Abstract
Purpose
COVID-19 broke out in late 2019 and rapidly spread around the world and became a pandemic. This highly contagious disease affects routine health care services and patients with cancer who are susceptible to it. Delivering brachytherapy on time is critical for patients with cancer to get better prognosis. The purpose of this study is to present workflow and standard for radiation centers to deliver brachytherapy and avoid cross-infection during the COVID-19 pandemic.
Methods and Materials
This study combined previous literature and guidelines of precaution with clinical experience in the COVID-19 pandemic.
Results
A workflow covering patients' screening, health care workers’ precaution, training, and other aspects of the whole brachytherapy procedure was established.
Conclusions
From the reopening of radiation center to mid-May in 2020, there is no hospital infection of COVID-19 in patients or health care workers. This recommendation is effective and helpful to other cancer centers.
Keywords: Brachytherapy, COVID-19, SARS-CoV-2, Workflow
Introduction
A novel respiratory syndrome known as coronavirus disease 2019 (COVID-19), which was caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in Wuhan, Hubei, China, in late 2019. It appeared to be more easily transmitted and infectious than SARS, especially among patients with cancer (1). By May 17, the virus had spread to 216 countries with 4,534,731 confirmed cases and 307,537 fatalities (2). SARS-CoV-2 has an incubation period, which allows infected patients to unknowingly transmit the virus (3).
Brachytherapy is often used in cases such as gynecologic, breast, prostate, skin, and head and neck cancers as a radical, adjuvant, or even a palliative treatment ([4], [5], [6]). Studies have revealed that increases in the duration of treatment impact the prognosis of cervix, uterus, and breast cancers (7). The RetroEMBRACE research on cervical cancer indicated that overall treatment times (OTTs) of less than 7 weeks were associated with a higher, 3-year local control rate (86–94%); by contrast, while an OTT of more than 7 weeks needed an additional 5 Gy to compensate the loss of local control (8). Even with chemotherapy, an OTT (treatment includes external-beam radiotherapy and brachytherapy) greater than 56 days had a pelvic failure rate of 26%, whereas an OTT less than 56 days had a 9% failure rate. Both failure rates were determined for locally advanced cervical cancer (9). Studies indicated that intervals from surgery to adjuvant radiotherapy greater than 8 weeks led to higher local recurrence rates in breast cancer (10). In uterine carcinomas, intervals greater than 9 weeks were associated with lower recurrence-free survival rates (11). In conclusion, the delay of brachytherapy, which is irreplaceable as a radical therapy for cervical cancer and an optional or adjuvant therapy for uterine, breast, and other cancers, will negatively affect the prognosis of patients (12,13). Although all hospital services are affected by COVID-19, it is important to maintain brachytherapy which benefits clinical outcomes and life expectancy of patients. The brachytherapy department of the Wuhan Union Hospital formulated the workflow of brachytherapy delivery to resume medical practice while minimizing the risk of COVID-19 hospital infection.
