The impact of diagnostic stewardship and testing algorithms on the utilization and performance of the FilmArray meningitis/encephalitis (ME) panel has received limited investigation. We performed a retrospective single-center cohort study assessing all individuals with suspected ME between February 2017 and April 2019 for whom the ME panel was ordered. Testing was restricted to patients with cerebrospinal fluid (CSF) pleocytosis. Positive ME panel results were confirmed before reporting through correlation with direct staining (Gram and calcofluor white) and CSF cryptococcal antigen or by repeat ME panel testing.
KEYWORDS: meningitis, encephalitis, multiplex PCR, diagnostic stewardship, FilmArray, ME panel
ABSTRACT
The impact of diagnostic stewardship and testing algorithms on the utilization and performance of the FilmArray meningitis/encephalitis (ME) panel has received limited investigation. We performed a retrospective single-center cohort study assessing all individuals with suspected ME between February 2017 and April 2019 for whom the ME panel was ordered. Testing was restricted to patients with cerebrospinal fluid (CSF) pleocytosis. Positive ME panel results were confirmed before reporting through correlation with direct staining (Gram and calcofluor white) and CSF cryptococcal antigen or by repeat ME panel testing. Outcomes included the ME panel test utilization rate, negative predictive value of nonpleocytic CSF samples, test yield and false-positivity rate, and time to appropriate deescalation of acyclovir. Restricting testing to pleocytic CSF samples reduced ME panel utilization by 42.7% (263 versus 459 tests performed) and increased the test yield by 61.8% (18.6% versus 11.5% positivity rate; P < 0.01) with the application of criteria. The negative predictive values of a normal CSF white blood cell (WBC) count for ME panel targets were 100% (195/195) for nonviral targets and 98.0% (192/196) overall. All pathogens detected in nonpleocytic CSF samples were herpesviruses. The application of a selective testing algorithm based on repeat testing of nonviral targets avoided 75% (3/4) of false-positive results without generating false-negative results. The introduction of the ME panel reduced the duration of acyclovir treatment from an average of 66 h (standard deviation [SD], 43 h) to 46 h (SD, 36 h) (P = 0.03). The implementation of the ME panel with restriction criteria and a selective testing algorithm for nonviral targets optimizes its utilization, yield, and accuracy.
INTRODUCTION
Infectious meningitis and encephalitis are potentially life-threatening conditions with significant morbidity and mortality (1, 2). They require rapid and accurate diagnosis to ensure effective therapy and to deescalate unnecessary antimicrobials. The FilmArray meningitis/encephalitis (ME) panel (BioFire Diagnostics, Salt Lake City, UT) is a sample-to-answer, on-demand, multiplex, real-time PCR assay for syndromic diagnosis of infectious meningitis and encephalitis from a small volume (200 μl) of cerebrospinal fluid (CSF) with less than 2 min of hands-on time and a 1-h assay time. The ME panel was approved by the U.S. Food and Drug Administration in 2015 for the detection of 14 meningitis/encephalitis pathogens (6 bacterial, 7 viral, and 1 fungal) that are commonly acquired by immunocompetent individuals in the community, by neonates during the perinatal period, and by immunocompromised hosts.
Despite its availability for near-patient syndromic testing, which simplifies ordering practices, questions remain about its accuracy. A pre-FDA prospective multicenter study showed that the ME panel has >95% sensitivity for most targets and >99% specificity for all targets; however, the positive predictive value (PPV) was low for a number of targets, including Escherichia coli K1, Streptococcus agalactiae, Streptococcus pneumoniae, cytomegalovirus (CMV), herpes simplex virus 1 (HSV-1), and Cryptococcus neoformans/C. gattii (3). A post-FDA retrospective study showed a positive percent agreement of 83 to 100% for the targets evaluated (4). A meta-analysis of ME panel accuracy showed overall (i.e., all targets) mean sensitivity of 90% (95% confidence interval [CI], 86 to 93%) and mean specificity of 97% (95% CI, 94 to 99%), with the highest proportion of false-positive results observed for bacterial targets (5).
Although many institutions have already implemented and some studies recommend the clinical adoption of the ME panel for near-patient testing, anticipating that it will improve clinical and antimicrobial stewardship outcomes (3, 6), the overutilization (7) and false-positive results (5) reported in published studies call for a more cautious, evidence-based approach to implementing the ME panel (6). Ideally, preanalytical and analytical interventions such as diagnostic stewardship and a testing algorithm, respectively, can be leveraged to maximize the utilization and accuracy of the assay (8). Such interventions would enhance health care efficiency and reduce the harm caused by false-positive results.
The ME panel was implemented at our institution for routine clinical use in February 2017. Prior to offering the assay for clinical testing, we developed testing criteria and a stringent testing algorithm to improve test utilization and mitigate false-positive results. Here, we describe the impact of our testing criteria and algorithm on the utilization, yield, and performance of the ME panel.
MATERIALS AND METHODS
Ethics.
Per the Stanford Institutional Review Board (IRB), this project constituted a quality improvement project and was exempt from IRB approval.
Study design.
We conducted a retrospective cohort study of pediatric and adult patients with suspected meningitis or encephalitis who had a test order for the ME panel from February 2017 to April 2019. This study evaluated the impact of testing criteria (pleocytosis) and the testing-and-reporting algorithm (see below) on the utilization, yield (positivity rate), and accuracy of the ME panel. Specific study outcomes included the ME panel utilization rate, negative predictive value (NPV) of normal CSF white blood cell (WBC) counts for ME panel targets, test yield, false-positive rate, and time (in hours) to the deescalation of acyclovir treatment.
