Abstract
The chicken egg vitelline membrane (CEVM) is an important structure for the transmembrane transport of egg yolk components, protection of the blastodisc, and separation of egg white and egg yolk. In this study, the N-glycoproteome of the CEVM was mapped and analyzed in depth. Total protein of the CEVM was digested, and the glycopeptides were enriched by a hydrophilic interaction liquid chromatography microcolumn and identified by nano liquid chromatography/tandem mass spectrometry. A total of 435 N-glycosylation sites on 208 N-glycoproteins were identified in CEVM. Gene Ontology enrichment analysis showed that CEVM N-glycoproteins are mainly involved in the regulation of proteinases/inhibitors and transmembrane transport of lipids. Mucin-5B is the primary N-glycoprotein in the CEVM. Comparison of the main N-glycoproteins between the CEVM and other egg parts revealed the tissue specificity of N-glycosylation of egg proteins. The results provide insights into protein N-glycosylation in the chicken egg, CEVM functions and underlying mechanisms.
Keywords: Chicken egg vitelline membrane, N-glycoproteome, Transmembrane transport of lipids
Graphical abstract
Highlights
-
•
A total of 435 N-glycosylation sites on 208 N-glycoproteins were identified in CEVM.
-
•
Mucin-5B is the primary N-glycoprotein in the CEVM.
-
•
CEVM N-glycoproteins are mainly involved in proteinases/inhibitors and lipid transport.
-
•
N-glycoproteomes between different egg parts revealed the tissue specificity of N-glycosylation.
1. Introduction
Chicken egg is a food rich in nutrients. Egg white and yolk differ in their nutrient compositions and show different functional properties during processing. The chicken egg vitelline membrane (CEVM) is an important structure separating the egg white and egg yolk. The rupture of the CEVM during storage is a sign that the egg is inedible. From the perspective of the hen, the primary function of the egg is reproduction. The egg white and yolk provide nutrients for embryonic development. The CEVM functions to protect the blastodisc. In addition, during the maturation of the hen follicle, the inner layer of the CEVM is the interface for the transmembrane transport of egg yolk material. The CEVM is the most important and most complex part of the chicken egg despite being thin (approximately 10 μm) and accounting for only for a small part (2% ~ 3%) of the total weight of the egg.
The in-depth exploration of the composition of the CEVM will help reveal its functions and the underlying mechanisms. Similar to egg white, the CEVM has a water content of 88%, and protein accounts for 87% of its dry matter. However, the protein profile of the CEVM is very different from that of egg white. In a previous CEVM proteome analysis, a total of 137 proteins were identified, and the relative abundance of CEVM proteins was estimated. The high-abundance CEVM proteins included some egg white proteins, such as ovalbumin, lysozyme, ovotransferrin, ovalbumin-related protein Y, and ovomucin, and the main proteins in egg yolk, such as apolipoprotein D and vitellogenin-2. Among the low-abundance CEVM proteins, some eggshell matrix-specific proteins were found, such as ovocleidin-17, ovocleidin-116, and ovocalyxin-36. Vitelline outer-membrane proteins and zona pellucida-related proteins were also major components of the CEVM [1]. These results reveal the diversity of protein sources in CEVM. However, our understanding of CEVM proteins remains incomplete. For example, the posttranslational modification of CEVM proteins has not been studied.
Recent research on the N-glycoproteomics of eggs has provided important information for the field of egg science. In our previous studies, the N-glycoproteomes of chicken egg white and egg yolk were investigated using the omics strategy, and many new glycoproteins and N-glycosites were experimentally identified and confirmed for the first time [2,3]. Furthermore, these studies revealed the heterogeneity of N-glycosylation of egg white proteins and its influence on protein properties, such as the isoelectric point and molecular weight [2], and the existence of a proteinase/inhibitor regulation system and complement/immune systems in chicken egg yolk [3]. Subsequent studies of duck and quail egg N-glycoproteomes revealed differences in N-glycosylation between species and provided insight into evolution and environmental adaptability [[4], [5], [6]]. Furthermore, a quantitative glycoproteome analysis of unhatched fertilized eggs and hatched eggs over a 12-day period indicated that N-glycoprotein variations affected the utilization of egg proteins by the chicken embryo during incubation [7]. These studies provided important structural information about egg N-glycoproteins and enhanced our understanding of the mechanisms of innate defense and embryo protection, egg protein functions, evolution, embryo development, and changes in egg characteristics during storage and processing.
