Little is known about the clinical characteristics of Clostridium difficile infection (CDI) in Asia in general, and Thailand specifically, with a few studies suggesting that the disease may be milder than elsewhere. This study aimed to describe CDI in Thailand, evaluate treatment options and their outcomes, and explore possible protective factors responsible for any unique disease characteristics. From 2015 to 2018, 469 patients were included in the study. All patients had their stools tested for the tcdB gene by direct PCR and detection of toxigenic C. difficile by culture.
KEYWORDS: Clostridium difficile infection, Thailand, clinical characteristics
ABSTRACT
Little is known about the clinical characteristics of Clostridium difficile infection (CDI) in Asia in general, and Thailand specifically, with a few studies suggesting that the disease may be milder than elsewhere. This study aimed to describe CDI in Thailand, evaluate treatment options and their outcomes, and explore possible protective factors responsible for any unique disease characteristics. From 2015 to 2018, 469 patients were included in the study. All patients had their stools tested for the tcdB gene by direct PCR and detection of toxigenic C. difficile by culture. C. difficile isolates were subjected to toxin gene profiling and ribotyping, and patient medical records were reviewed retrospectively. There were 248 and 221 patients included in CDI and control groups, respectively. The CDI group had a higher overall 30-day mortality rate than the control group (21% versus 14%, P = 0.046), but only 2 deaths (1%) were directly attributable to CDI. Metronidazole treatment was not inferior to vancomycin in this population, and vancomycin was associated with a higher 30-day mortality rate (P = 0.047). The prevalence of severe CDI and disease outcomes were not different between patients infected with A–B+ C. difficile and A+B+ C. difficile strains or between patients with and without colonization by nontoxigenic C. difficile. Besides C. difficile-specific tests, neither a single laboratory result nor a combination of results was predictive of CDI. In conclusion, CDI in Thailand was relatively mild, and metronidazole remained an effective treatment option for these mild infections.
INTRODUCTION
Clostridium difficile (also known as Clostridioides difficile) infection (CDI) has been a major public health problem in high-income countries for more than 2 decades. In the latest antimicrobial resistance threats report by the U.S. Centers for Disease Control and Prevention, C. difficile remains in the group of pathogens posing an urgent threat in the United States, having caused at least 12,800 deaths in 2017 (1). CDI was responsible for almost half the nosocomial gastrointestinal infections and around 8% of all nosocomial infections in a 2012 pan-European study (2). In both North America and Europe, CDI often results in severe colitis with high mortality, as well as a high recurrence rate among surviving patients (1, 3).
In Asia, the characteristics of CDI are less well described, although it appears that CDI in the region is associated with milder disease. Two clinical studies in Thailand (4) and South Korea (5) reported recurrence rates of 3% and 16%, respectively, compared to around 20% worldwide (6) with an increasing rate in North America (7). The mortality rate in Asia was also low (1% and 3% in the two studies) (4, 5). Previously, it was believed that milder presentations of CDI in Asia were due to the absence of “hypervirulent” C. difficile ribotypes (RTs), such as RT 027; however, more recent studies have suggested that C. difficile RT 027 does not necessarily cause more severe CDI (8). C. difficile RT 017 is often a dominant strain in Asia and has caused outbreaks of severe CDI globally (9). Thus, it is unlikely that these differences in C. difficile epidemiology would account for differences in clinical characteristics.
Recently, there has been an update in the Infectious Diseases Society of America (IDSA)/Society for Healthcare Epidemiology of America (SHEA) treatment guidelines for CDI. Vancomycin is now recommended as the sole agent of choice for the treatment of both mild and severe CDI (10); however, metronidazole continues to be the primary agent used in Thailand. Whether metronidazole treatment is inferior to vancomycin in this population remains unclear. Furthermore, recently in Thailand, there were outbreaks caused by vancomycin-resistant enterococci (VRE) in many tertiary care hospitals that were directly due to the overuse of vancomycin (11, 12). The 30-day mortality rate for VRE infection was 58% in one study (12), much higher than that of CDI in Thailand (4). Whether the presence of VRE has an impact on the outcomes of patients treated for CDI with vancomycin remains unknown.
This study describes the clinical characteristics of CDI in a tertiary care hospital in Thailand, the treatment regimens used, and the outcomes of disease. It also explores factors that may be responsible for milder disease presentations, such as colonization with nontoxigenic C. difficile (NTCD).
MATERIALS AND METHODS
Study population and specimen transfer.
