Diagnosis of Lyme neuroborreliosis (LNB) is challenging, as long as Borrelia-specific intrathecal antibodies are not yet detectable. The chemokine CXCL13 is elevated in the cerebrospinal fluid (CSF) of LNB patients. Here, we compared the performances of the Euroimmun CXCL13 enzyme-linked immunosorbent assay (CXCL13 ELISA) and the ReaScan CXCL13 lateral flow immunoassay (CXCL13 LFA), a rapid point-of-care test, to support the diagnosis of LNB. In a dual-center case-control study, CSF samples from 90 patients (34 with definite LNB, 10 with possible LNB, and 46 with other central nervous system [CNS] diseases [non-LNB group]) were analyzed with the CXCL13 ELISA and the CXCL13 LFA.
KEYWORDS: neuroborreliosis, Lyme disease, meningitis, Borrelia burgdorferi, biomarker, CSF, neuroborreliosis
ABSTRACT
Diagnosis of Lyme neuroborreliosis (LNB) is challenging, as long as Borrelia-specific intrathecal antibodies are not yet detectable. The chemokine CXCL13 is elevated in the cerebrospinal fluid (CSF) of LNB patients. Here, we compared the performances of the Euroimmun CXCL13 enzyme-linked immunosorbent assay (CXCL13 ELISA) and the ReaScan CXCL13 lateral flow immunoassay (CXCL13 LFA), a rapid point-of-care test, to support the diagnosis of LNB. In a dual-center case-control study, CSF samples from 90 patients (34 with definite LNB, 10 with possible LNB, and 46 with other central nervous system [CNS] diseases [non-LNB group]) were analyzed with the CXCL13 ELISA and the CXCL13 LFA. Classification of patients followed the European Federation of Neurological Societies (EFNS) guidelines on LNB. The CXCL13 ELISA detected elevated CXCL13 levels in all patients with definite LNB (median, 1,409 pg/ml) compared to the non-LNB controls (median, 20.7 pg/ml; P < 0.0001), with a sensitivity of 100% and a specificity of 84.8% (cutoff value, 78.6 pg/ml; area under the receiver operating characteristic [ROC] curve, 0.93). Similarly, the CXCL13 LFA yielded elevated CXCL13 levels in 31 patients with definite LNB (median arbitrary value, 223.5) compared to the non-LNB control patients (median arbitrary value, 0; P < 0.0001) and had a sensitivity and specificity of 91.2% and 93.5%, respectively (cutoff arbitrary value, 22.5; area under the ROC curve, 0.94). The correlation between the CXCL13 levels obtained by ELISA and LFA was strong (Spearman correlation coefficient r = 0.89; P < 0.0001). The CXCL13 ELISA and the CXCL13 LFA are comparable diagnostic tools for the detection of CXCL13 in the CSF of patients with definite LNB. The advantage of the CXCL13 LFA is the shorter time to result.
INTRODUCTION
Lyme neuroborreliosis (LNB) is the most common arthropod-borne central nervous system (CNS) infection in Europe and the United States (1). In Germany, the incidence of Lyme borreliosis ranges between 60,000 and >200,000 cases per year (2). Approximately 5 to 10% of patients with Lyme borreliosis develop LNB (3). In the United States, Borrelia burgdorferi sensu stricto is the predominant spirochete species responsible for neuroinfectious diseases, while in Europe, LNB is predominantly caused by Borrelia afzelii or Borrelia garinii (4, 5).
The diagnosis of acute LNB is based on specific neurological symptoms, cerebrospinal fluid (CSF) pleocytosis, and the presence of an elevated B. burgdorferi-specific CSF-to-serum antibody index (AI) (6–10). However, the B. burgdorferi-specific AI, which is the gold standard for the laboratory diagnosis of LNB, may be negative in 10 to 30% of patients when symptom duration is less than 6 weeks and can be positive for years following successful treatment (11, 12). Furthermore, antibiotic therapy is often initiated before the B. burgdorferi-specific AI is available (13), and therefore, treatment decisions during the early phase of disease mostly rely on clinical suspicion and CSF pleocytosis alone (14). To ensure timely LNB diagnosis and to prevent unnecessary antibiotic therapy, an early biomarker with high sensitivity and specificity is needed (15).
CXCL13 is such a diagnostic marker in acute LNB, since it is highly elevated in CSF (7, 16) even before intrathecally produced B. burgdorferi-specific antibodies are detectable (17). However, it is not unique to LNB and may also be increased in other neuroinfectious and neuroinflammatory disorders (18). Nevertheless, CXCL13 has the potential to accelerate the diagnosis of LNB. However, to do so, a rapid result and daily testing are mandatory. So far, enzyme-linked immunosorbent assays (ELISA) for CXCL13 measurement are available, but they are usually not performed on a daily basis, resulting in an undesirable delay of test results. Immunochromatographic assays, such as the ReaScan CXCL13 lateral flow immunoassay (CXCL13 LFA), may overcome this disadvantage. The CXCL13 LFA is a single-patient point-of-care test that can be performed without extensive laboratory equipment and provides results within 20 min.
So far, only one study has compared a CXCL13 lateral flow immunoassay with an ELISA. Pietikäinen et al. examined 13 patients with definite LNB and 81 controls. The CXCL13 levels determined by Pietikäinen et al. with both assays in 225 CSF samples (113 without clinical classification) correlated very well (correlation coefficient [r] = 0.896) (19). In order to verify these results in a larger cohort of LNB patients and to establish a cutoff value for the CXCL13 LFA, we conducted a comparative study on the Euroimmun CXCL13 ELISA (CXCL13 ELISA) and the CXCL13 LFA in patients with LNB and other neurological diseases.
MATERIALS AND METHODS
We performed a dual-center retrospective case-control study at two tertiary-care hospitals in Germany (University Medical Centers Nürnberg and Erlangen). The study was approved by the local institutional review board of the Paracelsus Medical University Nürnberg (IRB-2020-011). All consecutive CSF samples sent to the microbiology laboratory between June 2016 and December 2018 were stored if (i) a pathogen was detected in the CSF, (ii) the results of antibody detection suggested a CNS infection, or (iii) the CSF was sent for anti-B. burgdorferi antibody testing and the patient was diagnosed with an inflammatory CNS disease. CSF samples from patients without a definite diagnosis were not stored. The samples were tested in 2019 for CXCL13 using the ReaScan CXCL13 LFA (Reagena, Toivala, Finland) and the CXCL13 ELISA (Euroimmun AG, Lübeck, Germany). Some CSF samples could not be reanalyzed because they were completely used up during the initial diagnostic assessment.