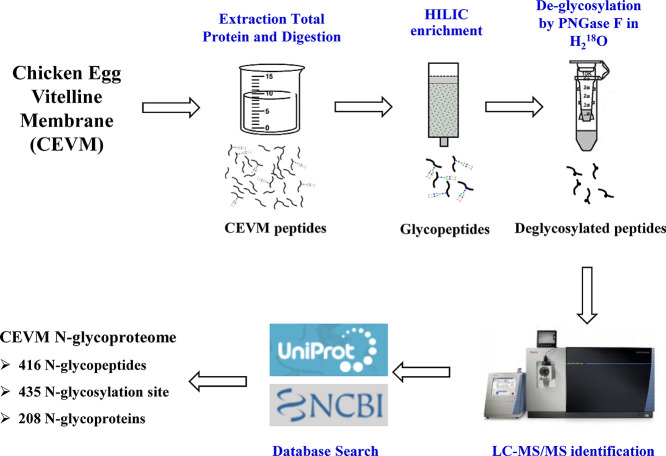
The study of the CEVM N-glycoproteome is expected to clarify the mechanisms underlying CEVM functions. Therefore, the present study aimed to characterize and analyze the CEVM N-glycoproteome. The total proteins of the CEVM were extracted and digested, and the glycopeptides were enriched by hydrophilic interaction liquid chromatography and subsequently deglycosylated by using PNGase F in H2 18O. The -18OH-labeled deglycopeptides were then identified by nano liquid chromatography/tandem mass spectrometry. In addition, bioinformatics analysis was employed to elucidate the functions of CEVM N-glycoproteins and the associated biological processes.
2. Materials and methods
2.1. CEVM sampling
Fresh unfertilized chicken eggs from hens of the Roman variety (40–50 weeks old, caged, on standard diets) were collected from Sichuan Sundaily Village Ecological Food Co., Ltd. (Mianyang, Sichuan) within 24 h of laying and used in this study [[8], [9], [10]]. The eggshells were broken, and the egg yolks were rolled on filter paper to remove the egg white [3,9]. A pipet tip was used to penetrate the CEVM; after the yolk flowed out, the CEVM was washed using PBS buffer (10 mmol/L, pH 7.2, containing 0.14 mol/L NaCl). The CEVMs were rinsed in PBS buffer and magnetically stirred (30 r/min) 5 times for 30 min each time. Twelve CEVMs were mixed as one biological replicate, and a total of 3 biological replicates were produced. The collected CEVM samples were frozen and stored in liquid nitrogen until further analysis.
2.2. Total protein extracted from the CEVM
Total protein was extracted according to previous methods [3,11]. Briefly, the frozen CEVM samples (one biological replicate consisting of 12 CEVMs) were ground with liquid nitrogen into powder. Then, the powder was transferred into a 5-mL centrifuge tube, and lysis buffer (8 mol/L urea, 1% protease inhibitor cocktail) was added at a mass ratio of 1:4 (sample:buffer). To maintain protein N-glycosylation during the extraction process, 10 μmol/L trichostatin A and 50 mmol/L nicotinamide were added. Then, ultrasonic extraction on ice was performed three times (150 W, 30 s each time). The supernatant was collected by centrifugation at 8000 ×g at 4 °C for 10 min. Finally, the protein concentrations of the extracted samples were determined with a BCA kit (P0010, Beyotime Institute of Biotechnology, Shanghai, China) [12,13].
2.3. Digestion and enrichment of glycopeptides
To digest the extracted CEVM proteins, 5 mmol/L dithiothreitol was added to the sample solution, and reduction was executed at 56 °C for 30 min. Then, the reduced proteins were alkylated with 11 mmol/L iodoacetamide for 15 min in darkness at room temperature. The sample was then diluted to a urea concentration of less than 2 mol/L by adding NH4HCO3 (100 mmol/L). Digestion was performed by adding trypsin at a mass ratio of 1:50 (trypsin:protein) overnight, followed by a second digestion at a mass ratio of 1:50 (trypsin:protein) for 4 h [14,15].