This study was conducted at Siriraj Hospital, a 2,061-bed tertiary care teaching hospital in central Thailand, from 2015 tot 2018. The study population included all diarrheal patients above 15 years of age who (i) had a complete medical record of the diarrheal episode, (ii) had a stool sample submitted for the detection of toxigenic C. difficile, and (iii) had a sufficient amount of stool sample for further testing at the reference laboratory. A diarrheal episode was defined as a new onset of ≥3 unformed stools within 24 h. The diarrheal episode was considered resolved when the patient started producing formed stools or the stool frequency decreased to ≤2 stools in 24 h. At the hospital, each stool sample was tested for the presence of the C. difficile tcdB gene using the BD MAX Cdiff assay (Becton, Dickinson, USA) and was then sent at ambient temperature by courier (transit time of <72 h) to a reference laboratory in Perth, Western Australia, Australia, for isolation and characterization of C. difficile. Patients whose diarrheal episodes were not due to other causes (e.g., other gastrointestinal infections, laxatives, or the effects of other drugs) who tested positive by both PCR detection of tcdB and culture of toxigenic C. difficile were classified in the CDI group. Patients who tested negative by either test or whose diarrheal episodes were due to other causes were classified in the control group.
C. difficile culture and strain characterization.
At the reference laboratory, stool samples were cultured anaerobically for C. difficile on ChromID C. difficile agar (bioMérieux, France) (13) and in an enrichment broth as previously described (14), and all C. difficile isolates were characterized using toxin gene profiling and PCR ribotyping (15–18). Because of the high prevalence of NTCD in South-East Asia (19, 20), each stool sample was also tested for the presence of NTCD. Briefly, the stool samples were inoculated into Robertson’s cooked meat broth supplemented with 5 mg/liter gentamicin, 8 mg/liter cefoxitin, and 250 mg/liter cycloserine (CMB-GCC) (PathWest Media, Australia) and incubated at 35°C for at least 5 days. After incubation, 1 ml of CMB-GCC was treated with 1 ml of absolute ethanol for 1 h. A 10-μl aliquot of the ethanol-shocked CMB-GCC was then inoculated onto horse blood agar and incubated anaerobically for 48 h. DNA extraction of all the growth on each plate was performed using 5% Chelex suspension (Sigma-Aldrich, Australia). Each DNA sample was screened for NTCD using lok1-lok3 PCR as described by Braun et al. (21). All stool samples testing positive by lok1-lok3 PCR were recultured, and a strain of NTCD from each sample was isolated and characterized as described above. These patients were considered to be colonized by NTCD.
Patient data collection.
All patient data were acquired from the Siriraj Hospital patient database. If the patient had multiple episodes of diarrhea, only the details of the first episode were recorded. Charlson’s comorbidity index was used to compare the severity of comorbid disease (22). The severity of CDI was evaluated using both Zar criteria (23) and the recommended IDSA/SHEA method (10). For the Zar criteria, each patient was given 1 point for each of the following parameters: body temperature > 38.3°C, age > 60 years, total white blood cell (WBC) count > 15,000 cells/mm3, and plasma albumin < 2.5 mg/dl. Each patient was also given 2 points if he or she required admission in an intensive care unit and another 2 points if pseudomembranous colitis (PMC) was identified. Patients were considered to have severe CDI if they had at least 2 points (23). For the recommendation by IDSA/SHEA, patients were considered to have severe CDI if they had an increase of plasma creatinine level of >1.5-fold from their baseline or if they had a total WBC count of >15,000 cells/mm3 (10). Laboratory results at the onset of diarrhea were recorded. Three outcomes were assessed, the 30-day mortality rate, the total length of stay (LOS) in the hospital among surviving patients, and the recurrence rate of CDI. Recurrent CDI was defined as a CDI episode which occurred after the resolution of the previous CDI episode.
Statistical analysis.
All statistical analyses were performed using IBM SPSS Statistics for Windows version 26.0 (IBM, New York, USA). A P value of ≤0.05 was considered statistically significant.
Human research ethics approval.
This study was approved by the Human Research Ethics Committee of The University of Western Australia (reference file RA/4/20/4704) and the Siriraj Institutional Review Board (protocol number 061/2558 [EC1]).
RESULTS
Patient characteristics.