We formulated the following specific practical guidelines for brachytherapy during COVID-19:
-
(1)
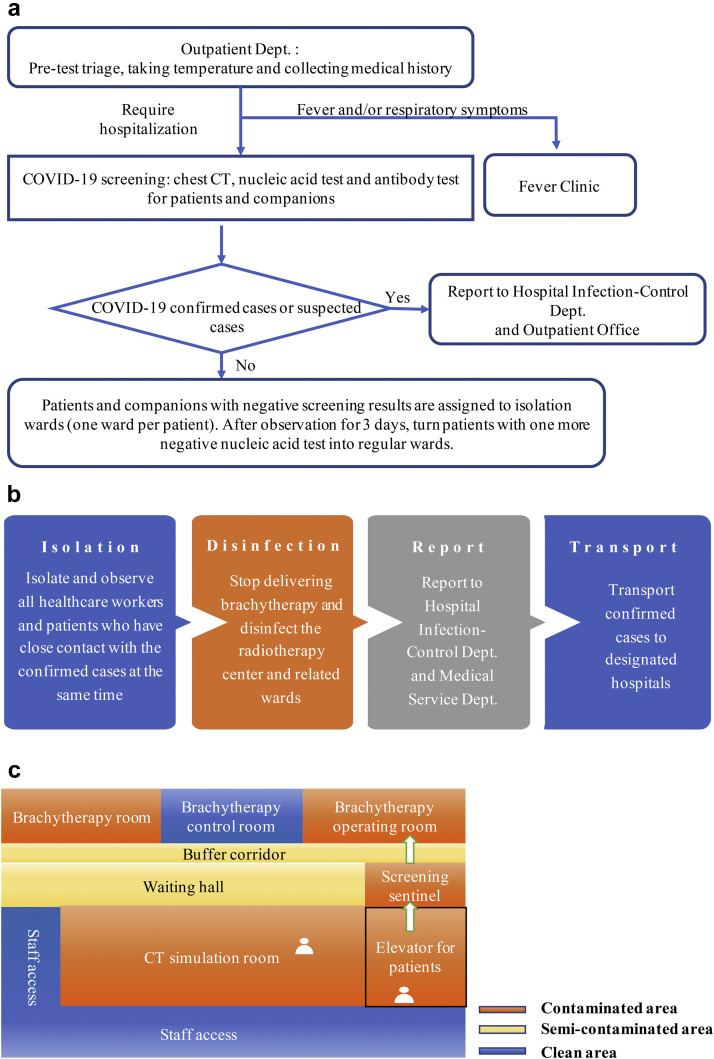
Patient screening: All patients receiving brachytherapy in our hospital as well as their companions are required to undergo COVID-19 screening, including chest computed tomography (CT), test for the specific IgM/IgG antibody against SARS-CoV-2, and a throat swab nucleic acid test. Brachytherapy is available to patients with negative screening results or those who are in recovery period from a COVID-19 infection. Patients and companions with negative screening results are assigned to isolation wards (one ward per patient). After observation for 3 days, we transfer patients into regular wards who have a negative nucleic acid retest result (sampling interval for at least 24 h) and exhibit no COVID-19 symptoms (Fig. 1 a). Isolation for more than 14 days after recovery, until each individual was no longer contagious, was required for COVID-19 infected cases (per guidelines given in The Diagnosis and Treatment Protocol of COVID-19, Temporary Sixth Edition) (14).
-
(2)
Verifying body temperature and maintaining interpersonal distance: Patients must verify their body temperature twice a day in the ward. While entering the brachytherapy center, patients are required to measure body temperature, present the chest CT scans along with nucleic acid and antibody test reports in addition to registration information. We have implemented an appointment system in which each patient is scheduled for a 1-h treatment. The patients are required to maintain an interpersonal distance greater than 2 m while waiting if there is a treatment overlap.
-
(3)
Establishment of an emergency response plan (Fig. 1b): For patients newly diagnosed with COVID-19 during brachytherapy treatment, immediate isolation and observation of all the health care workers and patients having had close contact with the confirmed cases is required. Delivery of brachytherapy is halted. The brachytherapy center and related wards are disinfected. We transport confirmed cases to designated hospitals as soon as possible while reporting the case to the hospital infection control and medical service departments.
-
(4)
Patient health education: Every hospitalized patient is required to wear a mask in public. In addition, we provide an informed consent form for patients. All the patients are informed of the risk of cross-infection during treatment, the personal protection and prevention policy of COVID-19, and are required to sign the form before receiving brachytherapy. The form also includes the measures we take to prevent cross-infection in brachytherapy center, such as new workflow and partitions of brachytherapy center, which relieve the anxiety of patients. Notification of the amended brachytherapy workflow implemented during the COVID-19 outbreak and necessary individual precautions to avoiding cross-infection of COVID-19 are available in the brachytherapy center clinical area.
-
(5)
Partitions: The brachytherapy center is divided into sections corresponding to different contamination levels (clean, semicontaminated, and contaminated areas). Each section is regularly disinfected as per protocol, with varied frequency, depending on contamination level. The protection level of each area is clearly defined using restrictions for the patients' routes and activity (Fig. 1c).
