Streptococcal serology is a cornerstone in the diagnosis of acute rheumatic fever (ARF), a postinfectious sequela associated with group A Streptococcus infection. Current tests that measure anti-streptolysin O (ASO) and anti-DNaseB (ADB) titers require parallel processing, with their predictive value limited by the low rate of decay in antibody response. Accordingly, our objective was to develop and assess the diagnostic potential of a triplex bead-based assay, which simultaneously quantifies ASO and ADB together with titers for a third antigen, SpnA.
KEYWORDS: immunoassay, DNaseB, SpnA, acute rheumatic fever, group A streptococcus, immunoassay, serology, streptolysin O
ABSTRACT
Streptococcal serology is a cornerstone in the diagnosis of acute rheumatic fever (ARF), a postinfectious sequela associated with group A Streptococcus infection. Current tests that measure anti-streptolysin O (ASO) and anti-DNaseB (ADB) titers require parallel processing, with their predictive value limited by the low rate of decay in antibody response. Accordingly, our objective was to develop and assess the diagnostic potential of a triplex bead-based assay, which simultaneously quantifies ASO and ADB together with titers for a third antigen, SpnA. Our previous cytometric bead assay was transferred to the clinically appropriate Luminex platform by coupling streptolysin O, DNaseB, and SpnA to spectrally unique magnetic beads. Sera from more than 350 subjects, including 97 ARF patients, were used to validate the assay and explore immunokinetics. Operating parameters demonstrate that the triplex assay produces accurate and reproducible antibody titers which, for ASO and ADB, are highly correlative with existing assay methodology. When ARF patients were stratified by time (days following hospital admission), there was no difference in ASO and ADB between <28 and 28+ day groups. However, for anti-SpnA, there was a significant decrease (P < 0.05) in the 28+ day group, indicative of faster anti-SpnA antibody decay. Anti-SpnA immunokinetics support very recent group A Streptococcus infection and may assist in diagnostic classification of ARF. Further, bead-based assays enable streptococcal serology to be performed efficiently in a high-throughput manner.
INTRODUCTION
Group A Streptococcus (GAS) (Streptococcus pyogenes) causes a broad range of diseases in humans, including skin and throat infections that may lead to more serious autoimmune sequelae such as acute rheumatic fever (ARF) and poststreptococcal glomerulonephritis (PSGN) (1). ARF precedes rheumatic heart disease (RHD), which is responsible for major morbidity and mortality in resource-poor countries and in certain indigenous populations in high-income countries (2). In particular, Māori and Pacific children in New Zealand, and Aboriginal children in Australia, have some of the highest incidences of ARF and RHD in the world (3, 4).
Accurate diagnosis of ARF is essential to ensure timely hospitalization, clinical management, and initiation of secondary prevention (monthly benzathine penicillin injections) to reduce the risk of RHD. The Jones criteria are a set of clinical guidelines that form the basis of ARF diagnosis; these criteria specifically include laboratory evidence of a previous GAS infection (5). Since ARF occurs 2 to 4 weeks after an initial GAS infection, the causative bacteria are often not detected by culture at the time of clinical presentation. Streptococcal serology, the measurement of antibodies produced in response to a GAS infection, is therefore commonly used as definitive laboratory evidence of infection (6). Current ARF guidelines require a patient to have elevated antibody titers to one of two GAS antigens—streptolysin O (SLO) and deoxyribonuclease B (DNaseB) (7, 8).
Demonstrating a rise in anti-SLO (ASO) and anti-DNaseB (ADB) antibody titers for the diagnosis of poststreptococcal diseases requires paired serum measurements at both the acute and convalescent stage of infection. This can be impractical to obtain in patients with suspected ARF (9), such that an upper limit of normal (ULN) cutoff is often applied in lieu of the gold standard twofold rise in titer (8, 10). ULN cutoffs are defined as the 80th percentile of ASO and ADB titers in a matched healthy population, and can be challenging to establish in areas where GAS is endemic, resulting in a broad range of ULN recommendations for ASO and ADB globally (9).
ASO is generally measured by an automated turbidimetric or nephelometric assay that utilizes an international standard of pooled sera (8, 11). While there is a nephelometric assay for ADB, it is commonly measured with an enzyme inhibition assay. This inhibition assay is only semiquantitative, and there are no international reference sera available for ADB, making these assays less standardized (8). With currently available methods, the titers for ASO and ADB need to be determined in two separate assays in parallel, which impacts laboratory efficiency. In addition to the limitation associated with parallel processing, the antigens themselves have inherent limitations. The gene encoding DNaseB shows allelic variation and is not universally present across all GAS strains. This can lead to false-negative results, where a lack of ADB response may be the result of a true GAS infection with a strain not carrying a DNaseB-encoding gene (12). Furthermore, the immunokinetics of ASO and ADB complicate interpretation. The titers to both antigens can stay elevated for many weeks after infection, and in some individuals, elevated titers have been observed 12 months after a GAS infection (13). This can lead to false-positive results, where elevated antibody titers do not necessarily represent a recent infection, but are the consequence of slow antibody decay following a GAS infection that occurred months or even years prior.