A total of 469 patients were included in the study, 248 patients in the CDI group and 221 patients in the control group. Eleven and nine patients in the CDI and control groups, respectively, underwent colonoscopy. PMC was confirmed in two patients in the CDI group and none in the control group. More patients in the CDI group were admitted to the hospital to undergo surgery (18% versus 11%, P = 0.047), and more patients in the control group were admitted for nonoperative procedures (10% versus 4%, P = 0.02). These differences did not contribute to the difference in the outcomes of the two groups (P = 0.71). Other basic characteristics were similar between both groups (Table 1). Compared to the culture method (14), the BD MAX Cdiff assay had a positive predictive value of 88% (95% confidence interval [CI], 84% to 91%) and a negative predictive value of 91% (95% CI, 87% to 95%).
TABLE 1.
Basic demographic characteristics of patients in the CDI and control groups
| Characteristic | Data for patients in: |
P value | |
|---|---|---|---|
| CDI group (n = 248) | Control group (n = 221) | ||
| Average age (yrs) ± standard deviation | 67.78 ± 2.04 | 65.57 ± 2.41 | 0.17 |
| Sex (no. of male patients) | 107 (43%) | 98 (44%) | 0.87 |
| Charlson’s comorbidity index (range)a | 2 (1–4) | 2 (1–4) | 0.60 |
| Outpatient cases | 16 (6%) | 10 (5%) | 0.48 |
| No. of cases in each department | |||
| Internal medicine | 192 (77%) | 180 (81%) | 0.34 |
| Surgery | 41 (17%) | 31 (14%) | 0.53 |
| Other | 15 (6%) | 10 (5%) | 0.60 |
| No. of cases that underwent colonoscopy | 11 (4%) | 9 (4%) | 0.97 |
| Reason for hospital visit (no. of patients) | |||
| Medical treatment | 168 (68%) | 154 (70%) | 0.72 |
| Surgery | 44 (18%) | 24 (11%) | 0.047 |
| Nonoperative procedure | 10 (4%) | 22 (10%) | 0.02 |
| Chemotherapy | 22 (9%) | 19 (8%) | 0.95 |
| Other | 4 (1%) | 2 (1%) | 0.69 |
| Province of residence (no. of patients) | |||
| Bangkok | 134 (54%) | 124 (56%) | 0.72 |
| Metropolitan regionb | 47 (19%) | 41 (19%) | 0.99 |
| Other | 67 (27%) | 56 (25%) | 0.76 |
| LOS (days) (range)a | 22 (11–40) | 20 (10–35.75) | 0.35 |
Charlson’s index and LOS are expressed as median (interquartile range).
Metropolitan region refers to 5 provinces surrounding Bangkok (Nakhon Pathom, Nonthaburi, Pathum Thani, Samut Prakan, and Samut Sakhon).
Comparison of the severity assessment methods.
Using the severity assessment method published by Zar et al. in 2007 (23) and the 2017 IDSA/SHEA guidelines (10), 115 (46%) and 87 (35%) CDI cases, respectively, were classified as severe in our patient population. The 30-day mortality rate was higher in severe CDI cases classified by both the Zar and IDSA/SHEA methods (P < 0.001 and 0.01, respectively). The categorical agreement between both methods was 69% (Cohen’s kappa = 0.37). Comparing the two methods, the Zar method was better at predicting 30-day mortality (McNemar’s P < 0.001). Thus, the Zar method was used to classify the severity of CDI throughout this study.
Comparison of the overall 30-day mortality rate.
