During the COVID-19 global pandemic, it is critical to preserve access to sexually transmitted infection care and treatment. Infection-control approaches should be utilized to minimize the risk to patients while maintaining access to important services.
Abstract
Coronavirus disease (COVID-19) is responsible for a global pandemic. It is important to balance the need for access to healthcare services, including testing and treatment for sexually transmitted infections. Sexually transmitted infection programs must consider how to use limited resources and implement novel approaches to provide continued access to care.
Coronavirus disease (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 was first described in December 2019 in China and is responsible for a global pandemic.1 Cases have exponentially increased in the United States (US).2 As the outbreak progresses, a critical question is how to maintain access to healthcare services, including care for sexually transmitted infections (STIs). STI rates have increased across the US3 and are associated with significant morbidity and cost.4 Efforts implemented to halt COVID-19 transmission include restrictions on travel, businesses, and mass gatherings.5 Many healthcare settings have canceled “nonessential” visits. As strict measures are implemented, preserving access to STI services is critical.
STI programs exist in different capacities, including programs run by state health departments, private clinics, and academic health centers. Programs may provide walk-in services and serve as safety nets for: populations with higher risk for STIs; racial, ethnic, and sexual minorities; and the uninsured.6 Clinic structure is important to consider when planning for COVID-19 infection control. Significant challenges in continuing to provide STI care during a pandemic include: (1) lack of personal protective equipment (PPE); (2) limited staff to screen and refer patients for signs or symptoms of COVID-19; (3) limited resources due to categorization as a “nonessential” service; (4) limited laboratory services when state health departments maximize testing resources; (5) Crowded waiting rooms; (6) Need for physical and bimanual examinations for the diagnosis and treatment of symptomatic STIs, which place the provider and patients in close contact for an extended period of time; and (7) other infection control issues (ability to regularly clean surfaces, appropriate ventilation, negative pressure rooms, and access to other diagnostic testing) (Fig. 1).
Figure 1.
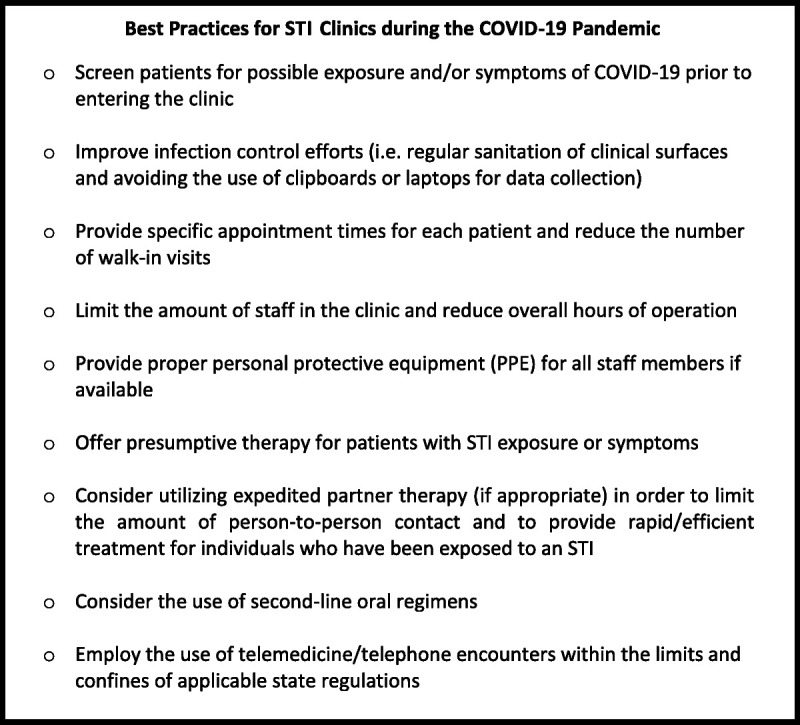
Best practices for STI clinics during the COVID-19 pandemic.
