Abstract
Patients with anxiety disorders suffer from impaired concentration, potentially as a result of stronger emotional interference on attention. Studies using behavioural measures provide conflicting support for this hypothesis. Elevated state anxiety may be necessary to reliably document differences in emotional interference in patients versus healthy controls. The present study examines the effect of experimentally induced state anxiety (threat-of-shock) on attention interference by emotional stimuli. Anxiety patients (n = 36) and healthy controls (n = 32) completed a modified affective Stroop task during periods of safety and threat-of-shock. Results indicated that in both patients and controls, threat decreased negative, but not positive or neutral, emotional interference on attention (both p < .001). This finding supports a threat-related narrowing of attention whereby a certain level of anxiety decreases task-irrelevant processing.
Keywords: anxiety disorder, attention control, cognition, state anxiety
1 |. INTRODUCTION
Patients with anxiety disorders suffer from pervasive worry and impaired concentration (Craske et al., 2017; Hoge, Ivkovic, & Fricchione, 2012) potentially due to poor cognitive control and/or overpowering emotional interference. Although everybody can experience attention interference by emotional stimuli, the presence of an anxiety disorder may enhance this effect (reviews Bishop, 2008; Cisler & Koster, 2010; Clarke & Johnstone, 2013). Empirically, however, studies of anxiety patients offer inconsistent evidence for this hypothesis (Balderston et al., 2017; Feng et al., 2018; Mitchell, Richell, Leonard, & Blair, 2006; Pergamin-Hight, Naim, Bakermans-Kranenburg, van IJzendoorn, & Bar-Haim, 2015).
Tasks such as the emotional Stroop are considered to measure emotional interference on cognitive control (Stickel et al., 2019; Williams, Mathews, & MacLeod, 1996; Blair et al., 2012; Klumpp et al., 2018; Minkova et al., 2017). During these tasks, emotional interference is qualified by the increase in attention resources (e.g. brain activation and reaction time) required during Stroop conflict trials in the presence of an emotional stimulus (Compton et al., 2003; Dresler, Attar, et al., 2012; Dresler, Ehlis, et al., 2012; Khanna et al., 2017; Liu, Yang, Jiang, & Li, 2018; Song et al., 2017; Williams et al., 1996). However, while some studies report emotional interference on cognitive control in anxiety patients at the neural, but not behavioural, level (Blair et al., 2012; Klumpp et al., 2018; Minkova et al., 2017), others demonstrate emotional interference on behavioural measures of cognitive control (for reviews, see Bishop, 2008; Cisler & Koster, 2010).
One explanation for the discrepancy in the literature is that emotional interference on cognitive control in anxiety patients may only be reliably documented in the context of elevated state anxiety. Indeed, the effect of state anxiety on cognition is complex. While anxiety can have a detrimental effect on cognitive control, a modest level of state anxiety may have the opposite effect (i.e. improve cognitive control; Easterbrook, 1959). By increasing attention to task-relevant stimuli and decreasing attention to task-irrelevant distractors, anxiety-induced narrowing of attention can improve cognitive control on some tasks (Booth & Sharma, 2009; Chajut & Algom, 2003; Hu, Bauer, Padmala, & Pessoa, 2012; Pessoa, Kastner, & Ungerleider, 2002).
In studies showing similar cognitive control in anxiety patients and healthy controls during emotional distractors, state anxiety may sufficiently narrow attention to eradicate group differences. In studies where anxiety patients exhibit worse attention than healthy controls, state anxiety may be too low to narrow attention.
The present study examines the effect of experimentally induced state anxiety (threat-of-shock) on emotional interference on cognitive control using a modified affective Stroop task in anxiety patients compared to healthy controls. The affective Stroop task uses emotional distractors to interfere with task-relevant processing (Fani et al., 2019; Hwang, White, Nolan, Sinclair, & Blair, 2014; Raschle et al., 2017; Robinson, Letkiewicz, Overstreet, Ernst, & Grillon, 2011; Vythilingam et al., 2007; White, Costanzo, Blair, & Roy, 2015). While other affective Stroop tasks present emotional distractors simultaneously as the target stimulus, the modified affective Stroop task presents emotional distractors before and after the stimulus (Blair et al., 2007; Mitchell et al., 2006). In so doing, changes in performance are considered to reflect emotional interference without the potential confound of a defensive response (e.g. freezing) (review Clarke & Johnstone, 2013).
Threat-of-shock is a well-established anxiety-induction procedure in both patients (Cha et al., 2014; Grillon, O’Connell, et al., 2017; Grillon, Robinson, et al., 2017; Vytal, Arkin, Overstreet, Lieberman, & Grillon, 2016) and healthy controls (Ernst, Lago, Davis, & Grillon, 2016; Lago et al., 2019; Robinson, Krimsky, & Grillon, 2013; Robinson, Vytal, Cornwell, & Grillon, 2013). Several studies have used threat-of-shock during attention tasks (review Robinson, Vytal, et al., 2013). Specific to emotional interference, Choi et al and Hu et al have investigated the influence of threat-of-shock on the Stroop effect (i.e. attention disruption) with opposing results (Choi, Padmala, & Pessoa, 2012; Hue et al., 2012). Choi et al found more attention interference during threat than safe trials, an effect that increased as a function of state anxiety (STAI, Spielberger State-Trait Anxiety Inventory; Spielberger, 1983; Choi et al., 2012). Hu et al, however, found a decrease in attention interference during threat-of-shock compared to safe (Hu et al., 2012). Studies have yet to use threat-of-shock to study emotional interference on attention in anxiety patients.
Thus, the aims of this study were to determine the effect of elevated state anxiety (induced by threat-of-shock) on cognitive control (i.e. measured during Stroop conflict trials) during an affective Stroop task as a putative measure of emotional interference of attention and to compare this effect between anxiety patients and healthy controls. Specifically, the study was designed to determine (a) whether inducing state anxiety modulates the effect of emotion on cognitive control and (b) whether this modulation differs between patients with anxiety disorders and healthy controls.
2 |. MATERIALS AND METHODS
2.1 |. Participants
Participants were recruited from the Washington, DC, metropolitan area through advertisements and flyers. A power analysis was conducted (G*Power 3.1) based on prior data which indicated an expected small effect size for behaviour measures (f = 0.1; Mitchell et al., 2006). Assuming a strong correlation among repeated measures (ρ = 0.8; estimated from previous behaviour measures), calculations indicated that the testing of 68 participants at an alpha of 0.05 would provide power of 0.95 to detect a significant 2 (Diagnosis) × 3 (Emotion) × 2 (Condition) interaction.