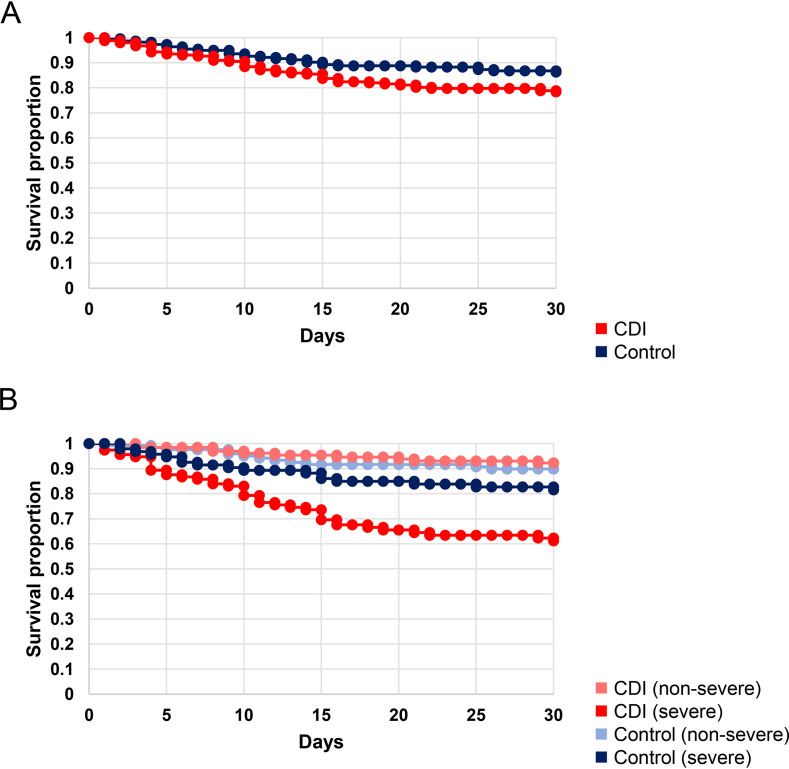
The overall 30-day mortality rate was significantly higher in the CDI group than the control group (21% versus 14%, P = 0.046). When stratified by severity using the Zar method, patients with severe CDI had a higher 30-day mortality rate than patients with nonsevere CDI (36% versus 8%, P < 0.001). After applying the Zar method to the control group (see discussion below), patients with severe CDI also had a higher 30-day mortality rate than patients in the control group with severe host status (36% versus 19%, P = 0.009) and those without (36% versus 10%, P < 0.001). Figure 1 shows the Kaplan-Meier survival curves for both groups. The most common cause of death in both groups was the complication of non-CDI nosocomial infection, followed by complications associated with the patients’ underlying diseases and other intrahospital complications. In the CDI group, CDI was the direct cause of death in two patients (1%).
FIG 1.
Kaplan-Meier curve comparing 30-day survival rates between CDI (red) and control (blue) groups. (A) Overall, CDI patients had a lower survival rate than the control group (P = 0.046). (B) When stratified by host severity according to Zar et al. (23), patients with severe CDI (dark red) had a much lower survival rate than the control group with severe host status (dark blue) (P = 0.009), as well as both patients with nonsevere CDI (light red) and the control group without severe host status (light blue) (P < 0.001 for both groups).
Comparison of the treatment outcomes between patients receiving metronidazole and vancomycin.
A total of 216 (87%) patients received specific treatment for CDI. Oral metronidazole was chosen as the sole therapeutic agent in 156 patients (63%). Oral vancomycin was given as the main treatment to 60 patients (24%), 28 of whom (11%) also briefly received oral metronidazole before switching to vancomycin. In addition to oral antimicrobial therapy, the inciting antimicrobials were discontinued in 19 patients (8%). The remaining patients (32, 13%) did not receive any specific treatment for CDI.
Table 2 compares the clinical outcomes of CDI patients treated with oral metronidazole (n = 156) and oral vancomycin (n = 60). Overall, metronidazole was not inferior to vancomycin in terms of recurrence rate and LOS. Interestingly, the metronidazole group had a lower 30-day mortality rate compared to the vancomycin group. Among 18 deaths in the vancomycin group, 15 (83%) were due to other nosocomial infections. Causative agents were identified in 11 cases, 1 of which was a strain of vancomycin-resistant Enterococcus faecium.
TABLE 2.
Clinical outcomes of CDI patients treated with metronidazole and vancomycin regimens
| Clinical outcome | Patient data for regimen (no. of patients): |
P value | |
|---|---|---|---|
| Metronidazole | Vancomycin | ||
| 30-day mortality rate | 26/156 (16%) | 18/60 (30%) | 0.047 |
| Recurrence rate | 16/156 (10%) | 5/60 (8%) | 0.80 |
| LOS (days) (range)a | 22 (10–38.75) | 22 (11–39) | 0.82 |
| Prolonged LOSb | 62/122 (51%) | 20/37 (54%) | 0.88 |
LOS is expressed as median (interquartile range).
Prolonged LOS is defined as LOS above the median LOS (21 days).
