The sexually transmitted infections (STIs) chlamydia (CT) and gonorrhea (NG) are often asymptomatic in women and undetected by syndromic management, leading to complications such as pelvic inflammatory disease, infertility, and ectopic pregnancy. Molecular testing, such as the GeneXpert CT/NG assay, is highly sensitive, but cost restraints preclude implementation of these technologies in resource-limited settings. Pooled testing is one strategy to reduce the cost per sample, but the extent of savings depends on disease prevalence.
KEYWORDS: chlamydia, gonorrhea, sexually transmitted diseases, algorithms, diagnosis, global health
ABSTRACT
The sexually transmitted infections (STIs) chlamydia (CT) and gonorrhea (NG) are often asymptomatic in women and undetected by syndromic management, leading to complications such as pelvic inflammatory disease, infertility, and ectopic pregnancy. Molecular testing, such as the GeneXpert CT/NG assay, is highly sensitive, but cost restraints preclude implementation of these technologies in resource-limited settings. Pooled testing is one strategy to reduce the cost per sample, but the extent of savings depends on disease prevalence. The current study describes a pooling strategy based on identification of sociodemographic and laboratory factors associated with CT/NG prevalence in a high-risk cohort of Zambian female sex workers and single mothers conducted from 2016 to 2019. Factors associated with testing positive for CT/NG via logistic regression modeling included city, younger age, lower education, long-acting reversible contraception usage, Trichomonas vaginalis infection, bacterial vaginosis, and incident syphilis infection. Based on these factors, the study population was stratified into high-, intermediate-, and low-prevalence subgroups and tested accordingly—individually, pools of 3, or pools of 4, respectively. The cost per sample was reduced from $18 to as low as $9.43 in the low-prevalence subgroup. The checklist tool and pooling approach described can be used in a variety of treatment algorithms to lower the cost per sample and increase access to molecular STI screening. This is particularly valuable in resource-limited settings to detect and treat asymptomatic CT/NG infections missed by traditional syndromic management.
INTRODUCTION
Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are curable, bacterial sexually transmitted infections (STIs). Symptoms in women can include discharge, discomfort, and painful urination (1, 2), but frequently, these infections are asymptomatic (3, 4). Untreated CT/NG can lead to severe complications such as pelvic inflammatory disease, ectopic pregnancy, infertility, and increased risk of HIV infection (1, 2). In the population under study, a Zambian cohort of high-risk women (HRW) including female sex workers (FSW) and single mothers with a child under age 5 (SM) (5–7), the prevalence of CT and/or NG infection was 15%.
In sub-Saharan Africa, syndromic management is the World Health Organization (WHO)-recommended approach to detecting STIs, although the WHO acknowledges that the sensitivity and specificity of this method are low for CT/NG infections (8). Syndromic management relies on clinical presentation for diagnosis and treatment. This is challenging because symptoms for CT/NG are often indistinguishable from those of other vaginal dysbioses such as Trichomonas vaginalis, bacterial vaginosis (BV), candida, or urinary tract infections. Furthermore, the high prevalence of asymptomatic presentations causes the majority of cases to remain undetected under this approach. Together, these factors result in missed diagnoses and suboptimal or incorrect therapy for all vaginal infections, which can deplete healthy flora, worsen other vaginal conditions, such as candida (9), or contribute to antibiotic resistance.
Molecular screening for CT/NG is more sensitive and more accurate than syndromic management; however, it is also much more expensive. One such platform is the GeneXpert CT/NG assay (Cepheid, Sunnyvale, CA, USA), which is a highly sensitive, highly specific (CT sensitivity, 99.5%, NG sensitivity, 100%; CT specificity, 99.1%; NG specificity, 99.9%) automated system to qualitatively detect genomic DNA of CT or NG in a clinical specimen via real-time PCR (10). At the time of study, each GeneXpert cartridge cost approximately $18 and is intended to test a single specimen in 90 min. In settings similar to our study, syndromic management performed poorly compared to molecular diagnostic methods (3) but was still preferred due to widespread lack of financial resources, laboratory equipment, and trained laboratory technicians.
One approach to increase the usage of highly sensitive molecular diagnostics is to reduce the cost per sample tested through specimen pooling (11–14). If the pool tests negative, all samples included are considered CT/NG negative, leading to substantial cost savings. If the pool tests positive, it must be deconvoluted by retesting each specimen individually, requiring additional test kits and incurring a higher cost per sample. The probability that a pool tests positive is a function of the prevalence of the disease in the population (12, 13); therefore, pooling recommendations should be population specific. In this study, we sought to strategically guide sample pooling by stratifying our study population into subgroups with different CT/NG prevalence using a scoring checklist. By pooling within subgroups of high, intermediate, and low CT/NG prevalence, we maximize the cost-saving potential of sample pooling.
MATERIALS AND METHODS
Population.
Data are from a cross-sectional study conducted from September 2016 to January 2019 adding CT/NG testing in a cohort of HIV-uninfected women in Lusaka and Ndola, Zambia (5). Participants included FSW recruited from community sex work hot spots and SM referred to the Zambia-Emory HIV Research Project (ZEHRP) by local postnatal care providers.
