Abstract
Background
Socioeconomic status (SES) disparities in the surgical management of patients with non-small cell lung cancer (NSCLC) are well described. Disparities in the receipt of adjuvant chemotherapy are poorly understood. We assessed the influence of SES on adjuvant chemotherapy after resection in patients with pN1 NSCLC.
Methods
The National Cancer Database was queried for cN0/N1 NSCLC patients who underwent surgical resection and had demonstrated pN1 disease. This cohort was further divided into those who received multiagent adjuvant chemotherapy (MAAC) vs surgery-only treatment. Factors associated with treatment assignment were examined, and long-term survival was compared.
Results
Of the 14,892 patients who underwent resection for pN1 disease, 8061 (54.1%) received MAAC. Patients were less likely to receive MAAC if they resided in rural areas (odds ratio, 1.23; 95% confidence interval [CI],1.11–1.37; P < .001), or were uninsured or on Medicaid (odds ratio, 1.23; 95% CI, 1.07–1.41; P [ .004). The propensity score-weighted 5-year survival was significantly higher for those receiving MAAC compared with surgery only (53.6% vs 39.5%, log-rank P < .001). Lower income (hazard ratio, 1.06; 95% CI, 1.00–1.12; P [ .044) and uninsured or Medicaid insurance status (hazard ratio, 1.22; 95% CI, 1.13–1.31; P < .001) were independently associated with increased mortality by Cox regression in the propensity score-weighted cohort.
Conclusions
pN1 NSCLC patients living in rural areas or who are uninsured or on Medicaid insurance are at increased risk of not receiving MAAC. Treatment with MAAC significantly improves long-term survival of pN1 patients. Efforts should be made to ensure these at-risk groups receive guideline-concordant care.
Lung cancer is the leading cause of cancer-associated mortality in the United States.1,2 Non-small cell lung cancer (NSCLC) constitutes more than 80% of the estimated 220,000 incident cases annually.1,2 For patients with pathologic N1 (pN1) disease, current National Comprehensive Cancer Network (NCCN) guidelines recommend complete surgical resection, followed by a platinum-based doublet adjuvant chemotherapy regimen.3,4 The evidence in support of this multimodal protocol emerged from several randomized controlled trials and subsequent meta-analyses, which collectively demonstrated a reduction in disease recurrence and an overall 5-year survival benefit of 5.4% for all patients treated with this algorithm.5–9
Stage-specific subgroup analyses presented in the Adjuvant Lung Cancer Project Italy (ALPI), Canada Clinical Trials Group (JBR.10), and the Adjuvant Navelbine International Trialist Association (ANITA) trials suggest that patients with pN1 NSCLC may experience an even greater survival advantage. Despite established evidence-based indications for multiagent adjuvant chemotherapy (MAAC) in pN1 patients, the extent to which these guidelines are followed and the factors mediating their adoption in clinical practice are unclear.
Socioeconomic status (SES) factors are important determinants of treatment and outcome disparities among lung cancer patients. Previous studies have demonstrated that patients with high-risk SES factors are less likely to undergo definitive surgery for early-stage NSCLC and palliative chemotherapy for advanced disease.10–13 However, there are currently no data concerning the impact of patient SES on the receipt of MAAC in the NSCLC population who meet indications for this therapy. Therefore, the objectives of this study were to assess the influence of SES factors on adjuvant chemotherapy use in a nationally representative cohort of pN1 NSCLC patients and to evaluate the effect of this treatment strategy on long-term survival. We hypothesized that SES factors significantly contribute to disparities in the use of MAAC after resection of pN1 NSCLC.
Patients and Methods
Data Source
This study is a retrospective observational study of NSCLC patients sourced from the National Cancer Database (NCDB). The NCDB is a hospital-based oncology registry sponsored by the American College of Surgeons and the American Cancer Society and captures approximately 70% of all patients with newly diagnosed cancer in the United States.14 This study was exempted from review by the University of Southern California Institutional Review Board.