The patients were categorized according to the European Federation of Neurological Societies (EFNS) guidelines on the diagnosis and management of European LNB as cases of definite or possible LNB or as patients without LNB (6). Briefly, a patient was grouped into the category of definite LNB if all the following criteria were fulfilled: (i) neurological symptoms suggestive of LNB (with other causes excluded), (ii) CSF pleocytosis (CSF cell count > 5 cells/μl), and (iii) B. burgdorferi-specific antibodies in the CSF (produced intrathecally). A patient was classified as possible LNB if only two of the three criteria were fulfilled. If criterion iii was initially negative, a possible case of LNB required the detection of B. burgdorferi-specific antibodies in serum after a period of 6 weeks (6). The classification of LNB patients was independent of the CXCL13 results. In the non-LNB control group, patients suffered from bacterial or viral CNS infection or inflammatory (autoimmune or malignant) CNS disease. The diagnosis of bacterial meningitis was based on culture growth or PCR and sequencing of the 16S rRNA gene. The diagnosis of tick-borne encephalitis was based on positive IgM antibody levels measured in serum. The diagnosis of enterovirus meningitis was based on PCR detection of enterovirus in CSF or feces. The diagnosis of varicella-zoster virus (VZV) or herpes simplex virus (HSV) encephalitis was based on the detection of the respective pathogen in CSF by PCR. The diagnosis of malignant melanoma and multiple sclerosis was obtained from the clinical information system. There were no healthy patients or patients with uncertain diagnosis included in the study.
The serologic workup for borreliosis comprised an initial screening test for anti-Borrelia antibodies using an ELISA for IgM and IgG (Virotech Diagnostics GmbH, Rüsselsheim, Germany, or Euroimmun AG, Lübeck, Germany), followed by confirmatory Western blot analysis (Borrelia Europe Line IgM and IgG [Virotech Diagnostics GmbH, Rüsselsheim, Germany] or Borrelia afzelii Western blot IgM and Borrelia Euroline plus VlsE IgG [Euroimmun AG, Lübeck, Germany]). All tests were performed according to the manufacturers’ instructions. In case of a positive confirmatory test, the B. burgdorferi-specific CSF/serum AI was determined by Reiber’s method (20). Afterward, samples were stored at −20°C until CXCL13 analyses were performed. The maximum numbers of freeze-thaw cycles were two for samples with a CXCL13 value below 500 pg/ml and three for samples with a CXCL13 value of >500 pg/ml.
The CXCL13 ELISA was performed according to the instructions of the manufacturer on an automated ELISA platform (Euroimmun Analyzer 1; Euroimmun AG, Lübeck, Germany) (Fig. 1A). Samples with concentrations above the upper validation limit (>500 pg/ml) were diluted with the calibration diluent buffer from the kit and reanalyzed. Samples with CXCL13 concentrations below the limit of valid detection (4.6 pg/ml) were given a value of 4.5 pg/ml. The manufacturer suggests the following interpretation of CXCL13 results: values of <20 pg/ml are in the normal range, values between 20 and 29 pg/ml are in the borderline range, values between 30 and 100 pg/ml are elevated, and values of >100 pg/ml are highly elevated and suspicious for acute LNB if corresponding clinical symptoms are present. Accordingly, the CXCL13 ELISA manufacturer’s cutoff value for the diagnosis of LNB is 100 pg/ml. The individuals performing the CXCL13 ELISA were blinded to the diagnoses of the patients and the CXCL13 LFA results. There were no indeterminate results in the CXCL13 ELISA.
FIG 1.
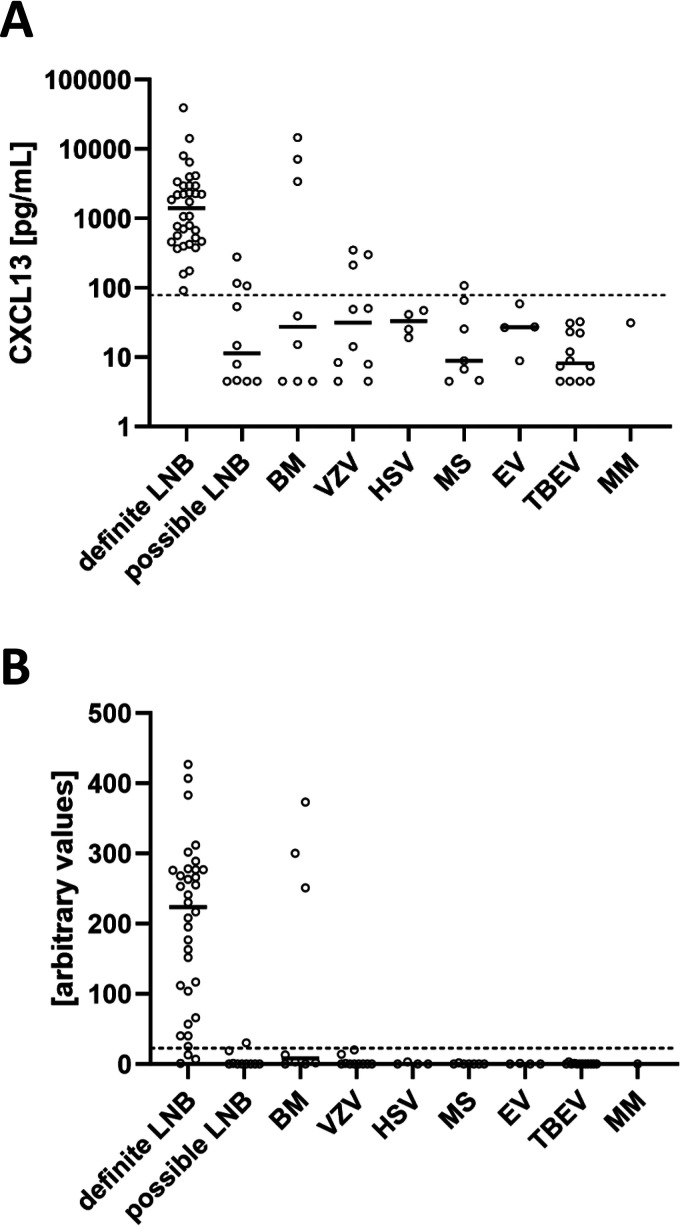
(A) CXCL13 concentrations in CSF determined by CXCL13 ELISA in different disease groups. Levels of CXCL13 in CSF of patients with definite and possible LNB were determined by CXCL13 ELISA. Non-LNB patients were stratified according to disease group. The horizontal lines indicate medians. Values lower than the detection limit were given a value of 4.5 pg/ml. The dashed line indicates the optimal cutoff value of the CXCL13 ELISA (78.6 pg/ml). VZV, varicella-zoster virus; HSV, herpes simplex virus; BM, bacterial meningitis; MS, multiple sclerosis; EV, enterovirus; TBEV, tick-borne encephalitis virus; MM, malignant melanoma. (B) CXCL13 concentrations in CSF determined by CXCL13 LFA in different disease groups. The dashed line indicates the optimal AV cutoff of 22.5.
