SARS-Cov 2 infection is responsible for pandemic COVID19, a new viral illness causing acute respiratory syndrome and cardiovascular events [1] in which inflammation and thrombotic events are frequent. It’s also known that exacerbated inflammation ad immune system dysregulation can cause severe clinical manifestations; in this process a crucial effector role is played by monocytes.
Coronary thrombosis occurring during acute coronary syndromes, similarly, are due to an inflammatory storm with increased number of circulating activated monocytes/macrophages [2]. We know that monocytes are involved in infections: persistent activation of circulating monocytes/macrophages, induced by the release of cytokines, above all IL-6, has been postulated [1]; moreover IL-6 overexpression seems to be related with disease severity and pro-coagulant profile. Prior observations documented that inflammation mediated by phagocytes in rat lungs is blocked by preincubation with anti-Mo1 monoclonal antibodies thus preventing pulmonary injury [3]; these antibodies react with CD11b/CD18 integrin complex, that represents a major adhesion on monocytes/macrophages and IL6 promotes interaction with ICAM, also acting on platelets, to favor thrombosis [4,5].
Our recent work [6] showed that, during the monitoring of patients infected by SARS-CoV-2 a reproducible decrease of peripheral Non-classical (NC) and intermediate (INT) monocyte was found in subjects with the most severe clinical status at admission suggesting that their decreasing may be considered prognostic indicators that may help in the early identification of patients with the worst and most rapid unfavorable outcome.
Here we describe our investigations in expression levels of CD11b on peripheral blood CD14+ monocytes, studied by flow cytometry, in a 64-year-old patient affected by COVID19 pneumonia who underwent to non -invasive ventilation (CPAP) then tracheal intubation due to an interstitial pneumonia conditioning acute respiratory failure. At admission the expression of CD11b was 8231 MFI (Mean Fluorescence Intensity) units. The patient was treated with enoxaparin and tocilizumab (in combination with hydroxychloroquine and antibiotic therapy with ceftriaxone and azithromycin). No steroids were used. CD11b expression fell to 4582 MFI units over 7 days. In accordance with this, IL6 values were also hyper – expressed (IL6 243,4 pg/mL; L < 7 pg/mL) while value after 8 days was 18 pg/mL, showing a reduction in value, congruent with the improvement of the patient (Fig. 1 , flow cytometric). Simultaneously with the improvement of data relating to the expression of IL6 and CD11b, as described in Fig. 2 , we assisted to a normalization of inflammatory markers indices, improvement in lymphopenia values of and resolution of respiratory insufficiency. Asthenia improved and fever disappeared and the patient was progressively weaned from oxygen therapy.
Fig. 1.
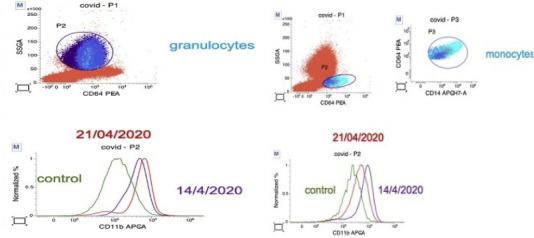
Flow cytometric displays of CD11b expression of patient’s monocytes and granulocytes (expressed as percentage of total) before and after treatment (forward and side scatter gating).
Fig. 2.
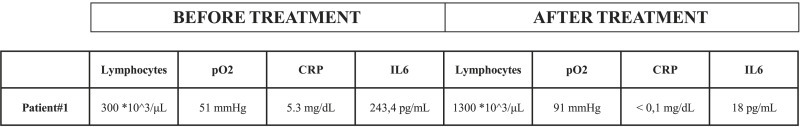
Main biochemical data of the patient before and after treatment.
The relationship between inflammatory process and heart disease has been debated: monocytes/macrophages recruitment express the CD11b/CD18 adhesion molecule, mediating their adhesion to the endothelial cells and leading to inflammation. Therefore, as reported in ischemic diseases and unstable angina, activated leukocytes and platelets potentiate each other’s effects, favoring the occurrence of thrombosis [1]. A paper of Altieri [7] clarify these aspects: binding of soluble clotting factor X to CD11b/CD18. The physiological significance of factor X and fibrinogen binding with activated CD11b/CD18 in vivo, is one of the possible bridges between inflammation and thrombosis. Autopsy studies in patients affected by SARS-CoV2 pneumonia demonstrate that inflammation, vasculitis and thrombosis are the pathogenetic mechanism of ARDS and subsequent death. So, in an effort to investigate the interface between coagulation and inflammation, we have mapped the expression of CD11b/CD18 in monocytes/macrophages of a COVID19 patient. In vivo acute inflammatory response, induced by the virus, and the subsequent cytokine release stimulates the up-regulation of CD11b/CD18 that favors thrombosis. We know that the role of anti IL6 drugs on the regulation of the expression of CD11bCD/18 on monocytes macrophages was previously studied in inflammation in atherosclerosis and in myocardial ischemia; our data, even in light of our recent work [6], and once completed, could help to demonstrate the hypothesis that the interaction between CD11b/CD18, endothelial cells platelets, factor X and fibrinogen plays a fundamental role in favoring inflammation and thrombosis. The overproduction of early response proinflammatory results in what has been described as an inflammatory storm, leading to an increased risk of vascular hyperpermeability, multiorgan failure, and death. Advances in cytokine biology and molecular biology leaded to the development of novel immunologic approaches to the treatment of COVID19 lung injury; increasing expression of CD11b/CD18 induced by COVID19 may play a key role in bridging inflammation and thrombosis.
Funding
This research received no external funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020 Apr 15 doi: 10.1016/j.jacc.2020.04.031. pii: S0735–1097(20)35008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Servi S., Mariani M., Mariani G., Mazzone A. C-reactive protein increase in unstable coronary disease cause or effect? J. Am. Coll. Cardiol. Oct 18 2005;46(8):1496–1502. doi: 10.1016/j.jacc.2005.05.083. Epub 2005 Sep. 28. [DOI] [PubMed] [Google Scholar]
- 3.Ismail G., Morganroth M.L., Todd R.F., III, Boxer L.A. Prevention of pulmonary injury in isolated perfused rat lungs by activated human neutrophils preincubated with anti-Mo1 monoclonal antibody. Blood. 1987;69:1167–1174. [PubMed] [Google Scholar]
- 4.Mazzone A., De Servi S., Ricevuti G., et al. Increased expression of neutrophil and monocyte adhesion molecules in unstable coronary artery disease. Circulation. Aug 1993;88(2):358–363. doi: 10.1161/01.cir.88.2.358. PMID: 8101771. [DOI] [PubMed] [Google Scholar]
- 5.Mazzone A., De Servi S., Mazzucchelli I., et al. Increased expression of CD11b/CD18 on phagocytes in ischaemic disease: a bridge between inflammation and coagulation. Eur. J. Clin. Invest. 1997;27(8):648–652. doi: 10.1046/j.1365-2362.1997.1610710. [DOI] [PubMed] [Google Scholar]
- 6.Gatti A., Radrizzani D., Viganò P., Mazzone A., Brando B. Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV-2 infection. Cytometry. 2020 doi: 10.1002/cyto.a.24188. 10.1002/cyto.a.24188. [published online ahead of print, 2020 Jul 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altieri D.C., Edgington T.S. The saturable high affinity association of factor X to ADP-stimulated monocytes defines a novel function of the Mac-1 receptor. J. Biol. Chem. 1988;263:7007–7015. [PubMed] [Google Scholar]