The recommended PPE for COVID-19 includes N95 respirators (or facemasks if unavailable), eye protections, gloves, and gowns.7 STI programs would never need this degree of PPE and may not stock PPE. The PPE is currently extremely limited across the US and STI clinics are not prioritized. To address limitations in PPE, many outpatient settings have implemented screening procedures for COVID-19. Common symptoms of COVID-19 include influenza-like illness with upper respiratory tract signs and symptoms (fever, myalgia, malaise, cough, sore throat, rhinitis); less common are gastrointestinal symptoms. Although severe cases present with dyspnea, leading to hospitalization and ventilation, most cases are mild (>80%)8 with a subset of infected asymptomatic individuals able to transmit the disease.9 Transmission of COVID-19 occurs through respiratory droplets.10 Exposed individuals may become infected when COVID-19 comes in contact with mucous membranes. The STI clinics may screen individuals for COVID-19 by having a staff member query patients before entering the clinic or taking a temperature. However, greater than 50% of infected individuals may be afebrile11 and median incubation period of fever onset is 5.7 days.12
Other options for infection control in STI programs include limiting walk-in appointments and hours of operation, and prioritizing in-person care for symptomatic individuals. Individuals with scheduled appointments may be screened over the phone for symptoms of COVID-19 before presenting to the clinic. Additionally, many states have implemented telephone triage and telemedicine. The Centers for Medicare and Medicaid Services (CMS) has released guidance on reimbursing telemedicine visits.13 When indicated, STI programs should consider providing counseling over the phone and should develop and implement a corresponding triage protocol that includes identification and referral for additional evaluation of individuals at risk for complications from undiagnosed or known STIs.14 Physical presentation to clinic should be limited to individuals needing treatment, or with other urgent concerns. Other infection control measures should be taken, including wiping down surfaces, handwashing, and using hand sanitizers with at least 60% alcohol.15
The importance of protecting STI program staff cannot be overemphasized, as any staff person exposed to COVID-19 should quarantine for 14 days.16 STI programs should reduce staffing to the minimum number needed to provide appropriate care. Staff should practice social distancing, work in individual spaces, and minimize contact with others. Staff who are at-risk of complications from COVID-19 should be re-assigned to duties not requiring face-to-face patient interaction, including telephone or online consultation. Importantly, no staff members should work if symptomatic for COVID-19. Some organizations may require staff to wear surgical facemasks, regardless of symptoms. However, this approach may be limited by lack of PPE.
Most STI programs are not set up to care for individuals with COVID-19. Referral systems should be implemented for patients who are identified with suspected COVID-19, with input from state health departments. Many health departments have recommended that patients with mild symptoms should self-isolate at home without presenting for testing, due to limited resources. Individuals with more concerning symptoms (i.e. dyspnea) should present to the emergency department. State health departments have maximized staffing resources for COVID-19 testing, which may limit laboratory capabilities for STI testing. However, maintaining access to STI services will limit individuals seeking care at emergency departments or urgent care, thereby increasing one's risk for COVID-19 and placing a greater burden on emergency care settings.
COVID-19 tends to most severely affect those who are 60 years or older.17 Adolescents and younger adults may have milder symptoms, but still may be able to transmit the disease to others.18 The burden of STIs is high among young adults. Individuals aged 15 to 24 years acquire half of all new STIs and 1 in 4 sexually active adolescent females has an STI.19 If STI services are suspended due to COVID-19, clinics risk not detecting and treating asymptomatic STIs, which could lead to increased diagnoses once the pandemic is over.
In worst-case scenarios, STI programs could consider using second-line oral medications. For patients presenting with urethritis/cervicitis, oral cefixime and azithromycin for empiric gonorrhea treatment, or doxycycline for syphilis treatment could be used; risks to patients of COVID-19 transmission should be carefully considered. For patients who are taking preexposure prophylaxis to prevent HIV transmission, clinics may use telemedicine visits to extend preexposure prophylaxis prescriptions when the medication is well-tolerated without regular laboratory, as recommended by the Centers for Disease Control and Prevention (CDC).20 In states where it is allowed, STI clinics may optimize expedited partner therapy to the fullest extent possible per CDC guidance.21 Finally, in cases where programs must close STI clinics, the CDC recommends and encourages the development of innovative testing and treatment approaches. STI programs should try to establish relationships with other clinics and pharmacies that can provide preferred treatments, and symptomatic patients and their known contacts could be referred to these sites for syndromic treatment. If this is not possible, STI programs may design and implement home or nonclinic-based testing programs.14
In conclusion, maintaining access to STI care is important during an infectious disease outbreak, such as COVID-19. STI clinic directors and staff should be aware of current protocols and standing orders in regard to providing clinical services in the context of COVID-19. Clinical and administrative staff should regularly review CDC recommendations cited above and other best practices, as this is a rapidly evolving topic of study. Preventative steps to minimize risk include appropriate infection control measures and other steps to reduce the risk for both staff and patients. Such approaches are necessary to maintain some access to STI services during a global pandemic such as COVID-19. To expand upon these efforts, the authors respectfully call on STI-focused organizations to support the dissemination of best practices and continued discourse on providing STI services in the context of the COVID-19 pandemic, including dissemination and discussion of recent and important CDC guidance on this topic. The authors recognize the efforts of the National Coalition of STD Directors and the CDC, and encourage other state and federal organizations to build upon this important work.