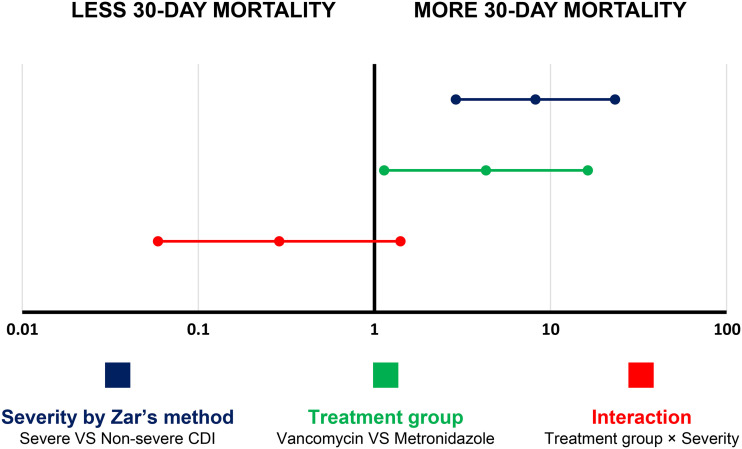
In a logistic regression analysis (Fig. 2), a higher 30-day mortality rate was associated with both severe disease (as assessed by the Zar method) (odds ratio [OR], 8.21 [2.90 to 23.24]; P < 0.001) and vancomycin treatment (as opposed to metronidazole treatment [OR], 4.30 [1.14 to 16.29]; P = 0.032) without significant interaction between these two parameters (OR, 0.29 [0.06 – 1.41]; P = 1.24).
FIG 2.
Univariate logistic regression analysis of factors associated with a 30-day mortality rate among CDI patients receiving appropriate treatment. A forest plot of the odds ratio shows that both severe CDI according to Zar’s method (dark blue) and treatment with vancomycin (green) were associated with a higher 30-day mortality rate without significant interactions between disease severity and treatment regimen (red).
Characteristics of C. difficile in the study groups.
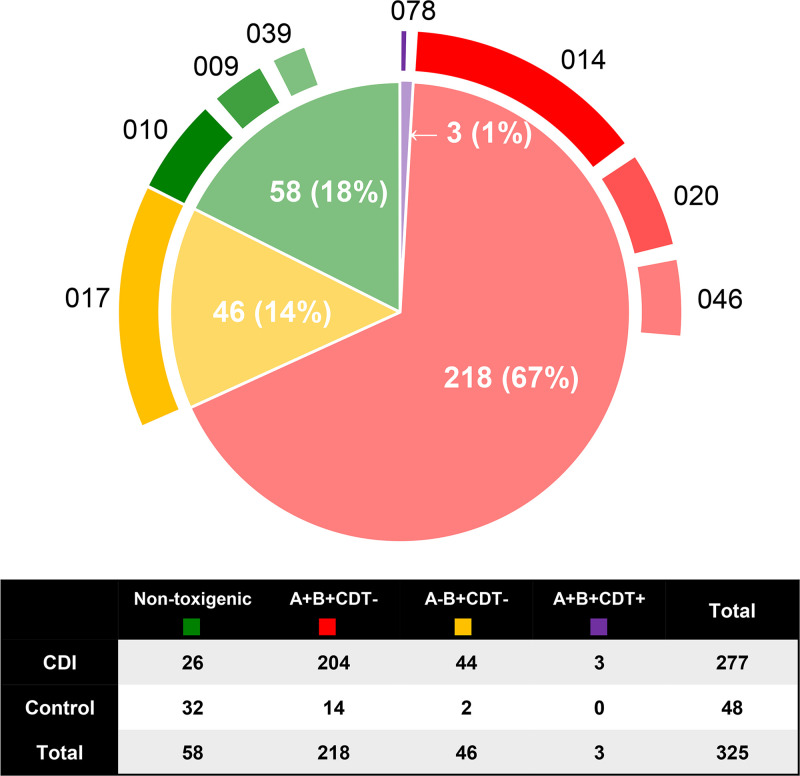
A total of 325 nonduplicate C. difficile strains were isolated (Fig. 3), 267 (82%) of which were toxigenic (250 [94%] isolated directly from ChromID agar and an additional 17 [6%] isolated from enrichment broth). The most common toxin profile was tcdA-positive, tcdB-positive, binary toxin-negative (A+B+CDT–, 67%), followed by A–B+CDT– (14%), while three strains (1%) were binary toxin-positive (A+B+CDT+). All A–B+CDT– C. difficile isolates belonged to RT 017, and one of the A+B+CDT+ C. difficile isolates was the epidemic strain RT 078.
FIG 3.
Summary of the C. difficile toxin profiles and common RTs of isolates recovered from both CDI and control patients. There were a total of 325 nonrepeating C. difficile strains categorized into A+B+CDT– C. difficile (218 strains [67%]; the most common RTs included RTs 014 [n = 46], 020 [n = 19], and 046 [n = 14]), A–B+CDT– C. difficile (46 strains [14%], all belonging to RT 017), A–B–CDT– C. difficile (58 strains [17%]; the most common RTs included RTs 010 [n = 16], 009 [n = 6], and 039 [n = 5]), and A+B+CDT+ C. difficile (3 strains [1%], one belonging to RT 078).