An experienced mental health nurse administered the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (SCID, First, 2002) to evaluate all participants. Clinical anxiety was considered a negative valence construct in accordance with the NIH Research Domain Criteria (RDoC, Kozak & Cuthbert, 2016). Therefore, individuals were enrolled in the anxiety group (ANX) if they were diagnosed with one or more anxiety disorders (Generalized Anxiety Disorder, Panic Disorder, or Social Anxiety Disorder). Participants were excluded if they met any of the following criteria: other current Axis I disorder, use of psycho-pharmacological medications, current substance use abuse, substance use dependence within the past six months, or any significant medical disease as determined by history, routine laboratories, electrocardiogram and neurological examination. Of the consented patients (n = 37), one participant was excluded for choosing not to perform the task due to threat-of-shock (n = 1). The remaining ANX participants were adults with generalized anxiety disorder (n = 12); social anxiety disorder (n = 8); generalized anxiety disorder and social anxiety disorder (n = 14); and generalized anxiety disorder, social anxiety disorder and panic disorder (n = 2) (Table 1).
TABLE 1.
Demographics and characteristics by group. Mean (SE). ANX group included all anxiety patients
| HC (n = 31) | ANX (n = 36) | GAD (n = 12) | SAD (n = 8) | GAD/SAD (n = 14) | GAD/SAD/PD (n = 2) | |
|---|---|---|---|---|---|---|
| Female | 26 | 30 | 12 | 6 | 10 | 2 |
| Male | 5 | 6 | 0 | 2 | 4 | 0 |
| Age (years) | 28.7 (1.3) | 28.8 (1.3) | 27.8 (2.3) | 26.5 (2.4) | 30.7 (2.4) | 29.5 (4.5) |
| STAI-trait* | 28.7 (0.9) | 51.0 (1.9) | 48.50 (2.1) | 45.0 (3.2) | 54.9 (3.7) | 63.0 (2.0) |
Abbreviations: ANX, anxiety patients; GAD/SAD/panic, comorbid GAD, SAD, and panic disorder; GAD/SAD, comorbid GAD and SAD; GAD, generalized anxiety disorder; HC, healthy controls; SAD, social anxiety disorder; STAI-trait, Spielberger State-Trait Anxiety Inventory.
Significant difference between groups (p < .001).
Of the recruited controls (n = 32), one participant was excluded for not finishing the task (n = 1). The final sample included 36 ANX participants and 31 healthy control (HC) participants. Groups were matched for gender (F(1,65) = 0.00, p = .95) and age (F(1,65) = 0.00, p = 1.0; Table 1). The ANX group had higher STAI-trait (F(1,65) = 103.14, p < .001) than the HC group. All participants gave written informed consent approved by the NIMH Combined Neuroscience Institutional Review Board and were compensated for their participation. This study was in accordance with the Declaration of Helsinki.
2.2 |. Shock
Anxiety was evoked by threat-of-shock (Vytal, Cornwell, Arkin, & Grillon, 2012). Shocks were delivered through electrodes (Biopac Systems, Goleta, CA, USA) placed on the left forearm. Prior to the task, shocks were administered at increasing intensities until a level that corresponded to subjective discomfort, but not pain, was reached. This intensity was used throughout the affective Stroop task.
2.3 |. Affective stroop task
2.3.1 |. Presentation
We used a commercially available system (E-Prime 2.0) to present a version of the previously described affective Stroop task (Blair et al., 2007). Participants watched as six trial types differing by task (incongruent, congruent) and emotion (negative, neutral, positive) were presented during condition blocks (safe, threat) on a computer screen (Figure 1).
FIGURE 1.
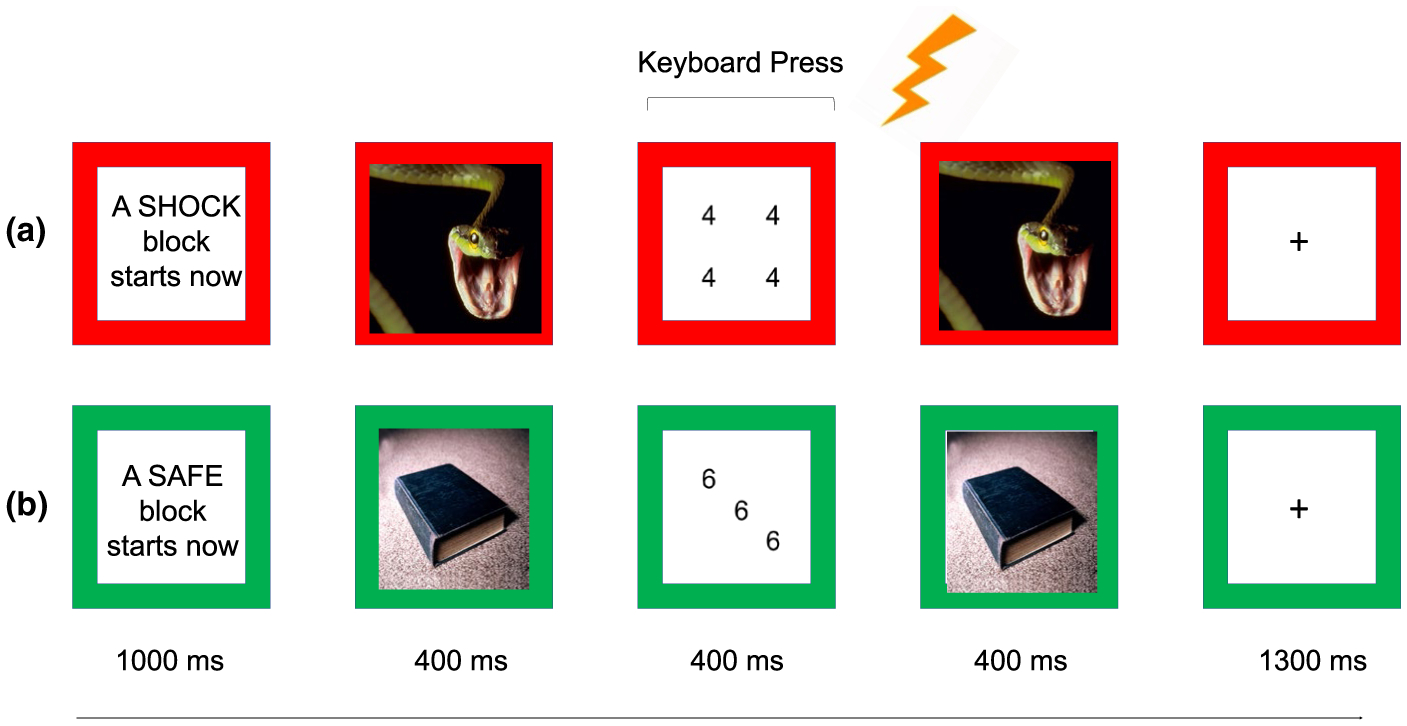
Affective Stroop task. (a) Example of threat trial. During threat, a participant could receive a shock (lightning bolt), as indicated by a red border. Participants pressed the key that corresponded to the numerosity of each number display. This example shows a negative congruent task trial (Arabic digit of 4, numerosity of 4). (b) Example of safe trial. During safe, a participant could not receive as shock, as indicated by a green border. This example shows a neutral incongruent task trial (Arabic digit of 6, Numerosity of 3)
Each trial began with a fixation point presented for 1,000 ms, which was then immediately followed by a picture stimulus presented for 400 ms, followed by a numerical display for 400 ms, followed by a repeat of the picture stimulus for 400 ms and followed by a blank screen for 1,300 ms. The short latency of the numerical display ensured that task-relevant processing continued into the second presentation of the picture stimulus. There were eight trials of each of the six trial types (negative congruent, negative incongruent, neutral congruent, neutral incongruent, positive congruent, positive incongruent) during each condition block (safe, threat). There were four condition blocks (2 safe, 2 threat) per run, and 2 runs per visit, for a total of 384 trials per participant (8 trials × 6 trial types × 4 blocks × 2 runs).
2.3.2 |. Task (incongruent, congruent)
Participants were told to count the number of digits (i.e. numerosity) presented on the numerical displays, which consisted of three, four, or five 3s, 4s, 5s or 6s. Participants then pressed the corresponding key on the keyboard (i.e. ‘3’ for numerosity of three). On incongruent trials, the numerosity of Arabic digits was inconsistent with the identity of the digit. On congruent trials, the Arabic digit was consistent with the numerosity.
2.3.3 |. Condition (safe, threat)
Trials were presented during blocks when subjects could not (safe) and could (threat) receive a shock. Each block began with an instruction screen presented for 800 ms that read ‘A SAFE block starts now’ or ‘A SHOCK block starts now’. Instruction screens as well as numerical displays had coloured borders indicating Condition (i.e. green for safe, red for threat). Each participant received a total of 2 shocks throughout the task.
2.3.4 |. Emotion
Pictures differed by negative, neutral or positive emotion and were selected from the International Affective Picture System (IAPS; Lang & Greenwald, 1988). Normative mean (SE) valence and arousal values on a nine-point scale were, respectively, 3.18 (0.71) and 5.90 (0.94) for negative, 4.90 (0.30) and 2.68 (0.37) for neutral, and 7.43 (0.47) and 5.17 (0.90) for positive pictures.
2.4 |. Questionnaires and retrospective ratings
Subjects completed the STAI-trait inventory during screening. After the affective Stroop task, participants rated subjective shock unpleasantness (‘How unpleasant were the electric shocks?’) on an analog scale ranging from 1 (not at all) to 10 (extremely). Subjects used the scale then to retrospectively rate their distress during negative pictures (‘How distressing were the negative pictures during threat/safe?’), pleasure during positive pictures (‘How pleasant were the positive pictures during threat/safe?’), as well as overall subjective difficulty with attention (‘How difficult was it to pay attention during threat/safe?’) and level of anxiety (‘How anxious were you during threat/safe?’). Subjects were not asked about their perception of the neutral stimuli.
2.5 |. Data analysis
All analyses were conducted in SPSS 21. All measures, conditions and data exclusions are reported. Due to specific focus on emotional interference on Stroop conflict trials, behavioural measures of interest included Stroop effect reaction time (difference score between incongruent and congruent accurate trials), as well as Stroop effect accuracy (difference score between incongruent and congruent trials).1 Stroop effect reaction time and accuracy were averaged within each emotion and condition and analysed with three-way Diagnosis (HC, ANX) × Emotion (negative, neutral, positive) × Condition (safe, threat) rANOVAs.
STAI-trait, shock-level, shock discomfort, retrospective distress during negative pictures and retrospective pleasure during positive pictures were analysed with one-way Diagnosis (HC, ANX) ANOVAs. Retrospective ratings of anxiety and difficulty with attention were analysed with twoway Diagnosis (HC, ANX) × Condition (safe, threat) rANOVAs. Post hoc analyses were tested using Bonferroni adjusted alpha levels of 0.008 per test (0.05/6).
3 |. RESULTS
3.1 |. Shock
Diagnosis did not affect shock level (F(65) = 2.96, p = .10) or shock discomfort (F(65) = 0.02, p = .89, Table 2).
TABLE 2.
Shock and retrospective ratings by group and condition. Mean (SE)
| mA | Discomfort | Distress*,† | Pleasure† | Anxiety*,† | Attention*,† | |
|---|---|---|---|---|---|---|
| HC | ||||||
| Safe | 9.2 (1.4) | 8.2 (0.3) | 3.3 (0.3) | 5.5 (0.5) | 2.0 (0.3) | 2.9 (0.4) |
| Threat | 4.2 (0.5) | 4.6 (0.5) | 6.7 (0.5) | 4.4 (0.5) | ||
| ANX | ||||||
| Safe | 6.5 (0.9) | 8.1 (0.3) | 4.8 (0.4) | 5.9 (0.4) | 3.5 (0.4) | 4.1 (0.4) |
| Threat | 6.1 (0.4) | 4.6 (0.4) | 8.1 (0.3) | 6.5 (0.4) | ||
Abbreviations: ANX, anxiety patients; Anxiety, subjective anxiety; Attention, subjective difficulty with attention; Discomfort, shock discomfort; Distress, distress during negative pictures; HC, healthy controls; mA, shock level in milliAmps; Pleasure, pleasure during positive pictures.
Significant difference between Groups (Distress p < .01, η2 = 0.1; Anxiety p = .001, η2 = 0.2; Attention p < .001, η2 = 0.2).
Significant difference between Conditions (Distress p < .001, η2 = 0.2; Pleasure p < .001, η2 = 0.2; Anxiety p < .001, η2 = 0.8; Attention p < .001, η = 0.3).