At the study visit, each participant completed a sociodemographic and risk behavior questionnaire, received a pelvic examination, and was tested for HIV and STIs. ZEHRP provided free treatment for STIs and referred HIV-infected women to government clinics for antiretroviral therapy. There were 825 unique clinic visits where molecular CT/NG testing was performed on the GeneXpert. On 26 occasions, the same individual was observed at more than one visit; however, these data were not excluded because five of the women had different CT/NG test results between visits, indicating that risk factors and test results vary over time.
Variables considered.
The outcome of interest was whether a sample would generate a positive pool and require subsequent deconvolution. On the GeneXpert CT/NG platform, tests for both pathogens are contained in the same cartridge, and therefore, positive CT and/or NG results were combined.
A composite variable for reported symptoms was created to capture participants who reported one or more of the following: cystitis/dysuria, vaginal itching, vaginal discharge, dyspareunia, lower abdominal pain, and acute/chronic/recurrent genital ulcer. Another composite variable was created to capture clinical signs observed on pelvic exam, which included on either the external or internal genitalia inguinal adenopathy, inflammation, ulceration, condyloma or warts, cervicitis, cervical discharge or pus, vaginal discharge, erosion or friability of the cervix or vagina, nonmenstrual bleeding, or adnexal tenderness or mass.
Covariates based on laboratory diagnostics included wet mount microscopy for T. vaginalis, sperm, and candida, HIV rapid test, and treatment for an incident syphilis infection determined by rapid plasma regain (RPR) and previous syphilis serology (15, 16). BV was classified by a composite variable of positive potassium hydroxide (KOH) whiff test and presence of clue cells.
Sociodemographic variables included self-report of illiteracy, education, year of birth, city of residence, unprotected sex in the past 1 to 3 months, history of transactional sex, number of partners in the past 1 to 3 months, pregnancy, and verified current long-acting reversible contraception (LARC) usage. Illiteracy was classified as unable to easily read English or Nyanja or Bemba (the most common languages spoken in Lusaka and Ndola, respectively). Education was divided into two categories based on completing secondary school or college versus no school or only primary school. Year of birth from the participant questionnaire was used to calculate age in 2016 and create age groups (18 to 24 and 25+). LARC included the copper intrauterine device (IUD) and the hormonal implant.
Logistic regression modeling.
The probability that the GeneXpert outcome is positive follows a binomial distribution. Thus, logistic regression modeling was used to predict the probability that an individual was infected with CT and/or NG.
To select variables for model inclusion, the frequencies of each predictor among the infected and uninfected groups were compared, overall as well as stratified by city, by chi-square, or by Fisher’s exact test. Variables for which the chi-square or Fisher’s exact test P value was below 0.05 in at least one city or overall were candidates for inclusion in the full multivariate logistic regression (MLR) model. Correlation coefficients between each of the predictors were all less than 0.5, and all variance inflation factors were below 10; therefore, no variables were removed due to collinearity.
For simple translation to a screening checklist, interaction terms were excluded and relevant variables were included in the MLR as main effects. Before mathematical model selection, each predictor was pragmatically evaluated by the time required to collate information necessary to inform pooling decisions. Model selection was then performed on the variables remaining in the MLR model using the R stepAIC function in both directions.
A composite score, weighted 1 point each, was created based on the risk factors included in the final reduced MLR model and categorized into high-, middle-, and low-scoring groups. These score categories were tested for their ability to predict the odds of CT/NG infection in a bivariate logistic regression model.
Comparing screening performance.
The sensitivity and specificity of syndromic management, based on symptoms only or signs and symptoms together, were calculated. Sensitivity was determined by dividing the number of true positives by the total number of individuals with either CT and/or NG. Specificity was calculated by dividing the number of true negatives by the total number of individuals who were CT and NG uninfected.
The positive predictive value (PPV) and negative predictive value (NPV) of the score groups were calculated. NPV was calculated for the low-scoring group by dividing the number of CT/NG-uninfected low-scoring women by the total number of low-scoring women. PPV was calculated for the high-scoring group by dividing the number of CT/NG-infected high-scoring women by the total number of high-scoring women.
Validation of pooling.
Pooling on the GeneXpert was validated using a commercially available CT and NG panel with high, medium, and low bacterial concentrations of various CT/NG strains (NATrol CT/NG panel; ZeptoMetrix, catalog no. NATCT/NGP-C). To address the concern that pooling might hinder the sensitivity of detecting samples with low-bacterial burden, we tested 1:5 and 1:10 dilutions of the commercial low-bacterial burden controls. For the 1:5 dilution, 250 μl of each low panel member was added to either 1,000 μl of 1× phosphate-buffered saline (PBS), a CT/NG-negative panel member, or a known CT/NG-negative sample for a total volume of 1.25 ml. For the 1:10 dilution, 125 μl of each low panel member was added to either 1,125 μl of 1× PBS, a CT/NG-negative panel member, or a known CT/NG-negative sample for a total volume of 1.25 ml. The diluted samples were then run in duplicate according to the manufacturer’s instructions.