To circumvent some of the limitations with current streptococcal serology, our laboratory recently developed a proof-of-concept multiplex immunoassay using cytometric bead array (CBA) technology (14). The assay simultaneously measured ASO and ADB titers as well as antibody titers to a third, novel GAS antigen, S. pyogenes nuclease A (SpnA, Spy0747), which is a highly conserved and immunogenic cell wall-anchored nuclease (15, 16). However, the CBA platform is not currently suitable for routine use in clinical laboratories due to a lack of automation and reliance on a flow cytometer for read-out. Accordingly, the aims of the current study were (i) to design and optimize the multiplex immunoassay technology for the clinically available Luminex bead-based platform and (ii) to assess the efficiency and accuracy of the resulting triplex assay in diagnosing ARF in a large clinical cohort. The Luminex system is based on spectrally unique magnetic beads that enable simultaneous measurement of antibodies against multiple analytes in a single reaction well. Compared with CBA, Luminex assays tend to be more reproducible and can be automated, and the machines are widely used in clinical diagnostic laboratories (17–19).
MATERIALS AND METHODS
Study subjects.
Human sera were obtained from several studies conducted in the North Island of New Zealand. Each had appropriate ethical approval, and all participants (or their parents or legal guardians) provided written informed consent. All ARF cases (n = 97) were diagnosed according to the New Zealand modification of the Jones criteria (7, 20). Sixteen cases were recruited via two small studies conducted at Waikato Hospital (2012 to 2015; ethics CEN/12/06/017) and Starship Hospital (2004 to 2006; ethics AKX/2002/08). The remaining 79 cases were recruited as part of the Rheumatic Fever Risk Factors (RF RISK) study (21). This nationwide study was conducted between 2014 and 2017 (ethics 14/NTA/53) and aimed to identify modifiable risk factors for ARF in New Zealand. Sera were also obtained from 120 healthy controls recruited into the RF RISK study and highly matched with the ARF cases for age, ethnicity, and social deprivation. Forty-five of these participants were excluded from analysis, as they reported a definite or probable sore throat or skin infection in the 4 weeks preceding enrollment, such that the final healthy control group comprised 75 participants. Demographics for all ARF cases and healthy controls are shown in Table 1. Finally, to enable comparison of the triplex assay with existing commercial serology assays, residual sera (n = 180) were stored following routine streptococcal serological testing in 2018 at Labtests, a community laboratory that serves the greater Auckland region (ethics 17/CEN/230). Demographic information was not collected for these samples.
TABLE 1.
Demographics of the study participants included in the calculation of experimental ULN values and the diagnostic sensitivity of each antigen
| Demographic characteristic | Value for children: |
|
|---|---|---|
| With ARF (n = 97) | Healthy (n = 75) | |
| Age (yr) | ||
| Median | 11 | 11.7 |
| Range | 4−20 | 6−18 |
| Male sex, n (%) | 67 (69) | 46 (61) |
| Ethnicity, n (%) | ||
| Māori | 42 (43) | 30 (40) |
| Pacific | 54 (56) | 43 (57) |
| Other | 1 (1) | 2 (1) |
Recombinant antigens.
Recombinant DNaseB (rDNaseB) and recombinant SpnA (rSpnA) were expressed and purified as previously described (14). The slo gene was cloned using GeneArt Gene Synthesis technology (ThermoFisher). DNA encoding a SLO double mutant (amino acids [aa] 32 to 571) with substitutions at amino acid sites P427L and W535F was synthesized and cloned into pET151/d-TOPO for expression of a detoxified SLO construct with an N-terminal His6 tag. The protein was expressed in Escherichia coli BL21AI cells after induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and 0.2% l-arabinose (Alfa Aeser) at 37°C for 2.5 h. rSLO was purified from E. coli cell lysate using Ni2+ nitrilotriacetic acid (NTA) immobilized metal affinity chromatography, and the His6 tag was cleaved using recombinant tobacco etch virus protease at a 1:20 ratio of protease to protein. The protein was subject to a final purification step using a Superdex200 10/300 column (GE Healthcare) in phosphate-buffered saline (PBS), and fractions containing rSLO were pooled following verification by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Bead coupling.
The three antigens were covalently coupled to MagPlex microspheres (beads) by carbodiimide chemistry using the xMAP Antibody Coupling kit (Luminex Corporation), according to the manufacturer’s instructions. Briefly, magnetic beads were washed with activation buffer, followed by a 20-min incubation with EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) and Sulfo-NHS (N-hydroxysulfosuccinimide). Each protein antigen was coupled to a spectrally distinct bead region; rSLO to region 72, rDNaseB to region 30, and rSpnA to region 78. Small-scale coupling reactions with protein concentrations ranging from 10 to 30 μg per 2.5 × 106 beads were performed by incubating the proteins with beads for 2 h at room temperature to determine optimal coupling concentrations for each protein. For large-scale reactions, 12.5 × 106 beads were incubated with proteins for 2 h at room temperature. Protein-coupled beads were washed and enumerated with a hemocytometer, and stored at 4°C protected from light until further use.
Triplex immunoassay overview.