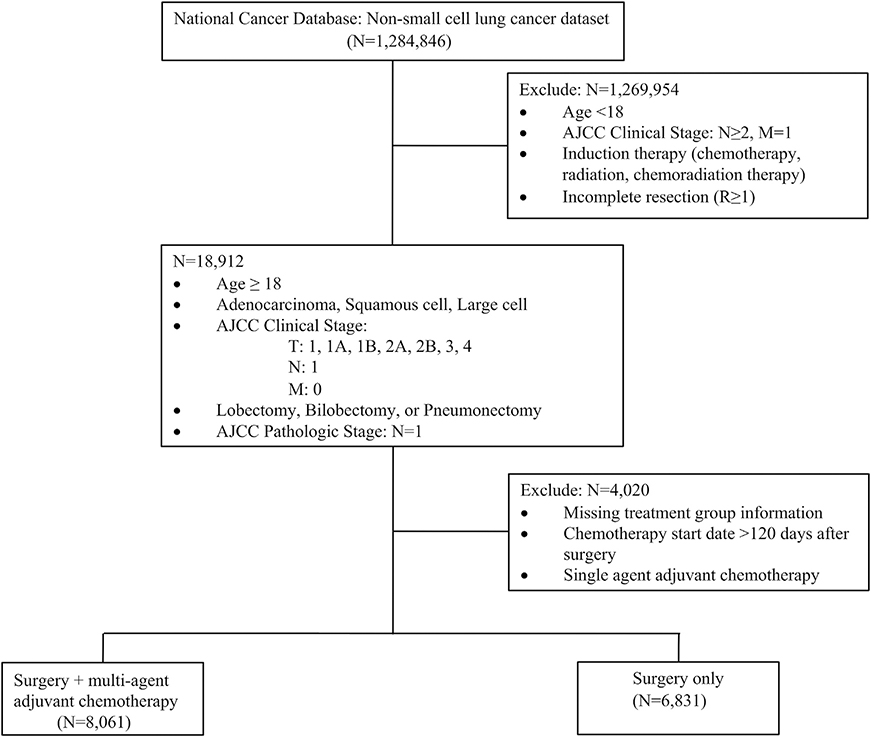
The NCDB participant user file was queried to identify patients who underwent lobectomy, bilobectomy, or pneumonectomy for clinical N0 or N1 NSCLC between 2004 and 2014. Clinical and pathologic staging was based on the 7th edition of the American Joint Commission on Cancer guidelines.15 Patients were excluded if they were younger than 18 years old, received chemotherapy, radiotherapy, or chemoradiotherapy before resection, received adjuvant radiotherapy, had clinical N2 staging, carcinoid tumor histology, underwent an operation categorized as local tumor destruction or not otherwise specified (NOS), did not have complete resection (R ≥1), or had missing pathologic staging data (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram demonstrating the study criteria for selection of the pathologic N1 cohort from the National Cancer Database. (AJCC, American Joint Commission on Cancer.)
From this cohort, patients with pathologic N1 disease (pN1) were selected and assigned to 1of2 groups according to the treatment received after surgery. Patients who underwent MAAC within 120 days after surgery were classified as “MAAC,” and patients who had no additional postoperative treatment were classified as “surgery only.” We excluded 334 patients who underwent single-agent adjuvant chemotherapy because this could also be interpreted as not being consistent with guidelines.The120-day interval was selected to ensure that chemotherapy was not administered for the treatment of recurrent disease.
Study Variables
Five socioeconomic status variables were evaluated: race/ ethnicity, median household income, education level, urban/rural area of residence, and insurance status. Race/ ethnicity was classified according to NCDB categories. Median household income and education data were sourced using estimates derived from patient ZIP codes and were dichotomized. Urban/rural residence was based on population estimates of the patient’s county of residence at the time of diagnosis. Insurance status was dichotomized to compare patients who were uninsured or on Medicaid with those patients with all other forms of insurance. Also included were the following demographic and clinical variables: age, sex, Charlson-Deyo comorbidity score, clinical and pathologic stage, tumor grade, and histologic subtype. Tumor histology was determined using International Classification of Diseases for Oncology codes for adenocarcinoma, squamous cell carcinoma, and other.16 Time from diagnosis to surgery was categorized at as 90 days or less and after 90 days to evaluate whether the timing of care influenced subsequent treatment strategies. Year of diagnosis was included to adjust for possible health care improvements over the study period.
Statistical Analysis
Descriptive analyses were performed to report the frequency counts and percentages for categorical variables. We used χ2 tests to analyze categorical variables for bivariate analysis and logistic regression to identify factors associated with treatment. Variance inflation factor was checked for collinearity and Hosmer-Lemeshow goodness of fit for model fitting.
Propensity score weighting was used to limit the confounding effects of baseline characteristics on survival analyses.17 The variables used in the propensity score model are listed in Supplemental Table 1. The weight (W) is generated using the following formula: W = (treatment group/propensity score) + (1 -treatment group/1 -propensity score), with the treatment group defined as “1” for surgery only, and “0” for MAAC.
