Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, mechanical ventilation, mortality
Abstract
Objectives:
Increasing time to mechanical ventilation and high-flow nasal cannula use may be associated with mortality in coronavirus disease 2019. We examined the impact of time to intubation and use of high-flow nasal cannula on clinical outcomes in patients with coronavirus disease 2019.
Design:
Retrospective cohort study.
Setting:
Six coronavirus disease 2019-specific ICUs across four university-affiliated hospitals in Atlanta, Georgia.
Patients:
Adults with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection who received high-flow nasal cannula or mechanical ventilation.
Interventions:
None.
Measurements and Main Results:
Among 231 patients admitted to the ICU, 109 (47.2%) were treated with high-flow nasal cannula and 97 (42.0%) were intubated without preceding high-flow nasal cannula use. Of those managed with high-flow nasal cannula, 78 (71.6%) ultimately received mechanical ventilation. In total, 175 patients received mechanical ventilation; 44.6% were female, 66.3% were Black, and the median age was 66 years (interquartile range, 56–75 yr). Seventy-six patients (43.4%) were intubated within 8 hours of ICU admission, 57 (32.6%) between 8 and 24 hours of admission, and 42 (24.0%) greater than or equal to 24 hours after admission. Patients intubated within 8 hours were more likely to have diabetes, chronic comorbidities, and higher admission Sequential Organ Failure Assessment scores. Mortality did not differ by time to intubation (≤ 8 hr: 38.2%; 8–24 hr: 31.6%; ≥ 24 hr: 38.1%; p = 0.7), and there was no association between time to intubation and mortality in adjusted analysis. Similarly, there was no difference in initial static compliance, duration of mechanical ventilation, or ICU length of stay by timing of intubation. High-flow nasal cannula use prior to intubation was not associated with mortality.
Conclusions:
In this cohort of critically ill patients with coronavirus disease 2019, neither time from ICU admission to intubation nor high-flow nasal cannula use were associated with increased mortality. This study provides evidence that coronavirus disease 2019 respiratory failure can be managed similarly to hypoxic respiratory failure of other etiologies.
The optimal management approach to respiratory failure in coronavirus disease 2019 (COVID-19) is not yet established. Early reports of high mortality in mechanically ventilated adults with COVID-19 raised concern that respiratory failure related to COVID-19 may be different from other causes of the acute respiratory distress syndrome (ARDS) (1–3). More recent reports have suggested that mortality rates and clinical features mimic those of ARDS from other causes (4–6). However, whether management of respiratory failure in COVID-19 should be similar to management in other causes of ARDS has not been studied.
Deciding if and when to intubate and mechanically ventilate a patient with respiratory failure is a complex decision based on both patient disease severity and provider judgment and may have significant implications for patient outcomes. For example, delayed mechanical ventilation has been associated with worsened clinical outcomes in ARDS (7). High respiratory drive leading to self-induced lung injury (SILI) has been posited as a potential mechanism underlying these observations (8). Similarly, use of high-flow nasal cannula, which can provide 30–60 L/min of supplemental oxygen and decrease dead space (9), may delay recognition of clinical deterioration, thereby prolonging time to intubation and worsening SILI. The role of high-flow nasal cannula and the optimal timing of intubation in COVID-19 are unknown. Despite concern that high respiratory drive might hasten disease progression and worsen static compliance (10), this has not been shown in several cohort studies of patients with COVID-19 (1, 5, 6, 11). Larger studies with longer follow-up of mechanically ventilated patients are needed to determine whether management of patients with COVID-19-associated respiratory failure, including timing of intubation and use of high-flow nasal cannula, is associated with clinical outcomes including static compliance and mortality.
To address these outstanding questions, we conducted a cohort study of patients with COVID-19 who received mechanical ventilation or high-flow nasal cannula at four university-affiliated hospitals. Our primary hypothesis was that longer time from ICU admission to intubation would be associated with increased mortality. In addition, we examined the impact of time from ICU admission to intubation on secondary clinical outcomes, including ICU length of stay, duration of mechanical ventilation, initial Pao2:Fio2 (P:F) ratio, and static compliance. Last, we determined whether use of high-flow nasal cannula prior to mechanical ventilation was associated with mortality.
MATERIALS AND METHODS
Study Setting and Patients
This retrospective cohort study was conducted within six COVID-specific ICUs at four Emory Healthcare hospitals in Atlanta, Georgia. These ICUs were all traditional critical care environments, including two medical ICUs, three medical-surgical ICUs, and one surgical ICU. This study includes all adults (≥ 18 yr) with a positive severe acute respiratory syndrome coronavirus 2 polymerase chain reaction test admitted to one of the COVID-designated ICUs from March 6, 2020, to May 7, 2020. No study sample size was prespecified. Patients were excluded if they were 1) intubated prior to transfer from another facility; 2) had a “do-not-intubate” order; or 3) remained in the ICU less than 12 hours. For patients with more than one COVID-19-associated ICU admission (n = 8), data from the first admission requiring either mechanical ventilation or high-flow nasal cannula were analyzed.
