Clinical justification for rapid antimicrobial susceptibility testing (AST) in Gram-negative rod (GNR) bacteremia is compelling; however, evidence supporting its value is sparse. We investigated the impact of rapid AST on clinical and antimicrobial stewardship outcomes in real-world practice. We performed a before-and-after quasi-experimental study from February 2018 to July 2019 at a tertiary hospital of the 24-h/day, 7-day/week implementation of the direct Vitek 2 AST method from positive blood culture broth for GNR bacteremia with electronic isolate-specific de-escalation comments and daytime antibiotic stewardship program (ASP) intervention.
KEYWORDS: antibiotic stewardship, Gram-negative bacteria, bacteremia, rapid diagnostic tests
ABSTRACT
Clinical justification for rapid antimicrobial susceptibility testing (AST) in Gram-negative rod (GNR) bacteremia is compelling; however, evidence supporting its value is sparse. We investigated the impact of rapid AST on clinical and antimicrobial stewardship outcomes in real-world practice. We performed a before-and-after quasi-experimental study from February 2018 to July 2019 at a tertiary hospital of the 24-h/day, 7-day/week implementation of the direct Vitek 2 AST method from positive blood culture broth for GNR bacteremia with electronic isolate-specific de-escalation comments and daytime antibiotic stewardship program (ASP) intervention. The primary outcome was time to appropriate antibiotic escalation or de-escalation, and secondary outcomes included time to oral antibiotic stepdown, hospital length of stay (LOS), all-cause 30-day mortality, 7-day incidence of acute kidney injury (AKI), and 30-day incidence of Clostridioides difficile infection (CDI). A total of 671 GNR isolates were included from 643 adult patients. Among patients for whom antibiotic change occurred after rapid AST result, rapid AST was associated with a trend in decreased time to escalation or de-escalation (hazard ratio, 1.22; 95% confidence interval [CI], 0.99 to 1.51; P = 0.06), with median times of 52.3 versus 42.2 h. Secondary outcomes were similar in both groups and include median time to oral antibiotic stepdown, LOS, all-cause mortality, and incidence of AKI and CDI. Rapid AST led to improved stewardship measures but did not impact clinical patient outcomes. These results highlight that multiple variables in addition to the timing of the AST result contribute to clinical outcome and that further intervention may be required to clinically justify rapid AST implementation.
INTRODUCTION
Gram-negative rod (GNR) bacteremia and sepsis are associated with significant morbidity and mortality that increase with delay in effective antibiotic therapy (1). Rapid methods for organism identification directly from positive blood cultures have emerged over the last decade, including matrix-assisted laser desorption–ionization-time of flight (MALDI-TOF) and rapid multiplexed PCR panels, with turnaround time of 1 to 5 h (2–4); however, standard phenotypic antimicrobial susceptibility testing (AST) requires 2 to 4 days given the time required for subculture and AST (5). As such, rapid AST methods directly from positive blood cultures have been developed, which include the FDA-cleared Accelerate Pheno system (6–8) and short incubation disk diffusion (9, 10). Rapid nucleic acid tests have also been developed for the identification of certain resistance mechanisms (4, 11, 12), but due to technical limitations and complexity of resistance mechanisms, they do not currently capture the full antimicrobial resistance profiles found in GNRs for which rapid phenotypic testing is necessary. Rapid AST methods were developed with the goals of optimizing the timing of initiation of appropriate antibiotics, reducing time to antibiotic de-escalation, facilitating timely transition to oral antibiotics, and limiting adverse patient outcomes and emergence of antibiotic resistance, all significant antibiotic stewardship program (ASP) objectives (13). However, little is known about the real-world clinical impact of these rapid AST procedures and whether the expense and effort associated with their implementation is justified by improved patient outcomes.
Vitek 2 (bioMérieux, Marcy l’Étoile, France) is an automated instrument that is FDA cleared for AST from isolated bacterial colonies. The Stanford Health Care Clinical Microbiology Laboratory developed and validated a simple and rapid sample processing method for Vitek 2 AST directly from BD Bactec FX (Becton, Dickinson, Franklin Lakes, NJ) blood culture bottles (BCBs) positive for GNRs (14). Hands-on time is about 10 min per sample, and mean turnaround time from AST setup to results is 9 h for Enterobacterales (formerly Enterobacteriaceae) and 12 h for Pseudomonas aeruginosa (7). Vitek 2 direct AST was implemented for routine care in November 2018. Here, we present the findings of our investigation on the impact of rapid AST on ASP measures and clinical outcomes in patients with GNR bacteremia in real-world clinical practice.
MATERIALS AND METHODS
Ethics.
This work was conducted as part of a hospital-sponsored quality improvement project, and thus institutional review board (IRB) approval was not required.
Study design.
This study was a before-and-after quasi-experimental study at Stanford Health Care (SHC) to compare ASP measures and clinical outcomes of direct Vitek 2 testing in patients with GNR bacteremia. The retrospective period included cases from 8 February 2018 to 9 October 2018, and the prospective period included cases from 1 November 2018 to 15 July 2019. Data collection was performed from 1 November 2018 to 30 August 2019, retrospectively for the preintervention period and prospectively for the intervention period. The primary outcome was the time from positive blood culture to appropriate escalation or de-escalation (based on antibiogram result and the protocol in Table S1 in the supplemental material), and secondary outcomes included time to oral antibiotic stepdown, all-cause 30-day mortality, length of stay (LOS), 7-day incidence of acute kidney injury (AKI), and 30-day incidence of Clostridioides difficile infection (CDI).