Kaplan-Meier and log-rank tests were used for time-toevent analysis before and after propensity score inverse weighting. Cox regression with propensity score inverse weighting was used to generate hazard ratios (HRs). Schoenfeld residuals were checked for proportional hazards assumption. Sensitivity analysis was performed by excluding surgery-only patients who died within 90-days postoperatively. Propensity scores were recalculated, and the weighted survival analysis was performed with the assumption that excluded patients had zero probability to be treated with adjuvant chemotherapy. A significance level of 0.05 was used for 2-sided tests. All analyses were performed using SAS 9.4 software (SAS Institute Inc, Cary, NC).
Results
Of the 14,892 patients who met the inclusion criteria, 8061 (54.1%) received MAAC after resection (Table 1). Multivariable logistic regression identified 2 SES factors predictive of treatment strategy (Table 2). After adjusting for demographic and clinical characteristics, patients with pN1 disease were less likely to receive MAAC if they resided in rural areas (odds ratio [OR], 1.23; 95% confidence interval [CI], 1.11–1.37; P < .001) or were uninsured or on Medicaid insurance (OR, 1.23; 95% CI, 1.07–1.41; P =.004). In addition to these factors, patients who were male (OR, 1.10; 95% CI, 1.02–1.19; P = .009), aged older than 69 years (OR, 3.05; 95% CI, 2.83–3.28; P < .001), had clinical N1 stage (OR, 1.11; 95% CI, 1.03–1.20; P =.005), had Charlson-Deyo comorbidity scores of 2+(OR, 1.35; 95% CI, 1.21–1.51; P < .001), had squamous cell carcinoma histology (OR, 1.25; 95% CI, 1.15–1.35; P < .001), underwent pneumonectomy (OR, 1.24; 95% CI, 1.12–1.38; P < .001), or underwent surgery greater than 90 days after diagnosis (OR, 1.79; 95% CI, 1.54–2.08; P < .001) were also less likely to receive MAAC after resection.
Table 1.
Demographic and Clinical Characteristics of pN1 Patients Receiving Multiagent Adjuvant Chemotherapy Compared With Surgery-Only Therapy
| MAAC (n = 8061) |
Surgery Only (n = 6831) |
||
|---|---|---|---|
| Characteristic | No. (%)a | No. (%)a | P Value |
| Sex | <.001 | ||
| Male | 4242 (52.6) | 3916 (57.3) | |
| Female | 3819 (47.4) | 2915 (42.7) | |
| Age, y | <.001 | ||
| ≤69 | 5553 (68.9) | 2995 (43.8) | |
| >69 | 2508 (31.1) | 3836 (56.2) | |
| Race/ethnicity | .01 | ||
| White | 6683 (82.4) | 5686 (83.2) | |
| Asian | 151 (1.9) | 121 (1.8) | |
| Black | 676 (8.3) | 471 (6.9) | |
| Hispanic | 147 (1.8) | 136 (2.0) | |
| Other | 64 (0.8) | 67 (1.0) | |
| Median household income | .007 | ||
| <$38,000 | 1437 (17.8) | 1326 (19.4) | |
| ≥$38,000 | 6560 (81.4) | 5400 (79.0) | |
| Education | <.001 | ||
| <20.9% no HS diploma | 6814 (84.5) | 5577 (81.6) | |
| >21% no HS diploma | 1185 (14.7) | 1156 (16.9) | |
| Charlson-Deyo score | <.001 | ||
| 0 | 4263 (52.9) | 3290 (48.2) | |
| 1 | 2858 (35.4) | 2468 (36.1) | |