-
(6)
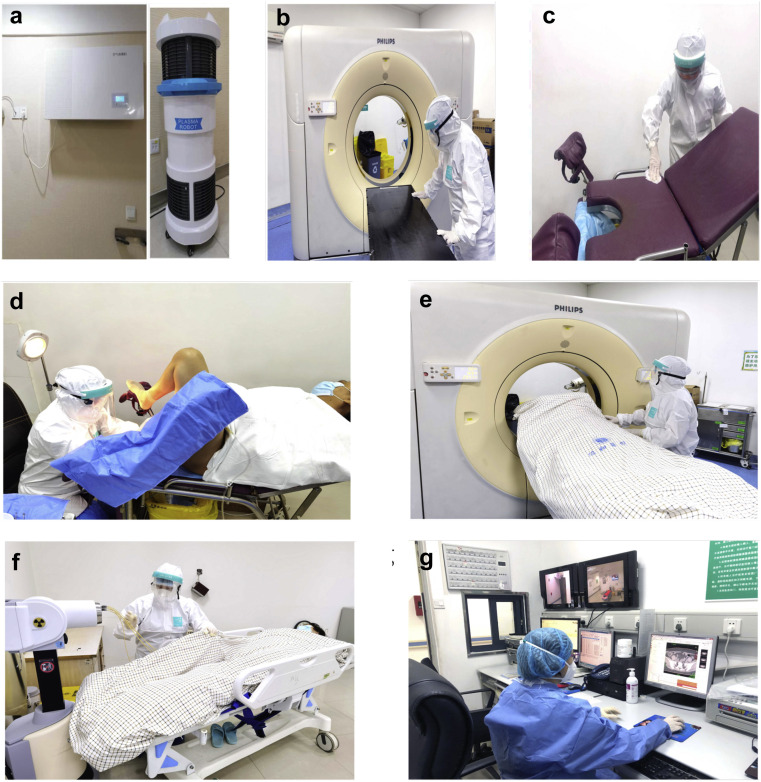
Disinfection of the treatment rooms: We continuously operate the air disinfection machine (Fig. 2 a). Subsequent to placing the applicator or delivering brachytherapy for each patient, the CT simulation room, brachytherapy operating room, and brachytherapy room are subject to surface disinfection (chlorine disinfectant, 1000 mg/L) (Figs. 2b and 2c).
-
(7)
Screening of health care workers: Our staff worked on a rotating shift, meaning that every shift lasted for 2 weeks, followed by a 2-week rest corresponding to the incubation period of COVID-19 is less than 14 days (15). Based on COVID-19 guidelines, every staff should undergo COVID-19 screening including test for the specific IgM/IgG antibody against SARS-CoV-2 and a throat swab nucleic acid test, before returning to their work. Moreover, chest CT is also required every 2 months. Only health care workers with negative screening results are permitted to return to their posts. We reduced our brachytherapy staff to a core team consisting of four health care workers: a brachytherapist, a radiation oncologist, a physicist, and a radiation therapist. They provided all the brachytherapy interventions for the day to minimize staff exposure. No observers, students, apprentices, or trainees were allowed in the brachytherapy operating room, brachytherapy room, or brachytherapy control room.
-
(8)
Staff training: Before returning to work, employees received training in COVID-19 prevention and protection. Staff learned the use of suitable personal protective equipment and different workflows for their roles.
-
(9)
Staff protection devices: As contamination is primarily through body fluids and not aerosols, Grade II precautions are required in the brachytherapy operating room, CT simulation room, and brachytherapy room. These Grade II precautions consist of using an antipenetration isolation gown, waterproof medical cap, antifog protective goggles, protective face shield, filtering facepiece 2 mask, latex gloves, and shoe covers (Figs. 2d–2f) (16); Grade I precautions are required in the brachytherapy control room. Grade I precautions consist of using a disposable gown, waterproof medical cap, filtering facepiece 2 mask, and latex gloves (Fig. 2g) (16).
Fig. 1.