We also validated pooling of known-positive clinical specimens, both urine and vaginal swabs, that had previously tested positive unpooled on the GeneXpert system. Five pools were designed to produce both a CT- and NG-positive result at 1:5 and 1:10 dilutions. Three pools contained only vaginal swab samples, one pool contained only urine, and another pool contained vaginal swab samples and urine. For samples positive for both CT and NG, a 1:5 dilution was created by mixing 300 μl of sample with 1,200 μl of 1× PBS or known CT/NG-negative sample, for a total volume of 1.5 ml. A 1:10 dilution was created by mixing 300 μl of the 1:5 dilution with 1,200 μl of 1× PBS or known CT/NG-negative sample, for a total volume of 1.5 ml. For samples that were positive for either CT or NG, a 1:5 dilution was created by mixing 300 μl of a CT-positive sample, 300 μl of a NG-positive sample, and 900 μl of 1× PBS or known CT/NG-negative sample, for a total volume of 1.5 ml. A serial 1:10 dilution was created as above. All dilutions were then run according to the manufacturer’s instructions.
Cost analysis.
At the time of study, each GeneXpert cartridge cost approximately $18. Cost per sample was calculated, cost per sample = (18 + [$18× c × s])/c, based on the previously published equation s = (1 – [1 – p]c), where s is the proportion of positive pools, p is the prevalence of disease, and c is the number of samples in each pool (12, 13).
The 1-year cost savings was based on a hypothetical clinic that screens 2,000 women per year. For each score category, the category’s cost per sample was multiplied by the expected number of women in that category based on the proportion of women in our study in that category. The sum of the costs for all categories was then subtracted from the total cost of individual testing. Technician time, wages, benefits, supplies, and treatment costs were not considered in the cost analysis.
Ethics.
The data used in this study were derived from a cross-sectional study approved by the Institutional Review Board of Emory University and the Research Ethics Committee of the University of Zambia. Written informed consent from each participant was completed before any study materials were gathered.
RESULTS
Syndromic management fails to detect the majority of CT/NG cases in this population.
Of the 825 total data entries with GeneXpert test results, there were 124 instances of either CT and/or NG infection—68 cases of CT only, 34 cases of NG only, and 22 CT/NG coinfections. Information on reported symptoms was available for 559 of these women, and a pelvic examination was performed on 530 women. Neither clinical signs (chi-square P = 0.48) nor reported symptoms (Fisher’s exact P = 0.43) were statistically significantly associated with CT/NG. All but three of the infected women for whom symptoms were available (91/94, 97%) reported no symptoms and would have been missed under traditional syndromic management.
Setting the GeneXpert CT/NG result as the gold standard (17), we calculated the sensitivity and specificity of syndromic management in this population to be 0.02 and 0.98, respectively, assuming that any symptoms reported were syndromes of the true infection (Table 1). Pelvic examination allowed us to consider clinical signs in addition to reported symptoms. Syndromic management plus exam had an increased sensitivity of 0.68 but a sacrifice in specificity to 0.29.
TABLE 1.
Prevalence of signs and symptoms combined by CT/NG statusa
| Statusb | CT only (n = 68) |
NG only (n = 34) |
CT/NG coinfected (n = 22) |
CT/NG uninfected (n = 701) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Asymptomatic, no clinical signs observed | 20 | 29 | 6 | 18 | 2 | 9 | 125 | 18 |
| Asymptomatic, clinical signs observed | 30 | 44 | 16 | 47 | 12 | 55 | 304 | 43 |
| Symptoms reported, no clinical signs observed | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 |
| Symptoms reported, clinical signs observed | 1 | 1 | 1 | 3 | 0 | 0 | 5 | 1 |
| Missing symptoms or signs | 17 | 25 | 11 | 32 | 8 | 36 | 263 | 38 |
CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae.
Syndromic management by symptoms only: sensitivity, 0.02; specificity, 0.98. Syndromic management by symptoms and/or clinical signs: sensitivity, 0.68; specificity, 0.29.
Sociodemographic factors and other STIs are associated with CT/NG infection.
Several sociodemographic factors and behaviors demonstrated statistically significant crude associations with CT/NG in at least one city (Table 2). These included ages 18 to 24 years (Ndola chi-square P < 0.01), not completing secondary school (Lusaka chi-square P = 0.02), reporting unprotected sex in the past 1 to 3 months (Lusaka chi-square P = 0.02), and using LARC (Ndola chi-square P = 0.02).
TABLE 2.