The CXCL13 LFA was also performed according to the instructions of the manufacturer (Fig. 1B). Briefly, 100 μl of CSF was pipetted into the conjugate tube, and the conjugate-CSF mixture was transferred to the LFA cassette. After 20 min, the test cassette was read with the ReaScan reader, giving results as arbitrary ReaScan values (AVs). According to the manufacturer, an AV of <40 corresponds to a CXCL13 value of <250 pg/ml, an AV from 40 to 90 corresponds to 250 to 500 pg/ml, and an AV of >90 corresponds to >500 pg/ml. The measuring range of the LFA is between AVs of 0 and 427. The manufacturer does not provide a cutoff value for diagnosis of LNB. The individuals performing the CXCL13 LFA were blinded to the diagnoses of the patients and the CXCL13 ELISA results. There were no indeterminate results in the CXCL13 LFA.
Statistics.
Statistical analyses and receiver operating characteristic (ROC) curves were performed using GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA). The Youden index was determined using Microsoft Excel. Comparison of means was performed using the Mann-Whitney U test. P values of <0.05 were considered significant.
RESULTS
In total, 90 CSF samples from 90 patients with suspicion of LNB were included in this study. Fifty-one patients were male (57%), and the mean age was 50 years (range, 0 to 89 years).
According to the EFNS guidelines, 34 patients (38%) were diagnosed with definite LNB and 10 patients (11%) with possible LNB, and 46 patients (51%) were classified as controls in a non-LNB group (see supplemental file 1). The clinical manifestations of the definite-LNB patients were facial nerve palsy in 17 cases (50%), other cranial nerve palsies in 3 cases (9%), and meningopolyradiculitis in 14 cases (41%). The possible-LNB group consisted of 5 patients with CSF pleocytosis and a negative AI, 3 patients with CSF pleocytosis and no AI (not performed due to initially negative serology or lack of material), and 2 patients with a positive AI and no CSF pleocytosis.
In the non-LNB group, patients suffered from the following diseases: tick-borne encephalitis (n = 12; 26%), VZV encephalitis (n = 10; 22%), bacterial meningitis (n = 8; 17%), multiple sclerosis (n = 7; 15%), enterovirus meningitis (n = 4; 9%), HSV encephalitis (n = 4; 9%), and malignant melanoma (n = 1; 2%). The clinical characteristics of the different study groups are summarized in Table 1.
TABLE 1.
Clinical characteristics of study groups
| Study group | n | Borrelia AI | CSF pleocytosis >5 cells/μl | % male | Agea (yr) | CSF cell count/μlb (range) | CSF lactatea (mmol/liter) | CSF proteina (mg/dl) |
|---|---|---|---|---|---|---|---|---|
| Definite LNB (total) | 34 | Positive | Yes | 68 | 54 | 161 (7–894) | 2.3 | 2,013 |
| Possible LNB | 5 | Negative | Yes | 40 | 47 | 19 (5–77) | 1.8 | 48 |
| 3 | NDc | Yes | 67 | 12 | 65 (26–200) | 1.7 | 35 | |
| 2 | Positive | No | 50 | 83 | 0 (0–0) | 2.1 | 62 | |
| Total | 10 | Total | 50 | 44 | 26 (0–200) | 1.8 | 47 | |
| Non-LNBd | ||||||||
| Bacterial meningitis | 8 | Negative | 6 yes, 2 ND | 50 | 55 | 2,020 (152–10,000) | 9.9 | 403 |
| VZV encephalitis | 10 | Negative | 9 yes, 1 no | 50 | 64 | 47 (1–722) | 2.5 | 109 |
| Multiple sclerosis | 7 | Negative | 6 yes, 1 no | 29 | 34 | 10 (4–24) | 1.8 | 40 |
| HSV encephalitis | 4 | Negative | 3 yes, 1 ND | 25 | 74 | 42 (30–60) | 3.3 | 73 |
| EV encephalitis | 4 | Negative | 4 yes | 75 | 10 | 123 (65–474) | 1.9 | 51 |
| TBEV encephalitis | 12 | Negative | 11 yes, 1 ND | 58 | 44 | 130 (22–285) | 2.2 | 67 |
| Malignant melanoma | 1 | Negative | Yes | 100 | 63 | 10 | 3.2 | 56 |
| Total | 46 | Negative | 40 yes, 2 no, 4 ND | 50 | 49 | 78.5 (1–10,000) | 3.3 | 119 |
Data are presented as means.
Data are presented as medians.
ND, not determined.
EV, enterovirus; TBEV, tick-borne encephalitis virus; VZV, varicella-zoster virus; HSV, herpes simplex virus.
CSF CXCL13 in patients with definite LNB versus non-LNB patients.
The median CXCL13 concentrations determined by the CXCL13 ELISA in the CSF of definite-LNB patients and non-LNB patients were 1,409 pg/ml (range, 91.4 to 39,370 pg/ml) and 20.7 pg/ml (range, 4.5 to 14,645 pg/ml), respectively (Table 2). Comparison of the mean CXCL13 concentrations in CSF determined by CXCL13 ELISA showed that the levels in definite-LNB patients were significantly higher than in non-LNB patients (P < 0.0001) (Fig. 2B).
TABLE 2.
CXCL13 levels of study groups as measured by ELISA and LFA
| Study group | n | Borrelia AI | CSF pleocytosis >5 cells/μl | CSF CXCL13 concna (range) |
No. of CXCL13-positive patientsb (% of total) |
||
|---|---|---|---|---|---|---|---|
| ELISA (pg/ml) | LFA (AV) | ELISA | LFA | ||||
| Definite LNB (total) | 34 | Positive | Yes | 1409 (91.4–39,370) | 223.5 (1–427) | 34 (100) | 31 (91) |
| Possible LNB | 5 | Negative | Yes | 4.6 (4.5–277.7) | 0 (0–19) | 2 (40) | 0 (0) |
| 3 | NDc | Yes | 53.5 (7.9–106) | 1 (0–30) | 1 (33) | 1(33) | |
| 2 | Positive | No | 9.65 (4.5–14.8) | 0 (0–0) | 0 (0) | 0 (0) | |
| Total | 10 | 11.35 (4.5–277.7) | 0 (0–30) | 3 (30) | 1 (10) | ||
| Non-LNBd | |||||||
| Bacterial meningitis | 8 | Negative | 6 yes, 2 ND | 27.15 (4.5–14,645) | 8 (0–373) | 3 (38) | 3 (38) |
| VZV encephalitis | 10 | Negative | 9 yes, 1 no | 31.5 (4.5– 350.6) | 0 (0–20) | 3 (30) | 0 (0) |
| Multiple sclerosis | 7 | Negative | 6 yes, 1 no | 8.9 (4.5–107) | 0 (0–2) | 1 (14) | 0 (0) |
| HSV encephalitis | 4 | Negative | 3 yes, 1 ND | 33.1 (19.2–47.3) | 0 (0–3) | 0 (0) | 0 (0) |
| EV encephalitis | 4 | Negative | 4 yes | 27 (8.9– 58.5) | 0 (0–1) | 0 (0) | 0 (0) |
| TBEV encephalitis | 12 | Negative | 11 yes, 1 ND | 8.15 (4.5–32.5) | 0 (0–3) | 0 (0) | 0 (0) |
| Malignant melanoma | 1 | Negative | Yes | 31.1 | 0 | 0 (0) | 0 (0) |
| Total | 46 | Negative | 40 yes, 2 no, 4 ND | 20.7 (4.5–14,645) | 0 (0–373) | 7 (15) | 3 (7) |
Data are presented as medians.