All decisions about clinical care were made by treating clinicians. Institutional guidelines initially recommended against the use of high-flow nasal cannula out of concern for aerosolization; however, that policy was changed on March 25, 2020, in light of evidence demonstrating minimal particle dispersion relative to other modalities of oxygen delivery (12). In contrast, institutional guidelines recommended against the use of noninvasive positive pressure ventilation throughout the study period.
Data Collection
Patient data were obtained from the electronic medical record (EMR; Cerner Millennium EMR, Kansas City, MO) and proprietary data collection software developed on the Oracle Apex platform (Redwood City, CA). Codified and continuous data elements were automatically extracted from the EMR. EMR documentation is consolidated nightly via an extract, transform, and load process and then indexed to support advanced analytics. Data were abstracted through June 22, 2020.
Variables of Interest
Age, sex, race, height, and weight were extracted as raw variables. Comorbidities, which were used to calculate the Elixhauser comorbidity index (13), were considered present if either documented in a patient’s problem list or as an International Classification of Diseases, 10th Revision code. Laboratory values were extracted as the closest value within 72 hours of ICU admission. Sequential Organ Failure Assessment (SOFA) scores were calculated using values closest to ICU admission.
Oxygen requirements and mechanical ventilation settings are typically charted every 4 hours by respiratory therapists. The level of supplemental oxygen administered was extracted from ICU admission until day 15 according to the following categories: room air (i.e., no supplemental oxygen), 1–6 L/min, 7–15 L/min, high-flow nasal cannula (i.e., heated and humidified oxygen at flow rates from 30 to 60 L/min), and mechanical ventilation. The P:F ratio was calculated at the time of intubation. Static compliance of the respiratory system was calculated by dividing the exhaled tidal volume over the difference between the plateau pressure and positive end-expiratory pressure (PEEP). Driving pressure was calculated by subtracting PEEP from plateau pressure.
Study Exposures and Outcomes
The primary exposure of time from ICU admission to intubation was categorized as less than 8 hours, 8–24 hours, and greater than or equal to 24 hours based on the data distribution and author consensus. Patients intubated prior to arrival in the ICU, for example, in the emergency department, were classified as having been intubated in the first 8 hours of ICU admission. Receipt of high-flow nasal cannula was defined as any receipt of this oxygen modality prior to mechanical ventilation. The primary outcome was in-hospital death after receipt of mechanical ventilation. Secondary outcomes included the duration of mechanical ventilation, ICU length of stay, and first P:F ratio and static compliance after intubation. Static compliance was stratified by initial value (< 40 and ≥ 40 mL/cm H2O) and examined longitudinally (14).
Statistical Analysis
Categorical and continuous variables were described as proportions and medians with interquartile ranges (IQRs), respectively. Differences were tested using either chi-square test or Fisher exact test, and Kruskal-Wallis test with a two-sided p value of less than 0.05 considered statistically significant. Noninteraction logistic regression models were created to determine the association between the time from ICU admission to intubation and mortality. Variables were included in the multivariate models on the basis of clinical relevance or a bivariate association with a p value of less than 0.1. The Hosmer-Lemeshow test was used to assess model goodness-of-fit. A Sankey plot was created to depict the oxygen requirements and clinical trajectory of patients over the first 15 days after ICU admission. Analyses were conducted using SAS Version 9.4 (SAS, Cary, NC) and R Version 4.0 (networkD3 and tidyverse packages) (15, 16) was used to create the Sankey plot.
Ethics
This study was approved by the Emory University Institutional Review Board with a waiver of informed consent. This article was prepared in accordance with the Reporting of studies Conducted using Observational Routinely-collected Data Guidelines (17).
RESULTS
Study Population
Between March 6, 2020, and May 7, 2020, there were 730 patients admitted to the study hospitals with COVID-19. Two-hundred eighty-four patients (38.9%) were admitted to the ICU and 231 (81.3%) were included in this analysis (Fig. 1). Fifty-three patients (18.7%) were excluded based on intubation prior to transfer from another facility (n = 35), do-not-intubate status (n = 11), or less than 12 hours of ICU admission (n = 7). Among the 231 patients included in our study, 25 (10.8%) did not require high-flow nasal cannula or mechanical ventilation, 109 (47.2%) received high-flow nasal cannula, and 97 (42.0%) received mechanical ventilation without first receiving high-flow nasal cannula. In total, 175 patients (75.7%) received mechanical ventilation at some point during their ICU stay. The median time from ICU admission to intubation was 8.1 hours (IQR, 0.3–20.1 hr). Seventy-six patients (43.4%) were intubated prior to (e.g., in the emergency department) or within 8 hours of ICU admission, 57 (32.6%) were intubated between 8 and 24 hours, and 42 (24.0%) were intubated greater than or equal to 24 hours after ICU admission. The median time to intubation among patients intubated greater than or equal to 24 hours after ICU admission was 2.3 days (IQR, 1.2–3.1 d) with a range of 1.0–8.3 days.