Prevalence of demographic factors and risk behaviors by CT and/or NG status and citya
| Factor or behavior | Overall |
Lusaka | Ndola | ||||
|---|---|---|---|---|---|---|---|
| Either CT and/or NG (n = 124) |
CT/NG uninfected (n = 701) |
χ2 P value either CT and/or NG vs uninfected | χ2 P value either CT and/or NG vs uninfected | χ2 P value either CT and/or NG vs uninfected | |||
| No. | % | No. | % | ||||
| Age group | <0.01 | 0.09 | <0.01 | ||||
| Age 18–24 | 91 | 73 | 412 | 59 | |||
| Age 25+ | 30 | 24 | 284 | 41 | |||
| Data missing | 3 | 2 | 5 | 1 | |||
| Literacy | 0.11 | 0.77 | 0.06 | ||||
| Reads English, Bemba, or Nyanja | 74 | 60 | 361 | 51 | |||
| Illiterate | 49 | 40 | 328 | 47 | |||
| Data missing | 1 | 1 | 12 | 2 | |||
| Education | 0.13 | 0.02 | 0.39 | ||||
| No school or primary school only | 85 | 69 | 426 | 61 | |||
| Secondary school or higher | 38 | 31 | 261 | 37 | |||
| Data missing | 1 | 1 | 14 | 2 | |||
| Sex in exchange for money | 0.80 | 0.93 | 0.80 | ||||
| Never | 59 | 48 | 339 | 48 | |||
| Ever | 64 | 52 | 350 | 50 | |||
| Data missing | 1 | 1 | 12 | 2 | |||
| Unprotected sex (last 1–3 mo) | 0.06 | 0.02 | 0.29 | ||||
| None | 29 | 23 | 201 | 29 | |||
| At least once | 62 | 50 | 272 | 39 | |||
| Data missing | 33 | 27 | 228 | 33 | |||
| Pregnant | 0.33b | 0.56b | 0.42b | ||||
| Not pregnant | 90 | 73 | 453 | 65 | |||
| Pregnant | 4 | 3 | 12 | 2 | |||
| Data missing | 30 | 24 | 236 | 34 | |||
| Uses LARC method | 0.15 | 0.29 | 0.02 | ||||
| No LARC | 72 | 58 | 449 | 64 | |||
| Uses implant or IUD | 48 | 39 | 224 | 32 | |||
| Data missing | 4 | 3 | 28 | 4 | |||
CT, Chlamydia trachomatis; IUD, intrauterine device; LARC, long-acting reversible contraception; NG, Neisseria gonorrhoeae.
Fisher’s exact P value.
Crude associations between CT/NG and several laboratory tests were also identified (Table 3). The STIs T. vaginalis and syphilis were significantly associated with testing positive for CT/NG (chi-square P < 0.01 and Fisher's exact P = 0.02, respectively), as was the vaginal dysbiosis BV (chi-square P = 0.02).
TABLE 3.
Prevalence of lab results by CT and/or NG status and citya
| Test and result | Overall |
Lusaka | Ndola | ||||
|---|---|---|---|---|---|---|---|
| Either CT and/or NG (n = 124) |
CT/NG uninfected (n = 701) |
Fisher’s P value either CT and/or NG vs uninfected | Fisher’s P value either CT and/or NG vs uninfected | Fisher’s P value either CT and/or NG vs uninfected | |||
| N | (%) | N | (%) | ||||
| Trichomonas vaginalis (microscopy) | <0.01b | 0.02 | 0.02 | ||||
| Positive | 13 | 10 | 23 | 3 | |||
| Negative | 106 | 85 | 651 | 93 | |||
| Data missing | 5 | 4 | 27 | 4 | |||
| HIV rapid test | 0.77 | 0.69 | 0.41 | ||||
| Positive or discrepant | 4 | 3 | 20 | 3 | |||
| Negative | 119 | 96 | 675 | 96 | |||
| Data missing | 1 | 1 | 6 | 1 | |||
| Sperm (microscopy) | 0.37b | 1.00 | 0.44b | ||||
| Positive | 5 | 4 | 43 | 6 | |||
| Negative | 109 | 88 | 609 | 87 | |||
| Data missing | 10 | 8 | 49 | 7 | |||
| Candida (microscopy) | 0.41 | 1.00 | 0.56 | ||||
| Positive | 2 | 2 | 24 | 3 | |||
| Negative | 100 | 81 | 555 | 79 | |||
| Data missing | 22 | 18 | 122 | 17 | |||
| BV (both KOH and clue cells) | 0.02b | 1.00 | <0.01b | ||||
| Positive | 37 | 30 | 147 | 21 | |||
| Negative | 62 | 50 | 425 | 61 | |||
| Data missing | 25 | 20 | 129 | 18 | |||
| New syphilis infection (based on RPR) | 0.02 | 1.00 | 0.01 | ||||
| Yes | 8 | 6 | 13 | 2 | |||
| No | 88 | 71 | 454 | 65 | |||
| Data missing | 28 | 23 | 234 | 33 | |||
BV, bacterial vaginosis; CT, Chlamydia trachomatis; KOH, potassium hydroxide; NG, Neisseria gonorrhoeae; RPR, rapid plasma reagin.
χ2 P value.
Developing a checklist to guide sample pooling.
Factors crudely associated with CT/NG infection in at least one city were pragmatically evaluated for feasibility in a scoring checklist. Age, education, and LARC usage are quickly obtained from the participant record and do not require nurse consultation or rely on self-report. For these reasons and due to missing data, the unprotected sex variable was pragmatically eliminated. Laboratory tests for T. vaginalis, syphilis, and BV can be performed in under 10 min, are low-cost, and do not demand highly advanced technical skill. These six variables were included in the full MLR model of CT/NG infection (Table 4). City was also added to the model to account for geographic differences observed in some crude associations, as interaction terms are not easily translated to a checklist. Following bidirectional model selection, no variables were eliminated. The seven risk factors contained in the final MLR model were then translated into a checklist tailored to the ZEHRP clinic flow (See Fig. S1 in the supplemental material).
TABLE 4.