Optimized cutoff values (78.6 pg/ml for ELISA and AV of 22.5 for LFA) were used for classification.
ND, not determined.
EV, enterovirus; TBEV, tick-borne encephalitis virus; VZV, varicella-zoster virus; HSV, herpes simplex virus.
FIG 2.
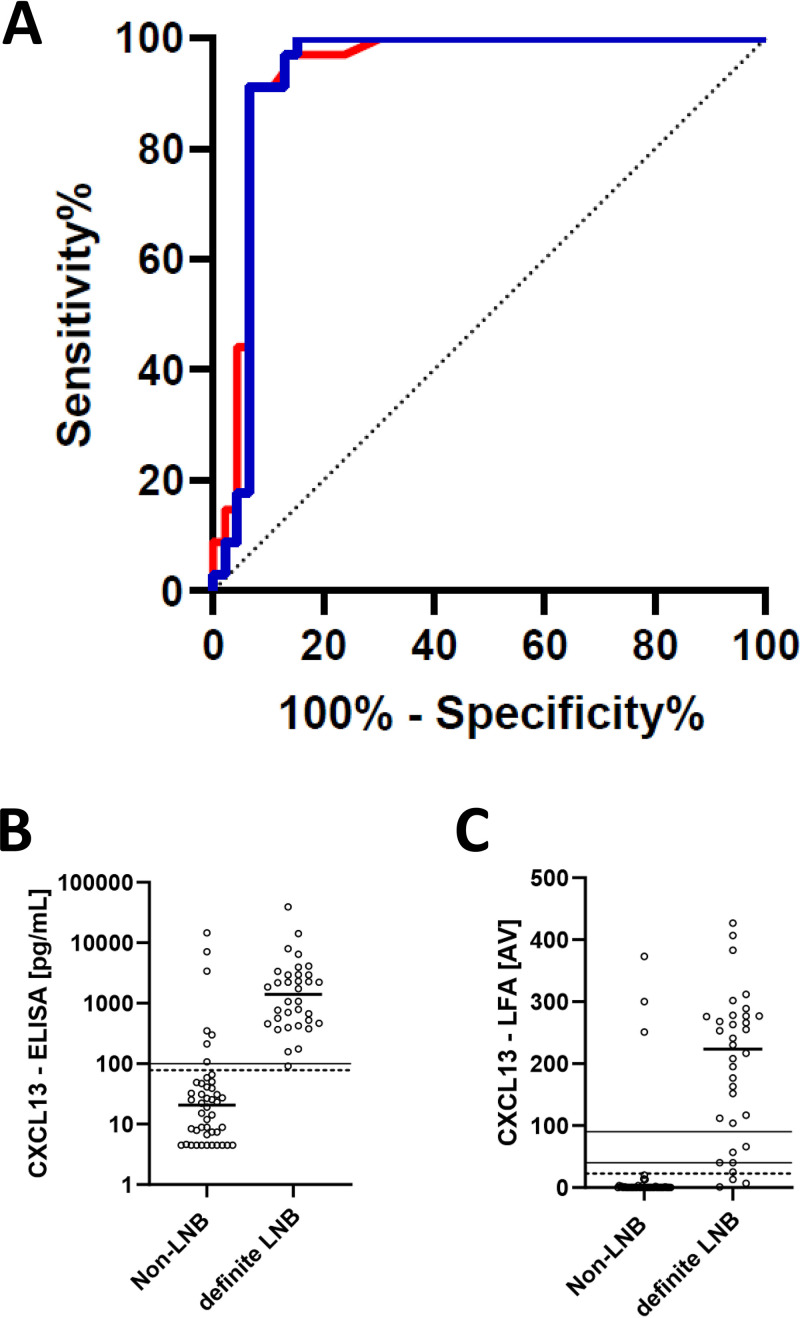
(A) ROC curves. Blue line, ROC curve for definite LNB versus non-LNB by ELISA (AUC = 0.9348); red line, ROC curve for definite LNB versus non-LNB by LFA (AUC= 0.9399); the dashed line represents the diagonal. (B and C) CXCL13 concentrations in the CSF of patients with definite LNB versus non-LNB patients. (B) The dashed line at 78.6 pg/ml indicates the optimized cutoff. The solid line at 100 pg/ml is the manufacturer’s cutoff. CXCL13 ELISA values below the detection limit were given a value of 4.5 pg/ml. (C) The dashed line indicates the optimal AV cutoff at 22.5. The solid lines indicate AVs of 40 and 90. Means were compared by a Mann-Whitney U test. The horizontal lines indicate medians.
The median CXCL13 concentrations determined by the CXCL13 LFA in the CSF of definite-LNB patients and non-LNB patients were AVs of 223.5 (range, 1 to 427) and 0 (range, 0 to 373), respectively. Again, the mean CXCL13 concentrations in CSF determined by CXCL13 LFA were significantly higher in patients with definite LNB than in non-LNB patients (P < 0.0001) (Fig. 2C).
Sensitivity and specificity were calculated for the CXCL13 ELISA by applying the manufacturer’s cutoff level (Table 3; see also Tables S1 to S3 in the supplemental material). The results were as follows: sensitivity, 97.1% (95% confidence interval [CI], 84.7% to 99.9%), and specificity, 84.8% (95% CI, 71.7% to 93.7%), for the CXCL13 ELISA, with a cutoff value of 100 pg/ml.
TABLE 3.
Diagnostic performance of CXCL13 ELISA and LFA with different cutoff values
| Test | Cutoff value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| CXCL13 ELISA | 100 pg/mla | 97.1 | 84.8 |
| 78.6 pg/mlb | 100 | 84.8 | |
| CXCL13 LFA | AV 22.5b | 91.2 | 93.5 |
Manufacturer’s cutoff value.
Optimized cutoff value.
The sensitivity and specificity of the CXCL13 LFA for the diagnosis of LNB could not be determined because the manufacturer did not provide a cutoff level.