Testing criteria and algorithm.
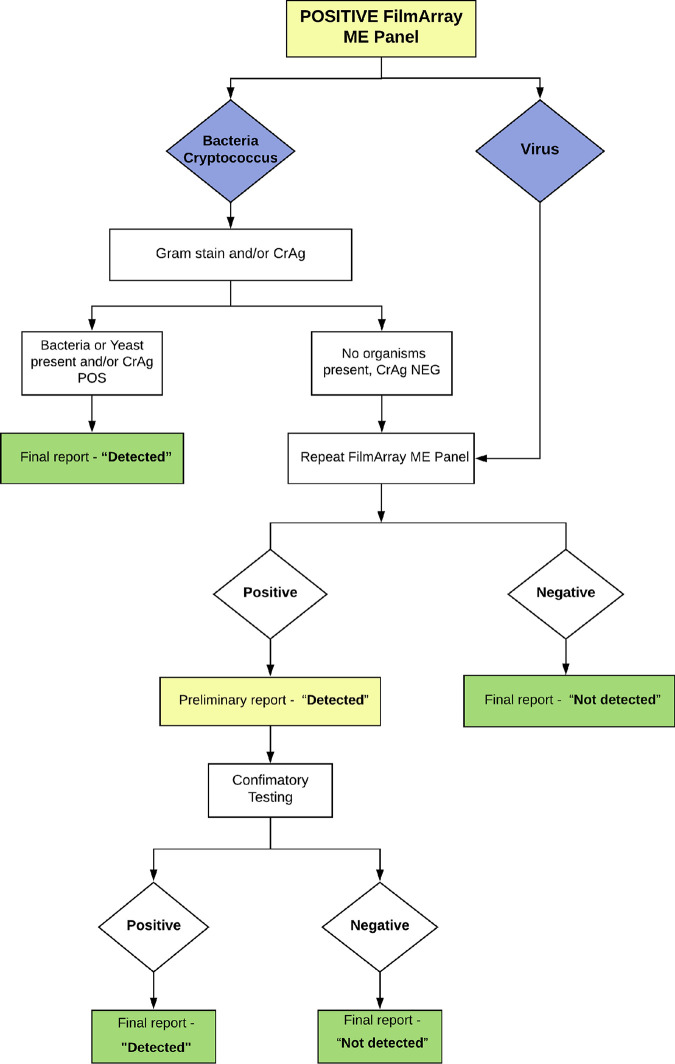
Specimen acceptance criteria and the testing algorithm for the ME panel were developed prior to assay implementation. An acceptable CSF specimen was defined as one collected via lumbar puncture with (i) a minimum volume of 0.7 ml, sufficient to repeat the ME panel for confirmatory testing if indicated, and (ii) an age-specific elevated WBC count (>30 WBCs/mm3 for 0 to 14 days of age, >20 WBCs/mm3 for 14 days to 1 year, >20 WBCs/mm3 for 1 to 12 years, and >5 WBCs/mm3 for >12 years) using manual or automated cell counts. The latter criterion was not applied for patients with low peripheral blood WBC counts or CSF specimens sent from outside hospitals for which the CSF WBC count was unavailable. A testing algorithm was developed for all positive ME panel results to correlate and confirm positive results with conventional assay results (Fig. 1). Positive results for bacterial targets and Cryptococcus targets were reported as positive in the electronic medical record (EMR) and called to the ordering provider if organisms with consistent morphology and staining were seen upon Gram and/or calcofluor white staining and/or the CSF cryptococcal antigen (CrAg) immunoassay was positive. The ME panel was repeated on a different BioFire module if direct stain and antigen results were negative for nonviral targets as well as for all positive viral targets. Targets that reverted from positive to negative were reported as negative in the EMR. Targets that remained positive were called to the ordering provider and reported in the EMR as preliminarily positive with a comment stating that confirmatory testing with a conventional assay for the respective targets will follow. Conventional tests were ordered by the laboratory if not already ordered by the provider. See the supplemental material for a description of conventional methods. Preliminarily positive results for targets with negative confirmatory conventional test results were amended to negative in the EMR and called to the ordering provider. Preliminary positive results confirmed by conventional testing were reported as such in the EMR.
FIG 1.
FilmArray ME panel testing-and-reporting algorithm for positive targets in this study.
Clinical adjudication.
Two investigators with neurology expertise (S. Dujari and C. A. Gold) performed an independent chart review of discordant results for patients with initially positive ME panel test results and negative conventional test results and for patients with positive conventional results for whom the ME panel order was canceled due to a lack of CSF pleocytosis to determine the likelihood of true infection and treatment delays.
Acyclovir deescalation.
Acyclovir treatment durations were compared in patients with suspected HSV encephalitis who tested negative for HSV before (May 2016 to December 2016) and after (February 2017 to March 2019) the implementation of the ME panel. Treatment duration was defined as the time elapsed (in hours) between the initiation of the first dose and the initiation of the last dose of acyclovir.
Statistical analyses.
The chi-square test was used to compare differences in positivity rates. The t test was used to compare acyclovir treatment durations. All statistical tests were computed for a two-sided type I error rate of 5%.