Logistic regression model of factors associated with either CT and/or NGa
| Predictor of CT and/or NG | No. | % | Multivariate final model (n = 496) |
|||
|---|---|---|---|---|---|---|
| Adj. odds ratio | Lower 95% CI | Upper 95% CI | Adj. P value | |||
| City | ||||||
| Ndola | 88 | 14 | Ref | |||
| Lusaka | 36 | 18 | 2.07 | 1.04 | 4.13 | 0.04 |
| Age group | ||||||
| Age 18–24 | 91 | 18 | 1.97 | 1.13 | 3.45 | 0.02 |
| Age 25+ | 30 | 10 | Ref | |||
| Education | ||||||
| No school or primary school only | 85 | 17 | 2.19 | 1.20 | 4.03 | 0.01 |
| Secondary school or higher | 38 | 13 | Ref | |||
| Unprotected sex (last 1–3 mo)b | ||||||
| None | 29 | 13 | ||||
| At least once | 62 | 19 | ||||
| Uses LARC method | ||||||
| No LARC | 72 | 14 | Ref | |||
| Uses implant or IUD | 48 | 18 | 1.65 | 0.99 | 2.75 | 0.06 |
| Trichomonas vaginalis (microscopy) | ||||||
| Positive | 13 | 36 | 3.96 | 1.53 | 10.28 | <0.01 |
| Negative | 106 | 14 | Ref | |||
| BV (Both KOH and clue cells) | ||||||
| Positive | 37 | 20 | 1.87 | 1.05 | 3.34 | 0.03 |
| Negative | 62 | 13 | Ref | |||
| New syphilis infection (based on RPR) | ||||||
| Yes | 8 | 38 | 4.58 | 1.63 | 12.93 | <0.01 |
| No | 88 | 16 | Ref | |||
Adj, adjusted; CI, confidence interval; CT, Chlamydia trachomatis; IUD, intrauterine device; KOH, potassium hydroxide; LARC, long-acting reversible contraception; NG, Neisseria gonorrhoeae; ref, reference group; RPR, rapid plasma reagin.
Pragmatically eliminated from the multivariate model.
In the study population, the distribution of scores was roughly normally distributed with a median of 2 and standard deviation of 1 (Fig. 1). While scores could range from 0 to 7, the actual scores of the participants only ranged from 0 to 5. The scores were divided into the categories low (0 to 1), middle (2 to 3), and high (4+). These score categories were statistically significantly associated with testing positive for CT/NG on the GeneXpert in a bivariate logistic regression model (Table 5).
FIG 1.
Checklist scores are normally distributed (n = 496). Checklist scores are roughly normally distributed (mean, 2.13; standard deviation [SD], 0.99) and categorized into low-scoring (0 to 1), mid-scoring (2 to 3), and high-scoring (4+) groups. Only women with nonmissing values for each score predictor were included.
TABLE 5.
Bivariate logistic regression model of composite score category and either CT and/or NG (n = 496)a
| Predictor of CT and/or NG | No. | % | Odds ratio | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|---|---|
| Score category | ||||||
| Low (0–1) | 10 | 8 | 0.44 | 0.21 | 0.87 | 0.02 |
| Mid (2–3) | 51 | 16 | Ref | |||
| High (4+) | 21 | 48 | 4.85 | 2.49 | 9.44 | <0.01 |
| NPV (low score) | 0.92 | |||||
| PPV (high score) | 0.48 |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; ref, reference group.
Furthermore, the checklist score categories stratified the population into groups with statistically significantly different CT/NG prevalence (χ2 = 38.50, P < 0.01) (Table 5). The prevalence of either CT and/or NG in the mid-scoring category was 16%, similar to the 15% prevalence in the overall population. CT/NG prevalence in the low-scoring and high-scoring categories were 8% and 48%, respectively.
Determining optimum pool size.
Based on the previously published equation (12, 13) and the prevalence of CT/NG in each of the score categories, the cost per sample was calculated for a range of potential pool sizes (Fig. 2). The optimum pool size was determined by the lowest cost per sample for each category; 4 for the low-scoring category and 3 for the mid-scoring category. All pool sizes for the high-scoring category resulted in a higher cost per sample than individual testing.
FIG 2.
Pooling low- and mid-scoring samples results in a lower cost per sample. The minimum cost per sample for the low-scoring category is for pools of 4 ($9.43 per sample). The minimum cost per sample for the mid-scoring category is for pools of 3 ($13.27 per sample). The cost per sample for the high-scoring category exceeds the unpooled cost per sample ($18 for all pool sizes of 2 to 10).
Pooling samples on the GeneXpert maintains high sensitivity.
Validation of sample pooling was conducted to ensure detection of low-bacterial burden specimens. Commercial positives with low bacterial load were tested in dilutions of 1:5 and 1:10 to simulate pool sizes of 5 and 10, respectively. All three low-bacterial burden CT strains were detected in 100% of the tests at both dilutions (Table S1). One low-bacterial NG strain (Z001) was detected in 100% of tests at both dilutions, while the other low-bacterial NG strain (Z017) was detected in only 66% of tests at both dilutions. Together, this results in 80% sensitivity at both the 1:5 and 1:10 dilutions for low-bacterial burden commercial NG strains.
Validation with known positive clinical specimens, urine and vaginal swabs, detected CT in 100% of tests at the 1:5 dilution and 83% of tests at the 1:10 dilution. One of the NG clinical samples was freeze-thawed at least twice prior to testing and was not detected at either dilution. Due to questionable sample integrity, this sample was removed from the analysis. Of the remaining NG samples, NG was successfully detected in 100% of the samples at both dilutions.
Projected cost savings using a guided sample pooling strategy.