Figure 1.
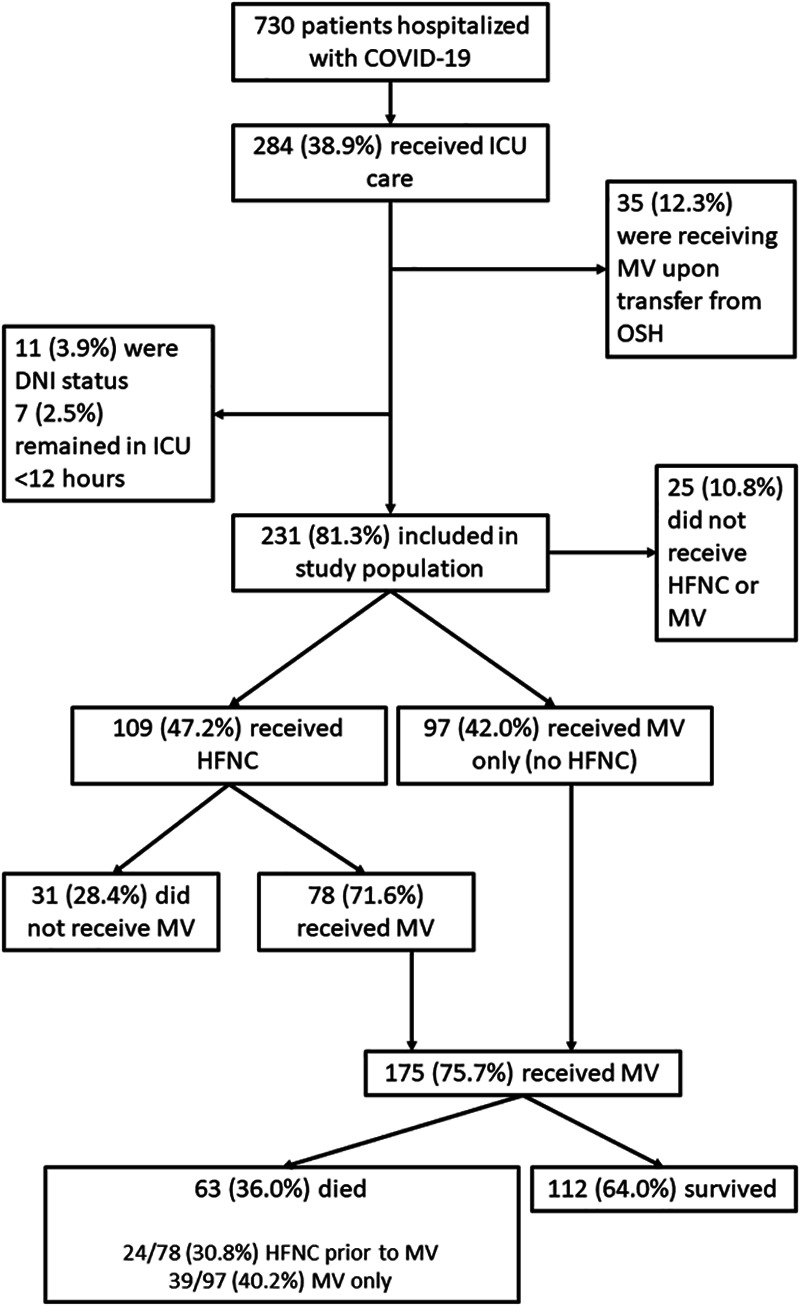
Flow diagram of study participants. COVID-19 = coronavirus disease 2019, DNI = do not intubate, FNC = high-flow nasal cannula, MV = mechanical ventilation, OSH = outside hospital.