A total of 44 (18%) patients were infected with A–B+ C. difficile. CDI due to A–B+ C. difficile did not differ from CDI due to A+B+ C. difficile in terms of case severity (severe CDI prevalence of 36% versus 49%, P = 0.19), 30-day mortality rate (23% versus 20%, P = 0.85), recurrence rate (11% versus 8%, P = 0.73), and LOS (median 18.5 versus 20 days, P = 0.41).
Colonization by NTCD was found in 26 (10%) CDI cases and 32 (14%) controls (P = 0.24). In the CDI group, the prevalence of severe CDI was slightly lower in the patients colonized by NTCD, although this difference was not statistically significant (27% versus 49%, P = 0.06). There were no differences in clinical outcomes between the two groups, including 30-day mortality (15% versus 21%, P = 0.61), recurrence rate (8% versus 9%, P = 1), and LOS (median 22 versus 19 days, P = 0.56).
DISCUSSION
Overall, CDI in Thai patients was associated with relatively mild disease. Most patients experienced watery diarrhea, and only 12% had evidence of red blood cells in the stool sample. Only two cases (1%) were confirmed to have PMC. Also, only two deaths (1%) were directly attributable to CDI within 30 days. The overall recurrence rate (10%) was also lower than that reported in other regions of the world (20%) (6). Even though CDI was relatively mild, patients in the CDI group still had a higher overall 30-day mortality rate compared to the control group, suggesting that CDI may have indirectly affected the patients’ outcomes. This could be either because CDI further complicated the patients’ hydration or nutritional status. It could also be due to a complication arising from the CDI treatment.
The Zar criteria are an effective tool for predicting 30-day mortality (23). However, the parameters used in this method are not specific for CDI, and it may predict the severity of the host status rather than of CDI, especially in cases where CDI is not the primary disease. Thus, the Zar criteria were applied to both CDI and control groups to evaluate the host status at the onset of diarrhea. After applying the Zar method (Fig. 1B), CDI was associated with higher 30-day mortality only in the group with a severely ill host status. This was supported by the logistic regression analysis in Fig. 2.
It remains unclear why CDI in Thailand is milder than that in other regions of the world. Initially, this was thought to be due to the lack of “hypervirulent” strains of C. difficile in South-East Asia; however, this may not be correct. Several studies have reported that differences in C. difficile RT do not necessarily predict severity of disease or outcome of CDI (5, 8, 24). This study provides further evidence to refute this idea, as it showed a high prevalence of C. difficile RT 017, an RT responsible for outbreaks of CDI around the world with a mortality rate as high as 38% in one study (25). In a nonoutbreak setting, RT 017 was also associated with severe CDI with high mortality in a study in Germany (26). In the present study, CDI due to RT 017 was similar to CDI due to other RTs and was mostly mild, as described in an earlier study in South Korea (5). Thus, it appears that when C. difficile RT 017 causes CDI outside Asia, the disease can be severe compared to CDI in Asia, which appears mild, suggesting perhaps that regional protective factors may be important.
To investigate one possible protective factor, the effect of colonization by NTCD on subsequent CDI severity was evaluated. In animal studies, colonization by NTCD can be protective against recurrence of CDI, most likely due to competition with toxigenic strains to colonize the colon (27, 28). Although the number of colonized patients was low in this study (26 cases, 10%) and there was no significant difference, there was a trend toward less severe disease in CDI patients colonized by NTCD. The control group in this study also had a slightly higher prevalence of colonization by NTCD. These findings suggest that NTCD may prevent colonization by toxigenic C. difficile and the subsequent development of CDI, as reported previously (27, 28); however, a larger study is needed to properly evaluate the effects of colonization by NTCD in humans.