Therefore, an ROC analysis was conducted for cases (definite LNB) versus controls (non-LNB) to further assess the diagnostic performance of the CXCL13 ELISA and the CXCL13 LFA (Fig. 2A). The optimal cutoff value (highest Youden index) for the diagnosis of definite LNB by the CXCL13 ELISA was 78.6 pg/ml (area under the ROC curve [AUC], 0.93; 95% CI, 0.87 to 1.00), with a sensitivity of 100% (95% CI, 89.7% to 100.0%) and a specificity of 84.8% (95% CI, 71.1% to 93.7%) (Table 3). The ROC analysis of the CXCL13 LFA gave an optimal cutoff AV of 22.5 (AUC, 0.94; 95% CI, 0.88 to 1), with a sensitivity and a specificity of 91.2% (95% CI, 76.3% to 98.1%) and 93.5% (95% CI, 82.1% to 98.6%), respectively. There was no statistically significant difference between the AUC of the CXCL13 ELISA and the CXCL13 LFA.
CSF CXCL13 in patients with possible LNB.
In the possible-LNB group, the median CXCL13 concentrations as measured by the ELISA and the LFA were 11.35 pg/ml (range, 4.5 to 277.7 pg/ml) and an AV of 0 (range, 0 to 30), respectively. Patients were divided into three groups according to the AI result/availability and CSF pleocytosis (Tables 1 and 2 and Fig. 1). Patients with positive CXCL13 levels (ELISA, n = 3; LFA, n = 1) were found only in the two groups with CSF pleocytosis. The absence of CSF pleocytosis precluded positive CXCL13 levels.
Non-LNB patients.
Characteristics such as CSF cell count, Borrelia-specific AI, and CXCL13 ELISA and CXCL13 LFA values of the different non-LNB disease groups are presented in Tables 1 and 2. Median levels of CXCL13 in CSF determined by CXCL13 ELISA, in descending order, were 33.1 pg/ml (range, 19.2 to 47.3 pg/ml) in HSV encephalitis (n = 4), 31.5 pg/ml (range, 4.5 to 350.6 pg/ml) in VZV encephalitis (n = 10), 31.1 pg/ml in the single patient with malignant melanoma, 27.15 pg/ml (range, 4.5 to 14,645 pg/ml) in bacterial meningitis (n = 8), 27 pg/ml (range, 8.9 to 58.5 pg/ml) in enterovirus infection (n = 4), 8.9 pg/ml (range, 4.5 to 107 pg/ml) in multiple sclerosis, and 8.15 pg/ml (range, 4.5 to 32.5 pg/ml) in tick-borne encephalitis (n = 12). In the bacterial meningitis group, there were three samples with very high levels of CXCL13 (ELISA, >500 pg/ml, and LFA, AVs of >200). These patients had bacterial meningitis due to Campylobacter fetus, Streptococcus intermedius, and Haemophilus influenzae. In contrast, four patients with meningitis caused by Streptococcus pneumoniae and one patient with Listeria monocytogenes meningitis had CXCL13 values below the ELISA and LFA cutoff values.
In the subgroup with VZV encephalitis, three patients showed CSF CXCL13 ELISA values above the cutoff value of 78.6 pg/ml. The corresponding results with the CXCL13 LFA were below the cutoff level of an AV of 22.5.
Correlation and agreement analysis between CXCL13 ELISA and CXCL13 LFA results.
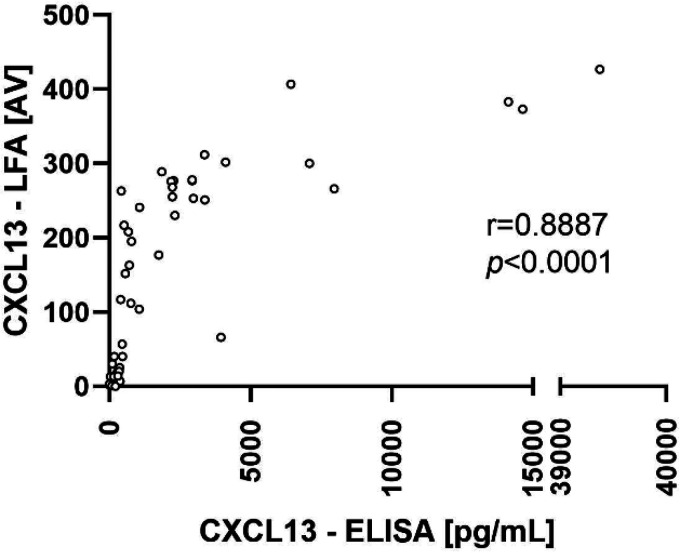
There was a significant correlation (Spearman correlation coefficient r = 0.89; P < 0.0001) between the CXCL13 levels determined with the CXCL13 ELISA and the CXCL13 LFA (Fig. 3).
FIG 3.
Correlation analysis of CXCL13 concentrations measured with the ELISA and the LFA. CXCL13 ELISA results are expressed as concentrations. CXCL13 LFA results are expressed as ReaScan AVs. Correlation between the results was calculated by Spearman rank correlation.
The categorical agreement analysis between the CXCL13 ELISA and the CXCL13 LFA using the optimized cutoff values gave the following results. In all CSF samples with a negative CXCL13 ELISA result, the LFA was negative, as well (100% agreement). However, the categorical agreement of the LFA with a positive CXCL13 ELISA result was only 80%.
Additionally, we performed an agreement analysis considering the manufacturer’s instructions for the LFA, which state that AVs of <40 correspond to a CXCL13 value of <250 pg/ml, AVs from 40 to 90 correspond to 250 to 500 pg/ml, and AVs of >90 correspond to >500 pg/ml. The agreement for the CXCL13 LFA compared to the CXCL13 ELISA was 90% in total, 98% for ELISA values of <250 pg/ml, 22% for ELISA values from 250 to 500 pg/ml, and 96% for ELISA values of >500 pg/ml.
DISCUSSION
CXCL13 CSF levels rise early in the course of LNB and decline during treatment (1). Borreliae that enter the CNS are recognized by monocytes, macrophages, or dendritic cells, which produce proinflammatory cytokines; in addition, several chemokines are released to attract other immune cells (21). CXCL13 is produced by antigen-presenting cells (22). It belongs to the CXC chemokine family and is a selective chemoattractant for B lymphocytes and follicular B helper T cells (also known as follicular T helper cells) via the chemokine receptor CXCR5 (23, 24). CXCL13 is involved in the development and maintenance of secondary lymphoid organs and is also produced during inflammation in nonlymphoid tissues, such as the CNS (25).