3.2 |. Affective stroop performance
3.2.1 |. Stroop effect reaction time (Incongruent-Congruent)
The three-way rANOVA revealed main effects of condition (F(1,65) = 20.53, p < .001; η2 = 0.24) and emotion (F(2,130) = 27.86, p < .001; η2 = 0.30) (Table 3, Figure 2). However, these effects were qualified by an interaction between Condition × Emotion (F(2,130) = 9.57, p < .001; η2 = 0.13). Decomposed by condition, during safe, there was greater Stroop effect on reaction time during negative compared to positive pictures (t(66) = 3.46, p < .001; 95% CI [9.18, 34.31]) but only at a trend for neutral pictures (Bonferroni corrected, t(66) = 2.74, p = .008; 95% CI [3.98, 25.36]). During Threat, there was less Stroop effect on reaction time during negative compared to neutral (t(66) = −5.12, p < .001; 95% CI [−44.05, −19.32]) and positive (t(66) = −5.93, p < .001; 95% CI [−46.45, −23.03]) pictures. Decomposed by emotion, condition affected Stroop effect reaction time during negative (threat less than safe, t(66) = −7.30, p < .001; 95% CI [−67.15, −38.31]) but not during neutral (t(66) = −1.51, p = .14; 95% CI [−14.84, 2.09]) or positive (t(66) = 0.66, p = .51; 95% CI [−7.66, 15.17]) pictures. There were no main effects or interactions of diagnosis on Stroop effect reaction time.
TABLE 3.
Reaction time (milliseconds) by group, emotion, Stroop effect (incongruent minus congruent), task and condition. Mean (SE)
| Stroop effect | Incongruent | Congruent | ||||
|---|---|---|---|---|---|---|
| Safe† | Threat‡ | Safe | Threat | Safe | Threat | |
| HC | ||||||
| Negative* | 74.6 (7.7) | 15.0 (5.1) | 576.7 (11.4) | 534.3 (13.3) | 502.1 (13.2) | 519.3 (12.1) |
| Neutral | 54.7 (6.4) | 47.0 (5.0) | 537.2 (16.0) | 542.6 (11.8) | 482.5 (11.7) | 495.6 (12.7) |
| Positive | 47.1 (6.9) | 48.2 (6.4) | 543.6 (12.6) | 555.4 (13.4) | 496.5 (11.4) | 507.2 (11.3) |
| ANX | ||||||
| Negative* | 59.1 (9.0) | 12.4 (6.4) | 643.4 (29.8) | 615.8 (33.1) | 584.3 (34.0) | 603.5 (32.5) |
| Neutral | 49.0 (7.7) | 43.7 (7.9) | 609.1 (31.7) | 620.6 (31.3) | 560.1 (34.2) | 576.9 (34.3) |
| Positive | 42.3 (5.2) | 48.4 (6.2) | 623.5 (32.2) | 636.5 (31.8) | 581.2 (34.3) | 588.1 (31.9) |
Abbreviations: ANX, anxiety patients; HC, healthy controls.
Collapsed within Groups:
Significant difference between safe and threat (p < .001, 95% CI [−67.2, −38.3]).
Significant difference between negative compared to positive pictures (p < .001; 95% CI [9.2, 34.3]), trend difference between negative compared to neutral pictures (Bonferroni corrected, p = .008; 95% CI [4.0, 25.4]).
Significant difference between negative compared to neutral (p < .001; 95% CI [−44.1, −19.3]) and positive pictures (p < .001, 95% CI [−46.5, −23.0]).
FIGURE 2.
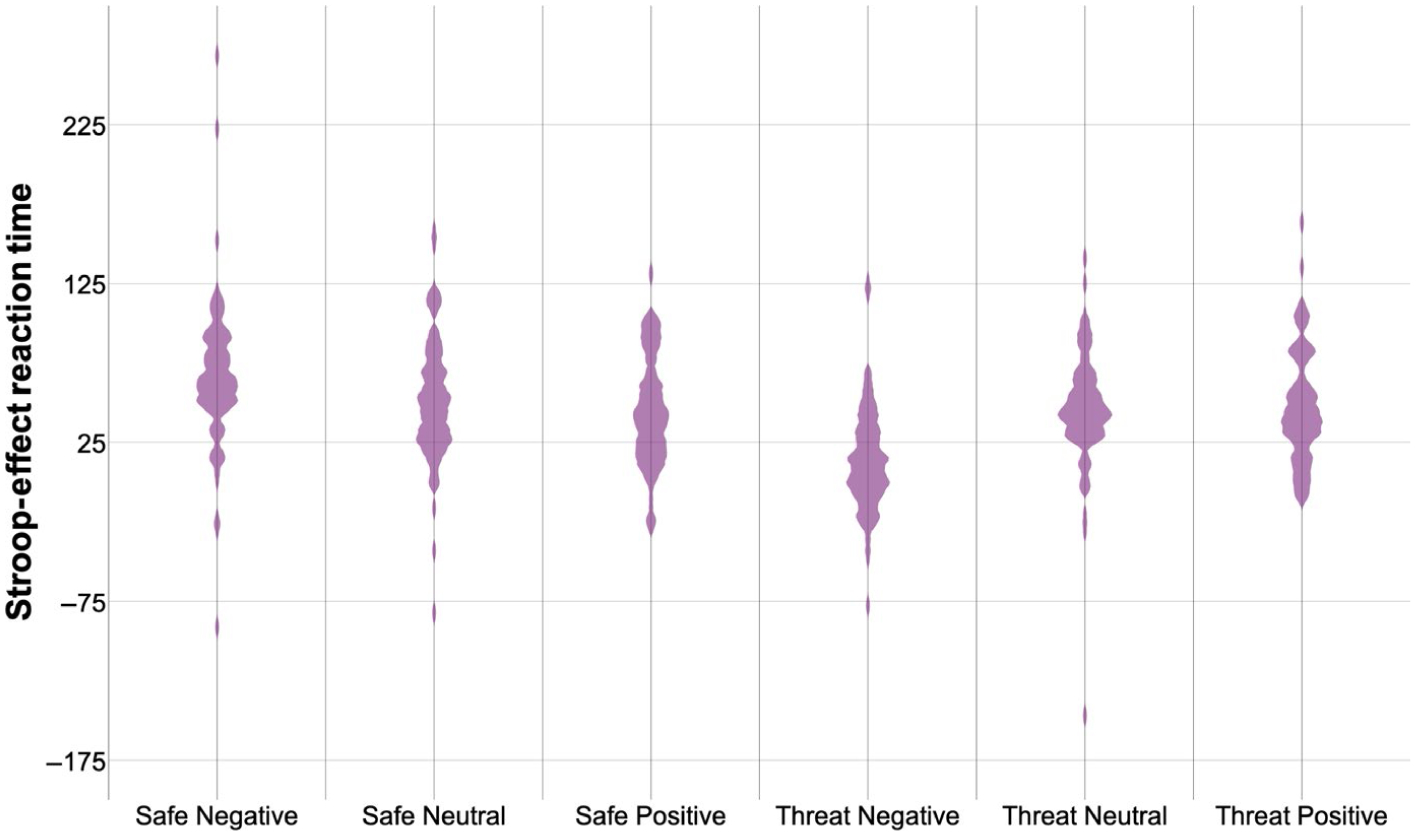
Stroop effect (incongruent minus congruent) reaction time. Violin plot of Stroop effect reaction time (incongruent minus congruent trials; ms) by condition (safe, threat) and emotion (negative, neutral, positive). Shaded areas represent histograms. For means and significant effects, please see Table 3
3.2.2 |. Stroop effect accuracy (Incongruent-Congruent)
There were no main effects or interactions of condition, emotion or diagnosis on Stroop effect accuracy (Table 4, Figure 3).