Serum samples were diluted 1:8,000 in assay buffer (AB) containing PBS supplemented with 0.5% IgG-free bovine serum albumin (BSA). The diluted sera (30 μl) were added into duplicate wells of a 96-well U-bottom plate (Greiner), and 30 μl of bead solution consisting of equal parts SLO-, DNaseB-, and SpnA-coupled beads was added to samples for a final concentration of 50 beads/μl/antigen. The plates were incubated for 1 h at room temperature and then washed twice with AB using a handheld magnet (Luminex Corporation). Phycoerythrin (PE)-labeled donkey anti-human IgG (Fcy-specific) detection antibody (Jackson Immunoresearch) was added to wells and incubated for 1.5 h at room temperature. Beads were washed twice, resuspended in 100 μl Drive Fluid (Luminex Corporation), and analyzed on a MagPix instrument (Luminex Corporation). All incubations were performed on a plate shaker at 800 rpm, protected from light.
Standard material was obtained by purifying IgG antibodies specific for rDNaseB, rSLO, and rSpnA from pooled human immunoglobulin (intravenous immunoglobulin [IVIG], Intragam P) as previously described (14). The specificity of the purified IgG antibodies was confirmed by an enzyme-linked immunosorbent assay (ELISA). A nine-point standard curve was created for each antigen by mixing known starting concentrations of specific IgG in AB (600 ng/ml for SLO, 400 ng/ml for DNaseB, and 250 ng/ml for SpnA) and performing a threefold dilution series. Standard curves were fitted using a five-parameter regression formula generated by the xPonent software version 4.2 (Luminex Corporation). Net median fluorescence intensity (MFI) values obtained for test sera were converted to concentration (micrograms per milliliter) based on the IgG standard curves.
Assay sensitivity and precision.
The lower limit of detection (LLOD) for each antigen was defined as the lowest concentration of analyte whose MFI response was greater than the blank plus 3 standard deviations (22) across 12 independent assays. Intraassay variability was determined by calculating the coefficient of variation (CV) (standard deviation divided by the mean) from 20 replicates of one sample in a single plate. Interassay variability was determined by calculating the CV from eight serum samples that exhibited various degrees of reactivity against the three antigens in eight independent assay runs (23).
Comparison with commercial assays.
ASO and ADB titers were determined at Labtests, Auckland, New Zealand, and LabPLUS, Auckland City Hospital, New Zealand. ASO titers were measured by the turbidimetric technique using the human anti-streptolysin O kit on a SPAplus analyzer (The Binding Site, CA, USA) and reported in international units (IU) per milliliter. ADB titers were measured by an enzyme inhibition assay following the manufacturer’s instructions, and titers were reported in units per milliliter (bioMérieux, Marcy l’Etoile, France). Both assays provide an inexact, titer range for samples with a low concentration of antibodies; <25 IU/ml for ASO and <100 U/ml for ADB. Mid-titer values of 12.5 IU/ml for ASO and 50 U/ml for ADB were therefore used to define samples that fell within these ranges.
Upper limit of normal.
The sensitivity of each antigen as a serological marker in ARF diagnosis was assessed by applying experimentally determined upper limit of normal (ULN) cutoffs that were calculated using a nonparametric method (24). ULN values were determined as the 80th percentile of titers in the 75 healthy controls recruited in the RF RISK study (21), with those that reported a sore throat or skin infection in the 4 weeks preceding enrollment having already been excluded. This exclusion is in line with previous studies that have removed individuals with recent streptococcal infections from ULN estimates (24, 25). ULN values were determined from titers measured with the triplex assay as well as titers measured with commercial serological methods.
Statistical analyses.
Differences in antibody responses between ARF cases and healthy controls for the three antigens were analyzed using Mann-Whitney U unpaired t test. For the comparison of the triplex measurements with commercial methods, the strength of correlation was calculated using Spearman’s r. Linear regression analysis was also performed on these data to obtain equations that enabled conversion of triplex concentrations (micrograms per milliliter) into internationally recognized units (IU per milliliter for ASO and units per milliliter for ADB). The diagnostic sensitivity of the three antigens (SLO, DNaseB, and SpnA) was compared using a chi-squared test. All statistical analyses were performed in GraphPad Prism (version 8.0), and a P value of ≤0.05 was considered significant.
RESULTS
Optimization and validation of the triplex Luminex assay.
In order to expand the utility of a bead-based assay for streptococcal serology, the assay was transferred from our previously described CBA beads (14) to the clinically relevant Luminex platform. The optimal coupling concentration for each of the highly purified (>95%) recombinant SLO, DNaseB, and SpnA antigens to spectrally unique magnetic beads was determined. This was defined as the concentration that gave the highest MFI across a panel of reference sera with known titers (previously determined by CBA) in single-plex assays. The optimal coupling concentrations were 12.5 μg of SLO and SpnA and 10 μg of DNaseB per 2.5 × 106 beads.