In this study, we showed that CXCL13 ELISA values are significantly elevated in patients with definite LNB (median, 1,409 pg/ml; range, 91.4 to 39,370 pg/ml) compared to control patients (median, 20.7 pg/ml; range, 4.5 to 14,645 pg/ml). With an optimal cutoff value of 78.6 pg/ml, the CXCL13 ELISA had a sensitivity of 100% and a specificity of 84.8%. Our data confirm previous studies that also reported highly elevated CXCL13 levels in LNB. In 2018, Rupprecht et al. performed a meta-analysis on 18 studies involving 618 patients with acute LNB and 2,326 control patients with other neurological diseases. The pooled sensitivity and specificity for CSF CXCL13 with an optimal cutoff value of 162 pg/ml were 89% and 96%, respectively (7). In this meta-analysis, the cutoff values determined by the included studies ranged from 18 to 1,229 pg/ml. One possible explanation for this strong heterogeneity could be the utilization of different CXCL13 assays. However, only two assays were used, namely, the R&D Systems ELISA (R&D Systems Europe, Abingdon, United Kingdom) in 15 studies and the Euroimmun ELISA (Euroimmun AG, Lübeck, Germany) in 3 studies. The cutoff values determined by the three studies using the same assay we did (Euroimmun ELISA) were 55 pg/ml, 93.8 pg/ml, and 200 pg/ml (18, 26, 27). The first study by Remy et al. retrospectively examined 53 children with LNB and 91 controls and found an optimal cutoff level of 55 pg/ml with a sensitivity of 96.7% and a specificity of 98.1%. The median CXCL13 concentration in the CSF of children with definite LNB was 774.7 pg/ml, and values ranged from 58.9 to 13,487 pg/ml (26). Wagner et al. reported a slightly higher optimal cutoff value of 93.8 pg/ml, leading to a sensitivity of 95% and a specificity of 97% in an unselected cohort of 459 patients, 20 of them having LNB. The median CXCL13 concentration in the CSF of LNB patients was 900 pg/ml, and values ranged from 10 to 6,500 pg/ml (18). Finally, Waiß et al. analyzed 10 patients with LNB and 53 controls and determined a sensitivity of 100% and a specificity of 92.4% using a literature-based cutoff value of 200 pg/ml. The median CXCL13 concentration in the CSF of LNB patients was 525.5 pg/ml, and values ranged from 254 pg/ml to >900 pg/ml (27). A direct comparison of these results to ours is difficult because there are significant differences in terms of study population and design. Remy et al., for example, examined only children and adolescents, and the median age was 8 years (26). In contrast, the median age of our patients was 55 years. An analysis of the literature on CXCL13 and the corresponding cutoff levels as a function of age was inconclusive. Therefore, it is currently unclear whether age accounts for the differences between the studies.
In contrast to our case-control design, Wagner et al. performed a cross-sectional study (18). According to the above-mentioned meta-analysis, the cutoff levels of cross-sectional studies were lower than those of case-control studies; thus, the different design cannot explain the lower cutoff value found by us (7).
Another difference between the three studies mentioned above and ours is the lower specificity of 84.8% found by us. Responsible for this are six false-positive patients with VZV encephalitis (n = 3; 30% of all VZV encephalitis patients) and bacterial meningitis (n = 3; 37.5% of all patients with bacterial meningitis). While the former showed moderately elevated CXCL13 levels (mean, 288 pg/ml), the CXCL13 levels of the latter were strongly increased (mean, 8,373 pg/ml). The causative bacterial pathogens were C. fetus and H. influenzae in two patients with purulent meningitis and S. intermedius in a patient with bacterial brain abscess caused by poor dental care. Previous studies also reported elevated CXCL13 CSF levels in VZV encephalitis (25, 26). From five of these samples, CSF cell counts were reported; all the patients had CSF pleocytosis. Consequently, CSF pleocytosis cannot aid as an additional discriminator between LNB and VZV encephalitis or bacterial meningitis. To judge the impact of these positive results on the CXCL13-assisted diagnosis of LNB, one has to take into consideration the most common CNS pathogens, e.g., S. pneumoniae, tick-borne encephalitis virus, enterovirus, VZV, herpes simplex virus, and Neisseria meningitidis (28). Only VZV seems to pose a frequent problem. However, VZV is efficiently detected in CSF using nucleic acid amplification techniques. Therefore, VZV encephalitis in patients with elevated CSF CXCL13 can easily be ruled out. Other, less common CNS disorders are more difficult to diagnose. Among these, neurosyphilis is probably the most challenging disease entity, because cross-reactivity can lead to weakly positive results in anti-Borrelia ELISAs and neurosyphilis is known to cause elevated CXCL13 levels (29, 30). Therefore, it is advisable to perform an additional serologic test to rule out Treponema pallidum infection if LNB is suspected and CXCL13 levels are elevated.
At this point, it must be emphasized that CXCL13 is a nonspecific marker for CNS inflammation and is not unique to CNS infection. CXCL13 levels are reported to be elevated in patients with various inflammatory CNS disorders (e.g., clinically isolated syndrome, multiple sclerosis, N-methyl-d-aspartate receptor encephalitis, or Rasmussen encephalitis) (31, 32). Although 60% of these noninfectious inflammatory CNS disorders do not cause CNS pleocytosis, they represent possible differential diagnoses for patients with suspected LNB (32). Consequently, the specificity of CXCL13 for the diagnosis of LNB is strongly dependent on the patient population, i.e., the clinical setting in which the test is used. A high number of patients with noninfectious inflammatory CNS disorders in the case mix would decrease the specificity of the CXCL13 assay, especially in the absence of CSF pleocytosis.
Two of the study patients were particularly interesting because they had positive AIs and lacked CSF pleocytosis. Their CXCL13 results were negative, strongly arguing against LNB. Thus, it may be assumed that antibiotic treatment would have been without effect. Perhaps nonspecific polyclonal activation was the reason for the elevated B. burgdorferi AIs.
One major outcome of our study is the careful comparative assessment of the performances of the CXCL13 ELISA and the CXCL13 LFA. While ELISA-based antigen tests usually show high sensitivity, they suffer from some drawbacks. Factors like a long assay turnaround time of 1 to 2 h; the need for appropriately equipped facilities and trained personnel; and last, but not least, low test frequencies for economic reasons restrict their immediate diagnostic impact. Immunochromatographic assays, like the CXCL13 LFA, may overcome these limitations. They are designed as fast point-of-care tests that can be performed on a single-patient basis without complicated laboratory equipment. In our hands, the CXCL13 LFA proved to be a robust test without invalid test results. The hands-on time and the time to result per test were 1 min and 20 min, respectively. The CXCL13 LFA enables instant testing around the clock and on weekends without the need for experienced personnel. For the LFA, we calculated an optimal cutoff AV of 22.5, leading to a sensitivity of 91.2% and a specificity of 93.5%. The agreement analysis of our CXCL13 ELISA and LFA results showed perfect agreement (100%) for values under 78.6 pg/ml and high agreement (80%) for values above 78.6 pg/ml. Overall, the correlation between the CXCL13 levels determined by the ELISA and the LFA was strong (r = 0.89; P < 0.0001) and in line with the results determined by Pietikäinen et al. (19). So far, the work by Pietikäinen et al. is the only study to compare a CXCL13 LFA and a CXCL13 ELISA. While we both used the same LFA, the CXCL13 ELISA was different (human CXCL13/BLC/BCA-1 Quantikine [R&D Systems, Minneapolis, MN, USA] versus CXCL13 ELISA [Euroimmun AG, Lübeck, Germany]). However, the main difference from the study by Pietikäinen et al. is that we calculated optimal cutoff values by ROC analysis for the CXCL13 ELISA, as well as for the CXCL13 LFA. In particular, the cutoff value for the CXCL13 LFA was extremely helpful, because the manufacturer does not provide one.
