These are unprecedented times. A novel coronavirus (SARS-Cov2) is circulating the globe and has much of the world's population homebound. At the same time, people continue to be exposed to, acquire, and/or display symptoms of sexually transmitted infections (STI) and need clinical care. The standards of care for 2 of the major STI pathogens are injectable medications, requiring face-to-face contact, and diagnostic testing usually requires patients to be evaluated in person. However, to mitigate the trajectory of COVID-19, we need to promote social distancing, including decreasing in-person visits for STI care. In this brief note, we present guidelines developed for STI management in King County, WA, during the COVID-19 epidemic. Our guidance expands upon the Centers for Disease Control and Prevention (CDC)'s Division of STD and Prevention “Dear Colleague” letter dated April 6, 2020, and offers ideas for operationalizing STI care at a distance. The National Coalition of STD Directors has collated additional ideas on their COVID-19 Command Center website.1
RECOMMENDATION 1: DEFER STI SCREENING VISITS
We screen patients for gonorrhea, chlamydia, syphilis, and HIV in order to prevent complications of these infections and to decrease community transmission. In some instances, screening visits are also opportunities to promote other care or prevention services, such as contraception or initiation of HIV preexposure prophylaxis. Screen positivity rates vary by the population and pathogen but are almost always lower than test positivity rates among known contacts to STI.2 For most patients, deferring screening a matter of months likely has little long-term impact on their health. The risk-benefit analysis for this decision involves weighing the individual and population risk of acquiring, becoming ill from, possibly hospitalized for, or spreading SARS-CoV-2 against the benefit of detecting gonorrhea, chlamydia or syphilis 1 to 3 months earlier. Because SARS-CoV-2 is such a highly infectious virus,3 attending a single clinic visit has the potential to pose significant risks to the patient as well as all the other individuals he or she may encounter during their time away from home, which may include time on public transportation, but will certainly include health care workers at the sexually transmitted disease clinic. These risks must be weighed against the typically small risk that a patient seeking STI screening test positive and will develop a serious complication—like pelvic inflammatory disease or neurosyphilis—in the time between a planned and delayed screening visit. Overall, we believe STI screening incurs a risk that exceeds its benefits during COVID-19. At the same time, depending on how long social distancing practices are necessary, clinics and programs may need to develop improved ways to screen people for STIs through the mail or through procedures that minimize potentially transmissible interactions.
RECOMMENDATION 2: TREAT PERSONS WITH KNOWN EXPOSURE TO STIs OR POSITIVE STI TEST RESULT VIA TELEPHONE OR TELEMEDICINE ENCOUNTERS WITH ORAL MEDICATIONS
Although CDC first-line recommended therapies for gonorrhea and syphilis both involve injectable medications, oral alternatives are available.4 The risk of a treatment failure with oral medications is low. The estimated efficacy of 800 mg cefixime for the treatment of urogenital gonorrhea is 97.9%,5–9 which is only slightly less efficacious than the estimated efficacy of 250 mg of ceftriaxone (99.1%).10 Although extragenital gonococcal infections treated with oral cephalosporins are more likely to fail treatment,5,11 the risk of a treatment failure needs to be weighed against the individual and population benefit of avoiding coronavirus transmission for a clinic visit.
Recommended oral therapies are in Table 1. Many of these regimens are already listed as alternative treatments by the 2015 CDC STD Treatment Guidelines.4 The gonorrhea treatments we recommend differ from the 2015 CDC STD Treatment Guidelines but are in line with drugs and doses recommended in the Division of STD and Prevention “Dear Colleague” letter and are based on expert opinion, recent gonococcal antimicrobial resistance surveillance showing rising levels of azithromycin resistance,12 and pharmacokinetic/pharmacodynamic data suggesting that a single 400-mg dose of cefixime may be inadequate in the treatment of gonorrhea and that a longer period of treatment is required to cure pharyngeal gonorrhea.13,14 In addition, given recent reports of limited cefixime availability, we offer an alternative oral cephalosporin.
TABLE 1.