Workflow and zoning in a brachytherapy center. (a) Workflow of outpatient admission screening. Both patients and companions are required to undergo chest CT scanning, antibody testing for specific IgM/IgG antibody against SARS-CoV-2, and a throat swab nucleic acid test. Ideally, we suggest that the patient should arrive without a companion; however, fixed one-to-one companionship is permitted due to necessity. (b) Diagram of the emergency response plan of newly diagnosed COVID-19 patients during brachytherapy. (c) Zoning of the brachytherapy center. We close nonessential access, restrict the area of patients' activity, and separate patients' passages and staff access.
Fig. 2.
Disinfection and personal protective equipment (PPE) in a brachytherapy center. (a) Air disinfection machine in brachytherapy room (left) and corridor (right). (b, c) Surface disinfection with 1000 mg/L chlorine disinfectant. (d) A brachytherapist uses PPE (anti-penetration isolation gown, waterproof medical cap, antifog protective goggles, protective face shield, FFP2 mask, latex gloves, and shoe covers) in the brachytherapy operating room with Grade II precaution. (e) Staff wears PPE in the CT simulation room with Grade II precaution. (f) A radiation therapist wears PPE in the brachytherapy room with Grade II precaution. (g) Radiation oncologists and physicists wear PPE (disposable gown, waterproof medical cap, FFP2 mask, and latex gloves) in brachytherapy control room with Grade I precaution.
Discussion
From the outbreak of COVID-19 to May 17, 2020, we have conducted brachytherapy for 124 patients (Table 1 ). In the early phase of the pandemic, there were confirmed cases with COVID-19 who received external radiotherapy, while, fortunately, no patients who received brachytherapy were diagnosed with COVID-19. Then, we implemented the described workflow. Once there were COVID-19 confirmed patients in the outpatient department who needed to receive brachytherapy, we initiated the workflow of outpatient admission screening. Therefore, there was no hospital-onset of COVID-19 transmission between patients and health care workers. This was while the aforementioned safety procedures were implemented. We based the workflow on the Infection Prevention and Control of Suspected COVID-19 Infection in Health Care guidelines, which is a temporary document provided by the World Health Organization detailing the measures to be taken for preventing and controlling MERS-CoV and acute respiratory infections (17). The amended workflow referred to the Prevention and Control Planning of COVID-19 (third edition) by the general office of the National Health Commission (18). Staff protection and education were based on the Technical Guidelines for Prevention and Control of Novel Coronavirus Infection in Medical Facilities (first edition) (19). The disinfection procedure was performed in accordance with the Regulation for Prevention and Control of Healthcare Associated Infection of Airborne Transmission Disease in Healthcare Facilities by the National Health and Family Planning Commission of People Republic of China (20). This suggests that the workflow and standards we developed for delivering brachytherapy, without nosocomial transmission, were effective and might facilitate the effective operation of other radiotherapy centers.
Table 1.
Clinical characteristics of the 124 patients who received brachytherapy
| Characteristics | Number of patients |
|---|---|
| Type of brachytherapy | |
| Intracavitary | 102 |
| Interstitial | 17 |
| Both | 5 |
| Primary disease site | |
| Cervical cancer | 109 |
| Radical radiotherapy | 86 |
| Postoperative adjuvant radiotherapy | 2 |
| Palliative radiotherapy | 21 |
| Endometrial cancer | 14 |
| Postoperative adjuvant radiotherapy | 14 |
| Ovarian cancer | 1 |
| Palliative radiotherapy | 1 |
The recent global outbreak of COVID-19 has created an extraordinary demand for medical care. Many hospitals have canceled less urgent procedures to minimize the exposure of patients and staff. This study adopts the precautions based on the current status of the COVID-19 pandemic. The overall workflow, which includes patient's screening and education, zone-design and disinfection of brachytherapy center, staff training and protection, balances the risk of necessary medical practice with the risk of potential cross-infection among patients and health care workers. Our practical guidelines are rational, safe, and based on the regular workflow of brachytherapy during the COVID-19 pandemic. However, the precautions regarding COVID-19 infection may change as a response to the evolution of information regarding the characteristics of COVID-19 and new clinical data. Further research is required to mitigate the risk of COVID-19 infection.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81974463) and the National Natural Science Foundation of China (grant no. 81902854).