Study population.
Consecutive adult (defined as ≥18 years old) inpatients at SHC with bloodstream infection (BSI) with Enterobacterales or Pseudomonas aeruginosa were included (see Table S2 in the supplemental material). Positive blood cultures with these organisms were included from all shifts. Stanford Hospital is a 613-bed adult tertiary care academic hospital with extensive programs for cancer care and hematopoietic stem cell and solid organ transplant. Exclusion criteria included patients with a previous BSI with the same organism in the preceding 7 days, blood cultures with mixed morphologies on Gram stain, and patients who died within 48 h of the positive blood culture Gram stain. Blood cultures that were only recognized to be polymicrobial after Gram stain were included in the study and analysis. Similarly, individuals with ≥1 positive blood culture with GNR were included in the study and analysis if at least 7 days had elapsed between the 2 collections.
AST and ASP.
All procedures were completed at the SHC Clinical Microbiology Laboratory by licensed technologists. Blood cultures were collected and incubated in the BD Bactec FX system. Once positive, a Gram stain was performed, reported in the electronic medical records (EMR) system, and called to the ordering provider within 1 h. Prior to the direct Vitek 2 implementation, all phenotypic AST was performed from rapid subculture (2 to 4 h for Enterobacterales and up to 6 h for Pseudomonas aeruginosa), followed by the standard setup on the MicroScan WalkAway plus system (Beckman Coulter, Brea, CA), with a total AST turnaround time of 1 to 2 days (preintervention). On 1 November 2018, the laboratory implemented direct Vitek 2 AST for all Enterobacterales- and Pseudomonas aeruginosa-positive blood cultures using a processing method previously described (intervention) (14). In brief, organism identification was performed by direct MALDI-TOF extraction from positive blood culture broth on a Bruker Biotyper instrument (Bruker, Billerica, MA) using a laboratory-developed lysis and differential centrifugation procedure (14). This procedure provides organism identification results within 30 min and is batched in the laboratory workflow such that results are available within 4 h. In parallel, the same steps of lysis and differential centrifugation were performed from positive blood culture broth, leading to a bacterial suspension that is set up on the Vitek 2 AST-GN81 card. Blood culture identification and susceptibility testing results were reported in the EMR system but not called to the ordering provider.
The ASP group at SHC during the study period consisted of two infectious disease (ID) physicians and two infectious disease pharmacists who provided services during regular working hours (8 a.m. to 5 p.m.) on weekdays. Comments specific to the isolate AST profile were developed and integrated into the Epic EMR system for real-time electronic reporting with AST results in addition to an email alert to the ASP group (Table S1). Emails were sent in real-time for each direct Vitek 2 AST result in the study. If sent during off-hours, the email would be read and acted on by the ASP team the next workday during regular working hours. Definitions of antibiotic escalation and de-escalation were established for this study (see Table S3 in the supplemental material). During working hours, the ASP group reviewed the case and communicated treatment recommendations directly with the treating team if appropriate. Patients for whom the infectious diseases service was already consulted and cases for which appropriate antibiotic change based on AST result and ASP assessment had already been performed by the primary team prior to case review were not approached by ASP.
Data collection.
Chart review was performed by three users for the ASP data (W.A., E.M., and L.M.) and by two users for the microbiological data (B. E. and C.A.H.). All data were reviewed by one user (C.A.H.). Data points collected included demographic and clinical characteristics, microbiologic data, ASP intervention data, admission details, and patient outcome data.
Data analysis.
The primary outcome was time to appropriate escalation and de-escalation in hours (time between Gram stain result to the first antibiotic change). Secondary outcomes included (i) time to oral antibiotic stepdown (time between Gram stain and oral antibiotic change for patients initially receiving intravenous [i.v.] antibiotics), (ii) LOS in hours (time between Gram stain to hospital discharge), (iii) all-cause 30-day mortality, (iv) incidence of AKI at 7 days (defined as a ≥0.3 rise in creatinine within 48 h), and (v) CDI incidence at 30 days (defined as predicted toxin-positive C. difficile PCR testing [15] with a compatible clinical syndrome).
Statistical analysis.
Statistical analysis was performed using R version 3.5.0. Time to escalation or de-escalation, time to oral antibiotic stepdown, and LOS were assessed by Cox proportional hazards regression analysis in both unadjusted and adjusted models, including the a priori-determined potential confounders of age, sex, Charlson comorbidity index, infection source, active empirical therapy, and febrile neutropenia. Censoring was performed based on mortality and on individuals who underwent antibiotic escalation or de-escalation before availability of AST results. All-cause 30-day mortality, AKI incidence, and CDI incidence were analyzed by logistic regression analysis, both crude and adjusted for the same potential confounders as above. A statistical threshold of P ≤ 0.05 was considered significant, and a complete case analysis was performed.
Assuming a two-sided alpha error of 0.05, power of 80%, and censorship rate of 0.01, and based on a time to de-escalation and escalation of 60 and 48 h in the preintervention and intervention groups, the required sample size per group was calculated to be 320 patients per group. A larger total number of individuals per group was screened to ensure enough samples to account for exclusions in each group and lack of antibiotic de-escalation or escalation after rapid AST result.
Data availability.