Next, beads coupled with each of the three antigens were mixed in equal parts, and the concentration of antibodies for each antigen was determined in a triplex format for the panel of reference sera. The antibody titers measured in the triplex format correlated strongly with the single-plex assays, indicating no cross-reactivity between the different beads (R2 values of >0.999 for all antigens in a linear regression analysis [data not shown]). After trying various test serum concentrations, a 1:8,000 dilution was found to give MFIs within the log-linear range for all three antigens. Comparison of the MFI at this dilution with the previous CBA beads for 33 samples showed significant correlations (P < 0.0001) for all three antigens (see Fig. S1 in the supplemental material), such that 1:8,000 was used in all subsequent triplex assays.
Following optimization, the assay operating characteristics were determined and are summarized in Table 2. The lower limit of detection (LLOD) was <0.1 ng/ml for all three antigens. The intraassay coefficient of variations (CVs) were <5%, and interassay CVs were <13% for each of the three antigens. These characteristics confirm that the Luminex-based triplex assay is a reliable and repeatable method for quantifying serum antibody titers to GAS antigens.
TABLE 2.
Summary of assay sensitivity and precision
| Assay characteristica | ASO | ADB | Anti-SpnA |
|---|---|---|---|
| Lower limit of detection (ng/ml) | 0.04 | 0.02 | 0.06 |
| Intraassay CV (%) | 4.8 | 4.6 | 4.5 |
| Interassay CV (%) | 12.4 | 12.5 | 11.6 |
CV, coefficient of variation.
Comparison with existing streptococcal serological methodologies.
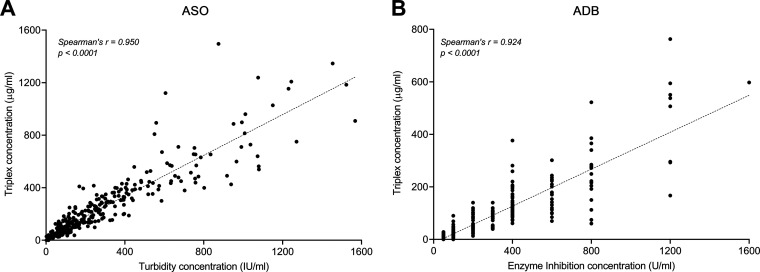
To compare the triplex assay with existing commercial methodologies, experimentally determined titers were compared with titers determined by the turbidimetric technique (ASO) and the semiquantitative enzyme inhibition assay (ADB). The 318 serum samples analyzed included residual sera from a community laboratory (n = 180) as well as sera from ARF cases and controls (n = 138) for which clinical titers were available. As seen in Fig. 1A, there is a highly significant correlation between the concentration of ASO IgG determined in the triplex assay and the commercial methodology (Spearman’s r = 0.950, P value of <0.0001). A significant correlation also exists between the concentration of ADB IgG determined by the triplex assay and the commercial methodology (Spearman’s r = 0.924, P value of <0.0001). However, as previously described (14), there is a lack of precision for the traditional ADB enzyme inhibition assay, which produces titer ranges rather than continuous values. This can be seen visually as a “stacking” of points in Fig. 1B.
FIG 1.
Scatterplots showing the correlation between the triplex Luminex assay and clinically available methodologies for ASO (n = 317) (A) and ADB (n = 318) (B). Spearman’s r and P values are shown. The dashed lines represent the linear regression equations (see also Table S1 in the supplemental material).
The lack of precise titers for ADB in the enzyme inhibition assay is further illustrated by plots of antibody titer distribution (Fig. S2). The continuous distribution of ASO is largely the same between the turbidimetric and triplex assay. In contrast, the discontinuous distribution of ADB titers in the enzyme inhibition assay is improved by the Luminex methodology. The triplex assay therefore offers increased precision for ADB, compared to traditional methods.
Streptococcal antibody titers in ARF.
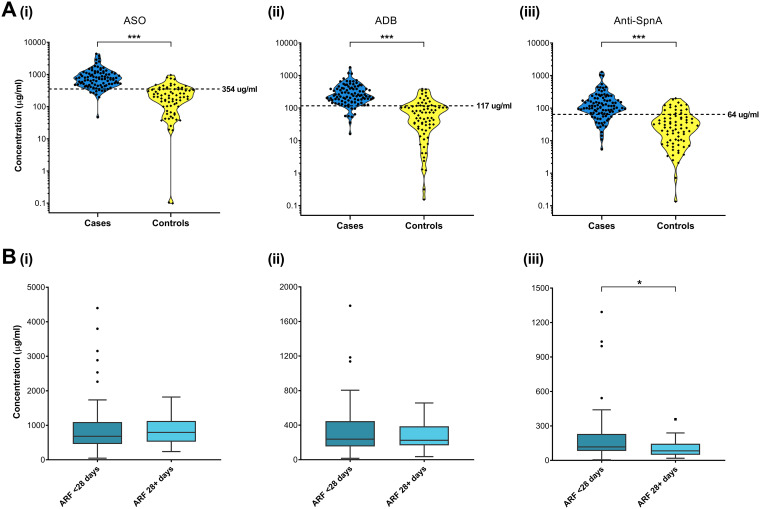
The ability of the triplex assay to detect streptococcal antibodies in ARF was assessed in sera obtained from 97 ARF cases and the 75 healthy controls (Table 1). As was expected, IgG titers against all three antigens are significantly elevated (P < 0.001) in children with ARF compared to healthy control children (Fig. 2A). Serum samples for each ARF case were obtained at various time points after hospital admission, with recruitment of some patients being delayed for more than 4 weeks. This provided an opportunity to assess the impact of time on serum antibody levels. ARF cases were stratified into two groups; those whose serum was collected <28 days after hospital admission (n = 72) and those whose serum was collected 28+ days after hospital admission (n = 25). As ARF tends to develop 2 to 4 weeks after an initial GAS infection, the 28-day stratification essentially grouped cases as those with a GAS infection in the preceding 6 to 8 weeks or greater. As shown in Fig. 2B, there is no significant difference in the median ASO and ADB titers between the <28 and 28+ day groups (P = 0.513 and 0.963, respectively). However, for anti-SpnA, there was a significant decrease (P < 0.05) in the median antibody titer in the 28+ day group compared with the <28 day group, suggesting a faster decay in anti-SpnA IgG over time.
