix, and the SagerStrong Foundation. M.K. provided consulting services to and received honoraria from AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, and Kisoji; received research funding and clinical trial support from AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, and AstraZeneca; and holds stock options in and receives royalties from Reata Pharmaceutical.
Correspondence: Naveen Pemmaraju, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0428, Houston, TX 77030; e-mail: npemmaraju@mdanderson.org.
References
- 1.Pagano L, Valentini CG, Pulsoni A, et al. ; GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell’Adulto, Acute Leukemia Working Party) . Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bueno C, Almeida J, Lucio P, et al. Incidence and characteristics of CD4(+)/HLA DRhi dendritic cell malignancies. Haematologica. 2004;89(1):58-69. [PubMed] [Google Scholar]
- 3.Ng AP, Lade S, Rutherford T, McCormack C, Prince HM, Westerman DA. Primary cutaneous CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma): a report of five cases. Haematologica. 2006;91(1):143-144. [PubMed] [Google Scholar]
- 4.Guru Murthy GS, Pemmaraju N, Atallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23. [DOI] [PubMed] [Google Scholar]
- 5.Pagano L, Valentini CG, Grammatico S, Pulsoni A. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188-202. [DOI] [PubMed] [Google Scholar]
- 6.Taylor J, Kim SS, Stevenson KE, et al. Loss-of-function mutations in the splicing factor ZRSR2 are common in blastic plasmacytoid dendritic cell neoplasm and have male predominance [abstract]. Blood. 2013;122(21). Abstract 741. [Google Scholar]
- 7.Beird HC, Khan M, Wang F, et al. Features of non-activation dendritic state and immune deficiency in blastic plasmacytoid dendritic cell neoplasm (BPDCN). Blood Cancer J. 2019;9(12):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaperot L, Bendriss N, Manches O, et al. Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood. 2001;97(10):3210-3217. [DOI] [PubMed] [Google Scholar]
- 9.Alayed K, Patel KP, Konoplev S, et al. TET2 mutations, myelodysplastic features, and a distinct immunoprofile characterize blastic plasmacytoid dendritic cell neoplasm in the bone marrow. Am J Hematol. 2013;88(12):1055-1061. [DOI] [PubMed] [Google Scholar]
- 10.Jardin F, Ruminy P, Parmentier F, et al. TET2 and TP53 mutations are frequently observed in blastic plasmacytoid dendritic cell neoplasm. Br J Haematol. 2011;153(3):413-416. [DOI] [PubMed] [Google Scholar]
- 11.Piccaluga PP, Sapienza MR, Fabio F, et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-KB pathway inhibition [abstract]. Blood. 2013;122(21). Abstract 2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Khoury JD, Miranda RN, et al. Immunophenotypic characterization of reactive and neoplastic plasmacytoid dendritic cells permits establishment of a 10-color flow cytometric panel for initial workup and residual disease evaluation of blastic plasmacytoid dendritic cell neoplasm [published online ahead of print 2 April 2020]. Haematologica. doi:10.3324/haematol.2020.247569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pemmaraju N, Konopleva M. Ask the hematologist: treating blastic plasmacytoid dendritic cell neoplasm. The Hematologist. 2018;15(5):1. Available at: https://pub.hematology.org/Thehematologist/Ask/8927.aspx. Accessed 6 June 2020.
- 14.Cruz NM, Sugita M, Ewing-Crystal N, et al. Selection and characterization of antibody clones are critical for accurate flow cytometry-based monitoring of CD123 in acute myeloid leukemia. Leuk Lymphoma. 2018;59(4):978-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugita M, Guzman ML. CD123 as a therapeutic target against malignant stem cells. Hematol Oncol Clin North Am. 2020;34(3):553-564. [DOI] [PubMed] [Google Scholar]
- 16.Sukswai N, Aung PP, Yin CC, et al. Dual expression of TCF4 and CD123 is highly sensitive and specific for blastic plasmacytoid dendritic cell neoplasm. Am J Surg Pathol. 2019;43(10):1429-1437. [DOI] [PubMed] [Google Scholar]
- 17.Ohe R, Aung NY, Shiono Y, et al. Detection of minimal bone marrow involvement of blastic plasmacytoid dendritic cell neoplastic cells - CD303 immunostaining as a diagnostic tool. J Clin Exp Hematop. 2018;58(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. [DOI] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia [published correction appears in Blood. 2016;128(3):462-463]. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 20.Martín-Martín L, López A, Vidriales B, et al. Classification and clinical behavior of blastic plasmacytoid dendritic cell neoplasms according to their maturation-associated immunophenotypic profile. Oncotarget. 2015;6(22):19204-19216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalle S, Beylot-Barry M, Bagot M, et al. Blastic plasmacytoid dendritic cell neoplasm: is transplantation the treatment of choice? Br J Dermatol. 2010;162(1):74-79. [DOI] [PubMed] [Google Scholar]