Enrichment of the CEVM glycopeptides was performed according to a previous study with minor modifications [3]. The digested CEVM peptides were reconstituted in 80% acetonitrile (with 1% trifluoroacetic acid) and then loaded into a hydrophilic interaction liquid chromatography (HILIC) microcolumn (Dalian Institute of Chemical Physics, Dalian, China). The glycopeptides were bound by the hydrophilic filter and retained in a HILIC microcolumn. After washing away the unbound peptides with 80% acetonitrile three times, the CEVM glycopeptides were eluted with 10% acetonitrile. The eluent containing the enriched glycopeptides was collected and lyophilized. The glycopeptides were resolved in 50 μL H2 18O, and deglycosylation was carried out by adding 200 units of PNGase F (Roche, 11365185001, Mannheim, Germany) and incubating overnight at 37 °C. Finally, the CEVM N-glycopeptides that had been deglycosylated and -18OH labeled were desalted through C18 ZipTips, and the sample was lyophilized for subsequent analysis.
2.4. LC-MS/MS
The enriched and deglycosylated CEVM N-glycopeptides were dissolved in water (0.1% formic acid) and loaded into a C18 column (Reprosil-Pur, 1.9 μm particles, inner diameter of 75 μm, length of 15 cm). Liquid chromatography separation and mass spectrometry were carried out by using an Orbitrap Fusion™ mass spectrometer equipped with an EASY-nLC 1000 UPLC system (Thermo Fisher Scientific, Bremen, Germany). Gradient separation was performed using buffer A (2% acetonitrile with 0.1% formic acid) and buffer B (90% acetonitrile with 0.1% formic acid) at a flow rate of 700 nL/min. Buffer B was increased stepwise from 5% to 22% in 40 min, from 22% to 35% in 8 min, and from 35% to 80% in 4 min and then held at 80% for 4 min. After UPLC separation, the deglycosylated CEVM N-glycopeptides were injected to a nanoelectrospray ionization source and ionized at 2000 V. The data-dependent acquisition mode was used, and the top 20 most abundant peptides from the MS were selected for MS/MS analysis. Fragmentation of precursor ions was achieved through high-energy collision-induced decomposition (35%). A mass range of 350–1550 m/z and a resolution of 70,000 were configured for MS, and a resolution of 15,000 was set for MS/MS.
2.5. Bioinformatics analyses
MS/MS data were parsed using MaxQuant software (1.5.2.8) and then searched for matches with the UniProt (Gallus gallus, 29,480 sequences) and NCBI (Gallus gallus, 48,708 sequences) databases. The following settings were employed: enzyme, trypsin; maximum number of missed cleavage sites, 2; precursor mass tolerance in the first search and main search, 20 ppm and 5 ppm, respectively; and fragment mass tolerance, 0.02 Da. Carbamidomethyl on Cys was configured as a fixed modification, and acetylation on the protein N-terminal and oxidation of Met were configured as variable modifications. Deamidation with 18O (N) and deamidation (NQ) were set as variable modifications for the identification of N-glycopeptides. The threshold false discovery rate was set as 1% for the identification of peptides, proteins and modification sites.
Gene Ontology (GO) annotation of the identified CEVM N-glycoproteins was achieved using the UniProt-Gene Ontology Annotation database (http://www.ebi.ac.uk/GOA). For GO enrichment analysis, a two-tailed Fisher's exact test was used to test the enrichment of each identified modified protein, and all proteins in the species database were set as the background. GO terms with a corrected p < 0.05 were considered significant.
3. Results and discussion
3.1. Characterization of the identified N-glycosylation sites in the CEVM
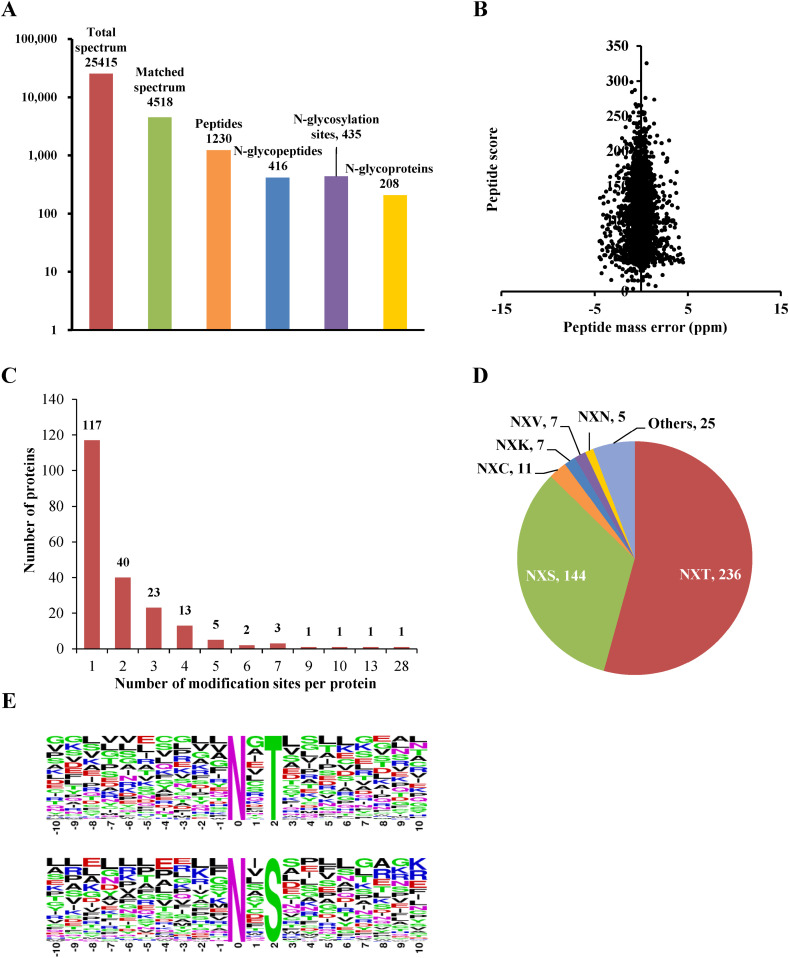
The CEVM proteins were extracted and digested, and the CEVM glycopeptides were enriched by a HILIC column and deglycosylated by PNGase F in H2 18O, resulting in an 18OH tag (with a 2.99 Da increase) for LC-MS/MS identification. The mass spectrometer produced 25,415 spectra, with a total of 4518 spectra matched to the aligned proteins (Gallus gallus); of the matched spectra, 1230 were identified as corresponding to N-glycopeptides. After clearing repeat sequences, a total of 416 unique CEVM N-glycopeptides were obtained, which contained 435 N-glycosylation sites and represented 208 glycoproteins (Fig. 1A, Table S1). The mass error of all peptide ions was less than 5 ppm (Fig. 1B), indicating the high precision of the detection results. Compared to the N-glycoproteomes of chicken egg white (26 N-glycoproteins, 71 N-glycosites) and yolk (86 glycoproteins, 217 N-glycosites) [2,3], the CEVM N-glycoproteome has a greater variety of N-glycoproteins and N-glycosites. Chicken egg white and yolk include some high-abundance proteins, such as ovalbumin in egg white and apolipoprotein B (the precursor protein of egg yolk low-density lipoprotein) in egg yolk, which may affect the throughput of proteomic analysis. However, in the analysis of the N-glycoproteome, the glycopeptides were first enriched before LC-MS/MS identification to offset the adverse effects of high-abundance proteins on the identification throughput. The results indicated that the kinds of glycoproteins in the yolk membrane were more diverse than those in egg white or egg yolk.
Fig. 1.
Characteristics of the identified N-glycoproteome of the chicken egg vitelline membrane (CEVM). (A) Statistical information from the CEVM N-glycoproteomic analysis. (B) Mass error distribution of the identified CEVM N-glycopeptides. (C) Number of N-glycosylation sites per identified CEVM N-glycoprotein. (D) Number of N-glycosylation sites matched with motifs (X ≠ P). (E) Sequences around motifs of N-X-T and N-X-S.
Most of the identified CEVM N-glycoproteins (117, representing 60.0% of the total) were single N-glycosylated, and 41 (19.6%) had two N-glycosylation sites. Another 51 (24.4%) CEVM N-glycoproteins were multiple N-glycosylated (Fig. 1C). Mucin-5B (α-ovomucin) was the most heavily N-glycosylated protein in the CEVM, with 28 N-glycosylation sites, followed by apolipoprotein B, with 13 N-glycosylation sites. Consistent with these results, mucin-5B and apolipoprotein B are the proteins with the most N-glycosylation sites in the N-glycoproteome of chicken egg white and yolk, respectively [2,3].
The posttranslational N-glycosylation modification site has sequence conservation, and the canonical motifs are N-X-[S/T] (where X is not proline). For the identified CEVM N-glycoproteins, 237 N-glycosylation sites (representing 54.2% of the total) matched N-X-T, with such sites being more abundant than those matching N-X-S (144 sites, 33.0%). Other atypical motifs included N-X-C (16 sites), N-X-K (7 sites), N-X-V (7 sites), and N-X-N (5 sites) (Fig. 1D). The sequence motifs around N-X-T and N-X-S sites (10 amino acids per side) were visualized by using Web-Logo (Fig. 1E). The proportion of canonical motifs N-X-[S/T] in the CEVM N-glycoproteome was very similar to those in the chicken egg white N-glycoproteome (55% for N-X-T and 32% for N-X-S) but much higher than those in the N-glycoproteomes of chicken egg yolk (35% for N-X-T and 29% for N-X-S), duck egg white (36% for N-X-T and 26% for N-X-S) and duck egg yolk (31% for N-X-T and 21% for N-X-S). Additionally, although the N-X-K motif only appeared in the CEVM N-glycoproteome, other motifs, such as N-X-C, N-X-V, and N-X-N, appeared in the N-glycoproteomes of egg white and yolk with high frequency.
3.2. GO annotation and analysis of CEVM N-glycoproteins
Although some egg N-glycoproteins have been well studied, the functions of most identified CEVM N-glycoproteins are unclear. In the present study, GO annotation and analysis were performed, and the identified N-glycoproteins were annotated and classified. Then, GO enrichment analyses were conducted to explore their functions or associated processes.
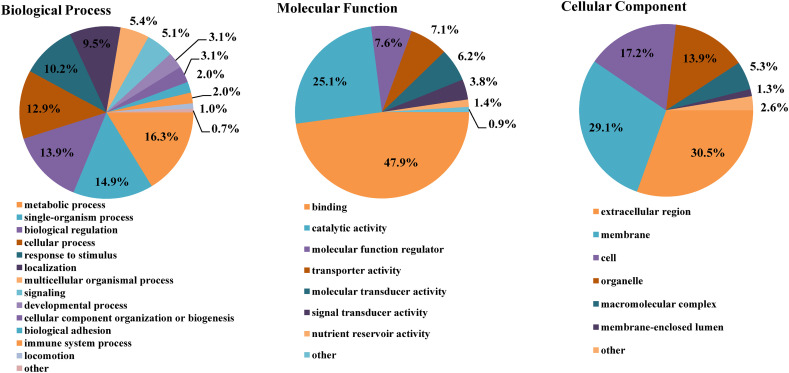
In the Gene Ontology (GO) annotation, the identified CEVM N-glycoproteins were divided into three categories: “biological processes”, “molecular functions”, and “cellular compositions”. A total of 99 CEVM N-glycoproteins were annotated in the category of “biological processes”, which are mainly involved in “metabolic process” (GO: 0008152, 48), “single biological process” (GO: 0044707, 44), “biological regulation” (GO: 0065007, 41), “cellular process” (GO: 0009987, 38), “response to stimulus” (GO: 0050896, 30), and “localization” (GO: 0051179, 28). The number of CEVM N-glycoproteins involved in the biological processes “signaling”, “developmental process” and “immune system process” was 15, 9, and 6, respectively (Fig. 2A). In the category “molecular function”, 155 CEVM N-glycoproteins were annotated, and most of them were annotated to “binding” (GO: 0005488, 101) and “catalytic activity” (GO: 0003824, 53). In addition, 15, 13, and 8 CEVM N-glycoproteins were annotated to “transporter activity”, “molecular transducer activity” and “signal transducer activity”, respectively (Fig. 2B). For the “cellular component” category, the annotated CEVM N-glycoproteins (90) were mainly associated with “extracellular region” (GO: 0005576, 46), followed by the formation of “membrane” (GO: 0016020, 44), “cell” (GO: 0005623, 26) and “organelle” (GO: 0043226, 21) (Fig. 2C).