Recommended Oral Therapies for Gonorrhea, Chlamydia, and Syphilis
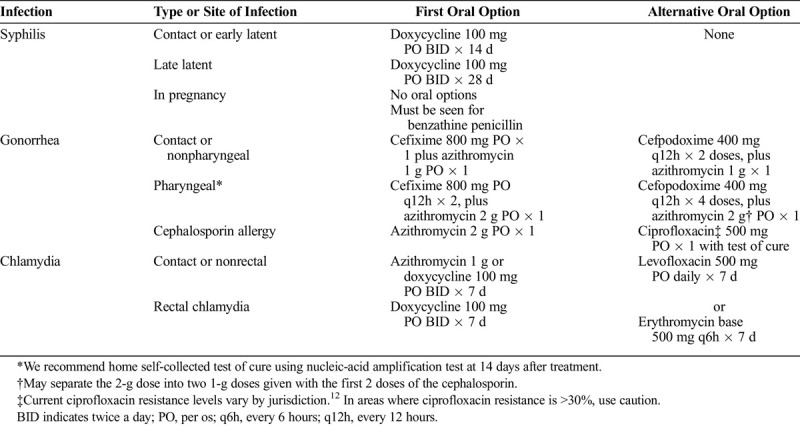
When empirically treating contacts to STI without simultaneously testing, there are risks. Patients may report contact to chlamydia but test positive for gonorrhea or syphilis, and thus would not have received appropriate treatment.15 Thus, it is important to counsel patients to seek full STI and HIV testing when social distancing restrictions are lifted.
RECOMMENDATION 3: TRIAGE PATIENTS WITH STI SYMPTOMS OVER THE PHONE OR USING TELEMEDICINE
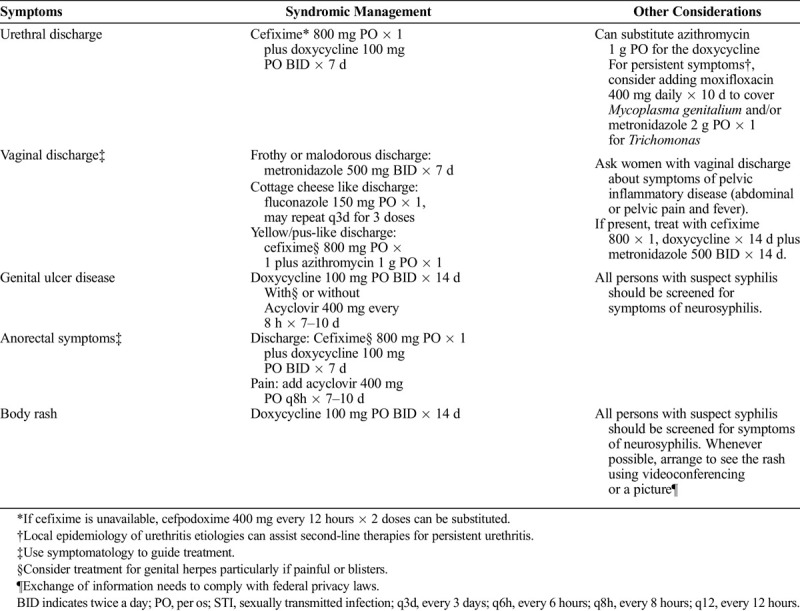
Under ideal circumstances, patients with urethral or vaginal discharge, or genital ulcer diseases, should be examined in person and point-of-care and laboratory tests used to aid diagnosis and management. However, clinicians often treat these syndromes before receiving any laboratory test results, and in much of the world, patients are treated without any diagnostic testing. We can avoid unnecessary exposures to SARS-CoV2 by having persons with symptoms of an STI call their clinic or medical provider, discuss their symptoms and exposures over the phone, and have treatment arranged without a face-to-face visit. Using local epidemiology and clinical symptomatology, clinicians can use their judgment for empiric treatment (Table 2). Patients will need clear counseling messages that medical care over the phone may be inaccurate and may result in overtreatment or undertreatment. Moreover, they will need clear guidelines for when to pursue additional evaluation. Lastly, patients treated syndromically should be counseled to seek STI screening once restrictions have been lifted.
TABLE 2.
Recommendations for Syndromic Management of STI Syndromes Over the Phone
RECOMMENDATION 4: CONTINUE TO PROVIDE IN-PERSON CLINICAL CARE FOR SELECTED PATIENTS WITH SYMPTOMS OF STI
There are some STI that will require in person visits: syphilis in pregnancy, complicated syphilis, and acute HIV. Pregnant women with syphilis require an injection with benzathine penicillin, which must be given by a health care worker. Although this usually occurs in a clinic setting, some jurisdictions may consider home visits. Complicated syphilis that encompasses ocular, otic, and neurosyphilis is a serious condition can lead to blindness and deafness if left untreated. The only recommended treatments are parenteral or daily intramuscular injections. In routine care, we recommend ophthalmologic evaluation for vision or eye symptoms and audiology referral for tinnitus or hearing loss in persons with syphilis. If both of those evaluations are negative for ocular or otic syphilis, then we arrange for the patient to have a lumbar puncture. In the era of COVID-19 and depending on the availability of services, it may be reasonable to defer at least some subspecialty evaluations (i.e., audiology) and treat selected patients empirically. On the other hand, it may be more reasonable to pursue a formal diagnosis before committing the patient to inpatient admission, outpatient peripherally inserted central venous catheter placement and aqueous penicillin, or daily trips to clinic for intramuscular procaine penicillin. Those decisions need to be made on a case-by-case basis.