RESULTS
Study population and test results.
During the study period, a total of 459 ME panel tests were ordered. Of these, 263 were for unique patients. The majority of patients were adults (76.8%) and admitted to the hospital (69.5%) or under evaluation in the emergency department (27.6%) at the time of testing (Table 1). CSF pleocytosis was present in 84% of 237 patients with CSF cell counts available (mean WBC count of 586 cells/mm3, standard deviation [SD] of 1,969 cells/mm3, and range of 0 to 18,300 cells/mm3) and in 97.8% of patients with a positive ME panel result (mean WBC count of 1,079 cells/mm3, SD of 2,548 cells/mm3, and range of 2 to 13,262 cells/mm3). In total, the ME panel was positive for 51 targets (37 viral, 11 bacterial, and 3 fungal) for 49 patients (18.6% positivity rate). For 47 patients, a single target was positive; for 2 patients, two targets were positive. Positive ME panel targets and corresponding conventional test results are shown in Table 2.
TABLE 1.
Characteristics of patients tested by the FilmArray ME panel
| Parameter | Value for group |
|
|---|---|---|
| All tested (n = 263) | Positive ME panel (n = 49) | |
| No. of patients of gender (%) | ||
| Female | 120 (45.6) | 24 (49) |
| Male | 143 (54.4) | 25 (51) |
| No. of patients of age (yr) (%) | ||
| <1 | 33 (12.5) | 7 (14.3) |
| 2–17 | 28 (10.6) | 2 (4.1) |
| 18–64 | 142 (54.0) | 30 (61.2) |
| >64 | 60 (22.8) | 10 (20.4) |
| No. of patients at hospital location (%)a | ||
| Inpatient | 141 (69.5) | 21 (52.5) |
| Emergency department | 56 (27.6) | 19 (47.5) |
| Outpatient | 6 (3.0) | 0 |
| CSF WBC count (cells/mm3)a | ||
| Mean (SD) | 586 (1,969) | 1,079 (2,548) |
| Range | 0–18,300 | 2–13,262 |
| No. of patients with pleocytosis present (%) | 199 (84.0) | 45 (97.8) |
Location and WBC counts were not available for 60 and 26 patients, respectively.
TABLE 2.
Positive FilmArray ME panel results and corresponding conventional test resultsa
| ME panel target | No. of specimens positive by the ME panel | Conventional test on CSF | No. of specimens positive by conventional test on CSF/total no. tested | Other testing | No. of specimens positive by another test/total no. tested |
|---|---|---|---|---|---|
| EV | 8 | Enterovirus RT-PCR | 8/8 | ND | |
| HPeV | 0 | NA | NA | ||
| HSV-1 | 4 | HSV-1 PCR | 4/4 | NA | |
| HSV-2 | 6 | HSV-2 PCR | 6/6 | NA | |
| VZV | 9 | VZV PCR | 9/9 | NA | |
| CMV | 1 | ND | CSF bacterial culture | 0/1 | |
| 16S rRNA PCR sequencing | 0/1 | ||||
| HHV-6 | 9 | HHV-6 PCR | 8/8b | ND | |
| E. coli K1 | 1 | Culture | 1/1 | ND | |
| H. influenzae | 2 | Culture | 1/2 | Blood culturec | 0/1 |
| 16S rRNA PCR sequencingc | 0/1 | ||||
| Neisseria meningitidis | 0 | NA | NA | ||
| S. pneumoniae | 3 | Culture | 0/3 | Blood culture | 3/3 |
| S. agalactiae | 5 | Culture | 2/5 | CSF S. agalactiae PCRd | 1/1 |
| Blood cultured | 0/1 | ||||
| Listeria monocytogenes | 0 | NA | NA | ||
| C. neoformans/C. gattii | 3 | Culture | 2/3 | CSF CrAge | 0/1 |
EV, enterovirus; HPeV, human parechovirus; RT-PCR, reverse transcription-PCR; ND, not done; NA, not applicable.
One was not tested with a conventional test.
The CSF culture-negative one was tested. The ME panel result was reproducible for H. influenzae.
Among the 3 CSF culture-negative samples, 1 was CSF PCR positive, 1 was blood culture negative, and 1 was not tested.
Culture-negative CSF was tested.
Utilization and yield of the ME panel.
In total, 196/459 (42.7%) ME panel orders during the study period were rejected due to a lack of CSF pleocytosis. Immunocompromising conditions were present in 60 (30.6%) patients, including malignancy (30 patients), bone marrow transplantation (21), solid-organ transplantation (7), and other (1). Of the 263 approved tests, 38 patients (14.4%) had a normal CSF WBC count due to peripheral blood leukopenia and referrals from outside hospitals with no CSF WBC counts available. Of all testing performed on CSF without pleocytosis, only 1/38 (2.6%; adult patient) yielded a positive result for human herpesvirus 6 (HHV-6), which was confirmed by HHV-6 PCR and adjudicated to not represent a clinically actionable infection. To further evaluate the appropriateness of our rejection criteria, the results of provider-ordered conventional tests for ME panel targets were retrospectively evaluated for all 196 rejected orders (Table 3). Four (2.0%) of the rejected specimens (all adult patients) tested positive by a conventional method, consisting of 1 varicella-zoster virus (VZV) PCR (cycle threshold [CT], 29.07), 1 CMV PCR (CT, 36.35), and 2 HHV-6 PCRs (CT values, 32.52 and 33.65). All nonviral targets were negative by microbiological culture and a CrAg test, yielding a nonviral NPV of 100% (95% CIs, 98.1% to 100% for culture and 92.3% to 100% for the CrAg test) and an overall NPV of 98.0% (95% CI, 94.9% to 99.4%) for all targets for CSF specimens with normal WBC counts. The four cases with positive herpesvirus results by conventional testing occurred in immunocompromised hosts and were all adjudicated to represent clinically actionable infections, with 3 of 4 rejections resulting in delayed initiation of antiviral therapy assuming that the ME panel would have detected these targets given their CT values if testing had been performed (Table 4).