Recently, it was suggested that metronidazole is inferior to vancomycin for the treatment of CDI (29). As a result, metronidazole was removed from the list of first-line agents for treating CDI in the 2017 IDSA/SHEA guidelines (10). However, in a publication that may have contributed to this recommendation, the prevalence of severe CDI was higher in the metronidazole group (92/278, 33% versus 65/259, 25%) (29). Metronidazole is known to be associated with lower efficacy in severe CDI (23), and the higher prevalence of severe cases in the metronidazole group may have contributed to its overall poorer performance. In the present study, metronidazole was not found to be inferior to vancomycin, though this study had 27% power to detect the inferiority of metronidazole according to the previous study (29), and further studies with sufficient power are needed to confirm this. Somewhat surprisingly, vancomycin was associated with a higher 30-day mortality rate in this study. Anecdotally, clinicians were more likely to prescribe vancomycin in cases that were perceivably more severe, many of whom died due to unrelated causes, resulting in the higher mortality rate in the vancomycin group. Also, vancomycin use poses a risk of prompting the emergence of VRE, which has recently caused outbreaks in Thailand (11, 12, 30). Indeed, a strain of VRE was later isolated from one of our CDI patients and likely contributed to the patient’s death. This study highlights a situation when the benefit of vancomycin treatment does not outweigh its risks (30). In such cases, clinicians should consider prescribing metronidazole for the treatment of CDI unless there is compelling evidence of severe CDI, such as the presence of PMC.
In conclusion, CDI in Thailand was associated with milder disease, and there is a possibility that the colonization by nontoxigenic C. difficile is protective against CDI. Metronidazole remained an effective drug for CDI and should still be considered a first-line agent.
ACKNOWLEDGMENTS
K.I. is a recipient of the Mahidol Scholarship from Mahidol University, Thailand.
T.V.R. has received grants from Cepheid, Merck, Otsuka, Sanofi, and Summit for work outside that in this report. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
K.I. isolated and characterized C. difficile strains, analyzed data, and prepared the manuscript. P.P. helped with isolation and characterization of C. difficile strains and revised the manuscript. T.L. collected patients’ records and revised the manuscript. P.K. and T.V.R. supervised the project and revised the manuscript.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). 2019. Antibiotic resistance threats in the United States, 2019. U.S. Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 2.Nagy E. 2018. What do we know about the diagnostics, treatment and epidemiology of Clostridioides (Clostridium) difficile infection in Europe? J Infect Chemother 24:164–170. doi: 10.1016/j.jiac.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. 2012. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 81:1–14. doi: 10.1016/j.jhin.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Thipmontree W, Kiratisin P, Manatsathit S, Thamlikitkul V. 2011. Epidemiology of suspected Clostridium difficile-associated hospital-acquired diarrhea in hospitalized patients at Siriraj Hospital. J Med Assoc Thai 94(Suppl 1):S207–S216. [PubMed] [Google Scholar]
- 5.Kim J, Kim Y, Pai H. 2016. Clinical characteristics and treatment outcomes of Clostridium difficile infections by PCR ribotype 017 and 018 strains. PLoS One 11:e0168849. doi: 10.1371/journal.pone.0168849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. 1997. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis 24:324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 7.Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis 40:1591–1597. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 8.Walk ST, Micic D, Jain R, Lo ES, Trivedi I, Liu EW, Almassalha LM, Ewing SA, Ring C, Galecki AT, Rogers MA, Washer L, Newton DW, Malani PN, Young VB, Aronoff DM. 2012. Clostridium difficile ribotype does not predict severe infection. Clin Infect Dis 55:1661–1668. doi: 10.1093/cid/cis786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imwattana K, Knight DR, Kullin B, Collins DA, Putsathit P, Kiratisin P, Riley TV. 2019. Clostridium difficile ribotype 017:- characterization, evolution and epidemiology of the dominant strain in Asia. Emerg Microbes Infect 8:796–807. doi: 10.1080/22221751.2019.1621670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. 2018. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 66:987–994. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 11.Chotiprasitsakul D, Santanirand P, Thitichai P, Rotjanapan P, Watcharananan S, Siriarayapon P, Chaihongsa N, Sirichot S, Chitasombat M, Chantharit P, Malathum K. 2016. Epidemiology and control of the first reported vancomycin-resistant Enterococcus outbreak at a tertiary-care hospital in Bangkok, Thailand. Southeast Asian J Trop Med Public Health 47:494–502. [PubMed] [Google Scholar]