TABLE 4.
Accuracy (% correct) by group, emotion, Stroop effect (incongruent minus congruent), task and condition. Mean (SE)
| Stroop effect | Incongruent | Congruent | ||||
|---|---|---|---|---|---|---|
| Safe | Threat | Safe | Threat | Safe | Threat | |
| HC | ||||||
| Negative | −0.1 (1.0) | −0.3 (0.8) | 94.2 (0.9) | 95.0 (0.7) | 94.3 (1.1) | 95.3 (0.7) |
| Neutral | −0.5 (1.0) | −0.4 (0.8) | 94.4 (1.1) | 95.8 (0.7) | 94.9 (0.8) | 96.2 (0.8) |
| Positive | 0.2 (1.3) | −0.6 (1.1) | 94.1 (1.0) | 94.3 (0.9) | 93.9 (0.8) | 94.9 (0.9) |
| ANX | ||||||
| Negative | −0.3 (1.0) | −0.6 (0.9) | 94.0 (0.7) | 95.3 (0.8) | 94.3 (0.8) | 95.9 (0.8) |
| Neutral | −0.3 (1.0) | −0.9 (0.8) | 94.8 (0.7) | 95.8 (0.7) | 95.1 (0.8) | 96.7 (0.5) |
| Positive | 0.8 (0.9) | −1.9 (1.0) | 96.5 (0.6) | 94.1 (1.0) | 95.7 (0.7) | 96.0 (0.8) |
Abbreviations: ANX, anxiety patients; HC, healthy controls.
FIGURE 3.
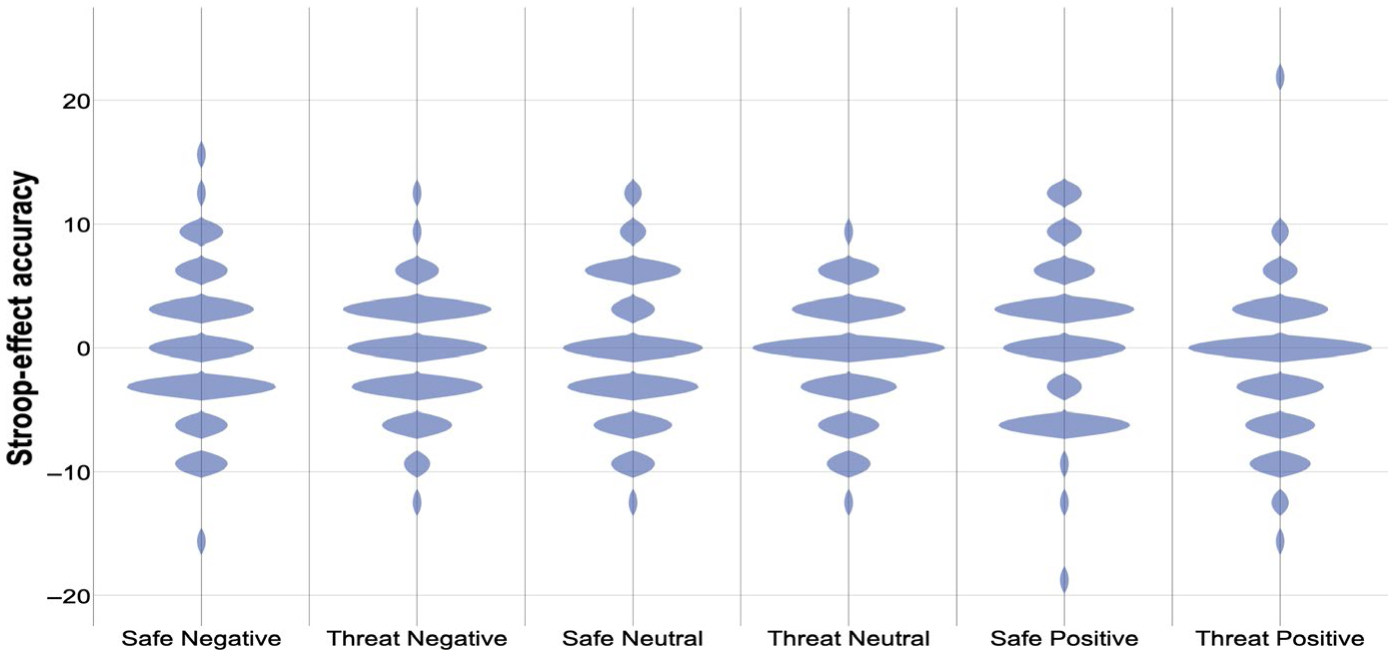
Stroop effect (incongruent minus congruent) accuracy. Violin plot of Stroop effect accuracy (incongruent minus congruent trials; % correct) by condition (safe, threat) and emotion (negative, neutral, positive). Shaded areas represent histograms. For means and significant effects, please see Table 4
3.3 |. Retrospective ratings
ANX participants reported more distress than HC participants during negative pictures (F(1,64)=8.99, p < .01; η2 = 0.12), but ratings of pleasure during Positive pictures did not differ between groups (F(1,64)=0.13, p=.72; η2 =0.00) (Table 2). Additionally, there was a main effect of diagnosis on both subjective anxiety and retrospective difficulty with attention, reflecting higher reports in the ANX than the HC group (respectively: F(1,65) = 12.40, p = .001; η2 = 0.16; F(1,65) = 14.89, p < .001; η2 = 0.19).