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
RESULTS
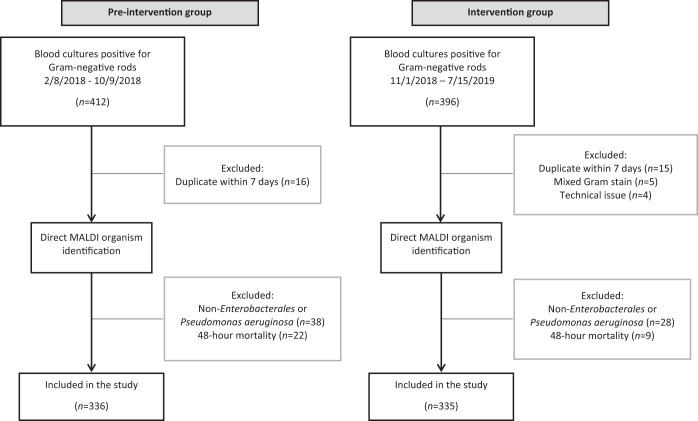
A total of 671 GNR bloodstream infections from 643 unique patients were included in total, with 336 samples in the preintervention group and 335 in the intervention group (Fig. 1). Clinical and demographic characteristics were similar in both groups, with the exceptions of a higher proportion of urinary infections and individuals in the emergency department in the intervention group and a higher proportion of active empirical antimicrobial therapy based on the AST result in the preintervention group (Table 1). About half of patients were immunocompromised (51.1%), most commonly from solid organ malignancy (26.4%), leukemia or lymphoma (14.8%), solid organ transplant (6.6%), or being recipients of a bone marrow transplant (1.9%). Across both groups, urinary source of infection was most common, followed by intraabdominal source. Median time from blood culture positivity to AST result communication in the preintervention group to ASP was 24.1 h (interquartile range [IQR], 21.5 to 29.4) for Enterobacterales and 25.8 h (IQR, 20.9 to 29.3) for Pseudomonas aeruginosa, compared to 12.3 h (IQR, 10.4 to 17.2) and 16.3 h (IQR, 14.7 to 19.4), respectively, in the intervention group.
FIG 1.
Schematic overview of blood culture selection for the preintervention and intervention groups.
TABLE 1.
Demographic characteristics in the preintervention and intervention groups
| Demographic characteristicc | Preintervention (n = 336) | Intervention (n = 335) | P value (LR)d |
|---|---|---|---|
| Sex, male (no. of patients [%]) | 178 (53.0) | 176 (52.5) | 0.9 |
| Age (yrs) (mean [SD]) | 63.9 (18.2) | 63.1 (17.7) | 0.5 |
| Charlson comorbidity index (mean [SD]) | 5.2 (2.8) | 5.1 (3.0) | 0.9 |
| Immunocompromised (no. of patients [%]) | 171 (50.9) | 172 (51.3) | 0.9 |
| Source of bacteremia (no. of patients [%]) | 0.04 | ||
| Urinary | 117 (34.8) | 131 (39.1) | |
| Intra-abdominal | 106 (31.6) | 104 (31.0) | |
| Central venous catheter | 19 (5.7) | 15 (4.5) | |
| Respiratory | 19 (5.7) | 21 (6.3) | |
| Surgical site infection | 23 (6.9) | 5 (1.5) | |
| Other | 10 (3.0) | 19 (5.7) | |
| Unknown | 42 (12.5) | 40 (11.9) | |
| Patient location at time of blood culture draw (no. of patients [%]) | 0.001 | ||
| Emergency department | 210 (62.5) | 225 (67.2) | |
| Inpatient ward | 89 (26.5) | 75 (22.4) | |
| Intensive care unit | 16 (4.8) | 11 (3.3) | |
| Other | 21 (6.3) | 24 (7.2) | |
| Mixed (polymicrobial) growth (no. of patients [%]) | 1 | ||
| Yes | 35 (10.4) | 34 (10.1) | |
| No | 301 (89.6) | 301 (89.9) | |
| Empiric antibiotic therapy (no. of patients [%]) | 0.3 | ||
| Piperacillin-tazobactam | 157 (46.7) | 153 (45.7) | |
| Ceftriaxone | 52 (15.5) | 69 (20.6) | |
| Cefepime | 44 (13.1) | 42 (12.5) | |
| No. (%) of isolates susceptible | |||
| Ceftriaxone | 263 (78.3) | 248 (74.0) | 0.2 |
| Ceftazidimea | 288 (85.7) | 293 (88.0) | 0.4 |
| Ciprofloxacin | 278 (82.7) | 256 (76.4) | 0.04 |
| TMP-SMX | 247 (73.5) | 226 (67.5) | 0.1 |
| Empirical antibiotic active against the GNR (no. of patients [%])b | 0.4 | ||
| Yes | 311 (92.6) | 294 (87.8) | |
| No | 25 (7.4) | 32 (9.6) | |
| Escalation (no. of patients [%]) | 118 (35.1) | 122 (36.4) | 0.7 |
| De-escalation (no. of patients [%]) | 151 (44.9) | 151 (45.1) | 1 |
| No antibiotic escalation or de-escalation (no. of patients [%]) | 67 (19.9) | 62 (18.5) | 0.6 |
Missing data for 2 isolates in intervention group.
Missing data for 9 isolates in intervention group.
Empirical, antibiotic at or around the time of Gram stain; GNR, Gram-negative rod; TMP-SMX, trimethoprim-sulfamethoxazole. No missing data were present unless otherwise noted.
LR, logistic regression.