- 12.Hemapanpairoa J, Changpradub D, Thunyaharn S, Santimaleeworagun W. 2019. Vancomycin-resistant enterococcal infection in a Thai university hospital: clinical characteristics, treatment outcomes, and synergistic effect. Infect Drug Resist 12:2049–2057. doi: 10.2147/IDR.S208298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry JD, Asir K, Halimi D, Orenga S, Dale J, Payne M, Carlton R, Evans J, Gould FK. 2010. Evaluation of a chromogenic culture medium for isolation of Clostridium difficile within 24 hours. J Clin Microbiol 48:3852–3858. doi: 10.1128/JCM.01288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Putsathit P, Morgan J, Bradford D, Engelhardt N, Riley TV. 2015. Evaluation of the BD Max Cdiff assay for the detection of toxigenic Clostridium difficile in human stool specimens. Pathology 47:165–168. doi: 10.1097/PAT.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, Suzuki K, Kim SM, Chong Y, Wasito EB. 1998. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol 36:2178–2182. doi: 10.1128/JCM.36.8.2178-2182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stubbs SL, Brazier JS, O’Neill GL, Duerden BI. 1999. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol 37:461–463. doi: 10.1128/JCM.37.2.461-463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato N, Ou CY, Kato H, Bartley SL, Brown VK, Dowell VR Jr, Ueno K. 1991. Identification of toxigenic Clostridium difficile by the polymerase chain reaction. J Clin Microbiol 29:33–37. doi: 10.1128/JCM.29.1.33-37.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stubbs S, Rupnik M, Gibert M, Brazier J, Duerden B, Popoff M. 2000. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol Lett 186:307–312. doi: 10.1111/j.1574-6968.2000.tb09122.x. [DOI] [PubMed] [Google Scholar]
- 19.Putsathit P, Maneerattanaporn M, Piewngam P, Kiratisin P, Riley TV. 2017. Prevalence and molecular epidemiology of Clostridium difficile infection in Thailand. New Microbes New Infect 15:27–32. doi: 10.1016/j.nmni.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley TV, Collins DA, Karunakaran R, Kahar MA, Adnan A, Hassan SA, Zainul NH, Rustam FM, Wahab ZA, Ramli R, Lee YY, Hassan H. 2018. High prevalence of toxigenic and non-toxigenic Clostridium difficile in Malaysia. J Clin Microbiol 56:e00170-18. doi: 10.1128/JCM.00170-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun V, Hundsberger T, Leukel P, Sauerborn M, von Eichel-Streiber C. 1996. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181:29–38. doi: 10.1016/s0378-1119(96)00398-8. [DOI] [PubMed] [Google Scholar]
- 22.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. 2011. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 23.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 24.Goorhuis A, Debast SB, Dutilh JC, van Kinschot CM, Harmanus C, Cannegieter SC, Hagen EC, Kuijper EJ. 2011. Type-specific risk factors and outcome in an outbreak with 2 different Clostridium difficile types simultaneously in 1 hospital. Clin Infect Dis 53:860–869. doi: 10.1093/cid/cir549. [DOI] [PubMed] [Google Scholar]
- 25.Alfa MJ, Kabani A, Lyerly D, Moncrief S, Neville LM, Al-Barrak A, Harding GK, Dyck B, Olekson K, Embil JM. 2000. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. J Clin Microbiol 38:2706–2714. doi: 10.1128/JCM.38.7.2706-2714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arvand M, Hauri AM, Zaiss NH, Witte W, Bettge-Weller G. 2009. Clostridium difficile ribotypes 001, 017, and 027 are associated with lethal C. difficile infection in Hesse, Germany. Euro Surveill 14:19403 https://www.eurosurveillance.org/content/10.2807/ese.14.45.19403-en. [DOI] [PubMed] [Google Scholar]
- 27.Gerding DN, Sambol SP, Johnson S. 2018. Non-toxigenic Clostridioides (formerly Clostridium) difficile for prevention of C. difficile infection: from bench to bedside back to bench and back to bedside. Front Microbiol 9:1700. doi: 10.3389/fmicb.2018.01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira Junior CA, Silveira Silva RO, Goncalves Cruz DS, Pires IH, Carvalho Guedes RM, Faria Lobato FC. 2019. The non-toxigenic strain of Clostridioides difficile Z31 can prevent infection by C. difficile in experimental model piglets. Anaerobe 55:24–28. doi: 10.1016/j.anaerobe.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, Gelone SP, Broom C, Davidson DM, Polymer Alternative for CDI Treatment investigators. 2014. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 59:345–354. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 30.Zilberman-Itskovich S, Youngster I, Lazarovitch T, Bondarenco M, Toledano L, Kachlon Y, Mengesha B, Strul N, Zaidenstein R, Marchaim D. 2019. Potential impact of removing metronidazole from treatment armamentarium of mild acute Clostridioides difficile infection. Future Microbiol 14:1489–1495. doi: 10.2217/fmb-2019-0157. [DOI] [PubMed] [Google Scholar]