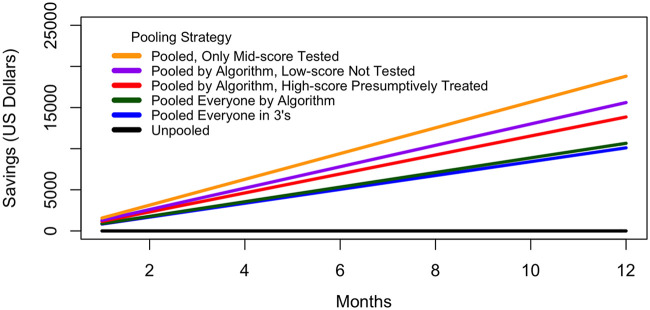
In a hypothetical STI clinic which tests approximately 2,000 participants per year from a comparable population, the cost savings of checklist-guided pooling varies based on clinical and testing decisions. After 1 year, more than $10,000 (28%) in test kits alone could be saved by testing all women in groups of 3 and over $10,600 (30%) if all women were tested following the proposed algorithm (Fig. 3).
FIG 3.
Projected 1-year cost savings with score-guided pooling strategies. Annual amount saved on GeneXpert test cartridges according to different applications of the scoring checklist based on a hypothetical STI clinic that screens 2,000 women per year with similar CT/NG prevalence and risk factors as the study population.
In resource-limited settings, if low-scoring women continue under syndromic management and high-scoring women are presumptively treated, the annual cost savings of only screening women in the middle-scoring category in pools of 3 exceeds $18,800 (52%). In this most limited testing scenario, 4.6% of women would be overtreated (92/2,000 women) and 2.1% of infections would be missed (41/2,000 women). However, even with limited pooled screening, 10.1% of women (202/2,000) would receive appropriate treatment for a subclinical CT/NG infection that would have otherwise gone undetected.
DISCUSSION
In this study, we proposed a population-specific score-based pooling algorithm to reduce the cost of molecular CT/NG testing for HRW in Zambia. We found that syndromic management performed with extremely low sensitivity in this population, highlighting the high prevalence of asymptomatic infections and underscoring the need for affordable molecular testing.
While the risk factors for CT and NG are distinct (18), the tests for these two pathogens are inseparable on the GeneXpert platform; therefore, the risk factors for both were combined on one scoring checklist. These factors included a variety of sociodemographic characteristics and results from low-cost, rapid laboratory tests associated with CT/NG prevalence among HRW in Zambia. The scoring checklist stratifies the study population into groups with different CT/NG prevalence and then recommends pooling within each stratum, thus maximizing the cost savings of sample pooling. This approach draws on the higher sensitivity of score-based methods (19, 20) and the WHO recommendation to tailor detection algorithms to risk factors within specific communities (8) and combines these with the cost-saving benefits of specimen pooling (11–14).
Pooling according to the proposed algorithm results in a 30% cost reduction from testing everyone individually and a 52% reduction if combined with syndromic management and presumptive treatment (21, 22). Although these estimates do not include increased treatment costs, it is expected that this cost would be minimal. In Zambia, CT/NG can be treated for less than $1. In terms of technician time and reagents, the rapid laboratory tests included on the checklist are routine at ZEHRP and do not increase to implement pooling.
An important consideration when implementing pooling is clinic volume. In order to operationalize pooling in groups of 3, without the checklist, a clinic would need at least 3 clients within a similar time frame in order to provide timely, same-day test results. At ZEHRP, clinic visits are scheduled so the number of samples available for pooling each day is predictable. Similarly, the clinic volume is high enough that pooling according to the checklist is feasible, or at minimum in pools of 2 or 3, which still represents a cost savings over individual testing. In the event of pool deconvolution, the time to result is extended by an additional 90 min. To adjust for this, ZEHRP has structured their clinic visits to begin with specimen collection and laboratory testing, which then runs in parallel to participant wait time, clinical consultation, and risk-reduction counseling. Clinic and laboratory staff were included in the development of the checklist and trained on its implementation; however, budgetary limitations and study-specific testing restrictions have not allowed its full-scale implementation.
A common concern with sample pooling is reduced sensitivity and missed detection of low-bacterial load infections. The GeneXpert CT/NG platform was developed to test first-catch urine and endocervical and vaginal swab specimens (10). A study comparing the GeneXpert CT/NG to the Aptima Combo 2 nucleic acid amplification test (NAAT) test demonstrated 100% sensitivity for pools of 4 vaginal swab samples (23). Other studies pooling CT/NG samples collected from various anatomical sites from the same individual observed discrepant results only for off-label sample types, such as rectal and pharyngeal swabs, but not for urine (24, 25). Bacterial load is known to vary between anatomical sites (26) and is thought to be higher in urine (25).
Our validation with low-bacterial burden commercial positives demonstrated perfect or acceptable sensitivity at a 1:5 dilution. Mathematical models suggest that point-of-care testing, even with moderate sensitivity (50%), is beneficial because same-day treatment of STIs reduces negative health consequences for the individual and breaks the chain of transmission to uninfected partners (27). Most women fall into the middle-scoring group and are recommended for testing in pools of 3, which would maintain 100% sensitivity for CT and result in a higher NG sensitivity than the 80% observed for 1:5 dilutions of known low-bacterial load samples. This is a substantial improvement to the 2% sensitivity of syndromic management in our study population and the 68% sensitivity of syndromic management plus gynecological exam.