TABLE 3.
Conventional test results for FilmArray ME panel targets in CSF specimens rejected for FilmArray ME panel testing due to normal CSF WBC counts
| Conventional test for ME panel targets | No. of specimens tested (%) | No. of specimens that tested positive (%) | NPV of normal CSF WBCs (95% CI) |
|---|---|---|---|
| Viral targets | |||
| Enterovirus PCR | 44 (22.4) | 0 | 100 (92.0–100) |
| HSV-1/2 PCR | 84 (42.9) | 0 | 100 (95.7–100) |
| VZV PCR | 43 (21.9) | 1b (2.3) | 97.7 (87.7–99.9) |
| CMV PCR | 33 (16.8) | 1b (3.0) | 97.0 (84.2–99.9) |
| HHV-6 PCR | 29 (14.8) | 2b (6.9) | 93.1 (77.2–99.1) |
| Nonviral targets | |||
| Culture | 195 (99.5) | 0 | 100 (98.1–100) |
| CrAg | 46 (23.5) | 0 | 100 (92.3–100) |
| All targets | 196a | 4 (2.0) | 98.0 (94.9–99.4) |
Not all specimens were tested by all conventional tests.
Cycle threshold values were 29.07 for VZV, 36.35 for CMV, and 32.52 and 33.65 for HHV-6.
TABLE 4.
Clinical investigation of cases rejected for FilmArray ME panel testing due to normal CSF WBC counts but positive with a conventional test
| Conventional test result | Clinical comorbidity(ies)a | Treatment decision | Impacts of ME panel rejection |
|---|---|---|---|
| HHV-6 detected | Lymphoma, status post-BMT | Foscarnet started | Diagnosis delayed 18 h,b treatment delayed 22 hb |
| HHV-6 detected | MDS, status post-BMT | Foscarnet started | Diagnosis delayed 133 h,b treatment delayed 136 hb |
| CMV detected | CNS vasculitis, on immunosuppressive therapy | Ganciclovir started | Diagnosis delayed 44 h,b treatment delayed 46 hb |
| VZV detected | Status post-liver transplant | Acyclovir continued | Diagnosis delayed 32 h,b no treatment delay |
BMT, bone marrow transplant; MDS, myelodysplastic syndrome; CNS, central nervous system.
Assumes that the Film Array ME panel would have detected the target detected by the conventional assay.
Taking into account the data presented above, the per-patient yield of the ME panel increased 61.8% from an estimated 11.5% (53/459) positivity rate with no testing restriction to 18.6% (49/263) with criteria in place (P < 0.01).
Effectiveness of testing algorithm.
To evaluate the effectiveness of a testing algorithm designed to mitigate false-positive results, we determined the rate of false-positive results with the ME panel with and without the testing algorithm (Fig. 1). The false-positive rate was calculated as the percentage of initially positive ME panel targets with a negative concurrent conventional test result and no evidence of infection on clinical adjudication. Concurrent conventional test results were available for 49 of the 51 positive ME panel targets for assessment of true positivity (Tables 2 and 5). Of these, 4 (8.2%) represented false-positive results, which included 2 for S. agalactiae, 1 for Haemophilus influenzae, and 1 for C. neoformans/C. gattii (Table 5). Forty-five positive targets with conventional test results (33 viral targets and 12 nonviral targets) had been successfully worked up per the testing algorithm (Table 6). All 12 nonviral targets had undergone repeat testing per the testing algorithm. The impact of our testing algorithm on the avoidance of false-positive results and the generation of false-negative results is shown in Table 6. When we applied the testing algorithm across all targets, including repeat testing of all 4 false-positive results, we avoided 3/4 (75%) false-positive results but generated 3/41 (7.3%) false-negative results. The former included an H. influenzae result that was reproducible when repeated on the ME panel but that had negative CSF and blood culture and negative 16S rRNA PCR in a patient whose CSF indices (WBC count, 432; neutrophils, 48%; lymphocytes, 43%; glucose, 41 mg/dl; protein, 73 mg/dl) were deemed by the emergency department provider most consistent with a viral process. The latter included 2 HHV-6 results and 1 C. neoformans/C. gattii results (Table 6). However, the false-negative C. neoformans/C. gattii result was avoidable and thus excluded because the patient had a concurrent positive CrAg test. Given that none of the false-positive results were attributable to viral targets, and the requirement for repeat testing generated false-negative results among viral targets, we devised a revised algorithm with repeat testing restricted to nonviral targets with negative direct stain and CrAg test results (see Fig. S1 in the supplemental material). In our study set, the revised algorithm avoided 75% of false-positive results without generating any false-negative results.
TABLE 5.
Positive FilmArray ME panel results and assessment for false positivity based on conventional test results and clinical adjudicationa
| ME panel target | No. of specimens that tested positive (%) | No. of false-positive specimens (%) |
|---|---|---|
| Viral targets (n = 35) | ||
| EV | 8 (16.3) | 0 |
| HPeV | 0 | NA |
| HSV-1 | 4 (8.2) | 0 |
| HSV-2 | 6 (12.2) | 0 |
| VZV | 9 (18.4) | 0 |
| CMV | 0 | NA |
| HHV-6 | 8 (16.3) | 0 |
| Nonviral targets (n = 14) | ||
| E. coli | 1 (2.0) | 0 |
| H. influenzae | 2 (4.1) | 1 (2.0) |
| N. meningitidis | 0 | NA |
| S. pneumoniae | 3 (6.1) | 0 |
| S. agalactiae | 5 (10.2) | 2 (4.1) |
| L. monocytogenes | 0 | NA |
| C. neoformans/C. gattii | 3 (6.1) | 1 (2.0) |
| All targetsb | 49 | 4c (8.2) |
The reference result was based on concurrent conventional test results and clinical adjudication. NA, not applicable.
Two targets were excluded because they did not have concurrent conventional test results.
All false-positive results had discrepant ME panel and conventional test results. None of the discrepant ME panel results were clinically adjudicated to represent true-positive results.
TABLE 6.
Performance of the FilmArray ME panel for positive targets with and without the testing algorithma
| Result category | No. of specimens with result/total no. of specimens tested (%) |
||
|---|---|---|---|
| Without testing algorithm | With testing algorithm (repeat testing for all positive targets) | With revised testing algorithm (selective repeat testing)b | |
| All targets (n = 45) | |||
| TP reported | 41/41 (100) | 38/41 (92.7) | 41/41 (100) |
| FP reported | 4/4 (100) | 1/4 (25) | 1/4 (25) |
| FP avoided | 0/4 (0) | 3/4 (75) | 3/4 (75) |
| PPV | 41/45 (91.1) | 38/39 (97.4) | 41/42 (97.6) |
| FN generated | NA | 3c /41 (7.3) | 0c /41 |
| Viral targets (n = 33) | |||
| TP reported | 33/33 (100) | 31/33 (93.9) | 33/33 (100) |
| FP reported | 0/0 (0) | 0/0 (0) | 0/0 (0) |
| FP avoided | NA | NA | NA |
| PPV | 33/33 (100) | 31/31 (100) | 33/33 (100) |
| FN generated | NA | 2/33 (6.1) | 0/33 |
| Nonviral targets (n = 12) | |||
| TP reported | 8/8 (100) | 7/8 (87.5) | 8/8 (100) |
| FP reported | 4/4 (100) | 1/4 (25) | 1/4 (25) |
| FP avoided | 0/4 (0) | 3/4 (75) | 3/4 (75) |
| PPV | 8/12 (66.7) | 7/8 (87.5) | 8/9 (88.9) |
| FN generated | NA | 1c /8 (12.5) | 0c /8 (0) |
The reference result was based on concurrent conventional test results and clinical adjudication. Only those targets with concurrent conventional test results and that were successfully worked up per the testing algorithm were included in this analysis. TP, true positive; FP, false positive; PPV, positive predictive value; NA, not applicable.
Repeat testing only for bacterial targets with a negative Gram stain result and the C. neoformans/C. gattii target with negative Gram stain, calcofluor white, and CrAg test results. There was no repeat testing for positive viral targets.
One false-negative C. neoformans/C. gattii result was avoidable because it had a positive CrAg test result. Therefore, it was reported as a true positive in the revised testing algorithm.
Impact on acyclovir deescalation.
A total of 38 patients prior to the implementation of the ME panel and 39 patients after implementation met the inclusion criteria for this analysis. There were no significant differences in gender or age between the groups (Table 7). There was a significant difference in the duration (hours) of treatment before (mean, 66 h; SD, 43 h) and after (mean, 46 h; SD, 36 h) the implementation of the ME panel (P = 0.03).
TABLE 7.
Duration of acyclovir treatment before and after implementation of the FilmArray ME panel
| Parametera | Value |
P value | |
|---|---|---|---|
| Pre-ME panel (n = 38) | Post-ME panel (n = 39) | ||
| No. of female patients (%) | 13 (34.2) | 18 (46.2) | 0.29 |
| Mean age (yr) (SD) | 61 (±22) | 52 (±19) | 0.06 |
| Median treatment time (h) (IQR) | 60 (32–89) | 32 (6–72) | 0.03 |
IQR, interquartile range.
DISCUSSION
Syndromic testing for meningitis and encephalitis with the ME panel offers an opportunity to provide rapid and actionable results to guide appropriate therapy, discontinue unnecessary antimicrobials, and avert additional unnecessary diagnostic workups. However, best practices to optimize the utilization and accuracy of the ME panel under routine clinical practice have not yet been defined. Furthermore, although many microbiology laboratories have considered implementing restriction criteria for ME panel testing, the lack of data in this area makes such implementations more challenging. Both meningitis and encephalitis are clinical syndromes associated with significant morbidity and mortality, particularly in immunocompromised patient populations (1, 2). Given the concern for false-positive ME panel results (5), we investigated the impact of preanalytical testing criteria (i.e., elevated CSF WBC count) and an analytical testing algorithm (i.e., repeating positive ME panel results with negative direct stain and CrAg results) on test utilization and accuracy in an academic health system. We show that the enforcement of testing criteria based on elevated CSF WBC counts appropriately reduced test utilization by 42.7% and increased the test yield by 61.8%, from an estimated 11.5% (53/459) positivity rate with no testing restriction to 18.6% (49/263) with criteria in place. The lower yield observed in the absence of preanalytical testing criteria is consistent with yields of 6.4% (45/705), 11.8% (121/1,025), 12.6% (89/708), and 13.1% (33/251) reported in studies where the ME panel was offered for patient care with no testing restriction (7, 9–11). The high NPV of a normal CSF WBC count across ME panel targets (98% overall and 100% for nonviral targets) in our study supports the inclusion of a normal CSF WBC count in the rejection criteria in our patient population, with the exception of immunocompromised patients unable to mount an inflammatory response. The enforcement of preanalytical testing criteria also improves health care efficiency and lowers costs without negatively impacting patient care. However, exceptions may be warranted for the detection of viral infections in suspected patients with normal CSF WBC counts (12, 13). We reported 4 patients with normal CSF WBC counts but positive conventional herpesvirus PCR results (1 VZV, 1 CMV, and 2 HHV-6), all of whom were immunocompromised, adjudicated to have had clinically actionable herpesvirus infections, and initiated or continued on antiviral treatment. A meta-analysis of ME panel accuracy showed a 1.5% false-negative rate after adjudication, with the highest proportion occurring for viral targets (HSV-1/2 and enterovirus) (5). Thus, exceptions for ME panel testing may be warranted in immunocompromised patients without CSF pleocytosis, even with normal peripheral blood WBC counts. The decision to discontinue antiviral treatment in high-risk patients should be based on virus-specific PCR assays with lower detection limits (4, 14), and repeat testing should be considered to accurately rule out viral infection (5).
Our finding on the high NPV (100%) of nonpleocytic CSF for nonviral targets is consistent with recent studies evaluating the correlation of pleocytosis to ME panel results. In a study where the majority of patients were adults, Boudet and colleagues observed pleocytosis in 85.5% (59/69) of patients with viral and 100% (16/16) of patients with bacterial infections (10). In a large pediatric study by Precit and colleagues, pleocytosis (≥5 WBCs/mm3) was observed in 54.4% (37/68) of patients with viral, 87% (20/23) of patients with bacterial, and 100% (2/2) of patients with cryptococcal infections diagnosed with the ME panel (11). In the latter study, the use of the ME panel result as the reference standard may have impacted the accuracy of nonviral targets given that false-positive ME panel results have been documented in the literature, with the highest proportion occurring for bacterial targets (5, 11). We also found 4 false-positive bacterial targets that were not confirmed with conventional phenotypic and molecular methods. Of the three positive bacterial targets in nonpleocytic samples reported by Precit et al. (E. coli, H. influenzae, and S. agalactiae), all occurred in neonates, and only S. agalactiae was confirmed upon Gram staining and microbiological culture of CSF and blood (11; Jennifer Dien Bard, personal communication). Altogether, our findings are consistent with those of previous ME panel studies and suggest that positive ME panel results for bacterial and fungal targets are rare in nonpleocytic samples, with the exception of neonates. However, as highlighted by Precit and colleagues and as discussed above, CSF pleocytosis may not be present in patients with viral ME infection, particularly in pediatric patients (11). Therefore, antiviral therapy, if relevant, should be administered, and more sensitive virus-specific PCR testing should be performed to rule out infection in high-risk patients, independent of CSF pleocytosis. This approach may prolong therapy at health systems without in-house testing and or long turnaround times.
The testing algorithm evaluated in this study required repeat testing of positive viral targets as well as positive bacterial and fungal targets not corroborated by direct staining or a CrAg test to confirm ME panel reproducibility. This algorithm reduced false-positive bacterial results by 75% (3/4) but generated three false-negative viral results. An optimized testing algorithm (see Fig. S1 in the supplemental material) in which repeat testing is restricted to nonviral targets with negative microbial stains and CrAg tests would have avoided 75% of false-positive results without generating any false-negative results. Given that viral targets represented >70% of the positive results in our population and account for the majority of positive targets in other diverse locales (7, 10, 15, 16), this selective algorithm also eliminates the majority of repeat tests required. While 100% of positive viral targets in our study represented true positives, false-positive ME panel viral targets are known to occur, and correlation of positive viral targets with clinical and imaging findings remains imperative (5). The inclusion of CSF pleocytosis in test criteria and pathogen staining and a CrAg test in the testing algorithm may delay the reporting of ME panel results, particularly in settings where near-patient testing is employed. However, we feel that it is essential to ensure the quality of positive results and improve the utilization of the ME panel.
The assessment of acyclovir deescalation in patients with negative HSV results before and after the implementation of the ME panel showed a statistically significant decrease in the duration of treatment with acyclovir, from a median duration of 60 h to 32 h. This finding is consistent with those of other studies showing a reduction in the time to acyclovir discontinuation with the ME panel and other rapid methods (17, 18). In addition to reducing acyclovir usage, this reduction may also help avert acyclovir-induced nephrotoxicity, which may have an even greater impact on health care spending (17). In a retrospective review of suspected cases of acyclovir-induced nephrotoxicity, the median number of days to creatinine elevation was 3 (interquartile range [IQR], 2 to 5), and the median number of days to peak creatinine levels was 3.5 (IQR, 2 to 7) (19). Thus, by reducing the duration of acyclovir treatment, the rapid results provided by the ME panel may also help reduce the risk of nephrotoxicity.