| 2+ | 940 (11.7) | 1073 (15.7) | |
| Clinical T Stage | .006 | ||
| 1 | 3287 (40.8) | 2594 (38.0) | |
| 2 | 3809 (47.2) | 3371 (49.3) | |
| 3 | 736 (9.1) | 656 (9.6) | |
| 4 | 229 (2.8) | 209 (3.1) | |
| Clinical N | .001 | ||
| 0 | 4631 (57.5) | 3742 (54.8) | |
| 1 | 3430 (42.5) | 3089 (45.2) | |
| Pathologic T stage | .001 | ||
| 1 | 2500 (31.0) | 1923 (28.2) | |
| 2 | 4167 (51.7) | 3634 (53.2) | |
| 3 | 1046 (13.0) | 931 (13.6) | |
| 4 | 348 (4.3) | 343 (5.0) | |
| Urban vs rural status | <.001 | ||
| Urban | 6784 (84.1) | 5510 (80.6) | |
| Rural | 1044 (13.0) | 1057 (15.5) | |
| Insurance status | .021 | ||
| Other | 7317 (90.8) | 6289 (92.1) | |
| Uninsured or Medicaid | 648 (8.01) | 482 (7.1) | |
| Facility type | .444 | ||
| Academic/research | 2792 (34.6) | 2324 (34.0) | |
| Community/integrated cancer | 5247 (65.1) | 4485 (65.7) | |
| Tumor grade | .295 | ||
| Well differentiated | 379 (4.7) | 365 (5.3) | |
| Moderately differentiated | 3627 (45.0) | 3019 (44.2) | |
| Poorly differentiated | 3629 (45.0) | 3093 (45.3) | |
| Other | 426 (5.3) | 354 (5.2) | |
| Histology | <.001 | ||
| Adenocarcinoma | 4873 (60.4) | 3650 (53.4) | |
| Squamous cell carcinoma | 2784 (34.5) | 2824 (41.3) | |
| Other | 404 (5.0) | 357 (5.2) | |
| Time between diagnosis and surgery | <.001 | ||
| ≤90 days | 7668 (95.1) | 6197 (90.7) | |
| >90 days | 393 (4.9) | 561 (8.2) | |
| Tumor size | .001 | ||
| <4 cm | 4784 (59.4) | 3876 (56.7) | |
| ≥4 cm | 3230 (40.1) | 2920 (42.8) | |
| Resection type | .012 | ||
| Bilobectomy/lobectomy | 6827 (84.7) | 5682 (83.2) | |
| Pneumonectomy | 1234 (15.3) | 1149 (16.8) | |
| Year of diagnosis | <.001 | ||
| 2006 | 392 (44.5) | 488 (55.5) | |
| 2007 | 488 (47.2) | 546 (52.8) | |
| 2008 | 826 (48.8) | 866 (51.2) | |
| 2009 | 917 (51.1) | 876 (48.9) | |
| 2010 | 931 (52.4) | 845 (47.6) | |
| 2011 | 1024 (54.5) | 855 (45.5) | |
| 2012 | 1128 (58.2) | 810 (41.8) | |
| 2013 | 1174 (60.4) | 770 (39.6) | |
| 2014 | 1181 (60.4) | 775 (39.6) |
Percentages may not add to 100% due to incomplete or missing data in the National Cancer Database.
Boldface indicates statistical significance (P < .05).
HS, high school; MAAC, Multiagent Adjuvant Chemotherapy.
Table 2.
Multivariable Logistic Regression for Treatment Assignment in the pN1 Cohort
| Multivariable Regression |
|||
|---|---|---|---|
| Covariatet | Odds Ratio | 95% CI | P Value |
| Sex | |||
| Male | 1.10 | 1.02–1.19 | .009 |
| Female | Reference | ||
| Age, y | |||
| ≤69 | Reference | ||
| >69 | 3.05 | 2.83–3.28 | <.001 |
| Race/ethnicity | |||
| White | Reference | ||
| Asian | 1.06 | 0.81–1.38 | .670 |
| Black | 0.92 | 0.80–1.05 | .214 |
| Hispanic | 1.11 | 0.85–1.44 | .436 |
| Other | 1.24 | 0.85–1.81 | .256 |
| Education | |||
| <20.9% no HS diploma | Reference | ||
| >21% no HS diploma | 1.12 | 1.00–1.25 | .051 |
| Charlson-Deyo score | |||