The screening algorithm proposed here has the potential to reduce the financial barrier of molecular diagnostic technology for STIs in resource-limited settings. Increasing accessibility and diagnostic capacity in developing countries can improve patient outcomes by allowing the identification and treatment of asymptomatic infections, thereby reducing the complications from untreated STIs, lowering the risk of HIV acquisition, and limiting the overuse of antibiotics, which drives pathogen drug resistance.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the ZEHRP staff for their care and dedication to the study participants and tireless efforts regarding data quality. Everyone from the recruitment, reception, lab, clinic, data, and administrative teams were essential to making this work possible. Thank you to the Rwanda-Zambia HIV Research Group staff at Emory for logistical and technical support. We extend our sincere gratitude for the generous contributions of all the ZEHRP study participants.
This study was supported by the International AIDS Vaccine Initiative (IAVI) and through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (DEL-15-006). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and is supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107752/Z/15/Z) and the UK government. The views expressed in this publication are those of the authors and not necessarily those of the AAS, NEPAD Agency, Wellcome Trust, or UK government. This study was made possible by the generous support of the American people through the United States Agency for International Development (USAID). Additional support was provided by the National Institute of Child Health and Development (NICHD R01 HD40125), the National Institute of Mental Health (NIMH R01 66767), the AIDS International Training and Research Program Fogarty International Center (D43 TW001042), the Emory Center for AIDS Research (P30 AI050409), the National Institute of Allergy and Infectious Diseases (NIAID R01 AI51231, NIAID R01 AI040951, NIAID R01 AI023980, NIAID R01 AI64060, and NIAID R37 AI51231), the Burroughs Wellcome Fund, and the Emory Global Health Institute.
Coauthors W.K., M.I., A.-M.V., T.S., E.H., and S.A. contributed to managing the research sites, data collection, and quality control. Coauthors S.C., R.P., K.M.W., A.T., and S.A. contributed to data quality, data analysis, and manuscript preparation.
None of the authors have any conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.CDC. 2014. Chlamydia: CDC fact sheet. https://www.cdc.gov/std/chlamydia/stdfact-chlamydia.htm.
- 2.CDC. 2014. Gonorrhea: CDC fact sheet. https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea.htm.
- 3.Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, Passmore JA, Grobler A, Sturm AW, Williamson C, Ronacher K, Walzl G, Abdool Karim SS. 2012. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis 206:6–14. doi: 10.1093/infdis/jis298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis SC, Mthiyane TN, Baisley K, McHunu SL, Ferguson JB, Smit T, Crucitti T, Gareta D, Dlamini S, Mutevedzi T, Seeley J, Pillay D, McGrath N, Shahmanesh M. 2018. Prevalence of sexually transmitted infections among young people in South Africa: a nested survey in a health and demographic surveillance site. PLoS Med 15:e1002512. doi: 10.1371/journal.pmed.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilembe W, Inambao M, Sharkey T, Wall KM, Parker R, Himukumbwa C, Tichacek A, Malama K, Visoiu AM, Price M, Chomba E, Allen S. 2019. Single mothers and female sex workers in Zambia have similar risk profiles. AIDS Res Hum Retroviruses 35:814–825. doi: 10.1089/AID.2019.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malama K, Sagaon-Teyssier L, Parker R, Tichacek A, Sharkey T, Kilembe W, Inambao M, Price MA, Spire B, Allen S. 2019. Client-initiated violence against Zambian female sex workers: prevalence and associations with behavior, environment, and sexual history. J Interpers Violence doi: 10.1177/0886260519860083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall KM, Kilembe W, Inambao M, Chen YN, McHoongo M, Kimaru L, Hammond YT, Sharkey T, Malama K, Fulton TR, Tran A, Halumamba H, Anderson S, Kishore N, Sarwar S, Finnegan T, Mark D, Allen SA. 2015. Implementation of an electronic fingerprint-linked data collection system: a feasibility and acceptability study among Zambian female sex workers. Global Health 11:27. doi: 10.1186/s12992-015-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. 2004. Syndromic management, p 3–4. In Guidelines for the management of sexually transmitted infections. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/bitstream/handle/10665/42782/9241546263_eng.pdf?sequence=1. [Google Scholar]
- 9.Seelig MS. 1966. Mechanisms by which antibiotics increase the incidence and severity of candidiasis and alter the immunological defenses. Bacteriol Rev 30:442–459. doi: 10.1128/MMBR.30.2.442-459.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cepheid. 2019. Xpert CT/NG package insert. Cepheid, Sunnyvale, CA: https://www.cepheid.com/Package%20Insert%20Files/Xpert-CTNG-US-ENGLISH-Package-Insert-301-0234--Rev-K.pdf. [Google Scholar]