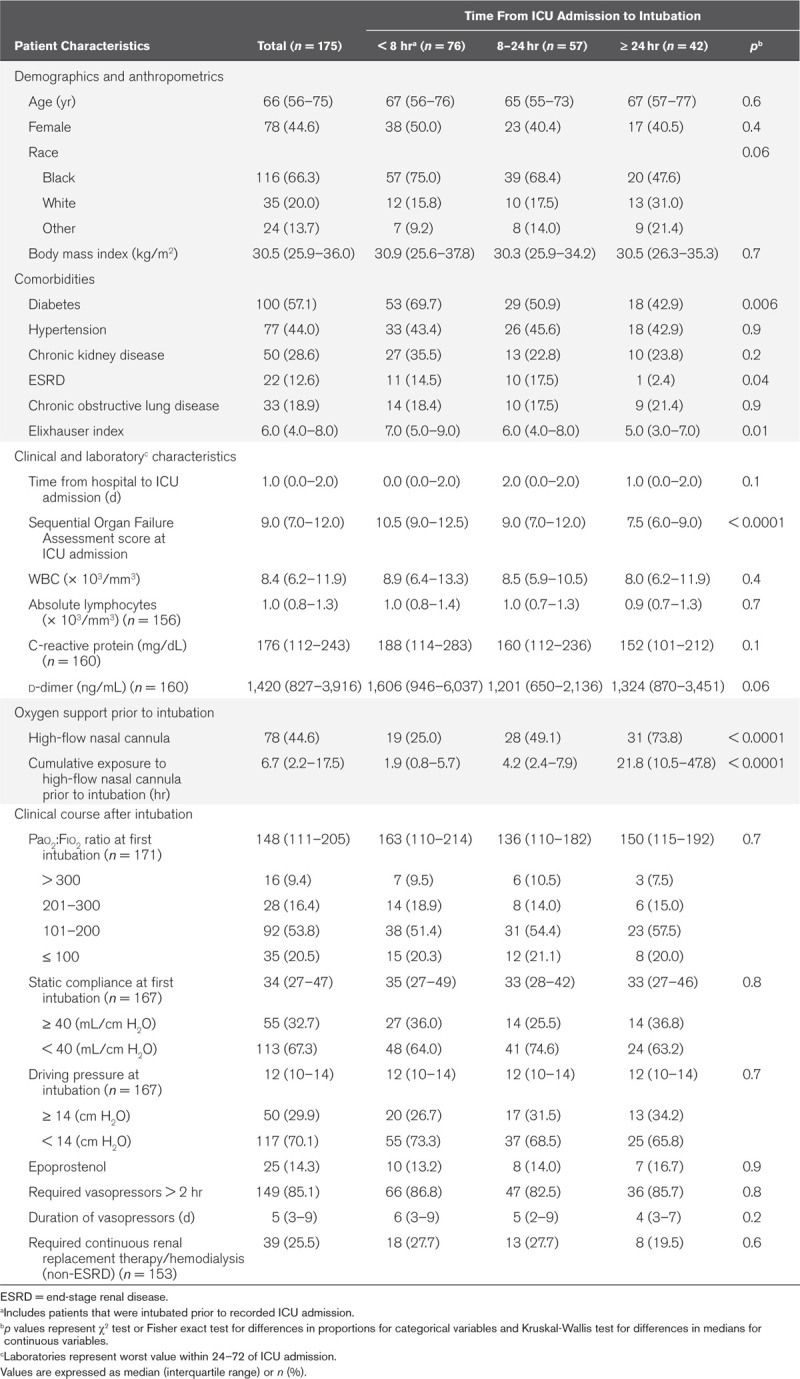
The comorbidities, demographic, clinical, and laboratory characteristics of 175 intubated patients overall and stratified by time from ICU admission to intubation are shown in Table 1. The median age was 66 years (IQR, 56–75 yr), 44.6% were female, 66.3% were Black, and 55.4% were obese (median body mass index [BMI] 30.5 kg/m2); these characteristics did not vary by time from ICU admission to intubation. Diabetes and hypertension were the most common comorbidities in our population at 57.1% and 44.0%, respectively; diabetes prevalence was higher among patients intubated less than 8 hours after ICU admission (p = 0.006). The median Elixhauser comorbidity index was 6.0 (IQR, 4.0–8.0). The Elixhauser index decreased from a median of 7.0 (IQR, 5.0–9.0) among those intubated within 8 hours of ICU admission to 5.0 (IQR, 3.0–7.0) for those intubated greater than 24 hours after ICU admission (p = 0.01). The median time from hospital to ICU admission was 1 day (IQR, 0–2 d) and was similar by time from ICU admission to intubation. The median SOFA score at ICU admission was 9.0 (IQR, 7.0–12.0) and significantly decreased from 10.5 (IQR, 9.0–12.5) among those intubated within 8 hours of ICU admission to 7.5 (IQR, 6.0–9.0) among those intubated greater than 24 hours after ICU admission (p < 0.0001). Median WBC count and absolute lymphocyte count did not differ by time to intubation category. d-dimer and C-reactive protein (CRP) levels were higher among those intubated within 8 hours of ICU admission but these differences were not statistically significant.
Table 1.
Demographic, Comorbidities, Clinical, and Laboratory Characteristics of Mechanically Ventilated Patients by Time From ICU Admission to Intubation
Supplemental Oxygen Prior to and Clinical Course After Intubation
The proportion of patients who received different levels of supplemental oxygen from ICU admission until day 15 is shown in Figure 2. Among the 109 patients initially managed with high-flow nasal cannula, 78 patients (71.6%) ultimately received mechanical ventilation. Patients who progressed from high-flow nasal cannula to mechanical ventilation were more likely to have chronic obstructive lung disease (24.4% vs 6.5%; p = 0.04), had a higher median SOFA score at admission to the ICU (9.0 vs 3.0; p < 0.0001), and higher median WBC count (8.7 vs 5.8; p = 0.01) than those who did not go on to receive mechanical ventilation (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F736). The proportion using high-flow nasal cannula prior to intubation was highest among those intubated greater than 24 hours after ICU admission (73.8%; p < 0.0001), with a median cumulative time of high-flow nasal cannula use of 21.8 hours (IQR, 10.5–47.8 hr) in this group. The median cumulative exposure to high-flow nasal cannula among those who failed high-flow nasal cannula and received intubation was 6.7 hours (IQR, 2.2–17.5 hr) (Table 1).