Suspicions for acute HIV, which may present similarly to COVID-19 with fever, body aches, and sore throat, require phlebotomy and laboratory diagnostics, namely, a fourth-generation HIV test and/or a quantitative HIV RNA. At present, we are unaware of home HIV testing that will allow for both of these tests. In addition, in patients at elevated risk of HIV infection who present with symptoms consistent with acute HIV, we recommend starting a 3 drug antiretroviral regimen immediately without waiting for confirmatory test results.
Finally, insofar as STD clinics and other clinicians involved in STD care see patients with HIV who are out of care or off antiretrovirals, we recommend either seeing those patients face-to-face or arranging for them to have an in-person clinical evaluation to initiate antiretroviral therapy. Ensuring that all people with HIV receive antiretroviral therapy is a priority, and clinicians should capitalize on all opportunities to promote the use of antiretroviral treatment, even in the era of COVID-19.
OPERATIONAL CONSIDERATIONS
It will be prudent for clinics to decide how to inform patients about changes to both clinical guidelines and the process for obtaining care. This will be unique to each setting. In Seattle, we elected to place notices on our clinic's website and on our front door. Other clinics may choose to inform patients through social media. In 2015, our clinic moved to an electronic health record and most of our patients have signed up for the electronic access and messaging to their chart. We sent all patients who have signed up for “MyChart” a message about deferred STI screening and how to seek care for exposure and symptoms of STI. In the first week of implementation, this change has been incredibly successful with as many patients calling the clinic as walking-in.
Public health STD clinics serve as safety clinics particularly for uninsured or underinsured individuals. Prescribing medications through commercial pharmacies may be cost-prohibitive for some patients. Cefixime in particular is a costly medication. In Seattle, we either mail medications to our patients directly or offer curbside pick-up during this period of social distancing. Although mailed meds may result in a few days of delay of treatment, it does allow us to provide medications to those who cannot afford them otherwise. Clinics without these options might help patients find prescription coupons. As part of our mailed and curbside medication program, we have implemented a home gonorrhea and chlamydia testing and test-of-cure program. Although we initiated this as a test-of-cure program only for pharyngeal gonorrhea treated with oral cephalosporins given the lack of clinical data with these regimens, we have since expanded it to test contacts and all persons treated with nonstandard regimens. Further developing mail-in laboratory capacity for STI testing—ideally including syphilis and HIV testing too—may be necessary if the COVID-19 pandemic persists beyond a few months.
We have also heard from the community that casual hook-ups have continued despite our governor's mandate of social distancing. It is possible that individuals do not understand the transmission modes of the novel coronavirus include kissing and sex. Counseling patients about the risk of COVID-19 during sex should be promoted during all clinical encounters for STI. New York Health Commission said it best: during COVID-19 the safest sex is with yourself, and the second best with someone you live with.16 All other sex should be avoided for the risk of COVID-19 transmission.
LIMITATIONS TO THESE APPROACHES
We recognize that our recommendations involve compromising current standards of care in the United States.4 The STI treatments we propose and the limitations in clinical evaluation are not ideal. However, these are unprecedented times, and we believe the benefits of diminishing in-person contact to decrease the spread of SARS-CoV-2 exceed the downsides associated with modifications in care we suggest. One of the major limitations from the public health perspective is the lack of diagnostic test results, which will limit epidemiologic data for surveillance. As an alternative, we could track the number of 14- and 28-day doxycycline courses provided as well as phone visit data to estimate the number of syphilis cases during this time of social distancing. Similarly, we can track cefixime and cefpodoxime prescribing. We can also accept that 2020 STI data are likely to be inaccurate.
These guidelines may also result in the overuse of antibiotics in some individuals. Syndromic management will likely result in individuals receiving medications for both gonorrhea and chlamydia that might not be needed if diagnostic testing were pursued. At the same time, current CDC STD Treatment Guidelines recommend empiric treatment for persons exposed to STI before their testing results, which likely overtreats a large number of individuals annually.4 Although some fear the use of oral cephalosporins may increase the risk of cephalosporin resistance, our hope is that these alternative treatment regimens will only be used for a brief period of time.
CHALLENGES TO THESE GUIDELINES
Drug shortages could undermine implementation of these guidelines. Already we have heard that cefixime is on backorder. A recent report—based on 6 cases—suggesting that the combination of hydroxychloroquine plus azithromycin many improve COVID-19 clinical outcomes17 has many scared that azithromycin will face a shortage as well. The CDC monitors drug shortages and maintains a list on their website.18 If these drug shortages occur, we will need to devise alternatives to our alternative treatments.
SUMMARY
During this extraordinary time, we need to find a balance between providing necessary STI care and maintaining physical distancing to prevent unnecessary loss of life from COVID-19. We believe that the STI management guidelines that we have shared earlier and implemented in our clinic strike the right balance.
Footnotes
Conflict of Interest and Sources of Funding: None declared.
REFERENCES
- 1.NCSD. Available at: https://www.ncsddc.org/resource/covid-command-center-for-std-programs/. Accessed April 23, 2020.
- 2.CDC. 2018 STD Surveillance Report. Atlanta, GA: Centers for Disease Control and Prevention, 2018. [Google Scholar]
- 3.Swerdlow DL, Finelli L. Preparation for possible sustained transmission of 2019 novel coronavirus: Lessons from previous epidemics. JAMA 2020; 323:1129–1130. [DOI] [PubMed] [Google Scholar]
- 4.CDC. 2015 STD Treatment Guidelines. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 5.Allen VG, Mitterni L, Seah C, et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 2013; 309:163–170. [DOI] [PubMed] [Google Scholar]
- 6.Handsfield HH, McCormack WM, Hook EW, 3rd, et al. A comparison of single-dose cefixime with ceftriaxone as treatment for uncomplicated gonorrhea. The Gonorrhea Treatment Study Group. N Engl J Med 1991; 325:1337–1341. [DOI] [PubMed] [Google Scholar]
- 7.Mroczkowski TF, Hook Iii EW, Jones RB, et al. Grepafloxacin versus cefixime as single-dose therapy for uncomplicated gonorrhea in women. Infect Dis Obstet Gynecol 1997; 5:370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portilla I, Lutz B, Montalvo M, et al. Oral cefixime versus intramuscular ceftriaxone in patients with uncomplicated gonococcal infections. Sex Transm Dis 1992; 19:94–98. [PubMed] [Google Scholar]
- 9.Singh AE, Gratrix J, Martin I, et al. Gonorrhea treatment failures with oral and injectable expanded spectrum cephalosporin monotherapy vs dual therapy at 4 Canadian sexually transmitted infection clinics, 2010–2013. Sex Transm Dis 2015; 42:331–336. [DOI] [PubMed] [Google Scholar]
- 10.Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis 1995; 20(Suppl 1):S47–S65. [DOI] [PubMed] [Google Scholar]
- 11.Gratrix J, Bergman J, Egan C, et al. Retrospective review of pharyngeal gonorrhea treatment failures in Alberta, Canada. Sex Transm Dis 2013; 40:877–879. [DOI] [PubMed] [Google Scholar]
- 12.CDC. STD Surveillance Report 2017: Gonococcal Isolate Surveillance Project (GISP) Supplement and Profiles. Atlanta, GA: US Department of Health and Human Services, 2019. [Google Scholar]
- 13.Connolly KL, Eakin AE, Gomez C, et al. Pharmacokinetic data are predictive of in vivo efficacy for cefixime and ceftriaxone against susceptible and resistant Neisseria gonorrhoeae strains in the gonnorrhea mouse model. Antimicrob Agents Chemother 2019; 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbee LA, Nayak SU, Blumer JL, et al. A phase 1 pharmacokinetic and safety study of extended-duration, high-dose Cefixime for cephalosporin-resistant Neisseria gonorrhoeae in the pharynx. Sex Transm Dis 2018; 45:677–683. [DOI] [PubMed] [Google Scholar]
- 15.Schillinger JA, Jamison K, Slutsker J, Blank S. Concurrent STI and HIV among MSM presenting as contact to chlamydia and gonorrhea: Implications for expedited partner therapy. International Society of STD Research World Congress. Vancouver, British Columbia, Canada 2019.
- 16.New York Health Commission. Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/imm/covid-sex-guidance.pdf. 2020. Accessed April 23, 2020.
- 17.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020; 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.CDC. Available at: https://www.cdc.gov/std/treatment/drug-notices.htm. Accessed April 23, 2020.