Fig. 2.
Gene Ontology annotation and classification of the identified N-glycoproteins in the chicken egg vitelline membrane.
In the GO enrichment analysis, a total of 30 GO terms were enriched, but many terms were related to each other and shared the same mapped N-glycoprotein (Table S2). A large number of enriched GO terms were related to proteases and their inhibition. On the one hand, “peptidase activity” (GO: 0008233), which was associated with 19 mapped N-glycoproteins, was enriched in the category “molecular function”. On the other hand, 4 enriched GO terms in the category “biological process” (GO: 0045861, GO: 0010951, GO: 0010466, and GO: 0052548) and 6 enriched GO terms in the category “molecular function” (GO: 0030234, GO: 0004857, GO: 0030414, GO: 0061134, GO: 0004866, and GO: 0061135; with a total of 16 mapped N-glycoproteins) were associated with the inhibition of proteases, represented by “negative regulation of proteolysis” and “endopeptidase inhibitor activity”. These results demonstrated that the proteinase/inhibitor regulation system exists in the CEVM, similar to previous findings in chicken and duck egg yolk [3,5].
In addition, 9 enriched GO terms were related to the sodium ion export process, represented by “positive regulation of sodium ion export from cell” (GO: 1903278). These enriched GO terms share the same mapped proteins (P33879 and F1NSV6), which are the β-1 and β-3 subunits of sodium/potassium-transporting ATPase. This ATPase can catalyze the ATP hydrolysis associated with the transmembrane exchange of Na+ and K+. This action provides the energy for nutrient transport by creating an electrochemical gradient. Similar ATPases were identified in a previous proteomic analysis of the CEVM [1]. Furthermore, “lipid transport” (GO: 0006869) was enriched, and the proteins mapped in this term included apolipoprotein B, vitellogenin-1, vitellogenin-2, vitellogenin-3, apovitellenin-1, very low-density lipoprotein receptor (VLDLR), and cell cycle control protein 50A (CC50A). Apovitellenin-1 is a very low-density lipoprotein (VLDL) component of laying hens. It acts as a potent lipoprotein lipase inhibitor and prevents triglyceride loss from VLDLs during their transport from the liver to the growing follicles (egg yolk). VLDLR, localized on the cell membrane, functions as a transmembrane transporter of VLDL into cells through endocytosis. CC50A is the beta subunit of the P4-ATPase flippase complex, which could hydrolysis of ATP associated with the transmembrane transport (from the outer to the inner membrane space) of aminophospholipids. Therefore, the above CEVM glycoproteins likely work together and play very important roles in the transmembrane transport of nutrients, especially lipids, from the circulatory system to chicken yolk (follicle). N-glycosylation modifications are likely very important for the above enzymes, transporters, receptor proteins, and lipoproteins because the covalently bonded N-glycans are expected to affect their folding and surface properties, thereby controlling their stability, activity, and substrate affinity.
3.3. The predominant N-glycoproteins in the CEVM
In the LC-MS/MS identification, “MS/MS count” referred to the number of MS/MS spectra that matched a given unique N-glycopeptide and was used to approximate the relative abundances of the N-glycopeptides. The “MS/MS count” corresponding to the 208 CEVM N-glycoproteins was 2304 in total. Mucin-5B (with an “MS/MS count” of 564, accounting for 24.5%) was the most abundant N-glycoprotein in the CEVM, followed by alpha-2-macroglobulin-like protein 1 (181, 7.9%), apolipoprotein B (154, 6.7%), ovomucoid (138, 6.0%), HEP21 (76, 3.3%) and clusterin (68, 3.0%). The “MS/MS count” of these 6 glycoproteins exceeded half of the total “MS/MS count” values, and these glycoproteins were high-abundance glycoproteins in the CEVM.