- 22.Taylor J, Haddadin M, Upadhyay VA, et al. Multicenter analysis of outcomes in blastic plasmacytoid dendritic cell neoplasm offers a pretargeted therapy benchmark. Blood. 2019;134(8):678-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pemmaraju N, Kantarjian HM, Khoury JD, et al. Long-term outcomes in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) [abstract]. Blood. 2017;130(suppl 1). Abstract 3855. [Google Scholar]
- 24.Aoki T, Suzuki R, Kuwatsuka Y, et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 2015;125(23):3559-3562. [DOI] [PubMed] [Google Scholar]
- 25.Roos-Weil D, Dietrich S, Boumendil A, et al. ; European Group for Blood and Marrow Transplantation Lymphoma, Pediatric Diseases, and Acute Leukemia Working Parties . Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013;121(3):440-446. [DOI] [PubMed] [Google Scholar]
- 26.Kharfan-Dabaja MA, Lazarus HM, Nishihori T, Mahfouz RA, Hamadani M. Diagnostic and therapeutic advances in blastic plasmacytoid dendritic cell neoplasm: a focus on hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(7):1006-1012. [DOI] [PubMed] [Google Scholar]
- 27.Pemmaraju N. Treatment advances in blastic plasmacytoid dendritic cell neoplasm. Clin Adv Hematol Oncol. 2019;17(4):207-209. [PubMed] [Google Scholar]
- 28.Pemmaraju N. Novel pathways and potential therapeutic strategies for blastic plasmacytoid dendritic cell neoplasm (BPDCN): CD123 and beyond. Curr Hematol Malig Rep. 2017;12(6):510-512. [DOI] [PubMed] [Google Scholar]
- 29.Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14(10):1777-1784. [DOI] [PubMed] [Google Scholar]
- 30.Han L, Qiu P, Zeng Z, et al. Single-cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3-ITD-mutated AML stem/progenitor cells. Cytometry A. 2015;87(4):346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankel AE, Ramage J, Kiser M, Alexander R, Kucera G, Miller MS. Characterization of diphtheria fusion proteins targeted to the human interleukin-3 receptor. Protein Eng. 2000;13(8):575-581. [DOI] [PubMed] [Google Scholar]
- 32.Thorpe PE, Ross WC, Cumber AJ, Hinson CA, Edwards DC, Davies AJ. Toxicity of diphtheria toxin for lymphoblastoid cells is increased by conjugation to antilymphocytic globulin [published correction appears in Nature. 1978;272(5652):472]. Nature. 1978;271(5647):752-755. [DOI] [PubMed] [Google Scholar]
- 33.Ross WC, Thorpe PE, Cumber AJ, Edwards DC, Hinson CA, Davies AJ. Increased toxicity of diphtheria toxin for human lymphoblastoid cells following covalent linkage to anti-(human lymphocyte) globulin or its F(ab’)2 fragment. Eur J Biochem. 1980;104(2):381-390. [DOI] [PubMed] [Google Scholar]
- 34.Frankel AE, McCubrey JA, Miller MS, et al. Diphtheria toxin fused to human interleukin-3 is toxic to blasts from patients with myeloid leukemias. Leukemia. 2000;14(4):576-585. [DOI] [PubMed] [Google Scholar]
- 35.Feuring-Buske M, Frankel A, Gerhard B, Hogge D. Variable cytotoxicity of diphtheria toxin 388-granulocyte-macrophage colony-stimulating factor fusion protein for acute myelogenous leukemia stem cells. Exp Hematol. 2000;28(12):1390-1400. [DOI] [PubMed] [Google Scholar]
- 36.Frolova O, Benito J, Brooks C, et al. SL-401 and SL-501, targeted therapeutics directed at the interleukin-3 receptor, inhibit the growth of leukaemic cells and stem cells in advanced phase chronic myeloid leukaemia. Br J Haematol. 2014;166(6):862-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander RL, Kucera GL, Klein B, Frankel AE. In vitro interleukin-3 binding to leukemia cells predicts cytotoxicity of a diphtheria toxin/IL-3 fusion protein. Bioconjug Chem. 2000;11(4):564-568. [DOI] [PubMed] [Google Scholar]
- 38.Alexander RL, Ramage J, Kucera GL, Caligiuri MA, Frankel AE. High affinity interleukin-3 receptor expression on blasts from patients with acute myelogenous leukemia correlates with cytotoxicity of a diphtheria toxin/IL-3 fusion protein. Leuk Res. 2001;25(10):875-881. [DOI] [PubMed] [Google Scholar]
- 39.Estrov Z, Kurzrock R, Talpaz M, Blake M, Gutterman JU. Granulocyte-macrophage colony-stimulating factor and interleukin-3 in combination: a potent and consistent myelodysplastic syndrome bone marrow stimulant in vitro. Ann Hematol. 1991;63(6):297-301. [DOI] [PubMed] [Google Scholar]