Across diagnosis, threat increased reported distress during negative pictures (F(1,64) = 17.38, p < .001; η2 = 0.21), decreased pleasure during positive pictures (F(1,64) = 16.22, p < .001; η2 = 0.20), increased subjective anxiety (F(1,65) = 220.77, p < .001; η2 = 0.77) and increased retrospective difficulty with attention (F(1,65) = 27.43, p < .001; η2 = 0.30). Diagnosis × Condition interactions were not significant.2
4 |. DISCUSSION
The objectives of this study were to determine whether changes in state anxiety modulates the effect of emotion on cognitive control, and, if so, whether this modulation differs as a function of clinical anxiety. To this end, state anxiety was successfully induced using threat-of-shock, a method that has been shown to reliably increase anxiety in both patients and healthy participants (Cha et al., 2014; Grillon, O’Connell, et al., 2017; Grillon, Robinson, et al., 2017; Robinson, Krimsky, et al., 2013; Robinson, Vytal, et al., 2013; Vytal et al., 2016), as evidenced by an increase in ratings of anxiety during the threat compared to the safe condition. Negative, neutral and positive pictures were used as emotional distractors to interfere with cognitive control. The Stroop effect was used as an index of cognitive control and was calculated as the performance difference on incongruent trials minus congruent trials.
Overall, the ANX and HC groups did not differ on emotional interference. The main findings were that the Stroop effect for negative pictures was reduced in the threat compared to the safe condition. In addition, during the safe condition, the Stroop effect was larger during the negative pictures compared to the neutral and positive pictures, while in the threat condition, the Stroop effect was smaller during the negative pictures compared to the neutral and positive pictures. Thus, the combination of threat and negative pictures decreased emotional interference on reaction time. Stroop effect accuracy was not modulated by condition or emotion.
As in other studies (Basten, Stelzel, & Fiebach, 2011; Blair et al., 2012, 2013), reaction time and accuracy results differed. In brief, investigators have suggested that reaction time quantifies processing resources, while accuracy quantifies performance. Therefore, an increase in reaction time without a decrease in accuracy may reflect activation of compensatory strategies prevent impairment of performance (Eysenck & Calvo, 1992; Eysenck, Derakshan, Santos, & Calvo, 2007; Mandrick, Peysakhovich, Rémy, Lepron, & Causse, 2016). Specific to this study, in the safe condition, participants may have maintained performance (Stroop effect accuracy) during negative pictures compared to positive pictures at the cost of more cognitive resources (increased Stroop effect reaction time).
Focusing on Stroop effect reaction time, the opposite effect of putative emotional interference on cognitive control during safe and threat suggests the involvement of distinct attention mechanisms. According to resource theories of anxiety, cognitive resources are finite (reviews Eysenck et al., 2007; Pessoa, 2009). Thus, emotional processing would affect cognitive control when resources are in high demand (i.e. during incongruent compared to congruent trials). Additionally, cognitive resources are preferentially allocated to emotional stimuli at the cost of goal-directed processing (i.e. task). This is consistent with the results in the safe condition: the Stroop effect was greater after negative compared to positive (and a trend compared to neutral) emotional distractors. However, the resource theories of anxiety cannot explain the results in the threat condition. Here, during threat-of-shock, cognitive control was improved during negative emotional distractors (compared to the safe condition and compared to the positive and neutral distractors in the threat condition). Thus, when cognitive resources are taxed to the extent that dual processing cannot take place, goal-directed processing may preferentially consume processing resources in the context of state anxiety. We have reported such prioritization, that is goal-processing at the expense of threat processing, during difficult (high-load) working memory tasks (Vytal et al., 2012). However, it is not clear why taxing cognitive resources would prevent interference from negative, but not positive, stimuli.
An alternative interpretation of the reduced attention interference by negative pictures during threat involves the reported narrowing of attention of anxiety, which increases cognitive performance on some tasks because of decreased attention to task-irrelevant distractors (Easterbrook, 1959; Hu et al., 2012; Pessoa et al., 2002). According to this interpretation, the decreased Stroop effect during threat and negative emotional distractors may reflect passing of an anxiety threshold, after which the ‘spotlight effect’ is engaged. In other words, anxiety caused by threat-of-shock and negative pictures surpassed, while anxiety caused by threat-of-shock with neutral or positive pictures fell below this threshold.
With regard to group effects, our data do not support a difference in emotional interference between ANX and HC. This finding is consistent with previous data from our laboratory and others (Balderston et al., 2017; Bishop, 2009; Carleton et al., 2015; Edwards, Burt, & Lipp, 2010; McClure et al., 2007; van Peer, Spinhoven, & Roelofs, 2010) but is contrary to other studies that report impaired attention with negative, but not neutral, distractors in patients compared to controls (Dresler, Attar, et al., 2012; Price, Eldreth, & Mohlman, 2011). Nevertheless, the current data suggest that emotional interference on attention may be decreased with state anxiety in anxiety patients, suggesting that a modest level of anxiety (neither too high, nor too low) may improve cognitive functioning.
A strength of this study includes an objective, reliable, cross-species induction of state anxiety, that is threat-of-shock, which permits the comparison of high and low state anxiety within subjects (Grillon, Baas, Lissek, Smith, & Milstein, 2004; Robinson et al., 2011; Vytal et al., 2012). Another strength lies in the affective Stroop task design (Blair et al., 2007). The display of emotional distractors prior to and after, as opposed to during, cognitive trials eliminates the likelihood that slowing of reaction time may reflect a defensive (i.e. freezing) response (Clarke & Johnstone, 2013).
Limiting this study, retrospective questionnaires did not measure subjective salience of emotional distractors. Subjects were asked to rate their distress during negative pictures, and pleasure during positive pictures, but this does not allow direct comparison of salience between negative, neutral and positive stimuli. Additionally, results may be confounded by the ANX group comprised of patients with generalized anxiety disorder, social anxiety disorder and/or panic disorder. Although the study design of considering anxiety as a negative valence construct was in accordance with the NIH Research Domain Criteria (RDoC, Kozak & Cuthbert, 2016), differences in symptomatology may reflect heterogeneous attention control processes.
In conclusion, our results support that induced state anxiety decreases negative emotional interference on cognitive control (i.e. Stroop effect) for both anxiety patients and healthy controls. These data support a narrowing of attention whereby a level of anxiety (i.e. in the context of threat-of-shock and negative emotional distractor together, but not with each individually) decreases task-irrelevant processing. Further, emotional interference of attention occurs independently of subjective anxiety and retrospective difficulty with attention. Future studies should replicate these findings and explore potential neural processes underlying these effects.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by the Intramural Research Program of the National Institute of Mental Health (ZIAMH002798), Protocol 03-M-0093, NCT00055224. We gratefully acknowledge Zhi-De Deng, Ph.D., for the graphic abstract figure.
Funding information
Intramural Research Program of the National Institute of Mental Health, Grant/Award Number: ZIAMH002798
Abbreviations:
- ANX
anxiety patients
- HC
healthy controls
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interests.