Figure 2.
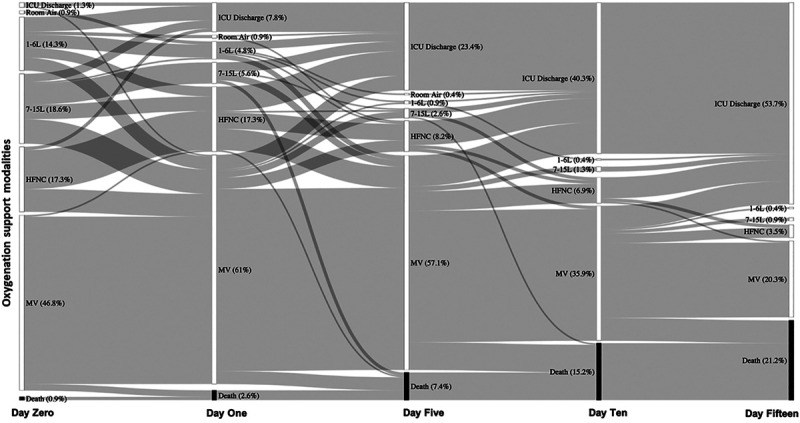
Sankey diagram depicting transitions between oxygen support modalities for 231 critically ill patients with coronavirus disease 2019. These data depict oxygen support and deaths through day 15, at which time 49 (78%) of deaths had occurred. HFNC = high-flow nasal cannula, MV = mechanical ventilation.
At the time of intubation, there was a normal distribution of static compliance and P:F ratio (Fig. 3). The median P:F ratio was 148 (IQR, 111–205). Sixteen percent of patients had a P:F ratio from 201 to 300, 53.8% had a P:F ratio from 101 to 200, and 20.5% had a P:F ratio less than or equal to 100. Median P:F ratios did not differ by group (p = 0.7). The median static compliance and driving pressure following intubation were 34 mL/cm H2O (IQR, 27–47 mL/cm H2O) and 12 cm H2O (IQR, 10–14 cm H2O), respectively, and did not differ by time from ICU admission to intubation.
Figure 3.
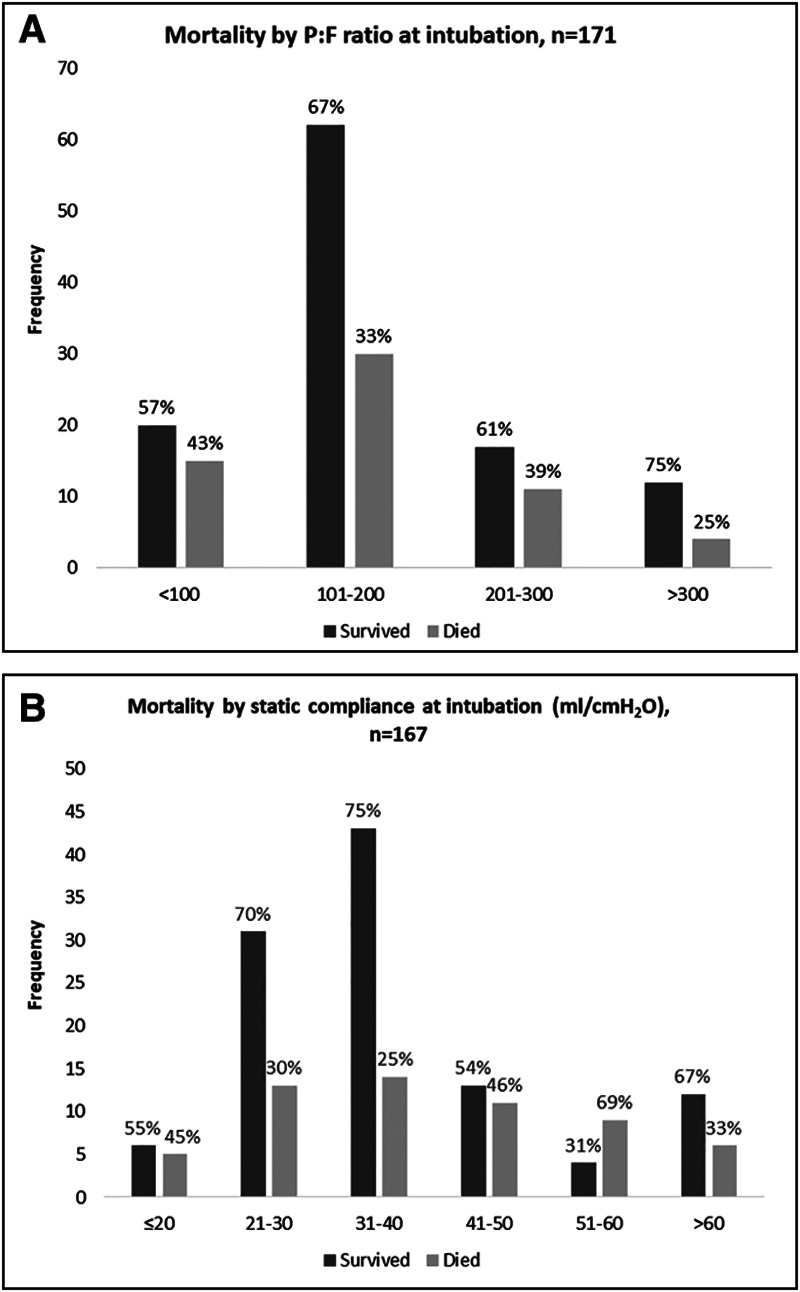
Respiratory system parameters at the time of intubation. Distribution of and mortality by (A) Pao2:Fio2 ratio and (B) static compliance.