FIG 2.
(A) Violin plots showing serum titers for ASO (i), ADB (ii), and anti-SpnA (iii) determined by the triplex Luminex assay in ARF cases (blue) and controls (yellow). Dashed lines represent experimentally determined ULN values. (B) Tukey box and whisker plots showing serum titers for ASO (i), ADB (ii), and anti-SpnA (iii) determined by the triplex assay for ARF patients stratified into serum collected <28 days after hospital admission (medium blue) and 28+ days after hospitalization (light blue). P values were determined by Mann-Whitney U unpaired two-tailed analysis. *, P ≤ 0.05; ***, P ≤ 0.001.
Utility of the triplex assay and SpnA in ARF diagnosis.
The ability of the triplex assay to detect a previous GAS exposure for ARF diagnosis was assessed using an upper limit of normal (ULN) approach. ULN was experimentally determined from the 75 healthy controls recruited in the RF RISK study, who had no recent history of sore throat or skin infection (Fig. 2A). The resulting ULN values of 354 μg/ml for SLO, 117 μg/ml for DNaseB, and 64 μg/ml for SpnA were applied to the ARF cases to determine the diagnostic sensitivity of each antigen (Table 3). There was no significant difference (P = 0.853) in sensitivity of each of the three antigens in detecting true-positive results in the <28-day ARF group. The sensitivity for each of the three antigens in this group was high, ranging from 85 to 88%. In contrast, in the 28+ day ARF group, there was a significant difference in sensitivity between the antigens (P = 0.001), with ASO and ADB very high at 92 and 96%, respectively, compared with anti-SpnA at 60%. This mirrors the significant decrease in anti-SpnA IgG titers seen between the <28 day and 28+ day ARF group (Fig. 2B) and is further evidence that anti-SpnA IgG decays faster than ASO and ADB.
TABLE 3.
Diagnostic sensitivity of ASO, ADB, and anti-SpnA in the triplex assaya
| Patient group | ASO | ADB | Anti-SpnA | P valueb |
|---|---|---|---|---|
| ARF <28 days (n = 72), n (%) | 63 (88) | 63 (88) | 61 (85) | 0.853 |
| ARF 28+ days (n = 25), n (%) | 23 (92) | 24 (96) | 15 (60) | 0.001 |
Sensitivity is defined as the number of ARF patients (n) with antibody titers above the experimentally determined ULN cutoffs. ARF patients were stratified into those with sera obtained <28 days after hospital admission and 28+ days after hospital admission.
The P values were determined by 3 × 2 χ2 test.
Impact of ULN values on diagnostic sensitivity.
The relationship between the experimentally determined ULN in this study and the ULN that are included in current ARF diagnostic guidelines was explored by conversion of triplex assay units (micrograms per milliliter) to the commonly reported clinical units for streptococcal serology (IU/ml for ASO and U/ml for ADB). The rationale for this conversion was based on the significant correlations between the ASO and ADB titers determined by the triplex assay and the commercial methodologies (Fig. 1). By solving the linear equation of the regression lines, the experimentally determined ULN for the 75 healthy controls equated to 412 IU/ml for ASO and 367 U/ml for ADB, respectively (Table S1). This is very similar to the ULN determined in the same 75 healthy controls when titers were measured using the commercially available assays in clinical laboratories (422 IU/ml for ASO and 400 U/ml for ADB), demonstrating the accuracy of interpolating the triplex assay units to clinical units.
Next, the linear regression approach was utilized to convert the ULN for ASO and ADB reported in ARF diagnostic guidelines in New Zealand (7) and Australia (25) to triplex assay units (Table S1). There are substantial differences in ULN applied in ARF diagnostic guidelines globally, which is exemplified by the differences between New Zealand and Australia. New Zealand guidelines stipulate a higher, all ages ULN, compared to the lower, age-specific ULN applied in Australia (9). These differences are reflected in sensitivity calculations for ASO and ADB in the triplex assay when the interpolated, clinical ULN are applied (Table S2). The sensitivity of ADB in the triplex assay is 51% when the current New Zealand cutoff is applied compared with 75% using Australian age-specific cutoffs, and 88% with the experimentally determined ULN. For ASO, sensitivity is 82% in the triplex assay using the current New Zealand cutoff compared with 97% using Australian age-specific cutoffs, and 88% with the experimentally determined ULN.