Footnotes
Conflict of Interest and Sources of Funding: None declared.
Acknowledgments: The authors thank Katherine Hsu and Sylvie Ratelle, STD/HIV Prevention Training Center of New England, Massachusetts Department of Public Health, for their helpful discussion on this article.
REFERENCES
- 1.World Health Organization. Situation Report 58: Coronavirus disease 2019 (COVID-19). https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2 Published March, 2020. Accessed March 19, 2020.
- 2.Cases in U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Published March 16, 2020. Accessed March 19, 2020.
- 3.STDs Continue to Rise in the U.S. Press Release. Centers for Disease Control and Prevention. https://www.cdc.gov/nchhstp/newsroom/2019/2018-STD-surveillance-report-press-release.html. Published October 8, 2019. Accessed March 19, 2020.
- 4.Centers for Disease Control and Prevention. CDC Fact Sheet: Incidence, Prevalence, and Cost of Sexually Transmitted Infections in the United States. https://www.cdc.gov/std/stats/sti-estimates-fact-sheet-feb-2013.pdf. Published February, 2013. Accessed March 19, 2020.
- 5.Schools, Workplaces & Community Locations. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/community/index.html. Published March 14, 2020. Accessed March 19, 2020.
- 6.Leichliter JS, Odonnell K, Kelley K, et al. Availability of safety-net sexually transmitted disease clinical services in the U.S., 2018. Am J Prev Med 2020; 58:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Infection Control: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html?CDC_AA_refVal=https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control.html. Published March 10, 2020. Accessed March 19, 2020.
- 8.Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020. Centers for Disease Control and Prevention. https://wwwcdcgov/mmwr/volumes/69/wr/mm6912e2htm Published March 18, 2020. Accessed March 19, 2020. [DOI] [PMC free article] [PubMed]
- 9.Cai J, Sun W, Huang J, et al. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg Infect Dis 2020; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Transmission of Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prepare/transmission.html. Published March 4, 2020. Accessed March 19, 2020.
- 11.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. NEJM 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Medicaid and Medicare Services. Fact sheet: Medicare Telemedicine Health Care Provider Fact Sheet. Published March, 2020. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed March 19, 2020.
- 14.Division of STD Prevention. Centers for Disease Control and Prevention. https://www.cdc.gov/std/dstdp/default.htm. Published January 2, 2020. Accessed April 17, 2020.
- 15.CDC Statement for Healthcare Personnel on Hand Hygiene during the Response to the International Emergence of COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/hcp-hand-sanitizer.html. Published March 14, 2020. Accessed March 19, 2020.
- 16.Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Published March 7, 2020. Accessed March 19, 2020.
- 17.Older Adults. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications/older-adults.html. Published March 18, 2020. Accessed March 19, 2020.
- 18.Information for Pediatric Healthcare Providers. Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html. Published March 12, 2020. Accessed March 19, 2020.
- 19.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018. Atlanta: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 20.Learn About PrEP. Centers for Disease Control and Prevention. https://www.cdc.gov/hiv/clinicians/prevention/prep.html. Published December 3, 2019. Accessed March 26, 2020.
- 21.Expedited Partner Therapy. Centers for Disease Control and Prevention. https://www.cdc.gov/std/ept/default.htm. Published August 26, 2019. Accessed March 26, 2020.