DATA AVAILABILITY STATEMENT
Data available upon request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
For results of 4-way Diagnosis (HC, ANX) x Emotion (Negative, Neutral, Positive) × Task (Congruent, Incongruent) × Condition (Safe, Threat) rANOVA please see Data S1.
Pearson correlations were conducted between Stroop-Effect reaction time and questionnaires/retrospective ratings as suggested by a reviewer. No correlations reached significance (all p > .05).
REFERENCES
- Balderston NL, Vytal KE, O’Connell K, Torrisi S, Letkiewicz A, Ernst M, & Grillon C (2017). Anxiety patients show reduced working memory related DLPFC activation during safety and threat. Depression and Anxiety, 34(1), 25–36. 10.1002/da.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U, Stelzel C, & Fiebach CJ (2011). Trait anxiety modulates the neural efficiency of inhibitory control. Journal of Cognitive Neuroscience, 23(10), 3132–3145. 10.1162/jocn_a_00003 [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2008). Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences, 1129, 141–152. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12(1), 92–98. 10.1038/nn.2242 [DOI] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, Devido J, Otero M, … Pine DS (2012). Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry, 72, 476–482. 10.1016/j.biopsych.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DGV, Morton J, Vythilingam M, Pessoa L, … Blair RJR (2007). Modulation of emotion by cognition and cognition by emotion. NeuroImage, 35, 430–440. 10.1016/j.neuroimage.2006.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, … Blair RJR (2013). Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychological Medicine, 43, 85–95. 10.1017/S0033291712000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, & Sharma D (2009). Stress reduces attention to irrelevant information: Evidence from the Stroop task. Motivation and Emotion, 33(4), 412–418. 10.1007/s11031-009-9141-5 [DOI] [Google Scholar]
- Carleton RN, Teale Sapach MJN, Oriet C, Duranceau S, Lix LM, Thibodeau MA, … Asmundson GJG (2015). A randomized controlled trial of attention modification for social anxiety disorder. Journal of Anxiety Disorders, 33, 35–44. 10.1016/j.janxdis.2015.03.011 [DOI] [PubMed] [Google Scholar]
- Cha J, Carlson JM, DeDora DJ, Greenberg T, Proudfit GH, & Mujica-Parodi LR (2014). Hyper-reactive human ventral teg-mental area and aberrant mesocorticolimbic connectivity in over-generalization of fear in generalized anxiety disorder. Journal of Neuroscience, 34(17), 5855–5860. 10.1523/JNEUROSCI.4868-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajut E, & Algom D (2003). Selective attention improves under stress: Implications for theories of social cognition. Journal of Personality and Social Psychology, 85(2), 231–248. 10.1037/0022-3514.85.2.231 [DOI] [PubMed] [Google Scholar]
- Choi JM, Padmala S, & Pessoa L (2012). Impact of state anxiety on the interaction between threat monitoring and cognition. NeuroImage, 59, 1912–1923. 10.1016/j.neuroimage.2011.08.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, & Koster EHW (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30, 203–216. 10.1016/j.cpr.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, & Johnstone T (2013). Prefrontal inhibition of threat processing reduces working memory interference. Frontiers in Human Neuroscience, 7(228), 1–17. 10.3389/fnhum.2013.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, … Heller W (2003). Paying attention to emotion: An fMRI investigation of cognitive and emotional stroop tasks. Cognitive, Affective and Behavioral Neuroscience, 3(2), 81–96. 10.3758/CABN.3.2.81 [DOI] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, & Wittchen H-U (2017). Anxiety Disorders. Nature Reviews Disease Primers, 3(17024), 1–18. 10.1038/nrdp.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler T, Attar CH, Spitzer C, Löwe B, Deckert J, Büchel C, … Fallgatter AJ (2012). Neural correlates of the emotional Stroop task in panic disorder patients: An event-related fMRI study. Journal of Psychiatric Research, 46, 1627–1634. [DOI] [PubMed] [Google Scholar]
- Dresler T, Ehlis A-C, Hindi Attar C, Ernst LH, Tupak SV, Hahn T, … Fallgatter AJ (2012). Reliability of the emotional Stroop task: An investigation of patients with panic disorder. Journal of Psychiatric Research, 46, 1243–1248. 10.1016/j.jpsychires.2012.06.006 [DOI] [PubMed] [Google Scholar]
- Easterbrook JA (1959). The effect of emotion on cue utilization and the organization of behavior. Psychological Review, 66(3), 183–201. 10.1037/h0047707 [DOI] [PubMed] [Google Scholar]
- Edwards MS, Burt JS, & Lipp OV (2010). Selective attention for masked and unmasked emotionally toned stimuli: Effects of trait anxiety, state anxiety, and test order. British Journal of Psychology, 101, 325–343. 10.1348/000712609X466559 [DOI] [PubMed] [Google Scholar]
- Ernst M, Lago T, Davis A, & Grillon C (2016). The effects of methylphenidate and propranolol on the interplay between induced-anxiety and working memory. Psychopharmacology (Berl), 233(19–20), 3565–3574. 10.1007/s00213-016-4390-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, & Calvo MG (1992). Anxiety and Performance: The Processing Efficiency Theory. Cognition and Emotion, 6(6), 409–434. 10.1080/02699939208409696 [DOI] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336–353. 10.1037/1528-3542.7.2.336 [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Clendinen C, Hardy RA, Surapaneni S, Blair JR, … Bradley B (2019). Attentional control abnormalities in posttraumatic stress disorder: Functional, behavioral, and structural correlates. Journal of Affective Disorders, 253, 343–351. 10.1016/j.jad.2019.04.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Becker B, Huang W, Wu X, Eickhoff SB, & Chen T (2018). Neural substrates of the emotion-word and emotional counting Stroop tasks in healthy and clinical populations: A meta-analysis of functional brain imaging studies. NeuroImage, 173, 258–274. 10.1016/j.neuroimage.2018.02.023 [DOI] [PubMed] [Google Scholar]
- First MB (2002). The DSM Series and Experience with DSM-IV. Psychopathology, 35, 67–71. 10.1159/000065121 [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, & Milstein J (2004). Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience, 118(5), 916–924. 10.1037/0735-7044.118.5.916 [DOI] [PubMed] [Google Scholar]
- Grillon C, O’Connell K, Lieberman L, Alvarez G, Geraci M, Pine DS, & Ernst M (2017). Distinct responses to predictable and unpredictable threat in anxiety pathologies: Effect of panic attack. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(7), 575–581. 10.1016/j.bpsc.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Robinson OJ, O’Connell K, Davis A, Alvarez G, Pine DS, & Ernst M (2017). Clinical anxiety promotes excessive response inhibition. Psychological Medicine, 47(3), 484–494. 10.1017/S0033291716002555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Ivkovic A, & Fricchione GL (2012). Generalized anxiety disorder: Diagnosis and treatment. BMJ, 345, e7500–e7500. 10.1136/bmj.e7500 [DOI] [PubMed] [Google Scholar]