Although the findings are promising, our study has certain limitations. First, our study was conducted at a single center, and hospitalized adults represented the majority of the study population; thus, the findings need to be reproduced at other institutions to confirm the generalizability of our findings. Second, nonpleocytic meningoencephalitis may be more common in infants and young children, particularly when associated with viral etiologies such as enteroviruses (11, 20–22). This population may have been underrepresented in our study due to the availability of a rapid, target-specific enterovirus PCR assay at our institution. Third, although the enforcement of preanalytical testing criteria lowered ME panel utilization, financial savings associated with test rejection could be countered by virus-specific PCR testing. We did not perform a cost-effectiveness analysis to accurately measure the financial impact of enforcing pleocytosis. Well-designed studies controlling for underlying conditions and familiarity of providers with the ME panel are needed to accurately assess the cost-effectiveness of enforcing pleocytosis. Fourth, we did not evaluate the clinical sensitivity of the ME panel. However, several studies have investigated the sensitivity of the ME panel with a meta-analysis showing a high overall sensitivity and the understanding that false-negative results occur (5, 23) when the pathogen load is below the limit of detection. Fifth, we did not investigate the impact of test approval by infectious diseases clinicians on improving the utilization and yield of the ME panel. Further studies are needed to evaluate such interventions. Finally, we did not investigate the impact of the ME panel on clinical outcomes such as length of stay. However, other groups have shown a shorter length of stay with the ME panel and attributed it to the faster availability of results (24).
In conclusion, infectious meningitis and encephalitis are serious conditions for which syndromic testing with the ME panel can provide rapid diagnosis and guide therapy. This study showed that clinical implementation of the ME panel with testing criteria and a selective testing algorithm for nonviral targets safely optimizes its utilization and yield while maximizing accuracy.
Supplementary Material
ACKNOWLEDGMENTS
We thank the clinical laboratory scientists at Stanford Health Care for performing all the tests.
No funding source was used for this study.
N.B. has served as ad hoc consultant to BioFire.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hasbun R, Rosenthal N, Balada-Llasat JM, Chung J, Duff S, Bozzette S, Zimmer L, Ginocchio CC. 2017. Epidemiology of meningitis and encephalitis in the United States, 2011-2014. Clin Infect Dis 65:359–363. doi: 10.1093/cid/cix319. [DOI] [PubMed] [Google Scholar]
- 2.Hasbun R, Wootton SH, Rosenthal N, Balada-Llasat JM, Chung J, Duff S, Bozzette S, Zimmer L, Ginocchio CC. 2019. Epidemiology of meningitis and encephalitis in infants and children in the United States, 2011-2014. Pediatr Infect Dis J 38:37–41. doi: 10.1097/INF.0000000000002081. [DOI] [PubMed] [Google Scholar]
- 3.Leber AL, Everhart K, Balada-Llasat J-M, Cullison J, Daly J, Holt S, Lephart P, Salimnia H, Schreckenberger PC, DesJarlais S, Reed SL, Chapin KC, LeBlanc L, Johnson JK, Soliven NL, Carroll KC, Miller J-A, Dien Bard J, Mestas J, Bankowski M, Enomoto T, Hemmert AC, Bourzac KM. 2016. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol 54:2251–2261. doi: 10.1128/JCM.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liesman RM, Strasburg AP, Heitman AK, Theel ES, Patel R, Binnicker MJ. 2018. Evaluation of a commercial multiplex molecular panel for diagnosis of infectious meningitis and encephalitis. J Clin Microbiol 56:e01927-17. doi: 10.1128/JCM.01927-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tansarli GS, Chapin KC. 2020. Diagnostic test accuracy of the BioFire FilmArray meningitis/encephalitis panel: a systematic review and meta-analysis. Clin Microbiol Infect 26:281–290. doi: 10.1016/j.cmi.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Dien Bard J, Alby K. 2018. Point-counterpoint: meningitis/encephalitis syndromic testing in the clinical laboratory. J Clin Microbiol 56:e00018-18. doi: 10.1128/JCM.00018-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radmard S, Reid S, Ciryam P, Boubour A, Ho N, Zucker J, Sayre D, Greendyke WG, Miko BA, Pereira MR, Whittier S, Green DA, Thakur KT. 2019. Clinical utilization of the FilmArray meningitis/encephalitis (ME) multiplex polymerase chain reaction (PCR) assay. Front Neurol 10:281. doi: 10.3389/fneur.