Mortality, Duration of Ventilation, and ICU Length of Stay
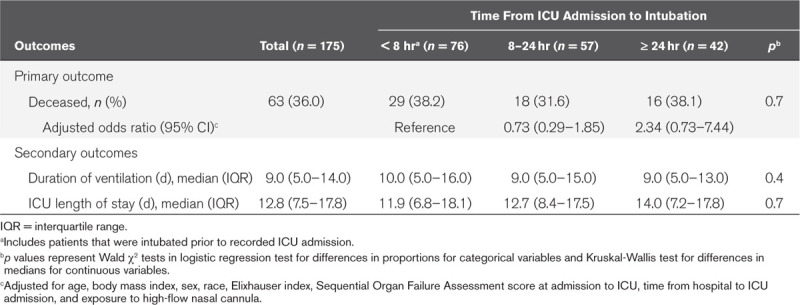
Among the 175 patients who received mechanical ventilation, 63 patients died (36.0%) (Table 2). Fifty-six died (89%) in the ICU. At the time of this report, all patients had either been transferred from the ICU or died. The median time from intubation to death was 10 days (IQR, 4–16 d). Mortality was 38.2%, 31.6%, and 38.1% among those intubated less than 8, 8–24, and greater than or equal to 24 hours after ICU admission, respectively (p = 0.7). After adjusting for age, sex, race, BMI, Elixhauser comorbidity index, SOFA score at ICU admission, time from hospital to ICU admission, and exposure to high-flow nasal cannula prior to intubation, longer time to intubation was not associated with increased odds of death (Table 2). Although mortality was lower among patients exposed to high-flow nasal cannula prior to intubation relative to those who did not receive high-flow nasal cannula prior to mechanical ventilation (30.8% vs 40.2%), this difference was not statistically significant (p = 0.2) and exposure to high-flow nasal cannula was not associated with death after multivariate adjustment (Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/CCM/F736). Sensitivity analyses incorporating the cumulative exposure to high-flow nasal cannula yielded similar results (data not shown). Increasing age, Elixhauser comorbidity index, SOFA at ICU admission, time from hospital to ICU admission, and lower BMI were associated with increased odds of death. Relative to Black patients in our study, White patients had lower odds of death (Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/CCM/F736). The median duration of mechanical ventilation was 9.0 days (IQR, 5.0–14.0 d) and median ICU length of stay was 12.8 days (IQR, 7.5–17.8 d), neither of which varied by time from ICU admission to intubation (Table 2).
Table 2.
Hospital Mortality, Duration of Ventilation, and ICU Length of Stay by Time from ICU Admission to Intubation, Atlanta, GA 2020
The proportion of death according to P:F ratios was 43% among those with a P:F ratio less than or equal to 100, 33% for those with a P:F ratio of 101–200 and 39% for those with a P:F ratio of 201–300 (Fig. 3). There was no apparent trend between initial static compliance and mortality, and a decreasing compliance was not associated with higher proportions of mortality (p = 0.2) (Fig. 3). Longitudinal trends in static compliance categorized by first compliance at intubation and stratified by death are shown in Supplemental Figure 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/F736). Static compliance remained stable over the duration of mechanical ventilation and trajectories did not vary among patients who survived and who died.
DISCUSSION
While mechanical ventilation is a lifesaving intervention for patients with respiratory failure, there is uncertainty as to whether the timing of intubation impacts patient outcomes in COVID-19. In this single-center cohort study, we found no association between time to intubation and either mortality, ventilator duration, or ICU length of stay, even after accounting for patient comorbidities, clinical presentation, and severity of illness. In addition, we found no association between the timing of intubation and subsequent oxygenation, as measured by initial P:F ratio, or static compliance.