DISCUSSION
In this study, a triplex Luminex assay that measures serum IgG concentration to three GAS antigens (SLO, DNaseB, and SpnA) with high efficiency and accuracy was developed and evaluated. The multiplex nature of the assay ensures that titers are measured simultaneously, in a single tube, from a very small volume of sera (2 μl). This provides efficiency advantages over current commercial methodology for ASO and ADB that include turbidimetric, nephelometric, or enzyme inhibition assays and require separate reactions with each antigen run in parallel assays (8, 9, 14). The accuracy of the triplex assay described here is highlighted by the significant correlation with ASO and ADB titers determined from the commercial assays currently used in the New Zealand clinical setting. Of note, the enzyme inhibition assay for ADB produces titer ranges, which results in a discontinuous distribution of values as observed in this study and previously (14, 25). In contrast, the newly developed triplex assay provides continuous values for all three antigens, resulting in enhanced precision for ADB titers in particular.
The predictive value of elevated ASO and ADB for the diagnosis of GAS immune sequelae is influenced by immunokinetics and by the low rate of antibody decay following GAS infection in some individuals (13). While induction of a long-lived antibody response is a desired feature of a vaccine antigen, it is not ideal for serological diagnostics where the goal is to identify individuals who have experienced a recent GAS infection. Indeed, the knowledge that SLO antibodies stay elevated for extended periods after infection (13), combined with the high sequence conservation across GAS strains (26), has provided a rationale for including SLO in combination vaccines (15, 27). This highlights a need to characterize additional GAS antigens, which are not being developed as vaccines, and have favorable immunokinetics for serological diagnostics. SpnA is not only highly conserved and immunogenic (15) but is unlikely to be used in a vaccine as it failed to provide protection in a mouse infection model (28).
This study has built on our previous findings regarding the potential utility of SpnA in streptococcal serology (14) by comparing the sensitivity of anti-SpnA with ASO and ADB in a larger cohort of ARF patients. While the sensitivities of all three antigens were comparable in cases whose serology was performed within 4 weeks of hospital admission, the significant reduction in anti-SpnA titers in cases for whom serology was delayed suggests that anti-SpnA IgG decays more rapidly following a GAS infection. The biological basis for the differences in IgG immunokinetics between GAS antigens is yet to be elucidated but may be driven by differences in CD4+ T-helper cells and long-term memory responses. Mechanism aside, the short-lived nature of the anti-SpnA IgG response following GAS infection is a highly desirable trait for diagnostic streptococcal serology as it decreases the likelihood of false-positive results.
The predictive value of streptococcal serology in the diagnosis of ARF is influenced by the ULN cutoff that is applied. This was clearly demonstrated in a recent audit of ARF patients in New Zealand and the Northern Territory of Australia, in which the high ULN cutoffs for ASO and ADB used in New Zealand resulted in undercounting of definite and probable cases (9). In the present study, the experimentally determined ULN was derived from 75 highly matched healthy children who did not have a recent sore throat or skin infection. When expressed in units commonly reported in the literature, these values are 412 IU/ml for ASO and 367 U/ml for ADB, respectively, which are markedly lower than those currently specified in the New Zealand guidelines. A greater proportion of ARF cases in our study thus met the experimentally determined ULNs for ASO and ADB, compared with when the ULN from current New Zealand guidelines were applied to our cohort. This highlights how crucial it is to have accurate ULNs for the diagnosis of ARF when paired sera are unable to be obtained (12). Moreover, it provides further evidence for New Zealand to consider updating ULN in future ARF guidelines, particularly given that the current ULN were determined over 35 years ago (7), and are higher than those recommended elsewhere in the world (9).
Previous studies of ASO and ADB in ARF have been inconsistent with respect to trends in the antigen-specific responses. A recent audit in New Zealand and the Northern Territory of Australia found ARF cases in both countries more likely to have elevated ASO than ADB, while an older Northern Territory study found that ADB was more likely to be elevated (29). Our previous study in a small ARF cohort (n = 16) found anti-SpnA and ASO were more likely to be elevated compared with ADB (14), yet in this study, there was no significant difference between the three antigens in patients for which sera were obtained within 4 weeks of hospital admission. This highlights the complexity of antigen-specific serological response following GAS infection and is consistent with a recent study in GAS pharyngitis, which found no clear pattern of immune responses against conserved GAS antigens, and where the need for a larger antigen panel to increase the sensitivity of assays to detect a recent GAS infection was described (30).
Limitations of this study include the relatively small sample size used in determining experimental ULN such that age-specific values were not practical to obtain, and an all-ages ULN was applied. In addition, paired sera from ARF cases were not available, meaning temporal responses in individuals could not be investigated. However, the significant reduction in anti-SpnA titers in ARF patients with delayed serology is strong evidence that the immunokinetics of anti-SpnA IgG differ from that of ASO and ADB, and further studies of participants with confirmed GAS pharyngitis and skin infections, which incorporate temporal samples, are planned.