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel R, Fang FC. 2018. Diagnostic stewardship: opportunity for a laboratory-infectious diseases partnership. Clin Infect Dis 67:799–801. doi: 10.1093/cid/ciy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naccache SN, Lustestica M, Fahit M, Mestas J, Dien Bard J. 2018. One year in the life of a rapid syndromic panel for meningitis/encephalitis: a pediatric tertiary care facility’s experience. J Clin Microbiol 56:e01940-17. doi: 10.1128/JCM.01940-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudet A, Pantel A, Carles MJ, Boclé H, Charachon S, Enault C, Stéphan R, Cadot L, Lavigne JP, Marchandin H. 2019. A review of a 13-month period of FilmArray meningitis/encephalitis panel implementation as a first-line diagnosis tool at a university hospital. PLoS One 14:e0223887. doi: 10.1371/journal.pone.0223887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Precit MR, Yee R, Pandey U, Fahit M, Pool C, Naccache SN, Dien Bard J. 2020. Cerebrospinal fluid findings are poor predictors of appropriate FilmArray meningitis/encephalitis panel utilization in pediatric patients. J Clin Microbiol 58:e01592-19. doi: 10.1128/JCM.01592-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert E, Alberola J, Bosque M, Camarena JJ, Clari MÁ, Domínguez Márquez MV, Gil-Fortuño M, Gimeno A, Nogueira JM, Ocete MD, Orta N, Prat J, Rodríguez JC, Valero I, Gimeno Cardona C, Navarro D. 2019. Missing cases of herpes simplex virus (HSV) infection of the central nervous system when the Reller criteria are applied for HSV PCR testing: a multicenter study. J Clin Microbiol 57:e01719-18. doi: 10.1128/JCM.01719-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson KE, Slechta ES, Killpack JA, Heyrend C, Lunt T, Daly JA, Hemmert AC, Blaschke AJ. 2016. Preclinical assessment of a fully automated multiplex PCR panel for detection of central nervous system pathogens. J Clin Microbiol 54:785–787. doi: 10.1128/JCM.02850-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez CA, Pinsky BA, Liu A, Banaei N. 2017. Delayed diagnosis of tuberculous meningitis misdiagnosed as herpes simplex virus-1 encephalitis with the FilmArray syndromic polymerase chain reaction panel. Open Forum Infect Dis 4:ofw245. doi: 10.1093/ofid/ofw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bårnes GK, Gudina EK, Berhane M, Abdissa A, Tesfaw G, Abebe G, Feruglio SL, Caugant DA, Jørgensen HJ. 2018. New molecular tools for meningitis diagnostics in Ethiopia—a necessary step towards improving antimicrobial prescription. BMC Infect Dis 18:684. doi: 10.1186/s12879-018-3589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarai B, Das P. 2019. FilmArray meningitis/encephalitis (ME) panel, a rapid molecular platform for diagnosis of CNS infections in a tertiary care hospital in North India: one-and-half-year review. Neurol Sci 40:81–88. doi: 10.1007/s10072-018-3584-y. [DOI] [PubMed] [Google Scholar]
- 17.Evans M, Merkel KG, Harder J, Rose DT. 2020. Impact of the implementation of a rapid meningitis/encephalitis multiplex polymerase chain reaction panel on IV acyclovir duration: multicenter, retrospective cohort of adult and pediatric patients. Diagn Microbiol Infect Dis 96:114935. doi: 10.1016/j.diagmicrobio.2019.114935. [DOI] [PubMed] [Google Scholar]
- 18.Van TT, Mongkolrattanothai K, Arevalo M, Lustestica M, Dien Bard J. 2017. Impact of a rapid herpes simplex virus PCR assay on duration of acyclovir therapy. J Clin Microbiol 55:1557–1565. doi: 10.1128/JCM.02559-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richelsen RKB, Jensen SB, Nielsen H. 2018. Incidence and predictors of intravenous acyclovir-induced nephrotoxicity. Eur J Clin Microbiol Infect Dis 37:1965–1971. doi: 10.1007/s10096-018-3332-5. [DOI] [PubMed] [Google Scholar]
- 20.Lumley SF, Pritchard D, Dutta A, Matthews PC, Cann K. 2018. Multiplex PCR reveals high prevalence of enterovirus and HHV6 in acellular paediatric cerebrospinal fluid samples. J Infect 77:249–257. doi: 10.1016/j.jinf.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Tan NW, Lee EY, Khoo GM, Tee NW, Krishnamoorthy S, Choong CT. 2016. Cerebrospinal fluid white cell count: discriminatory or otherwise for enteroviral meningitis in infants and young children? J Neurovirol 22:213–217. doi: 10.1007/s13365-015-0387-2. [DOI] [PubMed] [Google Scholar]
- 22.Yun KW, Choi EH, Cheon DS, Lee J, Choi CW, Hwang H, Kim BI, Park KU, Park SS, Lee HJ. 2012. Enteroviral meningitis without pleocytosis in children. Arch Dis Child 97:874–878. doi: 10.1136/archdischild-2012-301884. [DOI] [PubMed] [Google Scholar]
- 23.Piccirilli G, Chiereghin A, Gabrielli L, Giannella M, Squarzoni D, Turello G, Felici S, Vocale C, Zuntini R, Gibertoni D, Maraolo AE, Ambretti S, Lazzarotto T. 2018. Infectious meningitis/encephalitis: evaluation of a rapid and fully automated multiplex PCR in the microbiological diagnostic workup. New Microbiol 41:118–125. [PubMed] [Google Scholar]
- 24.DiDiodato G, Bradbury N. 2019. Cerebrospinal fluid analysis with the BioFire FilmArray meningitis/encephalitis molecular panel reduces length of hospital stay in patients with suspected central nervous system infections. Open Forum Infect Dis 6:ofz119. doi: 10.1093/ofid/ofz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.