Several recent reports have confirmed that survival among patients with respiratory failure from COVID-19 is comparable to that of other viral pneumonias and ARDS (4, 5, 11). However, these reports did not analyze outcomes according to timing of intubation. Concern for large swings in transpulmonary pressures and SILI have been put forth as a justification for early intubation in COVID-19 (3, 10, 18). While a prospective, randomized trial would be required to definitively address whether timing of intubation affects outcomes, our analysis provides reassurance that delays in intubation are not significantly associated with further lung injury in this vulnerable population of critically ill patients. More importantly, these data suggest that triggers for intubation need not be altered for patients with COVID-19; rather, providers can adhere to standard practice for ARDS management.
Although a shorter time to intubation from ICU admission was not associated with decreased mortality, it was associated with the presence of diabetes mellitus and a higher Elixhauser comorbidity index. Time to intubation also trended toward a significant association with CRP and d-dimer, as patients with shorter time to intubation had higher levels of these biomarkers. This is consistent with the observation that COVID-19-related lung disease may be driven by exuberant inflammation (19). Nevertheless, even after adjusting for severity of illness, mortality in the cohort did not differ by time to intubation and the overall mortality for those who received mechanical ventilation was 36%.
The role for high-flow nasal cannula in the management of COVID-19 has also been uncertain. High-flow nasal cannulas have been increasingly used in recent years for hypoxic respiratory failure, following a randomized trial showing a mortality benefit, alongside physiologic benefits of both increasing airway pressures and decreasing physiologic dead space and work of breathing (9, 20, 21). However, high failure rates of noninvasive positive pressure ventilation alongside limited experience with high-flow nasal cannulas during the severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks led to initial guidance from the World Health Organization to limit the use of these modalities in COVID-19 (22–24). In our cohort, among the nearly half of the patients who were initially managed with high-flow nasal cannula, 28% did not require escalation to mechanical ventilation. For the 72% of patients who were intubated after high-flow nasal cannula, there was no associated increase in mortality. These findings indicate that a limited trial of high-flow nasal cannula in COVID-19 may help a subset of patients avoid intubation.
Our data also demonstrate a normal distribution of static compliance on initiation of mechanical ventilation. The median compliance of 33 mL/cm H2O is congruent both with the compliance reported in large clinical studies of ARDS and with recent reports of COVID-19 (5, 11, 25). Furthermore, initial compliance did not significantly differ according to the timing of intubation, nor did compliance trajectories appreciably diverge for those who survived as compared to those who died.
Our analysis has several limitations. As a retrospective study, we are unable to account for unmeasured confounders that may have influenced decisions regarding intubation. Deciding when to intubate, and whether to intubate at all, are complex decisions driven by many factors including disease severity as well as institutional culture, clinician preference, patient and family preference, and ventilator availability (26). In this study, we attempted to control for disease severity but were unable to control for more subjective factors, including clinician preference, that contributes to decisions regarding intubation. Variability in factors driving decisions around intubation makes it unlikely that time to intubation alone will be able to predict clinical outcomes across a range of practice settings. In addition, only 24% of the patients who received mechanical ventilation in our cohort were intubated after more than 24 hours in the ICU, which limits our ability to estimate the impact of intubations that occurred multiple days after ICU admission. Nevertheless, our analysis was strengthened by the inclusion in our regression model of multiple covariates reflecting patient comorbidities and severity of illness. Ultimately, a prospective, randomized trial would be required to address the possibility of unmeasured confounders and more definitively answer the question of whether timing of intubation affects clinical outcomes.
Another limitation is our reliance on the EMR for data, such that we were only able to analyze documented oxygen supplementation and respiratory parameters. However, it is unlikely that inconsistencies in clinical documentation would result in systematic bias in our data or analysis. Furthermore, time to intubation and mortality, our primary exposure and outcome, are major clinical events that are less likely to have documentation errors.
With the emergence and global spread of COVID-19, the decision of whether or not to intubate has been made increasingly difficult. On the one hand, there is a desire to avoid aerosolization with modalities such as noninvasive positive pressure ventilation and also avoid prolonged delays in emergent intubations due to additional time needed for donning of personal protective equipment. On the other hand, premature intubations and prolonged periods of mechanical ventilation carry risks including ventilator-associated events and complications associated with sedation. Our analysis of a large cohort of critically ill patients with COVID-19 suggests that a trial of noninvasive strategies, including high-flow nasal cannula, in an attempt to avoid intubation is not associated with increased mortality or worse pulmonary physiology among those who eventually require mechanical ventilation. Clinicians caring for patients with COVID-19 can feel comfortable following standard practice for intubation and initiation of mechanical ventilation. Further research is needed to guide strategies to improve ventilator-associated mortality among patients with respiratory failure from COVID-19.