Our study has some limitations. First, it was a retrospective case-control study, and we could not retrieve data on antibiotic therapy before lumbar puncture for all patients. Because it is known that CSF CXCL13 decreases rapidly during antibiotic therapy (12), early antibiotic treatment could have diminished the CXCL13 values in our study. Second, the CSF samples were stored at −20°C before performing the CXCL13 assays. Freeze-thaw cycles might have caused degradation of CXCL13, thereby leading to decreased concentrations. However, Hytönen et al. demonstrated that up to five freeze-thaw cycles did not significantly affect the levels of CXCL13 (25). Our samples did not undergo more than three freeze-thaw cycles (see Materials and Methods), so it may be assumed that our samples were of high quality. Third, the numbers of non-LNB patients included in our study were small for several subgroups of diseases (e.g., CNS malignancies).
A question that remains to be answered is how CXCL13 measurement is optimally used in daily routine. Is it necessary, or will antibiotic therapy be started anyway in case of a typical clinical picture and CSF pleocytosis, irrespective of the CXCL13 result? In our experience, which is solely based on European LNB, it is not possible to diagnose LNB from the clinical picture alone. Furthermore, CSF pleocytosis is also present in other CNS infections and inflammatory disorders. With the CXCL13 LFA, a bedside test is now available that provides a result within 20 min. In case of a negative CXCL13 test, the diagnosis of LNB is unlikely, and in combination with a normal CSF cell count, it is virtually ruled out. In addition, the amount of money saved by avoiding ceftriaxone therapy until receipt of the antibody results after 3 days is sufficient to perform approximately 6 CXCL13 LFAs. Thus, the extra financial burden to the health care system of additional CXCL13 testing is limited and serves to spare antibiotics in times in which we face increasing numbers of multidrug-resistant bacteria worldwide. To include the CXCL13 LFA in the workflow of LNB diagnosis, we suggest the following algorithm for patients with a clinical picture compatible with LNB. Initially, CSF samples are analyzed for leukocyte counts and CXCL13 levels. In cases of pleocytosis and strongly increased CXCL13 values, antibiotic therapy should be initiated due to a high probability of LNB. If CXCL13 levels are negative, therapy for LNB may be withheld, irrespective of the CSF cell count, and alternative diagnoses should be considered. In any case, the CSF/serum Borrelia AI should be determined in parallel.
Conclusions.
The CXCL13 levels in CSF samples measured by the CXCL13 ELISA and the CXCL13 LFA were significantly elevated in patients with definite LNB compared to the non-LNB control group. The CXCL13 ELISA had slightly higher sensitivity (100%) but lower specificity (84.8%) than the CXCL13 LFA (91.2% and 93.5%, respectively) using optimized cutoff levels. Some patients with VZV encephalitis and bacterial CNS infection might also have elevated CXCL13 levels. The CXCL13 LFA proved to be an easy-to-use point-of-care test with reliable diagnostic results for the identification of patients with definite LNB and could potentially replace ELISA. To maximize the sensitivity and specificity of the LFA, a cutoff AV of 22.5 seems appropriate.
Supplementary Material
ACKNOWLEDGMENT
The kits for the CXCL13 LFA were provided by bestbion dx (Cologne, Germany).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bremell D, Mattsson N, Edsbagge M, Blennow K, Andreasson U, Wikkelso C, Zetterberg H, Hagberg L. 2013. Cerebrospinal fluid CXCL13 in Lyme neuroborreliosis and asymptomatic HIV infection. BMC Neurol 13:2. doi: 10.1186/1471-2377-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauer S, Kastenbauer S. 2018. Neuroborreliose, S3 Leitlinie. Deutsche Gesellschaft für Neurologie, Leitlinien für Diagnostik und Therapie in der Neurologie. https://www.dgn.org/leitlinien. Accessed 19 June 2019.
- 3.Aguero-Rosenfeld M, Stanek G. 2019. Borrelia, p 1066–1078. In Caroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 4.Rupprecht TA, Kirschning CJ, Popp B, Kastenbauer S, Fingerle V, Pfister HW, Koedel U. 2007. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect Immun 75:4351–4356. doi: 10.1128/IAI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wormser GP, Halperin JJ. 2013. Toward a better understanding of European Lyme neuroborreliosis. Clin Infect Dis 57:510–512. doi: 10.1093/cid/cit322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mygland A, Ljostad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I. 2010. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 17:8–16. doi: 10.1111/j.1468-1331.2009.02862.x. [DOI] [PubMed] [Google Scholar]
- 7.Rupprecht TA, Manz KM, Fingerle V, Lechner C, Klein M, Pfirrmann M, Koedel U. 2018. Diagnostic value of cerebrospinal fluid CXCL13 for acute Lyme neuroborreliosis. A systematic review and meta-analysis. Clin Microbiol Infect 24:1234–1240. doi: 10.1016/j.cmi.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Barstad B, Tveitnes D, Noraas S, Selvik Ask I, Saeed M, Bosse F, Vigemyr G, Huber I, Oymar K. 2017. Cerebrospinal fluid B-lymphocyte chemoattractant CXCL13 in the diagnosis of acute Lyme neuroborreliosis in children. Pediatr Infect Dis J 36:e286–e292. doi: 10.1097/INF.0000000000001669. [DOI] [PubMed] [Google Scholar]
- 9.Tjernberg I, Henningsson AJ, Eliasson I, Forsberg P, Ernerudh J. 2011. Diagnostic performance of cerebrospinal fluid chemokine CXCL13 and antibodies to the C6-peptide in Lyme neuroborreliosis. J Infect 62:149–158. doi: 10.1016/j.jinf.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Wutte N, Berghold A, Loffler S, Zenz W, Daghofer E, Krainberger I, Kleinert G, Aberer E. 2011. CXCL13 chemokine in pediatric and adult neuroborreliosis. Acta Neurol Scand 124:321–328. doi: 10.1111/j.1600-0404.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- 11.Koedel U, Fingerle V, Pfister HW. 2015. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol 11:446–456. doi: 10.1038/nrneurol.2015.121. [DOI] [PubMed] [Google Scholar]
- 12.Koedel U, Pfister HW. 2017. Lyme neuroborreliosis. Curr Opin Infect Dis 30:101–107. doi: 10.1097/QCO.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 13.Rupprecht TA, Pfister HW, Angele B, Kastenbauer S, Wilske B, Koedel U. 2005. The chemokine CXCL13 (BLC): a putative diagnostic marker for neuroborreliosis. Neurology 65:448–450. doi: 10.1212/01.wnl.0000171349.06645.79. [DOI] [PubMed] [Google Scholar]
- 14.Ljostad U, Mygland A. 2008. CSF B-lymphocyte chemoattractant (CXCL13) in the early diagnosis of acute Lyme neuroborreliosis. J Neurol 255:732–737. doi: 10.1007/s00415-008-0785-y. [DOI] [PubMed] [Google Scholar]
- 15.Kingwell K. 2011. Infectious disease: CXCL13 is a potential biomarker for Lyme neuroborreliosis. Nat Rev Neurol 7:244. doi: 10.1038/nrneurol.2011.50. [DOI] [PubMed] [Google Scholar]
- 16.Picha D, Moravcova L, Smiskova D. 2016. Prospective study on the chemokine CXCL13 in neuroborreliosis and other aseptic neuroinfections. J Neurol Sci 368:214–220. doi: 10.1016/j.jns.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 17.Gyllemark P, Forsberg P, Ernerudh J, Henningsson AJ. 2017. Intrathecal Th17- and B cell-associated cytokine and chemokine responses in relation to clinical outcome in Lyme neuroborreliosis: a large retrospective study. J Neuroinflammation 14:27. doi: 10.1186/s12974-017-0789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner JN, Weis S, Kubasta C, Panholzer J, von Oertzen TJ. 2018. CXCL13 as a diagnostic marker of neuroborreliosis and other neuroinflammatory disorders in an unselected group of patients. J Neurol 265:74–81. doi: 10.1007/s00415-017-8669-7. [DOI] [PubMed] [Google Scholar]
- 19.Pietikäinen A, Oksi J, Hytonen J. 2018. Point-of-care testing for CXCL13 in Lyme neuroborreliosis. Diagn Microbiol Infect Dis 91:226–228. doi: 10.1016/j.diagmicrobio.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Reiber H. 2016. Knowledge-base for interpretation of cerebrospinal fluid data patterns. Essentials in neurology and psychiatry. Arq Neuropsiquiatr 74:501–512. doi: 10.1590/0004-282X20160066. [DOI] [PubMed] [Google Scholar]
- 21.Cerar T, Ogrinc K, Lotric-Furlan S, Kobal J, Levicnik-Stezinar S, Strle F, Ruzić-Sabljic E. 2013. Diagnostic value of cytokines and chemokines in Lyme neuroborreliosis. Clin Vaccine Immunol 20:1578–1584. doi: 10.1128/CVI.00353-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Burgel ND, Bakels F, Kroes AC, van Dam AP. 2011. Discriminating Lyme neuroborreliosis from other neuroinflammatory diseases by levels of CXCL13 in cerebrospinal fluid. J Clin Microbiol 49:2027–2030. doi: 10.1128/JCM.00084-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senel M, Rupprecht TA, Tumani H, Pfister HW, Ludolph AC, Brettschneider J. 2010. The chemokine CXCL13 in acute neuroborreliosis. J Neurol Neurosurg Psychiatry 81:929–933. doi: 10.1136/jnnp.2009.195438. [DOI] [PubMed] [Google Scholar]
- 24.Moser B, Schaerli P, Loetscher P. 2002. CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol 23:250–254. doi: 10.1016/s1471-4906(02)02218-4. [DOI] [PubMed] [Google Scholar]
- 25.Hytönen J, Kortela E, Waris M, Puustinen J, Salo J, Oksi J. 2014. CXCL13 and neopterin concentrations in cerebrospinal fluid of patients with Lyme neuroborreliosis and other diseases that cause neuroinflammation. J Neuroinflammation 11:103. doi: 10.1186/1742-2094-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remy MM, Schobi N, Kottanattu L, Pfister S, Duppenthaler A, Suter-Riniker F. 2017. Cerebrospinal fluid CXCL13 as a diagnostic marker of neuroborreliosis in children: a retrospective case-control study. J Neuroinflammation 14:173. doi: 10.1186/s12974-017-0948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waiß C, Kindler W, Ströbele B, Aspöck C, Oberndorfer S. 2017. CXCL-13 as a biomarker in the diagnostics of neuroborreliosis. Nervenarzt 88:635–641. doi: 10.1007/s00115-017-0292-4. [DOI] [PubMed] [Google Scholar]
- 28.Erdem H, Inan A, Guven E, Hargreaves S, Larsen L, Shehata G, Pernicova E, Khan E, Bastakova L, Namani S, Harxhi A, Roganovic T, Lakatos B, Uysal S, Sipahi OR, Crisan A, Miftode E, Stebel R, Jegorovic B, Fehér Z, Jekkel C, Pandak N, Moravveji A, Yilmaz H, Khalifa A, Musabak U, Yilmaz S, Jouhar A, Oztoprak N, Argemi X, Baldeyrou M, Bellaud G, Moroti RV, Hasbun R, Salazar L, Tekin R, Canestri A, Čalkić L, Praticò L, Yilmaz-Karadag F, Santos L, Pinto A, Kaptan F, Bossi P, Aron J, Duissenova A, Shopayeva G, Utaganov B, Grgic S, Ersoz G, Wu AKL, Lung KC, Bruzsa A, Radic LB, Kahraman H, Momen-Heravi M, Kulzhanova S, Rigo F, Konkayeva M, Smagulova Z, Tang T, Chan P, Ahmetagic S, Porobic-Jahic H, Moradi F, Kaya S, Cag Y, Bohr A, Artuk C, Celik I, Amsilli M, Gul HC, Cascio A, Lanzafame M, Nassar M. 2017. The burden and epidemiology of community-acquired central nervous system infections: a multinational study. Eur J Clin Microbiol Infect Dis 36:1595–1611. doi: 10.1007/s10096-017-2973-0. [DOI] [PubMed] [Google Scholar]
- 29.Mothapo KM, Verbeek MM, van der Velden LB, Ang CW, Koopmans PP, van der Ven A, Stelma F. 2015. Has CXCL13 an added value in diagnosis of neurosyphilis? J Clin Microbiol 53:1693–1696. doi: 10.1128/JCM.02917-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dersch R, Hottenrott T, Senel M, Lehmensiek V, Tumani H, Rauer S, Stich O. 2015. The chemokine CXCL13 is elevated in the cerebrospinal fluid of patients with neurosyphilis. Fluids Barriers CNS 12:12. doi: 10.1186/s12987-015-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magliozzi R, Marastoni D, Rossi S, Castellaro M, Mazziotti V, Pitteri M, Gajofatto A, Monaco S, Benedetti MD, Calabrese M. 2019. Increase of CSF inflammatory profile in a case of highly active multiple sclerosis. BMC Neurol 19:231. doi: 10.1186/s12883-019-1455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liba Z, Nohejlova H, Capek V, Krsek P, Sediva A, Kayserova J. 2019. Utility of chemokines CCL2, CXCL8, 10 and 13 and interleukin 6 in the pediatric cohort for the recognition of neuroinflammation and in the context of traditional cerebrospinal fluid neuroinflammatory biomarkers. PLoS One 14:e0219987. doi: 10.1371/journal.pone.0219987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.