- 11.Papp JR, Schachter J, Gaydos C, Van Der Pol B. 2014. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae: 2014. MMWR Recommend Rep 63:1–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Kacena KA, Quinn SB, Howell MR, Madico GE, Quinn TC, Gaydos C. 1998. Pooling urine samples for ligase chain reaction screening for genital Chlamydia trachomatis infection in asymptomatic women. J Clin Microbiol 36:481–485. doi: 10.1128/JCM.36.2.481-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kacena KA, Quinn SB, Hartman SC, Quinn TC, Gaydos C. 1998. Pooling of urine samples for screening for Neisseria gonorrhoeae by ligase chain reaction: accuracy and application. J Clin Microbiol 36:3624–3628. doi: 10.1128/JCM.36.12.3624-3628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie MJ, McNiven M, Yee T, Yee T, Schiemer U, Bowden FJ. 2004. Pooling of clinical specimens prior to testing for Chlamydia trachomatis by PCR is accurate and cost saving. J Clin Microbiol 42:4866–4867. doi: 10.1128/JCM.42.10.4866-4867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dionne-Odom J, Karita E, Kilembe W, Henderson F, Vwalika B, Bayingana R, Li Z, Mulenga J, Chomba E, Del Rio C, Khu NH, Tichacek A, Allen S. 2013. Syphilis treatment response among HIV-discordant couples in Zambia and Rwanda. Clin Infect Dis 56:1829–1837. doi: 10.1093/cid/cit146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dionne-Odom J, Karita E, Kilembe W, Henderson F, Vwalika B, Bayingana R, Li Z, Mulenga J, Chomba E, del Rio C, Khu NH, Tichacek A, Allen S. 2013. Reply to Yang et al. Clin Infect Dis 57:1212–1213. doi: 10.1093/cid/cit353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Causer LM, Guy RJ, Tabrizi SN, Whiley DM, Speers DJ, Ward J, Tangey A, Badman SG, Hengel B, Natoli LJ, Anderson DA, Wand H, Wilson D, Regan DG, Shephard M, Donovan B, Fairley CK, Kaldor JM. 2018. Molecular test for chlamydia and gonorrhoea used at point of care in remote primary healthcare settings: a diagnostic test evaluation. Sex Transm Infect 94:340–345. doi: 10.1136/sextrans-2017-053443. [DOI] [PubMed] [Google Scholar]
- 18.Connolly S, Wall KM, Parker R, Kilembe W, Inambao M, Visoiu AM, Sharkey T, Hunter E, Allen S. 2020. Sociodemographic factors and STIs associated with Chlamydia trachomatis and Neisseria gonorrhoeae infections in Zambian female sex workers and single mothers. Int J STD AIDS 31:364–374. doi: 10.1177/0956462419894453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuylsteke B, Laga M, Alary M, Gerniers MM, Lebughe JP, Nzila N, Behets F, Van Dyck E, Piot P. 1993. Clinical algorithms for the screening of women for gonococcal and chlamydial infection: evaluation of pregnant women and prostitutes in Zaire. Clin Infect Dis 17:82–88. doi: 10.1093/clinids/17.1.82. [DOI] [PubMed] [Google Scholar]
- 20.Mayaud P, Grosskurth H, Changalucha J, Todd J, West B, Gabone R, Senkoro K, Rusizoka M, Laga M, Hayes R. 1995. Risk assessment and other screening options for gonorrhoea and chlamydial infections in women attending rural Tanzanian antenatal clinics. Bull World Health Organ 73:621–630. [PMC free article] [PubMed] [Google Scholar]
- 21.Steen R, Chersich M, Gerbase A, Neilsen G, Wendland A, Ndowa F, Akl EA, Lo Y-R, de Vlas SJ. 2012. Periodic presumptive treatment of curable sexually transmitted infections among sex workers: a systematic review. AIDS 26:437–445. doi: 10.1097/QAD.0b013e32834ed991. [DOI] [PubMed] [Google Scholar]
- 22.WHO. 2008. Periodic presumptive treatment for sexually transmitted infections: experience from the field and recommendations for research. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/bitstream/handle/10665/43950/9789241597050_eng.pdf;jsessionid=8F6F700FD3454718BDB0B3970D5C2098?sequence=1. [Google Scholar]
- 23.Dize L, West SK, Mkocha H, Quinn TC, Gaydos CA. 2015. Evaluation of pooled ocular and vaginal swabs by the Cepheid GeneXpert CT/NG assay for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae compared to the GenProbe Aptima Combo 2 Assay. Diagn Microbiol Infect Dis 81:102–104. doi: 10.1016/j.diagmicrobio.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badman SG, Bell SFE, Dean JA, Lemoire J, Coffey L, Debattista J, Redmond AM, Williams OD, Gilks CF, Whiley DM. 2020. Reduced sensitivity from pooled urine, pharyngeal and rectal specimens when using a molecular assay for the detection of chlamydia and gonorrhoea near the point of care. Sex Health 17:15–21. doi: 10.1071/SH19028. [DOI] [PubMed] [Google Scholar]
- 25.Speers DJ, Chua IJ, Manuel J, Marshall L. 2018. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis from pooled rectal, pharyngeal and urine specimens in men who have sex with men. Sex Transm Infect 94:293–297. doi: 10.1136/sextrans-2017-053303. [DOI] [PubMed] [Google Scholar]
- 26.Bissessor M, Tabrizi SN, Fairley CK, Danielewski J, Whitton B, Bird S, Garland S, Chen MY. 2011. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control. J Clin Microbiol 49:4304–4306. doi: 10.1128/JCM.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickerman P, Watts C, Alary M, Mabey D, Peeling RW. 2003. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex Transm Infect 79:363–367. doi: 10.1136/sti.79.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.