ACKNOWLEDGMENTS
We would like to extend our most profound thanks and gratitude to our colleagues in the Emory Critical Care Center and Emory Healthcare who have worked so hard to provide excellent clinical care during this global pandemic. Emory COVID-19 Quality and Clinical Research Collaborative Members (in alphabetical order): Max W. Adelman, Scott Arno, Sara C. Auld, Theresa Barnes, William Bender, James M. Blum, Gaurav Budhrani, Stephanie Busby, Laurence Busse, Mark Caridi-Scheible, David Carpenter, Nikulkumar Chaudhari, Craig M. Coopersmith, Gordon Dale, Lisa Daniels, Johnathan A. Edwards, Jane Fazio, Babar Fiza, Eliana Gonzalez, Ria Gripaldo, Charles Grodzin, Robert Groff, Alfonso C. Hernandez- Romieu, Max Hockstein, Dan Hunt, Craig S. Jabaley, Jesse T. Jacob, Colleen Kraft, Greg S. Martin, Samer Melham, Nirja Mehta, Chelsea Modlin, David J. Murphy, Jung Park, Deepa Patel, Cindy Powell, Amit Prabhaker, Jeeyon Rim, Ramzy Rimawi, Chad Robichaux, Nicholas Scanlon, Milad Sharifpour, Bashar Staitieh, Michael Sterling, Jonathan Suarez, Colin Swenson, Nancy Thakkar, Alexander Truong, Hima Veeramachaneni, Alvaro Velasquez, Aimee Vester, Michael Waldmann, Max Weinmann, Thanushi Wynn, and Joel Zivot.
Supplementary Material
Footnotes
Drs. Hernandez-Romieu and Adelman contributed equally to this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
This work was supported by the following grants: National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases K23 AI134182 (to Dr. Auld), NIH/Clinical and Translational Science Award UL1TR002378.
Dr. Blum’s institution received funding from the National Institutes of Health (NIH), and he received funding from Clew Medical. Drs. Blum and Auld received support for article research from the NIH. Dr. Martin disclosed he currently serves as president-elect for the Society of Critical Care Medicine. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was performed at Emory Healthcare and Emory University, both in Atlanta, GA.
REFERENCES
- 1.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323:1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46:1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auld SC, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020. May 26. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am J Respir Crit Care Med 2020; 201:1560–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos LD, Paulus F, Vlaar APJ, et al. Subphenotyping ARDS in COVID-19 patients: Consequences for ventilator management. Ann Am Thorac Soc 2020 May 12. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kangelaris KN, Ware LB, Wang CY, et al. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit Care Med 2016; 44:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 2017; 195:438–442 [DOI] [PubMed] [Google Scholar]
- 9.Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: Mechanisms of action and clinical implications. Chest 2015; 148:253–261 [DOI] [PubMed] [Google Scholar]
- 10.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 323; 22:2329–2330 [DOI] [PubMed] [Google Scholar]
- 11.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J 2019; 53:1802339. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27 [DOI] [PubMed] [Google Scholar]
- 14.Maiolo G, Collino F, Vasques F, et al. Reclassifying acute respiratory distress syndrome. Am J Respir Crit Care Med 2018; 197:1586–1595 [DOI] [PubMed] [Google Scholar]
- 15.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw 2019; 4:1686 Available at: 10.21105/joss.01686. Accessed August 7, 2020 [DOI] [Google Scholar]
- 16.Allaire JJ, Gandrud C, Russell K, et al. networkD3: D3 JavaScript Network Graphs from R. R package version 0.4. 2017. Available at: https://CRAN.R-project.org/package=networkD3. Accessed August 7, 2020. [Google Scholar]
- 17.Benchimol EI, Smeeth L, Guttmann A, et al. RECORD Working Committee: The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grieco DL, Menga LS, Eleuteri D, et al. Patient self-inflicted lung injury: Implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol 2019; 85:1014–1023 [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frat JP, Thille AW, Mercat A, et al. FLORALI Study Group; REVA Network: High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372:2185–2196 [DOI] [PubMed] [Google Scholar]
- 21.Vargas F, Saint-Leger M, Boyer A, et al. Physiologic effects of high-flow nasal cannula oxygen in critical care subjects. Respir Care 2015; 60:1369–1376 [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization: Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance, 28 January 2020. 2020. Available at: https://apps.who.int/iris/handle/10665/330893. Accessed May 28, 2020
- 23.Alraddadi BM, Qushmaq I, Al-Hameed FM, et al. Saudi Critical Care Trials Group: Noninvasive ventilation in critically ill patients with the Middle East respiratory syndrome. Influenza Other Respir Viruses 2019; 13:382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160:389–397 [DOI] [PubMed] [Google Scholar]
- 25.Guérin C, Reignier J, Richard JC, et al. PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 26.Wunsch H. Mechanical ventilation in COVID-19: Interpreting the current epidemiology. Am J Respir Crit Care Med 2020; 202:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