Conclusion.
In summary, this study describes the development and evaluation of a Luminex-based triplex assay that simultaneously measures ASO, ADB, and anti-SpnA in very small volumes of patient sera. The diagnostic utility of anti-SpnA was confirmed in a large cohort of ARF patients, with an immunokinetic profile that suggests a recent GAS exposure. As such, this triplex assay has the potential to improve both the efficiency and accuracy of streptococcal serology for the diagnosis of poststreptococcal sequelae.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Maurice Wilkins Centre for Biodiscovery and Return on Science. A.L.W. is supported by a University of Auckland Doctoral Scholarship. The RF RISK study, from which some samples were obtained, was funded by the Health Research Council of New Zealand (HRC) Rheumatic Fever Research Partnership (Ministry of Health, Te Puni Kōkiri, Cure Kids, Heart Foundation, and HRC). D.A.W. is funded by an Investigator Grant from the National Health and Medical Research Council (GNT1174555).
We thank Anita Bell previously of the Waikato District Health Board Public Health Unit, Lisa Stamp from Labtests, and Edwin Mitchell from the University of Auckland for assistance with patient recruitment and sample collection. All members of the RF RISK study are gratefully acknowledged.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. 2014. Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev 27:264–301. doi: 10.1128/CMR.00101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA. 2017. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 3.Milne RJ, Lennon DR, Stewart JM, Hoorn SV, Scuffham PA. 2012. Incidence of acute rheumatic fever in New Zealand children and youth. J Paediatr Child Health 48:685–691. doi: 10.1111/j.1440-1754.2012.02447.x. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, Sable C, Steer A, Wilson N, Wyber R, Zühlke L. 2016. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primer 2016:15084. doi: 10.1038/nrdp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaton A, Carapetis J. 2015. The 2015 revision of the Jones criteria for the diagnosis of acute rheumatic fever: implications for practice in low-income and middle-income countries. Heart Asia 7:7–11. doi: 10.1136/heartasia-2015-010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wannamaker LW, Ayoub EM. 1960. Antibody titers in acute rheumatic fever. Circulation 21:598–614. doi: 10.1161/01.cir.21.4.598. [DOI] [PubMed] [Google Scholar]
- 7.Heart Foundation of New Zealand. 2014. New Zealand guidelines for rheumatic fever: diagnosis, management and secondary prevention of acute rheumatic fever and rheumatic heart disease: 2014 update. Heart Foundation of New Zealand, Auckland, New Zealand: https://www.heartfoundation.org.nz/shop/marketing/non-stock-resources/diagnosis-management-rheumatic-fever-guideline.pdf. [Google Scholar]
- 8.Steer AC, Smeesters PR, Curtis N. 2015. Streptococcal serology: secrets for the specialist. Pediatr Infect Dis J 34:1250–1252. doi: 10.1097/INF.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 9.Jack S, Moreland NJ, Meagher J, Fittock M, Galloway Y, Ralph AP. 2019. Streptococcal serology in acute rheumatic fever patients: findings from 2 high-income, high-burden settings. Pediatr Infect Dis J 38:e1–e6. doi: 10.1097/INF.0000000000002190. [DOI] [PubMed] [Google Scholar]
- 10.Shet A, Kaplan EL. 2002. Clinical use and interpretation of group A streptococcal antibody tests: a practical approach for the pediatrician or primary care physician. Pediatr Infect Dis J 21:420. doi: 10.1097/00006454-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Spaun J, Bentzon MW, Larsen SO, Hewitt LF. 1961. International Standard for Antistreptolysin-O. Bull World Health Organ 24:271–279. [PMC free article] [PubMed] [Google Scholar]
- 12.Parks T, Smeesters PR, Curtis N, Steer AC. 2015. ASO titer or not? When to use streptococcal serology: a guide for clinicians. Eur J Clin Microbiol Infect Dis 34:845–849. doi: 10.1007/s10096-014-2303-8. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DR, Kurlan R, Leckman J, Kaplan EL. 2010. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clin Infect Dis 50:481–490. doi: 10.1086/650167. [DOI] [PubMed] [Google Scholar]
- 14.Hanson-Manful P, Whitcombe AL, Young PG, Atatoa Carr PE, Bell A, Didsbury A, Mitchell EA, Dunbar PR, Proft T, Moreland NJ. 2018. The novel group A Streptococcus antigen SpnA combined with bead-based immunoassay technology improves streptococcal serology for the diagnosis of acute rheumatic fever. J Infect 76:361–368. doi: 10.1016/j.jinf.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Bensi G, Mora M, Tuscano G, Biagini M, Chiarot E, Bombaci M, Capo S, Falugi F, Manetti AGO, Donato P, Swennen E, Gallotta M, Garibaldi M, Pinto V, Chiappini N, Musser JM, Janulczyk R, Mariani M, Scarselli M, Telford JL, Grifantini R, Norais N, Margarit I, Grandi G. 2012. Multi high-throughput approach for highly selective identification of vaccine candidates: the group A Streptococcus case. Mol Cell Proteomics 11:M111.015693. doi: 10.1074/mcp.M111.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang A, Khemlani A, Kang H, Proft T. 2011. Functional analysis of Streptococcus pyogenes nuclease A (SpnA), a novel group A streptococcal virulence factor. Mol Microbiol 79:1629–1642. doi: 10.1111/j.1365-2958.2011.07550.x. [DOI] [PubMed] [Google Scholar]