- 40.Testa U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankel AE, Woo JH, Ahn C, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124(3):385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angelot-Delettre F, Roggy A, Frankel AE, et al. In vivo and in vitro sensitivity of blastic plasmacytoid dendritic cell neoplasm to SL-401, an interleukin-3 receptor targeted biologic agent. Haematologica. 2015;100(2):223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frankel AE, Konopleva M, Hogge D, et al. Activity and tolerability of SL-401, a targeted therapy directed to the interleukin-3 receptor on cancer stem cells and tumor bulk, as a single agent in patients with advanced hematologic malignancies [abstract]. J Clin Oncol. 2013;31(suppl 15). Abstract 7029. [Google Scholar]
- 44.Pemmaraju N, Lane AA, Sweet KL, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380(17):1628-1637. [DOI] [PubMed] [Google Scholar]
- 45.Fanny D, Frankel AE, Seilles E, et al. Preclinical studies Of SL-401, a targeted therapy directed to the interleukin-3 receptor (IL3-R), in blastic plasmacytoid dendritic cell neoplasm (BPDCN): potent activity in BPDCN cell lines, primary tumor, and in an in vivo model [abstract]. Blood. 2013;122(21). Abstract 3942. [Google Scholar]
- 46.Maeda T, Murata K, Fukushima T, et al. A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol. 2005;81(2):148-154. [DOI] [PubMed] [Google Scholar]
- 47.Christie AL, Li Y, Togami K, et al. Blastic plasmacytoid dendritic cell neoplasm (BPDCN) patient-derived xenografts are faithful genomic and phenotypic models of primary leukemia and respond to the IL3 receptor targeting agent SL-401 in vivo [abstract]. Blood. 2015;126(23). Abstract 3797. [Google Scholar]
- 48.Togami K, Pastika T, Stephansky J, et al. DNA methyltransferase inhibition overcomes diphthamide pathway deficiencies underlying CD123-targeted treatment resistance. J Clin Invest. 2019;129(11):5005-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frankel A, Liu JS, Rizzieri D, Hogge D. Phase I clinical study of diphtheria toxin-interleukin 3 fusion protein in patients with acute myeloid leukemia and myelodysplasia. Leuk Lymphoma. 2008;49(3):543-553. [DOI] [PubMed] [Google Scholar]
- 50.FitzGerald DJ. Targeted diphtheria toxin to treat BPDCN. Blood. 2014;124(3):310-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsen EA, Whittaker S, Kim YH, et al. ; Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer . Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheson BD, Bennett JM, Kopecky KJ, et al. ; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia . Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. [published correction appears in J Clin Oncol. 2004;22(3):576]. J Clin Oncol. 2003;21(24):4642-4649. [DOI] [PubMed] [Google Scholar]
- 53.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeong GH, Lee KH, Lee IR, et al. Incidence of capillary leak syndrome as an adverse effect of drugs in cancer patients: a systematic review and meta-analysis. J Clin Med. 2019;8(2):E143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreitman RJ, Pastan I. Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin Cancer Res. 2011;17(20):6398-6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.FitzGerald DJ, Wayne AS, Kreitman RJ, Pastan I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71(20):6300-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19(2):376-388. [DOI] [PubMed] [Google Scholar]
- 58.McCann S, Akilov OE, Geskin L. Adverse effects of denileukin diftitox and their management in patients with cutaneous T-cell lymphoma. Clin J Oncol Nurs. 2012;16(5):E164-E172. [DOI] [PubMed] [Google Scholar]
- 59.Zheng G, Schmieg J, Guan H, Ali SZ. Blastic plasmacytoid dendritic cell neoplasm: cytopathologic findings. Acta Cytol. 2012;56(2):204-208. [DOI] [PubMed] [Google Scholar]
- 60.Therapeutics Stemline, Inc. Elzonris (tagraxofus-ezrs). http://www.elzonris.com/hcp/?gclid=CjwKCAjw5Ij2BRBdEiwA0Frc9Sb7kczaXF2m2ZCWiGD7QEMPzf8ej6QSMdkcfIerKCv-vGVwfXR6FRoClogQAvD_BwE. Accessed 6 June 2020.
- 61.Jen EY, Gao X, Li L, et al. FDA approval summary: tagraxofusp-erzs for treatment of blastic plasmacytoid dendritic cell neoplasm. Clin Cancer Res. 2020;26(3):532-536. [DOI] [PubMed] [Google Scholar]
- 62.Gondek LP. Hitting the bullseye with a nonlethal payload: resistance in CD123-positive malignancies. J Clin Invest. 2019;129(11):4590-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montero J, Stephansky J, Cai T, et al. Blastic plasmacytoid dendritic cell neoplasm is dependent on bcl2 and sensitive to venetoclax. Cancer Discov. 2017;7(2):156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401-407. [DOI] [PubMed] [Google Scholar]
- 65.Pemmaraju N, Konopleva M, Lane AA. More on blastic plasmacytoid dendritic-cell neoplasms. N Engl J Med. 2019;380(7):695-696. [DOI] [PubMed] [Google Scholar]
- 66.Agha ME, Monaghan SA, Swerdlow SH. Venetoclax in a patient with a blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2018;379(15):1479-1481. [DOI] [PubMed] [Google Scholar]