Footnotes
Financial disclosure: All authors declare no conflict of interest.
References
- 1.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO WHO. Coronavirus disease (COVID-19) outbreak situation. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at:
- 3.Heyd C.P., Desiato V.M., Nguyen S.A. Tracheostomy protocols during COVID-19 pandemic. Head Neck. 2020;42:1297–1302. doi: 10.1002/hed.26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guinot J.L., Rembielak A., Perez-Calatayud J. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother Oncol. 2018;126:377–385. doi: 10.1016/j.radonc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Bussu F., Tagliaferri L., Mattiucci G. HDR interventional radiotherapy (brachytherapy) in the treatment of primary and recurrent head and neck malignancies. Head Neck. 2019;41:1667–1675. doi: 10.1002/hed.25646. [DOI] [PubMed] [Google Scholar]
- 6.Chargari C., Deutsch E., Blanchard P. Brachytherapy: An overview for clinicians. CA Cancer J Clin. 2019;69:386–401. doi: 10.3322/caac.21578. [DOI] [PubMed] [Google Scholar]
- 7.Williams V.M., Kahn J.M., Harkenrider M.M. COVID-19 impact on timing of brachytherapy treatment and strategies for risk mitigation. Brachytherapy. 2020;19:401–411. doi: 10.1016/j.brachy.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanderup K., Fokdal L.U., Sturdza A. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol. 2016;120:441–446. doi: 10.1016/j.radonc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Song S., Rudra S., Hasselle M.D. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer. 2013;119:325–331. doi: 10.1002/cncr.27652. [DOI] [PubMed] [Google Scholar]
- 10.Huang J., Barbera L., Brouwers M. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 11.Cattaneo R., Hanna R.K., Jacobsen G. Interval between hysterectomy and start of radiation treatment is predictive of recurrence in patients with endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:866–871. doi: 10.1016/j.ijrobp.2013.11.247. [DOI] [PubMed] [Google Scholar]
- 12.Wortman B.G., Creutzberg C.L., Putter H. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br J Cancer. 2018;119:1067–1074. doi: 10.1038/s41416-018-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holschneider C.H., Petereit D.G., Chu C. Brachytherapy: a critical component of primary radiation therapy for cervical cancer: From the Society of Gynecologic Oncology (SGO) and the American Brachytherapy Society (ABS) Gynecol Oncol. 2019;152:540–547. doi: 10.1016/j.ygyno.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 14.General office of the National Health Commission The Diagnosis and treatment protocol of COVID-19 (the temporary Sixth edition) http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf Available at:
- 15.Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25(5):2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K., Lai X., Liu Z. Suggestions on the prevention of COVID-19 for health care workers in department of otorhinolaryngology head and neck surgery. World J Otorhinolaryngol Head Neck Surg. 2020 doi: 10.1016/j.wjorl.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Infection prevention and control of suspected COVID-19 infection in health care: Temporary document. https://apps.who.int/iris/bitstream/handle/10665/330674/9789240000957-chi.pdf Available at:
- 18.General office of the National Health Commission The prevention and control planning of COVID-19 (the third edition) http://www.nhc.gov.cn/jkj/s7923/202001/470b128513fe46f086d79667db9f76a5/files/8faa1b85841f42e8a0febbea3d8b9cb2.pdf Available at:
- 19.General office of the National Health Commission Technical guidelines for prevention and control of novel coronavirus infection in medical facilities, the first edition. http://www.nhc.gov.cn/yzygj/s7659/202001/b91fdab7c304431eb082d67847d27e14.shtml Available at:
- 20.National Health and Family Planning Commission of People Republic of China Regulation for prevention and control of healthcare associated infection of airborne transmission disease in healthcare facilities. https://www.whcdc.org/wcs/Upload/201808/5b84eeb4d8b5b.pdf Available at: