Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, myoclonus, neurocritical care, neuroinfectious diseases
Abstract
Objectives:
To describe the risk factors for and outcomes after myoclonus in a cohort of patients with coronavirus disease 2019.
Design:
Multicenter case series.
Setting:
Three tertiary care hospitals in Massachusetts, Georgia, and Virginia.
Patients:
Eight patients with clinical myoclonus in the setting of coronavirus disease 2019.
Interventions & Measurements and Main Results:
Outcomes in patients with myoclonus were variable, with one patient who died during the study period and five who were successfully extubated cognitively intact and without focal neurologic deficits. In five cases, the myoclonus completely resolved within 2 days of onset, while in three cases, it persisted for 10 days or longer. Seven patients experienced significant metabolic derangements, hypoxemia, or exposure to sedating medications that may have contributed to the development of myoclonus. One patient presented with encephalopathy and developed prolonged myoclonus in the absence of clear systemic provoking factors.
Conclusions:
Our findings suggest that myoclonus may be observed in severe acute respiratory syndrome coronavirus 2 infected patients, even in the absence of hypoxia. This association warrants further evaluation in larger cohorts to determine whether the presence of myoclonus may aid in the assessment of disease severity, neurologic involvement, or prognostication.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that has caused a global pandemic of coronavirus disease 2019 (COVID-19) (1). SARS-CoV-2 has been identified in the cerebrospinal fluid (CSF) of patients with clinical meningoencephalitis in rare cases (2–6), and both SARS-CoV-2 and related coronaviruses have been isolated in neural tissue, including the brainstem, cerebrum, and thalamus (7–9).
Myoclonus is an involuntary movement common among critically ill patients. It is characterized by sudden, brief, and sometimes repetitive muscle contractions variably involving the face, extremities, and trunk and can be seen in association with specific neurologic disorders or can result secondarily from a host of toxic or metabolic triggers, including both mild and severe hypoxic brain injury and sedating medications (10). The significance of myoclonus in neuroprognostication is highly variable and depends on the underlying etiology and both clinical and neurophysiologic features (11).
Myoclonus has been observed in patients infected with other neuroinvasive RNA viruses (12). However, myoclonus has only rarely been reported in patients with COVID-19 (13). Here, we describe eight cases of COVID-19 complicated by myoclonus.
MATERIALS AND METHODS
This study was either approved or exempted by the institutional review boards of Boston Medical Center, Emory University Hospital, and Inova Fairfax Medical Campus.
RESULTS
Patient Characteristics
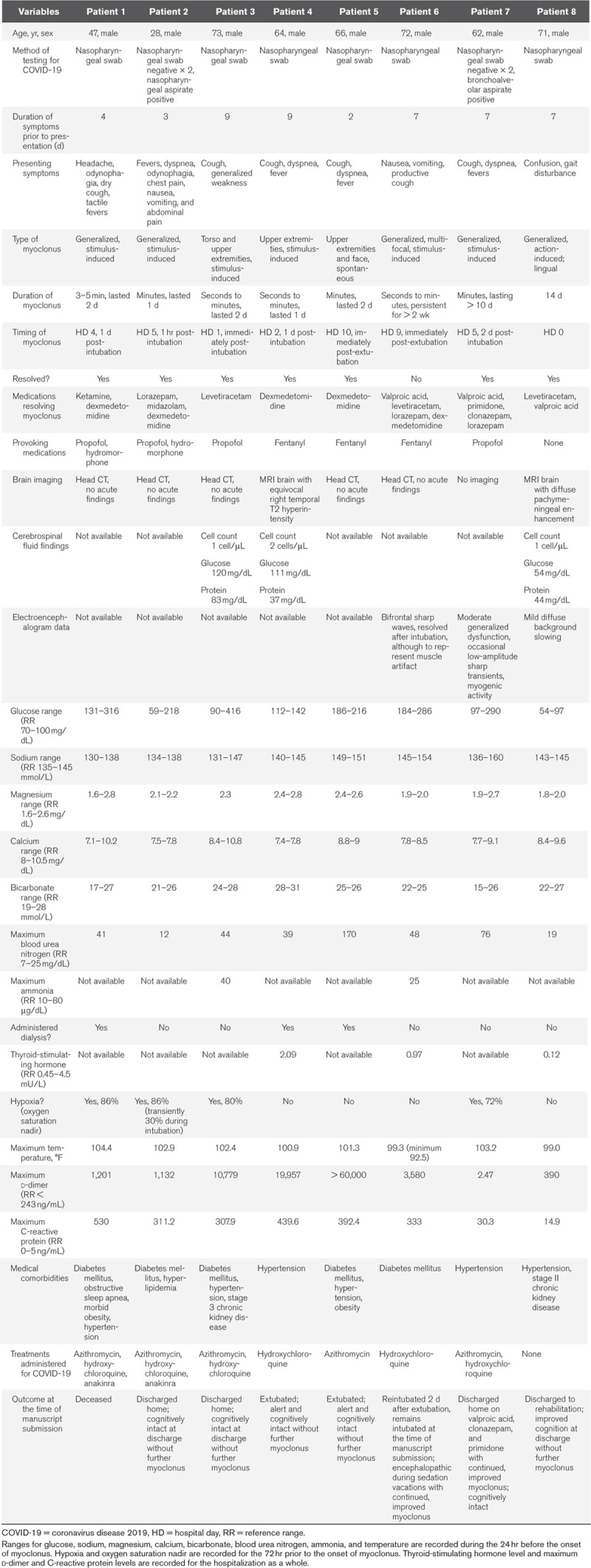
All patients were male, ranging in age from 28 to 73 (Table 1). Common comorbidities included diabetes mellitus in five of eight patients, hypertension (five patients), obesity (two patients), and chronic kidney disease (two patients). Obstructive sleep apnea was present in one patient.
Table 1.
Characteristics and Outcomes of Patients With Myoclonus and Coronavirus Disease 2019
Features of COVID-19
Presenting symptoms of COVID-19 included headache, odynophagia, fever, dyspnea, chest pain, nausea, vomiting, abdominal pain, and both dry and productive cough. One patient did not have respiratory or gastrointestinal symptoms but developed confusion and gait disturbance prior to presentation (case 8). In all cases, COVID-19 was identified based on characteristic clinical features and positive polymerase chain reaction testing for SARS-CoV-2 from nasopharyngeal or respiratory specimens. In two cases (numbers 2 and 7), two successive nasopharyngeal swab samples tested negative for SARS-CoV-2, and the diagnosis was made only with a third sample (nasopharyngeal aspirate in case 2 and bronchoalveolar lavage in case 7). Patients presented with between 2 and 9 days of respiratory and systemic symptoms. In six cases, patients had an elevated temperature during the 24 hours prior to the onset of myoclonus (100.9–104.4°F). In all cases, patients had elevated C-reactive protein (14.9–530 mg/L) and d-dimer (2.47 to > 60,000 ng/mL) levels during their hospitalization. One patient also had significant spontaneous hypothermia during the 24 hours prior to myoclonus onset (92.5°F). Seven patients had respiratory failure requiring intubation during their hospitalization, while one patient was not critically ill and did not require intensive care. Treatments administered for COVID-19 included hydroxychloroquine (six patients), azithromycin (five patients), and anakinra (two patients).
Myoclonus Risk Factors and Onset
In four of eight cases, the onset of myoclonus was within 24 hours of intubation, while in two cases, it occurred immediately post-extubation, and in one case, it occurred 2 days after intubation. In four cases, significant hypoxia was documented during the 72 hours before the onset of myoclonus (oxygen saturation range 30–86% by pulse oximeter). Metabolic derangements were noted during the 24 hours before the onset of myoclonus in all cases, including hypo- and hyperglycemia (glucose range 54–416 mg/dL), hypo- and hypernatremia (sodium range 130–160 mmol/L), and uremia (blood urea nitrogen range 12–170 mg/dL). Three of eight cases required renal replacement therapy for renal failure. In seven cases, neuroactive medications with the potential to provoke myoclonus were used for sedation, including propofol, hydromorphone, and fentanyl.
Myoclonus Semiology and Duration
In seven cases, myoclonus was stimulus- or action-induced, with just one case of spontaneous myoclonus. In five cases, myoclonus was generalized (Supplemental Video 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F731; and Supplemental Video 2, Supplemental Digital Content 2, http://links.lww.com/CCM/F732 [legend, Supplemental Digital Content 3, http://links.lww.com/CCM/F733]), while in one case, it was limited to the upper extremities and in the remaining two, the torso or face were also involved. The duration of myoclonus ranged from 1 to 14 days. Two patients (cases 6 and 7) continued to have myoclonus at the time of manuscript submission, including one patient who was discharged home.
Ancillary Neurologic Testing
Seven of eight patients had brain imaging, including five patients with CT and two with MRI (cases 4 and 8). No acute findings were seen on any of the head CTs, which were all done without contrast. The MRI in case 4 showed a temporal T2 hyperintensity of unclear significance without associated enhancement with gadolinium administration. The MRI in case 8 showed diffuse pachymeningeal enhancement. Twenty-four hour electroencephalography was obtained in three cases during myoclonus (cases 6, 7, and 8) and revealed significant myogenic activity and generalized cerebral dysfunction in all cases. CSF studies were obtained in three cases (3, 4, and 8) and revealed normal nucleated cell counts in all cases, with mildly elevated protein of 83 in case 3. SARS-CoV-2 testing was not available from CSF.
Myoclonus Treatments and Outcomes
Medications used with improvement of myoclonus included valproic acid, primidone, clonazepam, lorazepam, levetiracetam, dexmedetomidine, midazolam, and ketamine. Outcomes were variable, with one patient who was deceased at the time of manuscript submission, one who remained intubated, three who were discharged home cognitively intact, one who was discharged to rehabilitation with cognitive improvement, and two who were extubated, with improved mental status and without focal neurologic deficits. In five cases, the myoclonus completely resolved within 2 days of onset, while in three cases (cases 6, 7, and 8), it persisted for 10 days or longer. The presence of hypoxia did not clearly correlate with either the duration of myoclonus or the functional outcome: one patient who did not experience hypoxia remains intubated with continued myoclonus, while three patients who did experience hypoxia were discharged home cognitively intact. One patient continued to have myoclonus at discharge, although it was significantly improved on valproic acid, clonazepam, and primidone. A second patient who remains intubated continues to have myoclonus during sedation breaks.
DISCUSSION
Initial reports suggest that 5% of patients with COVID-19 will become critically ill, requiring intensive care (14). Limited literature suggests that critically ill COVID-19 patients are more likely to have neurologic complications, including cerebrovascular events, impaired consciousness, and skeletal muscle injury (15, 16). It is unclear whether these complications represent true CNS invasion by the virus or whether they result more generally from critical illness. More recent reports have described encephalitis or meningoencephalitis in patients with COVID-19 with or without respiratory symptoms (2–6). In these cases, SARS-CoV-2 has been identified in the CSF. Notably, early studies have found that the rates of both mortality and critical illness are higher in male patients with COVID-19 than in their female counterparts, a finding reflected in the demographics of the all-male cohort described here (17).
Myoclonus in critically ill patients can result from a range of etiologies, including medication toxicity, cerebral hypoxia, traumatic brain or spinal cord injury, metabolic derangements, and inflammatory and infectious encephalitides (10). The myoclonus observed in patients with COVID-19 may reflect multiple contributing factors. Myoclonus has been reported in the setting of other neuroinvasive RNA viruses (9, 10), and nervous system involvement by COVID-19 may contribute. In our series, metabolic derangements occurred in all cases, consistent with preliminary reports suggesting that hyponatremia and early renal failure may be common features of COVID-19 (18). Seven of the patients included in our series had some exposure to sedating medications with the potential to precipitate myoclonus. Finally, four of the patients in our series experienced periods of significant hypoxia, although the presence of hypoxia did not correlate with either functional outcome or duration of myoclonus. Notably, one patient who was not critically ill and did not require intubation developed prolonged myoclonus and encephalopathy in the setting of pachymeningeal enhancement on MRI.
Myoclonus occurs in 20% of cardiac arrest patients within 72 hours of cardiopulmonary resuscitation (19), but less is known about the frequency of myoclonus in patients who experience hypoxia without cardiac arrest. Preliminary reports suggest that paucisymptomatic hypoxia may be common in patients with COVID-19, even prior to hospital presentation (20). Patients with COVID-19 may therefore experience silent and prolonged hypoxia prior to admission that could contribute to the occurrence of myoclonus in this particular patient population.
Although ancillary neurologic testing, including neuroimaging, electroencephalography, and CSF studies, may be helpful in characterizing the etiologies of myoclonus, availability of these studies during a pandemic may be limited. In our series, CSF studies and electroencephalography were available for just three patients, while MRI brain was available for only two. Given limited resources, critical care providers must rely on clinical findings to determine whether to pursue available ancillary testing. In our experience, for patients with movements consistent with a diagnosis of myoclonus and without concern for seizure (suggested by a nonfocal neurologic examination and normal consciousness), electroencephalography may be safely deferred. Conversely, in situations where a patient has altered consciousness and movements that cannot be clinically distinguished between myoclonus and seizure, a trial of an anti-seizure medication may be considered, as this could potentially treat both conditions. For patients with altered consciousness and myoclonic movements who do not respond to anti-seizure medication trial, we strongly recommend pursing electroencephalography.
The significance of myoclonus in neuroprognostication for critically ill patients is highly variable, as is treatment responsiveness. Both are determined largely by etiology and neuroanatomic localization; cortical myoclonus caused by toxic or metabolic encephalopathy may be partially or completely reversible with medication cessation or correction of metabolic derangements, while myoclonus associated with cerebral infection or hypoxic injury is more often treatment refractory (10). Although myoclonic status epilepticus following hypoxic brain injury was previously thought to be associated with a high mortality rate, more recent studies suggest that a minority of patients with myoclonus after hypoxic brain injury have favorable outcomes, more often in those without epileptiform activity and with a preserved background rhythm (21), although a broad range of outcomes can also be seen depending on the electrographic pattern observed (22). A less common post-hypoxic action myoclonus, Lance-Adams syndrome, may occur after a cardiopulmonary arrest and is associated with a favorable neurologic prognosis (23).
In our cohort, outcomes after myoclonus were highly variable, with one deceased patient and five who were successfully extubated cognitively intact without focal neurologic deficits. In five cases, the myoclonus completely resolved within 2 days of onset, while in three cases, it persisted for 10 days or longer.
In cases of COVID-19, myoclonus may be multifactorial and represent a combination of hypoxia, medication toxicity, and direct or para-infectious complications of the virus itself. Our findings suggest that myoclonus may be observed in SARS-CoV-2 infected patients regardless of respiratory failure. This association warrants further evaluation in larger cohort studies to determine whether the presence of myoclonus may aid in the assessment of disease severity, neurologic involvement, or prognostication.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.World Health Organization: Coronavirus Disease 2019 (COVID-19) Situation Report-61. World Health Organization. 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200321-sitrep-61-covid-19.pdf?sfvrsn=ce5ca11c_2. Accessed June 2, 2020
- 2.Ye M, Ren Y, Lv L. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun 2020; 88:945–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun 2020; 87:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020; 94:55–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poyiadji N, Shahin G, Noujaim D, et al. COVID -19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020; 296:201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benameur K, Agarwal A, Auld S, et al. Encephalopathy. and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis J. 2020 doi: 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin Infect Dis 2005; 41:1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383:590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netland J, Meyerholz DK, Moore S, et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008; 82:7264–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutter R, Ristic A, Rüegg S, et al. Myoclonus in the critically ill: Diagnosis, management, and clinical impact. Clin Neurophysiol 2016; 127:67–80 [DOI] [PubMed] [Google Scholar]
- 11.Freund B, Kaplan PW. Post-hypoxic myoclonus: Differentiating benign and malignant etiologies in diagnosis and prognosis. Clin Neurophysiol Pract 2017; 2:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallivalappil B, Ali A, Thulaseedharan NK, et al. Dissecting an outbreak: A clinico-epidemiological study of Nipah virus infection in Kerala, India, 2018. J Glob Infect Dis 2020; 12:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, et al. Generalized. myoclonus in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 30;382:1708-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. medRxiv. 2020 [Google Scholar]
- 16.Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc Neurol 2020;svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maleki Dana P, Sadoughi F, Hallajzadeh J, et al. An insight into the sex differences in COVID-19 patients: What are the possible causes? Prehosp Disaster Med 2020; 35:438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong X, Chi Z, Liu G, et al. Analysis of early renal injury in COVID-19 and diagnostic value of multi-index combined detection. medRxiv. 2020 [Google Scholar]
- 19.Bouwes A, van Poppelen D, Koelman JH, et al. Acute posthypoxic myoclonus after cardiopulmonary resuscitation. BMC Neurol 2012; 12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cascella M, Rajnik M, Cuomo A, et al. StatPearls. 2020. Features, evaluation and treatment coronavirus (COVID-19). Jan-. Available at: https://www.ncbi.nlm.nih.gov/books/NBK554776/. Accessed August 6, 2020. [PubMed] [Google Scholar]
- 21.Seder DB, Sunde K, Rubertsson S, et al. Neurologic outcomes and postresuscitation care of patients with myoclonus following cardiac arrest. Crit Care Med 2015; 43:965–972 [DOI] [PubMed] [Google Scholar]
- 22.Elmer J, Rittenberger JC, Faro J, et al. Pittsburgh Post-Cardiac Arrest Service: Clinically distinct electroencephalographic phenotypes of early myoclonus after cardiac arrest. Ann Neurol 2016; 80:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lance JW, Adams RD. The syndrome of intention or action myoclonus as a sequel to hypoxic encephalopathy. Brain 1963; 86:111–136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