| 0 | Reference | ||
| 1 | 1.08 | 0.99–1.17 | .053 |
| 2+ | 1.35 | 1.21–1.56 | <.001 |
| Clinical T Stage | |||
| 1 | Reference | ||
| 2 | 0.98 | 0.88–1.09 | .691 |
| 3 | 1.01 | 0.85–1.21 | .884 |
| 4 | 0.87 | 0.67–1.13 | .304 |
| Clinical N | |||
| 0 | Reference | ||
| 1 | 1.11 | 1.03–1.20 | .005 |
| Pathologic T Stage | |||
| 1 | Reference | ||
| 2 | 1.05 | 0.93–1.17 | .440 |
| 3 | 1.16 | 0.98–1.37 | .083 |
| 4 | 1.20 | 0.96–1.49 | .102 |
| Median household income | |||
| <$38,000 | 1.08 | 0.97–1.21 | .150 |
| ≥$38,000 | Reference | ||
| Urban vs rural status | |||
| Urban | Reference | ||
| Rural | 1.23 | 1.11–1.37 | <.001 |
| Insurance status | |||
| Not insured or Medicaid | 1.23 | 1.07–1.41 | .004 |
| Other | Reference | ||
| Facility type | |||
| Academic/research | Reference | ||
| Community/integrated cancer | 0.99 | 0.92–1.07 | .821 |
| Tumor grade | |||
| Well differentiated | Reference | ||
| Moderately differentiated | 0.91 | 0.77–1.07 | .255 |
| Poorly differentiated | 0.92 | 0.77–1.09 | .319 |
| Other | 0.91 | 0.73–1.15 | .430 |
| Histology | |||
| Adenocarcinoma | Reference | ||
| Squamous cell carcinoma | 1.25 | 1.15–1.35 | <.001 |
| Other | 1.04 | 0.88–1.24 | .637 |
| Time between diagnosis and surgery | |||
| ≤90 days | Reference | ||
| >90 days | 1.79 | 1.54–2.08 | <.001 |
| Tumor size | |||
| <4 cm | Reference | ||
| ≥4 cm | 0.98 | 0.89–1.07 | .627 |
| Resection type | |||
| Bilobectomy/lobectomy | Reference | ||
| Pneumonectomy | 1.24 | 1.12–1.38 | <.001 |
| Year of diagnosis | |||
| 2006 | Reference | ||
| 2007 | 0.88 | 0.72–1.08 | .227 |
| 2008 | 0.84 | 0.69–1.01 | .063 |
| 2009 | 0.77 | 0.64–0.92 | .005 |
| 2010 | 0.71 | 0.59–0.85 | <.001 |
| 2011 | 0.66 | 0.55–0.80 | <.001 |
| 2012 | 0.54 | 0.45–0.65 | <.001 |
| 2013 | 0.49 | 0.41–0.59 | <.001 |
| 2014 | 0.52 | 0.43–0.62 | <.001 |
Boldface indicates statistical significance (P < .05).
CI, confidence interval; HS, high school.
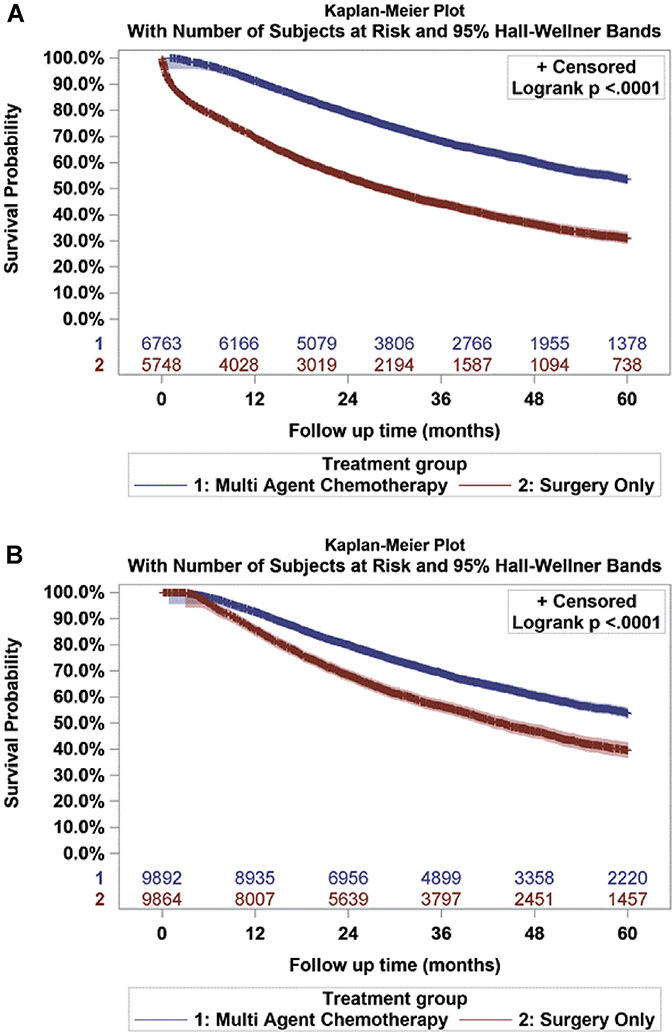
Survival data are presented in Figure 2. In the propensity score-weighted cohort, the 5-year overall survival rate after a 90-day mortality correction was significantly greater for those receiving MAAC compared with surgery alone (53.6% vs 39.5%, log-rank P < .001) (Figure 2). This is consistent with the results from the propensity score-weighted Cox regression, which showed that the receipt of surgery-only treatment was associated with an increased risk of mortality when compared with the addition of MAAC (HR, 2.11; 95% CI, 2.02–2.19; P < .001). In addition to treatment type, lower income (HR, 1.06; 95% CI, 1.00–1.12; P = .044) and uninsured or Medicaid insurance status (HR, 1.22; 95% CI, 1.13–1.31; P < .001) were independently associated with increased risk of mortality. Other significant demographic-, clinical-, and tumor-related variables predictive of increased mortality are summarized in Table 3.