The 4 most common pathogens in both groups were Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae complex, and Pseudomonas aeruginosa (Fig. 2). The most common antimicrobial agent administered empirically at the time of Gram stain was piperacillin-tazobactam (46.2%), followed by ceftriaxone (18.0%) and cefepime (12.8%). Recommended antibiotics for de-escalation included ceftriaxone, ciprofloxacin, or trimethoprim-sulfamethoxazole (TMP-SMX), depending on the isolate susceptibility profile (Table S1) and individual patient characteristics. Primary and secondary outcomes in the preintervention and intervention groups are shown in Table 2. Results were similar between the adjusted and unadjusted models for all endpoints. There was a trend for shorter time to appropriate escalation or de-escalation in the intervention group (hazard ratio [HR], 1.22; 95% CI, 0.99 to 1.51; P = 0.06), with median times of 52.3 versus 42.2 h (Fig. 3). When restricted to cases with an ASP interventional recommendation that was followed by the primary team, median time to appropriate escalation or de-escalation in the intervention group was reduced compared to that in the intervention group as a whole (HR, 1.68; 95% CI, 1.25 to 2.26; P = 0.001), with median times of 52.3 versus 38.4 h. Antibiotic escalation occurred in a similar time frame in both groups, with median times of 46.6 versus 45.0 h. The most commonly used antibiotic for escalation was oral ciprofloxacin (9.4%), followed by ertapenem (8.1%) and meropenem (7.3%). Antibiotic escalation was more frequent in the intervention subgroup of 47 ceftriaxone-nonsusceptible E. coli and K. pneumoniae isolates than in the rest of the intervention group (54.0 versus 30.2%; P = 0.0001). There was a trend for a shorter time to antibiotic de-escalation in the intervention group, with median times of 52.2 versus 43.4 h. The most commonly used antibiotic for de-escalation was cefepime (15.3%), followed by oral ciprofloxacin (9.4%) and oral levofloxacin (3.1%). Oral ciprofloxacin was used for both escalation and de-escalation, based on the definitions in Table S3. Time to oral antibiotic stepdown was similar in both groups, and the most commonly used agents for oral antibiotic stepdown were ciprofloxacin (55.7%), levofloxacin (17.9%), amoxicillin-clavulanic acid (11.3%), and TMP-SMX (6.9%).
FIG 2.
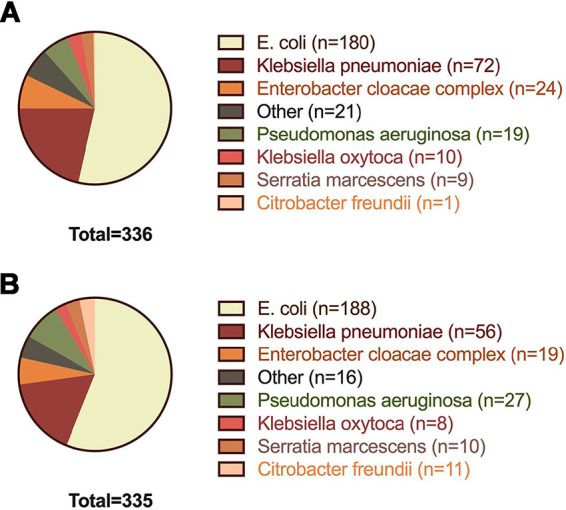
Gram-negative rod species distribution in the preintervention (A) and intervention (B) groups.
TABLE 2.
Primary and secondary outcomes in the preintervention and intervention groups
| Outcome parametera | Preintervention | Intervention | Unadjusted HRb (Cox) or OR (LR)c | Unadjusted P value (Cox or LR)c | Adjustedd HR (Cox) | Adjustedd P value (Cox or LR) |
|---|---|---|---|---|---|---|
| Time to appropriate escalation or de-escalation in h (median [95% CI]) | 52.3 (48.2–60.0) | 42.2 (37.4–48.0) | 1.24 (1.02–1.51) | 0.03 | 1.22 (0.99–1.51) | 0.06 |
| Time to appropriate escalation in h (median [95% CI]) | 46.6 (37.9–64.4) | 45.0 (33.0–60.7) | 1.23 (0.96–1.57) | 0.1 | 1.28 (0.98–1.68) | 0.08 |
| Time to appropriate de-escalation in h (median [95% CI]) | 52.2 (47.5–60.6) | 43.4 (37.4–54.5) | 1.27 (0.92–1.74) | 0.1 | 1.17 (0.83–1.65) | 0.4 |
| Time to oral antibiotic stepdown in h (median [95% CI]) | 105.8 (87.2–147.4) | 132.3 (102.8–195.7) | 0.92 (0.74–1.14) | 0.4 | 0.87 (0.69–1.10) | 0.2 |
| LOS in h (median [95% CI]) | 96.1 (86.0–107.2) | 115.2 (99.8–127.9) | 1.02 (0.87–1.19) | 0.8 | 0.92 (0.77–1.09) | 0.4 |
| 30-day all-cause mortality (no. [%]) | 24 (7.1) | 16 (4.8) | 0.65 (0.34–1.25) | 0.2 | 0.83 (0.39–1.77) | 0.6 |
| 7-day AKI incidence (no. [%]) | 58 (17.3) | 44 (13.1) | 0.72 (0.47–1.11) | 0.1 | 0.82 (0.51–1.31) | 0.4 |
| 30-day C. difficile incidence (no. [%]) | 7 (2.1) | 6 (1.8) | 0.86 (0.29–2.58) | 0.8 | 1.08 (0.32–3.60) | 0.9 |
AKI, acute kidney injury; CI, confidence interval; LOS, length of stay.
HR, hazard ratio; Cox, Cox proportional hazards model.
OR, odds ratio; LR, logistic regression.
Adjusted for age, sex, Charlson comorbidity index, empirical active therapy, infection source, and febrile neutropenia.
FIG 3.
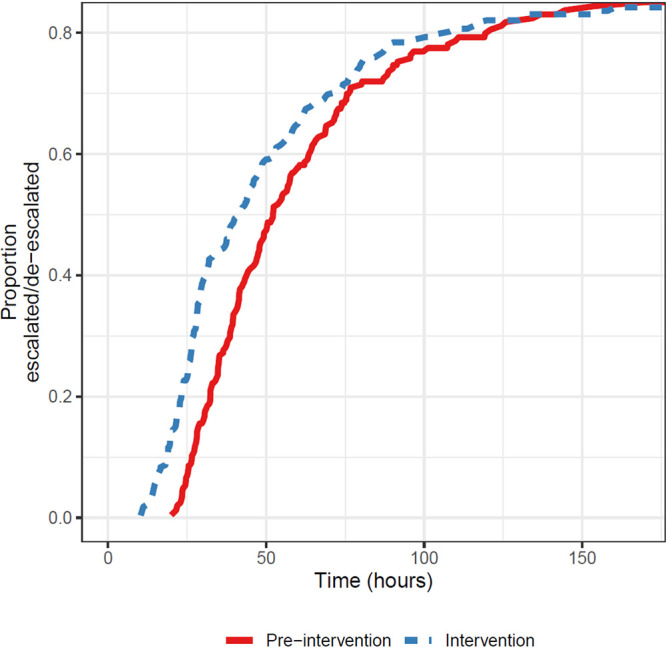
Kaplan-Meier curves of time to appropriate escalation or de-escalation of antibiotic therapy.
Secondary outcomes were similar in the two groups and included LOS (HR, 1.02; 95% CI, 0.87 to 1.19; P = 0.8), with median times of 96.1 h in the preintervention group versus 115.2 h in the intervention group. In addition, the two groups showed a similar 7-day incidence of AKI (17.3% in the preintervention versus 13.1% in the intervention group; P = 0.1), all-cause 30-day mortality (7.1% versus 4.8%; P = 0.2), and 30-day CDI incidence rate (2.1% versus 1.8%; P = 0.8).
Prior to the start of the rapid method, the ASP was minimally involved in real-time review and/or therapeutic recommendations for GNR bacteremia. In contrast, the ASP group directly intervened for therapeutic recommendations in 114 (34.0%) of cases in the intervention group. Of these, the acceptability of recommendations was high, with 97 (85.1%) of their recommendations being followed. The remaining recommendations were not followed mainly due to physician preference (64.7%) or treatment of concurrent febrile neutropenia (17.7%). The ASP group did not intervene in 221 (66.0%) cases. This was due to the infectious disease (ID) team already consulting on the case (31.2%), the treating team having made the appropriate antibiotic change per ASP review prior to ASP involvement (20.4%), or the patient no longer being hospitalized at the time of ASP review (22.2%).
DISCUSSION
The recognition that early initiation of appropriate antibiotic therapy is associated with improved outcomes in patients with serious infections, as well as the fact that early de-escalation reduces exposure to unnecessarily broad antibiotic therapy, has led to a desire to shorten the time required to complete antimicrobial susceptibility testing (16–20). This has generated growing interest and technology development designed to shorten the turnaround time of antimicrobial susceptibility testing for positive blood cultures as adoption of the rapid methods may facilitate achievement of important ASP targets. Implementation of rapid AST systems, however, requires significant investment of resources in instrument acquisition, reagent costs, and staffing. Given the paucity of evidence regarding GNR bacteremia, it is currently unclear if the clinical impact of rapid testing justifies the testing cost. Antimicrobial prescribing is a complex process influenced by multiple variables in addition to timing of AST result availability. These include availability of expert clinical advice by, e.g., ASP teams, willingness of the treating team to modify therapy, severity of clinical illness, drug allergies, potential adverse effects, and a number of host factors, such as immunocompromise (21). Thus, understanding the role that each of these variables plays in facilitating improved outcomes with rapid direct AST may be essential for justifying clinical implementation of rapid AST.
In this study, we showed that implementation of rapid AST for GNR bacteremia with isolate-specific de-escalation comments provided electronically and part-time ASP intervention was associated with a trend toward reduction in time to appropriate escalation and de-escalation of therapy but no changes in clinical outcomes. Thus, a bundled approach with more intensive ASP intervention may be required to leverage the full potential of rapid AST technology. The Pheno system (Accelerate Diagnostics Inc., Tucson, AZ) was the first rapid phenotypic AST commercial platform to receive FDA clearance, and it produces AST results within 7 h (6–8). Similarly, rapid disk diffusion testing methods from positive blood culture broth have been developed (9, 10, 22, 23). Real-world impact on patient outcomes has been examined in small studies that included Gram-negative bacteremia and showed improved time to optimal antimicrobial therapy but no change in clinical outcomes or antibiotic consumption (24–26). Selection of positive blood cultures from the daytime shift only and a dedicated team of investigators with full ASP intervention may explain shorter intervals for antibiotic change. Other published studies have considered the theoretical benefit of rapid testing with the Accelerate Pheno system and projected it to be beneficial (8, 27–30); however, this may not reflect real-world challenges in implementation of rapid AST strategy.
Approximately half of the participants included in this study were immunocompromised, including due to solid organ malignancy and leukemia or lymphoma; these two patient populations are at high risk for febrile neutropenia and bloodstream infection (31–33). Clinicians are often reluctant to de-escalate antibiotic therapy in neutropenic patients with bacteremia. Thus, patient characteristics, as well as clinician practice, are important factors to consider in assessing the clinical impact of rapid AST. Multicenter studies with representation of community hospitals are needed to measure the clinical impact of rapid AST in different patient settings, as impact may be greater in lower-risk populations. Furthermore, this study used rapid subculture-based AST in the preintervention period; greater impact would be expected in settings with AST methods with a longer turnaround time and/or with higher rates of drug-resistant GNR isolates.
In this study, the intervention subgroup with ceftriaxone-nonsusceptible E. coli or Klebsiella pneumoniae isolates was significantly more likely to have undergone antibiotic escalation compared to the rest of the intervention group (54.0 versus 30.2%), most commonly to a carbapenem antibiotic. The MERINO trial demonstrated a significant reduction in 30-day mortality in bloodstream infection patients treated with meropenem compared to piperacillin-tazobactam for such isolates (34). This finding bears important implications for antimicrobial stewardship, as patients who would previously have been considered candidates for antibiotic de-escalation are now maintained or escalated on a carbapenem antibiotic, limiting the potential impact of full phenotypic rapid AST. In addition, recent data support shortening duration of therapy for uncomplicated GNR bacteremia to 7 days, which should also be considered in assessing the overall impact of rapid AST testing (35).
This study’s main strength is its comprehensive nature in presenting the largest number of Gram-negative rod isolates in a rapid AST clinical impact study to date, representing diverse clinical settings and patient populations with a high proportion of immunocompromised hosts and real-world conditions. The primary outcome analysis was restricted to individuals with an antibiotic change occurring after AST result availability, which better assesses the impact of the rapid AST method itself. In addition, this study assesses impact of the direct Vitek 2 procedure, a method that is inexpensive, robust, and broadly relevant, given it makes use of an instrument that is already widely in use in many laboratories. Furthermore, the multipronged approach was designed to optimize clinical impact and enable full potential of the method. This study showed the effectiveness of individual case-by-case email communication with an ASP and the feasibility of pursuing rapid testing continuously on all laboratory shifts.
However, this study presents limitations. First, this quasi-experimental study design cannot account for confounding factors or changes in practice over time the same way a prospective, randomized controlled trial would have. Furthermore, since this intervention was rolled out as a bundled approach, we were unable to isolate the impact of the rapid AST method alone. Previously published data have shown a positive impact from approaches combining a rapid diagnostic for identification and AST with either ASP, ID pharmacist, or fellow intervention (11, 24, 36, 37), with this combination approach yielding better results than those with conventional testing with ASP intervention alone (24) or with the rapid diagnostic method alone (11, 24). Second, the study was powered statistically for the primary outcome only. Nonetheless, the secondary clinical outcomes were considered important to include, given that they may reveal trends that warrant further assessment in future studies. The lack of clinical impact is consistent with that seen in previous studies (24, 25, 38). Third, ASP intervention was only performed during regular weekday working hours, which may contribute to the prolonged time to appropriate antibiotic change. Delay in time to escalation may also occur from delay in switch to oral ciprofloxacin from ceftriaxone, a common clinical scenario in this study. Given that ASP involvement in this study was associated with a lower time to antibiotic change, an intensified ASP approach may have led to greater clinical impact (39). Fourth, the direct Vitek 2 method is at least 2 h slower than the Accelerate Pheno System; thus, more rapid intervention could have been possible with this technology. However, in the case of both techniques, real-world turnaround time is slower (an additional 3 to 7 h) than published validation results (6, 8, 14, 38). Finally, this study used an AST method in the preintervention period that provided results within 1 to 2 days, which may have limited clinical impact compared to a more standard method with a 2- to 4-day turnaround time.
In summary, this study showed that the impact of rapid AST testing led to improved antibiotic stewardship measures but did not impact length of stay and other clinical outcome measures. These results highlight that variables in addition to the timing of AST result, including antibiotic stewardship program availability, contribute to clinical outcome and that further investigation is needed to identify key factors that may increase the clinical impact of rapid AST.
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniel A. Green for critical review of the manuscript, as well as the Stanford Clinical Laboratory scientists for their contribution to this project.
This work was supported by the Stanford University Department of Pathology.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 2.Lagace-Wiens PR, Adam HJ, Karlowsky JA, Nichol KA, Pang PF, Guenther J, Webb AA, Miller C, Alfa MJ. 2012. Identification of blood culture isolates directly from positive blood cultures by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry and a commercial extraction system: analysis of performance, cost, and turnaround time. J Clin Microbiol 50:3324–3328. doi: 10.1128/JCM.01479-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Gaudio F, Indelicato S, Indelicato S, Tricoli MR, Stampone G, Bongiorno D. 2018. Improvement of a rapid direct blood culture microbial identification protocol using MALDI-TOF MS and performance comparison with SepsiTyper kit. J Microbiol Methods 155:1–7. doi: 10.1016/j.mimet.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 4.She RC, Bender JM. 2019. Advances in rapid molecular blood culture diagnostics: healthcare impact, laboratory implications, and multiplex technologies. J Appl Lab Med 3:617–630. doi: 10.1373/jalm.2018.027409. [DOI] [PubMed] [Google Scholar]
- 5.Khan ZA, Siddiqui MF, Park S. 2019. Current and emerging methods of antibiotic susceptibility testing. Diagnostics (Basel) 9:49. doi: 10.3390/diagnostics9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pancholi P, Carroll KC, Buchan BW, Chan RC, Dhiman N, Ford B, Granato PA, Harrington AT, Hernandez DR, Humphries RM, Jindra MR, Ledeboer NA, Miller SA, Mochon AB, Morgan MA, Patel R, Schreckenberger PC, Stamper PD, Simner PJ, Tucci NE, Zimmerman C, Wolk DM. 2018. Multicenter evaluation of the Accelerate PhenoTest BC kit for rapid identification and phenotypic antimicrobial susceptibility testing using morphokinetic cellular analysis. J Clin Microbiol 56:e01329-17. doi: 10.1128/JCM.01329-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantel A, Monier J, Lavigne JP. 2018. Performance of the Accelerate Pheno system for identification and antimicrobial susceptibility testing of a panel of multidrug-resistant Gram-negative bacilli directly from positive blood cultures. J Antimicrob Chemother 73:1546–1552. doi: 10.1093/jac/dky032. [DOI] [PubMed] [Google Scholar]
- 8.Marschal M, Bachmaier J, Autenrieth I, Oberhettinger P, Willmann M, Peter S. 2017. Evaluation of the Accelerate Pheno system for fast identification and antimicrobial susceptibility testing from positive blood cultures in bloodstream infections caused by Gram-negative pathogens. J Clin Microbiol 55:2116–2126. doi: 10.1128/JCM.00181-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Committee on Antimicrobial Susceptibility Testing. 2018. Breakpoints validated for EUCAST short incubation disk diffusion directly from positive blood culture bottles. http://www.eucast.org/rapid_ast_in_blood_cultures/breakpoints_for_short_incubation/.
- 10.Chandrasekaran S, Abbott A, Campeau S, Zimmer BL, Weinstein M, Thrupp L, Hejna J, Walker L, Ammann T, Kirn T, Patel R, Humphries RM. 2018. Direct-from-blood-culture disk diffusion to determine antimicrobial susceptibility of Gram-negative bacteria: preliminary report from the Clinical and Laboratory Standards Institute Methods Development and Standardization Working Group. J Clin Microbiol 56:e01678-17. doi: 10.1128/JCM.01678-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salimnia H, Fairfax MR, Lephart PR, Schreckenberger P, DesJarlais SM, Johnson JK, Robinson G, Carroll KC, Greer A, Morgan M, Chan R, Loeffelholz M, Valencia-Shelton F, Jenkins S, Schuetz AN, Daly JA, Barney T, Hemmert A, Kanack KJ. 2016. Evaluation of the FilmArray blood culture identification panel: results of a multicenter controlled trial. J Clin Microbiol 54:687–698. doi: 10.1128/JCM.01679-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan CA, Watz N, Budvytiene I, Banaei N. 2019. Rapid antimicrobial susceptibility testing by VITEK®2 directly from blood cultures in patients with Gram-negative rod bacteremia. Diagn Microbiol Infect Dis 94:116–121. doi: 10.1016/j.diagmicrobio.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Senchyna F, Gaur RL, Gombar S, Truong CY, Schroeder LF, Banaei N. 2017. Clostridium difficile PCR cycle threshold predicts free toxin. J Clin Microbiol 55:2651–2660. doi: 10.1128/JCM.00563-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corl KA, Zeba F, Caffrey AR, Hermenau M, Lopes V, Phillips G, Merchant RC, Levy MM, LaPlante KL. 2019. Delay in antibiotic administration is associated with mortality among septic shock patients with Staphylococcus aureus bacteremia. Crit Care Med 48:525–532. doi: 10.1097/CCM.0000000000004212. [DOI] [PubMed] [Google Scholar]
- 17.Andersson M, Ostholm-Balkhed A, Fredrikson M, Holmbom M, Hallgren A, Berg S, Hanberger H. 2019. Delay of appropriate antibiotic treatment is associated with high mortality in patients with community-onset sepsis in a Swedish setting. Eur J Clin Microbiol Infect Dis 38:1223–1234. doi: 10.1007/s10096-019-03529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, Cardozo C, Albasanz-Puig A, Marco F, Laporte-Amargos J, Moreno-Garcia E, Domingo-Domenech E, Chumbita M, Martinez JA, Soriano A, Carratala J, Garcia-Vidal C. 2019. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis 70:1068–1074. doi: 10.1093/cid/ciz319. [DOI] [PubMed] [Google Scholar]
- 19.Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. 2017. Daily cost of delay to adequate antibiotic treatment among patients surviving a hospitalization with community-onset Acinetobacter baumannii pneumonia or sepsis. Crit Care 21:130. doi: 10.1186/s13054-017-1719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt-Hieber M, Teschner D, Maschmeyer G, Schalk E. 2019. Management of febrile neutropenia in the perspective of antimicrobial de-escalation and discontinuation. Expert Rev Anti Infect Ther 17:983–995. doi: 10.1080/14787210.2019.1573670. [DOI] [PubMed] [Google Scholar]
- 21.Szymczak JE, Newland JG. 2018. In Barlam TF, Neuhauser MM, Tamma PD, Trivedi KK (ed), Practical implementation of an antibiotic stewardship program. Cambridge University Press, Cambridge, UK. [Google Scholar]
- 22.Périllaud C, Pilmis B, Diep J, Péan de Ponfilly G, Vidal B, Couzigou C, Mizrahi A, Lourtet-Hascoët J, Le Monnier A, Nguyen Van J-C. 2019. Prospective evaluation of rapid antimicrobial susceptibility testing by disk diffusion on Mueller-Hinton rapid-SIR directly on blood cultures. Diagn Microbiol Infect Dis 93:14–21. doi: 10.1016/j.diagmicrobio.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Stokkou S, Geginat G, Schluter D, Tammer I. 2015. Direct disk diffusion test using European Clinical Antimicrobial Susceptibility Testing breakpoints provides reliable results compared with the standard method. Eur J Microbiol Immunol (Bp) 5:103–111. doi: 10.1556/EUJMI-D-15-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehren K, Meissner A, Jazmati N, Wille J, Jung N, Vehreschild JJ, Hellmich M, Seifert H. 2019. Clinical impact of rapid species identification from positive blood cultures with same-day phenotypic antimicrobial susceptibility testing on the management and outcome of bloodstream infections. Clin Infect Dis 70:1285–1293. doi: 10.1093/cid/ciz406. [DOI] [PubMed] [Google Scholar]
- 25.Pilmis B, Thy M, Diep J, Krob S, Perillaud C, Couzigou C, Vidal B, Mizrahi A, Lourtet-Hascoet J, Le Monnier A, Nguyen Van JC. 2019. Clinical impact of rapid susceptibility testing on MHR-SIR directly from blood cultures. J Antimicrob Chemother 74:3063–3068. doi: 10.1093/jac/dkz271. [DOI] [PubMed] [Google Scholar]
- 26.Elliott G, Malczynski M, Barr VO, Aljefri D, Martin D, Sutton S, Zembower TR, Postelnick M, Qi C. 2019. Evaluation of the impact of the Accelerate Pheno system on time to result for differing antimicrobial stewardship intervention models in patients with gram-negative bloodstream infections. BMC Infect Dis 19:942. doi: 10.1186/s12879-019-4591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider JG, Wood JB, Schmitt BH, Emery CL, Davis TE, Smith NW, Blevins S, Hiles J, Desai A, Wrin J, Bocian B, Manaloor JJ. 2019. Susceptibility Provision Enhances Effective De-escalation (SPEED): utilizing rapid phenotypic susceptibility testing in Gram-negative bloodstream infections and its potential clinical impact. J Antimicrob Chemother 74:i16–i23. doi: 10.1093/jac/dky531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sofjan AK, Casey BO, Xu BA, Amadio J, Restrepo A, Alam MJ, Garey KW. 2018. Accelerate PhenoTest™ BC kit versus conventional methods for identification and antimicrobial susceptibility testing of Gram-positive bloodstream isolates: potential implications for antimicrobial stewardship. Ann Pharmacother 52:754–762. doi: 10.1177/1060028018765486. [DOI] [PubMed] [Google Scholar]
- 29.Charnot-Katsikas A, Tesic V, Love N, Hill B, Bethel C, Boonlayangoor S, Beavis KG. 2017. Use of the Accelerate Pheno system for identification and antimicrobial susceptibility testing of pathogens in positive blood cultures and impact on time to results and workflow. J Clin Microbiol 56:e01166-17. doi: 10.1128/JCM.01166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henig O, Kaye KS, Chandramohan S, Cooper CC, Lephart P, Salimnia H, Taylor M, Pogue JM. 2018. The hypothetical impact of Accelerate Pheno on time to effective therapy and time to definitive therapy for bloodstream infections due to drug-resistant Gram-negative bacilli. Antimicrob Agents Chemother 63:e01477-18. doi: 10.1128/AAC.01477-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramphal R. 2004. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis 39(Suppl 1):S25–S31. doi: 10.1086/383048. [DOI] [PubMed] [Google Scholar]
- 32.Gustinetti G, Mikulska M. 2016. Bloodstream infections in neutropenic cancer patients: a practical update. Virulence 7:280–297. doi: 10.1080/21505594.2016.1156821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 34.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). 2018. Effect of Piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, Neuberger A, Ghanem-Zoubi N, Santoro A, Eliakim-Raz N, Pertzov B, Steinmetz T, Stern A, Dickstein Y, Maroun E, Zayyad H, Bishara J, Alon D, Edel Y, Goldberg E, Venturelli C, Mussini C, Leibovici L, Paul M, Bacteremia Duration Study G. 2019. Seven versus 14 days of antibiotic therapy for uncomplicated Gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 69:1091–1098. doi: 10.1093/cid/ciy1054. [DOI] [PubMed] [Google Scholar]
- 36.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, Musser JM. 2014. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69:216–225. doi: 10.1016/j.jinf.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Trenholme GM, Kaplan RL, Karakusis PH, Stine T, Fuhrer J, Landau W, Levin S. 1989. Clinical impact of rapid identification and susceptibility testing of bacterial blood culture isolates. J Clin Microbiol 27:1342–1345. doi: 10.1128/JCM.27.6.1342-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee RKL, Virk A, Rajapakse NS, Schuetz A, Dylla B, Earley M, Lok J, Kohner P, Ihde S, Cole N, Hines L, Reed K, Garner O, Chandrasekaran S, de St Maurice AM, Kanatani M, Curello J, Arias R, Swearingen W, Doernberg SB, Patel RMD, Patel R. 2019. Randomized clinical trial evaluating clinical impact of RAPid IDentification and antimicrobial susceptibility testing for Gram-Nnegative bacteremia (RAPIDS-GN). Open Forum Infect Dis 6. doi: 10.1093/cid/ciaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel R, Fang FC. 2018. Diagnostic stewardship: opportunity for a laboratory-infectious diseases partnership. Clin Infect Dis 67:799–801. doi: 10.1093/cid/ciy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.