- 17.Binnicker MJ. 2015. Multiplex molecular panels for diagnosis of gastrointestinal infection: performance, result interpretation, and cost-effectiveness. J Clin Microbiol 53:3723–3728. doi: 10.1128/JCM.02103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satterly NG, Voorhees MA, Ames AD, Schoepp RJ. 2017. Comparison of MagPix assays and enzyme-linked immunosorbent assay for detection of hemorrhagic fever viruses. J Clin Microbiol 55:68–78. doi: 10.1128/JCM.01693-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richens JL, Urbanowicz RA, Metcalf R, Corne J, O’Shea P, Fairclough L. 2010. Quantitative validation and comparison of multiplex cytokine kits. J Biomol Screen 15:562–568. doi: 10.1177/1087057110362099. [DOI] [PubMed] [Google Scholar]
- 20.Atatoa-Carr P, Lennon D, Wilson N, New Zealand Rheumatic Fever Guidelines Writing Group. 2008. Rheumatic fever diagnosis, management, and secondary prevention: a New Zealand guideline. N Z Med J 121:59–69. [PubMed] [Google Scholar]
- 21.Baker MG, Gurney J, Oliver J, Moreland NJ, Williamson DA, Pierse N, Wilson N, Merriman TR, Percival T, Murray C, Jackson C, Edwards R, Foster Page L, Chan Mow F, Chong A, Gribben B, Lennon D. 2019. Risk factors for acute rheumatic fever: literature review and protocol for a case-control study in New Zealand. Int J Environ Res Public Health 16:4515. doi: 10.3390/ijerph16224515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker HN, Murphy R, Lopez E, Garcia C. 2012. Conversion of a capture ELISA to a Luminex xMAP assay using a multiplex antibody screening method. J Vis Exp 2012(65):4084. doi: 10.3791/4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giménez-Lirola LG, Jiang Y-H, Sun D, Hoang H, Yoon K-J, Halbur PG, Opriessnig T. 2014. Simultaneous detection of antibodies against Apx toxins ApxI, ApxII, ApxIII, and ApxIV in pigs with known and unknown Actinobacillus pleuropneumoniae exposure using a multiplexing liquid array platform. Clin Vaccine Immunol 21:85–95. doi: 10.1128/CVI.00451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danchin MH, Carlin JB, Devenish W, Nolan TM, Carapetis JR. 2005. New normal ranges of anti-streptolysin O and anti-deoxyribonuclease B titres for Australian children. J Paediatr Child Health 41:583–586. doi: 10.1111/j.1440-1754.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- 25.Steer AC, Vidmar S, Ritika R, Kado J, Batzloff M, Jenney AWJ, Carlin JB, Carapetis JR. 2009. Normal ranges of streptococcal antibody titers are similar whether streptococci are endemic to the setting or not. Clin Vaccine Immunol 16:172–175. doi: 10.1128/CVI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies MR, McIntyre L, Mutreja A, Lacey JA, Lees JA, Towers RJ, Duchêne S, Smeesters PR, Frost HR, Price DJ, Holden MTG, David S, Giffard PM, Worthing KA, Seale AC, Berkley JA, Harris SR, Rivera-Hernandez T, Berking O, Cork AJ, Torres R, Lithgow T, Strugnell RA, Bergmann R, Nitsche-Schmitz P, Chhatwal GS, Bentley SD, Fraser JD, Moreland NJ, Carapetis JR, Steer AC, Parkhill J, Saul A, Williamson DA, Currie BJ, Tong SYC, Dougan G, Walker MJ. 2019. Atlas of group A streptococcal vaccine candidates compiled using large-scale comparative genomics. Nat Genet 51:1035–1043. doi: 10.1038/s41588-019-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reglinski M, Lynskey NN, Choi YJ, Edwards RJ, Sriskandan S. 2016. Development of a multicomponent vaccine for Streptococcus pyogenes based on the antigenic targets of IVIG. J Infect 72:450–459. doi: 10.1016/j.jinf.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radcliff FJ, Fraser JD, Proft T. 2015. Vaccination with Streptococcus pyogenes nuclease A stimulates a high antibody response but no protective immunity in a mouse model of infection. Med Microbiol Immunol 204:185–191. doi: 10.1007/s00430-014-0353-2. [DOI] [PubMed] [Google Scholar]
- 29.Carapetis JR, Currie BJ. 2001. Rheumatic fever in a high incidence population: the importance of monoarthritis and low grade fever. Arch Dis Child 85:223–227. doi: 10.1136/adc.85.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hysmith ND, Kaplan EL, Cleary PP, Johnson DR, Penfound TA, Dale JB. 2017. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of group A streptococci. J Pediatric Infect Dis Soc 6:187–196. doi: 10.1093/jpids/piw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.