Figure 2.
(A) Unadjusted Kaplan-Meier survival curve for pN1 patients receiving multiagent adjuvant chemotherapy after surgery (n = 6763) vs surgery only (n = 5748). (B) Adjusted Kaplan-Meier survival curve comparing propensity score-weighted multiagent adjuvant chemotherapy (n = 9892) vs surgery-only (n = 9864) cohorts after a 90-day mortality adjustment in surgery-only patients.
Table 3.
Propensity Score-Weighted Cox Regression of Factors Associated With Increased Risk of Mortality in pN1 Patients
| Propensity Score-Weighted Cox Regression |
|||
|---|---|---|---|
| Covariate | Hazard Ratio | 95% CI | P Value |
| Treatment | |||
| Multiagent adjuvant chemotherapy | Reference | ||
| Surgery only | 2.11 | 2.02–2.19 | <.001 |
| Sex | |||
| Male | 1.28 | 1.23–1.33 | <.001 |
| Female | Reference | ||
| Age, y | |||
| ≤69 | Reference | ||
| >69 | 1.30 | 1.25–1.35 | <.001 |
| Race/ethnicity | |||
| White | Reference | ||
| Asian | 0.87 | 0.73–1.03 | .096 |
| Black | 0.96 | 0.89–1.03 | .235 |
| Hispanic | 0.97 | 0.84–1.12 | .670 |
| Other | 0.89 | 0.71–1.11 | .299 |
| Education | |||
| <20.9% no HS diploma | Reference | ||
| >21% no HS diploma | 1.02 | 0.96–1.09 | .485 |
| Charlson-Deyo score | |||
| 0 | Reference | ||
| 1 | 1.14 | 1.10–1.19 | <.001 |
| 2+ | 1.40 | 1.32–1.49 | <.001 |
| Clinical T stage | |||
| 1 | Reference | ||
| 2 | 0.94 | 0.88–0.99 | .047 |
| 3 | 1.06 | 0.97–1.17 | .212 |
| 4 | 1.12 | 0.98–1.28 | .086 |
| Clinical N | |||
| 0 | Reference | ||
| 1 | 1.07 | 1.03–1.11 | .001 |
| Pathologic T stage | |||
| 1 | Reference | ||
| 2 | 1.11 | 1.04–1.19 | .001 |
| 3 | 1.52 | 1.39–1.67 | <.001 |
| 4 | 1.54 | 1.38–1.73 | <.001 |
| Median household income | |||
| <$38,000 | 1.06 | 1.01–1.12 | .044 |
| ≥$38,000 | Reference | ||
| Urban vs rural status | |||
| Urban | Reference | ||
| Rural | 1.03 | 0.97–1.09 | .282 |
| Insurance status | |||
| Not insured or Medicaid | 1.22 | 1.13–1.31 | <.001 |
| Other | Reference | ||
| Facility type | |||
| Academic/research | Reference | ||
| Community/integrated cancer | 1.11 | 1.07–1.16 | <.001 |
| Tumor grade | |||
| Well differentiated | Reference | ||
| Moderately differentiated | 1.18 | 1.07–1.30 | .001 |
| Poorly differentiated | 1.24 | 1.13–1.37 | <.001 |
| Other | 1.24 | 1.09–1.41 | .001 |
| Histology | |||
| Adenocarcinoma | Reference | ||
| Squamous cell carcinoma | 0.93 | 0.89–0.98 | .002 |
| Other | 0.99 | 0.91–1.08 | .835 |
| Time between diagnosis and surgery | |||
| ≤90 days | Reference | ||
| >90 days | 1.08 | 1.01–1.17 | .047 |
| Tumor size | |||
| <4 cm | Reference | ||
| ≥4 cm | 1.13 | 1.22–1.35 | <.001 |
| Resection type | |||
| Bilobectomy/lobectomy | Reference | ||
| Pneumonectomy | 1.12 | 1.06–1.18 | <.001 |
| Year of diagnosis | |||
| 2006 | Reference | ||
| 2007 | 0.97 | 0.88–1.07 | .557 |
| 2008 | 0.96 | 0.88–1.04 | .317 |
| 2009 | 0.87 | 0.80–0.95 | .002 |
| 2010 | 0.92 | 0.85–1.01 | .067 |
| 2011 | 0.85 | 0.78–0.93 | <.001 |
| 2012 | 0.98 | 0.90–1.07 | .702 |
| 2013 | 0.92 | 0.84–1.01 | .095 |
| 2014 | ... | ... | ... |
Boldface indicates statistical significance (P < .05).
CI, confidence interval; HS, high school.
Comment
This study specifically evaluated the relationship between SES and the receipt of adjuvant chemotherapy for NSCLC. Previous reports on disparities in the NSCLC population have primarily focused on treatment inequity in the context of upfront care, demonstrating that patients with high-risk SES are less likely to undergo curative surgery for early-stage disease and palliative chemotherapy for advanced disease.12,18–20 An unresolved question from these studies is whether SES factors similarly contribute to treatment disparities after the receipt of initial therapy. Therefore, by defining the impact of SES factors on adjuvant chemotherapy use, this analysis provides a clinically relevant perspective on the relationship between SES and continuity of care and addresses a major gap in the literature on treatment disparities in the NSCLC population.
This study demonstrates that rural residence and Medicaid or no insurance are independently associated with the failure to receive MAAC after surgery. The finding that rural patients are at increased risk of not receiving guideline-concordant treatment is consistent with trends in prior studies. Using both state and national registries, several investigators have described similar treatment and outcome disparities in lung cancer patient cohorts residing in rural areas.11,18,21,22
In an analysis of more than 348,000 lung cancer patients in the Surveillance, Epidemiology, and End Results (SEER) program database, Atkins and colleagues22 demonstrated a positive correlation between patient residential rurality and lung cancer incidence and mortality. Importantly, the investigators found that patients with early-stage I NSCLC residing in the most rural counties experienced a median survival time that was 12 months shorter than stagematched patients residing in the most urban counties.22 One reason for this survival disparity argued by Atkins and colleagues22 and others11,18,21 is that rural patients are persistently less likely to undergo surgical resection than their urban counterparts. The present analysis suggests that the disadvantaged status of rural residents extends beyond the receipt of initial surgical intervention and includes adjuvant chemotherapy treatments.
This analysis did not identify race/ethnicity as a significant predictor of treatment strategy in pN1 patients. This finding is particularly interesting, because much of the literature examining SES disparities in lung cancer has been centered on racial/ethnic inequity in disease management and outcomes.10,23,24 One explanation for this discrepancy is the difference in the types of treatments studied here compared with previous reports. While previous studies have focused on first-line therapies, this analysis examined treatment disparities after resection and suggests that patient race/ethnicity may serve as a barrier to accessing initial therapies but not the receipt of subsequent treatments. Alternatively, these discordant findings may be due to demographic differences between the hospital-based NCDB, National Program of Cancer Registries, and SEER databases.25 Whether race/ethnicity is found to impact the receipt of adjuvant chemotherapy in other national registry cohorts has yet to be determined.
The timely delivery of appropriate therapies is critical for maximizing overall survival in cancer patients. Despite more than a decade of evidence in support of multimodal therapy for pN1 patients, only 54.1% of pN1 patients in this study received MAAC in accordance with NCCN guidelines. This observation is in keeping with other NCDB reports, which have described similarly low rates of postoperative chemotherapy among patients with pathologic nodal disease.26 The reasons for the underuse of MAAC are not clear, but one explanation may be the perception of a limited survival benefit (5%−6%) conferred by this treatment strategy.9
The consequence of poor adherence to MAAC treatment guidelines in the current study was a significant reduction in overall survival. In the propensity score-weighted cohort, pN1 patients treated with MAAC had a 14.1% overall survival benefit compared with patients receiving surgery-only treatment. This survival benefit is consistent with, although considerably greater than, the 5% to 6% estimate commonly reported for NSCLC patients.9 One possible explanation for the greater survival observed in this study may pertain to the more homogeneously staged cohort used in this analysis. This explanation is consistent with the primary data reported in the ALPI and JBR.10 clinical trials, which showed an approximately 13% and 20% survival advantage among stage II NSCLC patients receiving MAAC, respectively.6,27 Additionally, ANITA trial investigators reported a 16% overall survival benefit among pN1 NSCLC patients receiving MAAC.8
Although none of these individual trials were specifically designed or powered to evaluate MAAC outcomes in stage-specific cohorts, a meta-analysis of 4 separate trials by Douillard and colleagues28 supported these trends by demonstrating a statistically significant 11.6% survival benefit in stage II and a 14.7% survival benefit in stage III NSCLC patients treated with adjuvant vinorelbine and cisplatin compared with observation after surgery. Although this study reinforces the known therapeutic advantage of adjuvant chemotherapy for the treatment of NSCLC, it also suggests that pN1 patients may stand to benefit greater than previously perceived from this treatment strategy.
This study had several limitations related to its retrospective and observational nature. First, although the NCDB is well suited to investigate national patterns of cancer treatment and outcomes, many of the SES variables within this data set lack sufficient granularity to yield information about individual treatment decisions.
In addition, because data such as physiologic tolerance, overall functional status, and disease-specific survival are not included in the NCDB, we were unable to test whether the difference in overall survival between MAAC and surgery-only cohorts is driven by nonlung cancerrelated deaths.
Finally, this study focused on the impact of SES factors on the initiation of adjuvant therapy for pN1 patients but did not evaluate the effect of SES on treatment attrition. Future studies should also consider the impact of SES factors on the completion of appropriate therapy.
In conclusion, this study identified multiple SES and non-SES factors associated with the failure to receive adjuvant chemotherapy after curative resection for pN1 NSCLC. Given that our central hypothesis was centered on socioeconomic disparities, we focused our analysis on SES-related factors. The findings presented here largely support the hypothesis and implicate certain SES factors as critical determinants of treatment and survival disparities in pN1 patients. Overall, this work underscores the importance of SES as a driver of treatment discrepancies for patients with lung cancer and argues that such factors should be meaningfully considered in efforts to improve access and delivery of appropriate therapies to the NSCLC population.
Supplementary Material
Footnotes
The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data.
The Supplemental Table can be viewed in the online version of this article [https://dx.doi.org/10.1016/j.athoracsur.2019.11.059] on https://www.annalsthoracicsurgery.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Houston KA, Mitchell KA, King J, et al. Histologic lung cancer incidence rates and trends vary by race/ethnicity and residential county. J Thorac Oncol. 2018;13:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504535. [DOI] [PubMed] [Google Scholar]
- 4.Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(Suppl):e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. [DOI] [PubMed] [Google Scholar]
- 6.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non–small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. [DOI] [PubMed] [Google Scholar]
- 7.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7: 719–727. [DOI] [PubMed] [Google Scholar]
- 9.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–3559. [DOI] [PubMed] [Google Scholar]
- 10.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. [DOI] [PubMed] [Google Scholar]
- 11.Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. [DOI] [PubMed] [Google Scholar]
- 13.Ebner PJ, Ding L, Kim AW, et al. The effect of socioeconomic status on treatment and mortality in non-small cell lung cancer patients. Ann Thorac Surg. 2020;109:225–232. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual (7th ed). New York, NY: Springer; 2010. [Google Scholar]
- 16.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology: 3rd ed Geneva, CH: World Health Organization; 2000. [Google Scholar]
- 17.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson AM, Hines RB, Johnson JA, et al. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer. 2014;83:401–407. [DOI] [PubMed] [Google Scholar]
- 19.Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non-small cell lung cancer among black and white Medicare beneficiaries, 2000–2003. J Natl Med Assoc 2011;103:711–718. [DOI] [PubMed] [Google Scholar]
- 20.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest 2000;117: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 21.Crowell R, Goetz T, Wiggins C, et al. Regional disparities in treatment and survival of early stage non-small cell lung cancer. Ethn Dis. 2007;17:358–364. [PubMed] [Google Scholar]
- 22.Atkins GT, Kim T, Munson J. Residence in rural areas of the United States and lung cancer mortality: disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc 2017;14:403–411. [DOI] [PubMed] [Google Scholar]
- 23.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. [DOI] [PubMed] [Google Scholar]
- 24.Ellis L, Canchola AJ, Spiegel D, et al. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol 2018;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerro CC, Robbins AS, Phillips JL, et al. Comparison of cases captured in the National Cancer Data Base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–1765. [DOI] [PubMed] [Google Scholar]
- 26.Bott MJ, Patel AP, Verma V, et al. Patterns of care in hilar node-positive (N1) non-small cell lung cancer: a missed treatment opportunity? J Thorac Cardiovasc Surg 2016;161: 1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. CancerSpectrum Knowl Environ. 2003;95:1453–1461. [DOI] [PubMed] [Google Scholar]
- 28.Douillard J-Y, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5:220–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.