- Hu K, Bauer A, Padmala S, & Pessoa L (2012). Threat of bodily harm has opposing effects on cognition. Emotion, 12(1), 28–32. 10.1037/a0024345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, White SF, Nolan ZT, Sinclair S, & Blair RJR (2014). Neurodevelopmental changes in the responsiveness of systems involved in top down attention and emotional responding. Neuropsychologia, 62, 277–285. 10.1016/j.neuropsychologia.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna MM, Badura-Brack AS, McDermott TJ, Embury CM, Wiesman AI, Shepherd A, … Wilson TW (2017). Veterans with post-traumatic stress disorder exhibit altered emotional processing and attentional control during an emotional Stroop task. Psychological Medicine, 47(11), 2017–2027. 10.1017/S0033291717000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Kinney KL, Kennedy AE, Shankman SA, Langenecker SA, Kumar A, & Phan KL (2018). Trait attentional control modulates neurofunctional response to threat distractors in anxiety and depression. Journal of Psychiatric Research, 102, 87–95. 10.1016/j.jpsychires.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH research domain criteria initiative: Background, issues and pragmatics. Psychophysiology, 53, 286–297. [DOI] [PubMed] [Google Scholar]
- Lago TR, Hsiung A, Leitner BP, Duckworth CJ, Balderston NL, Chen KY, … Ernst M (2019). Exercise modulates the interaction between cognition and anxiety in humans. Cognition and Emotion, 33(4), 863–870. 10.1080/02699931.2018.1500445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, & Greenwald MK (1988). The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgements (Techical No. 1A & 1B). Gainesville, FL: University of Florida. [Google Scholar]
- Liu X, Yang Y, Jiang S, & Li J (2018). The facilitating effect of positive emotions during an emotional Stroop task. NeuroReport, 29(11), 883–888. 10.1097/WNR.0000000000001048 [DOI] [PubMed] [Google Scholar]
- Mandrick K, Peysakhovich V, Rémy F, Lepron E, & Causse M (2016). Neural and psychophysiological correlates of human performance under stress and high mental workload. Biological Psychology, 121, 62–73. 10.1016/j.biopsycho.2016.10.002 [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, … Pine DS (2007). Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry, 64, 97–106. 10.1001/archpsyc.64.1.97 [DOI] [PubMed] [Google Scholar]
- Minkova L, Sladky R, Kranz GS, Woletz M, Geissberger N, Kraus C, … Windischberger C (2017). Task-dependent modulation of amygdala connectivity in social anxiety disorder. Psychiatry Research Neuroimaging, 262, 39–46. 10.1016/j.pscychresns.2016.12.016 [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Richell RA, Leonard A, & Blair RJR (2006). Emotion at the expense of cognition: Psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology, 115(3), 559–566. 10.1037/0021-843X.115.3.559 [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Naim R, Bakermans-Kranenburg MJ, van IJzendoorn MH, & Bar-Haim Y (2015). Content specificity of attention bias to threat in anxiety disorders: A meta-analysis. Clinical Psychology Review, 35, 10–18. 10.1016/j.cpr.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Pessoa L (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13(4), 160–166. 10.1016/j.tics.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, & Ungerleider LG (2002). Attentional control of the processing of neural and emotional stimuli. Cognitive Brain Research, 15, 31–45. [DOI] [PubMed] [Google Scholar]
- Price RB, Eldreth DA, & Mohlman J (2011). Deficient prefrontal attentional control in late-life generalized anxiety disorder: An fMRI investigation. Translational Psychiatry, 1, e46. 10.1038/tp.2011.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Fehlbaum LV, Menks WM, Euler F, Sterzer P, & Stadler C (2017). Investigating the neural correlates of emotion-cognition interaction using an affective Stroop task. Frontiers in Psychology, 8(1489), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, & Grillon C (2013). The impact of induced anxiety on response inhibition. Frontiers in Human Neuroscience, 7(69), 1–5. 10.3389/fnhum.2013.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, & Grillon C (2011). The effect of induced anxiety on cognition: Threat of shock enhances aversive processing in healthy individuals. Cognitive, Affective & Behavioral Neuroscience, 11, 217–227. 10.3758/s13415-011-0030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, & Grillon C (2013). The impact of anxiety upon cognition: Perspectives from human threat of shock studies. Frontiers in Human Neuroscience, 7(203), 1–21. 10.3389/fnhum.2013.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Zilverstand A, Song H, d’Oleire Uquillas F, Wang Y, Xie C, … Zou Z (2017). The influence of emotional interference on cognitive control: A meta-analysis of neuroimaging studies using the emotional Stroop task. Scientific Reports, 7(1), 2088. 10.1038/s41598-017-02266-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto: Consulting Psychologists Press. [Google Scholar]
- van Peer JM, Spinhoven P, & Roelofs K (2010). Psychophysiological evidence for cortisol-induced reduction in early bias for implicit social threat in social phobia. Psychoneuroendocrinology, 35, 21–32. 10.1016/j.psyneuen.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Stickel S, Eickhoff S, Goecke TW, Schneider F, Quinete NS, Lang J, … Chechko N (2019). Cumulative cortisol exposure in the third trimester correlates with postpartum mothers’ neural response to emotional interference. Biological Psychology, 143, 53–61. 10.1016/j.biopsycho.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Vytal KE, Arkin NE, Overstreet C, Lieberman L, & Grillon C (2016). Induced-anxiety differentially disrupts working memory in generalized anxiety disorder. BMC Psychiatry, 16(62), 1–9. 10.1186/s12888-016-0748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal KE, Cornwell B, Arkin N, & Grillon C (2012). Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology, 49, 842–852. 10.1111/j.1469-8986.2012.01358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Blair KS, McCAFFREY D, Scaramozza M, Jones M, Nakic M, … Blair R j r (2007). Biased emotional attention in post-traumatic stress disorder: A help as well as a hindrance? Psychological Medicine, 37, 1445–1455. 10.1017/S003329170700092X [DOI] [PubMed] [Google Scholar]
- White SF, Costanzo ME, Blair JR, & Roy MJ (2015). PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. NeuroImage: Clinical, 7, 19–27. 10.1016/j.nicl.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, & MacLeod C (1996). The emotional stroop task and psychopathology. Psychological Bulletin, 120(1), 3–24. 10.1037/0033-2909.120.1.3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