3.3.1. Mucin-5B and Mucin-6
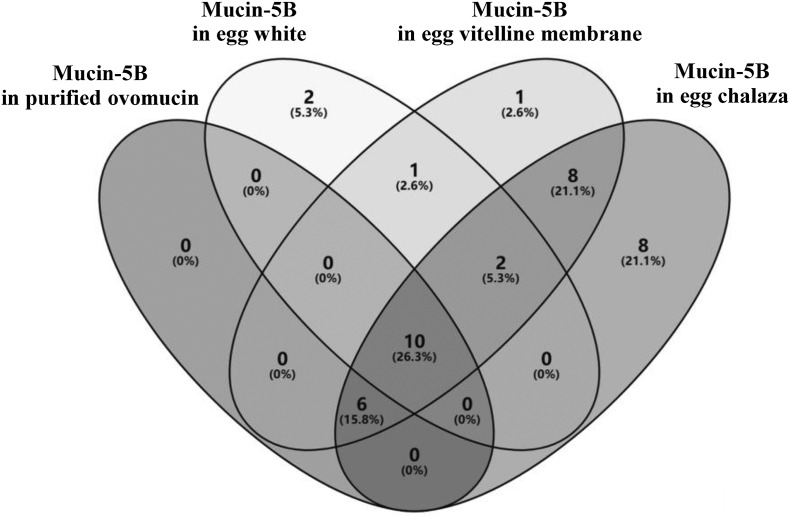
As the alpha subunit of chicken egg white ovomucin, mucin-5B is considered the most important glycoprotein in egg white and has been studied in detail. The N-glycosylation characteristics of mucin-5B have been investigated by analyzing the purified egg white ovomucin, and 16 N-glycosites have been identified [16]. Mucin-5B has also been identified in N-glycoproteomic analyses of egg white (with 15 N-glycosites) and chalaza (with 34 N-glycosites) [2]. However, mucin-5B is absent from the N-glycoproteomes of egg yolk and eggshell matrix, indicating that the distribution of mucin-5B is tissue specific [3,10]. In the CEVM, mucin-5B was identified with 28 N-glycosites and a total “MS/MS count” of 564. Interestingly, the N-glycosite locations of mucin-5B from different egg parts are quite different (Fig. 3 ). There may be several reasons for this difference. 1) The analysis method differed among studies. 2) The relative content of mucin-5B differs among egg parts, and a higher relative content would generally be beneficial for improving the depth of identification. 3) The N-glycosylation of mucin-5B is heterogeneous. Regardless, the four kinds of mucin-5B from different egg parts share 10 N-glycosites: N381, N528, N680, N772, N855, N1036, N1219, N1371, N1452, and N1964. These common N-glycosites might be very important for the folding and function of mucin-5B. Mucin-5B from the CEVM and chalaza shared the most N-glycosites (26), indicating that their N-glycosylation patterns are similar. This finding may be related to the close relationship between the CEVM and chalaza: chalaza formation starts from the CEVM, and chalaza is anchored at the poles of the CEVM.
Fig. 3.
Comparison of the N-glycosites of mucin-5B from different egg parts.
The beta subunit of ovomucin, mucin-6, was also identified in the CEVM, with 7 N-glycosites and a total “MS/MS count” of 57. Two N-glycosites (N223 and N930) of mucin-6 were first reported in an N-glycosylated characterization of purified ovomucin [16]. Another 2 N-glycosites (N 1108 and N1133) of mucin-6 were identified in an N-glycoproteomic analysis of chicken egg white [2]. In the present study, in addition to these 4 sites previously identified, 3 new N-glycosites (N387, N959, and N1020) of ovomucin were found in the CEVM.
The highly glycosylated ovomucin in egg white is considered the main reason for the high viscosity of egg whites, and ovomucin undergoes disaggregation during storage, accompanied by the thinning of egg white [17]. Furthermore, it was previously found that the abundances of mucin-5B and mucin-6 in thick egg white were 18.9% and 400.0% higher, respectively, than those in thin egg white (p < 0.01). The higher content of mucin-5B/mucin-6 in thick egg white could provide more skeleton structure during the formation of heat-induced gel and might regulate the microstructure of the gel, resulting in a “softer and tougher” gel texture [18]. These findings suggest that the abundance of ovomucin is related to the viscosity and texture properties of egg white. Therefore, it could be inferred that the high abundances of mucin-5B and mucin-6 in the CEVM might enhance CEVM mechanical strength by increasing its viscosity and toughness. Similar to the thinning of egg white, the decrease in mechanical strength of CEVM during storage might also related to the disaggregation of ovomucin. This is an important speculation that needs to be verified by future study.
Ovomucin (mucin-5B and mucin-6) has been identified as absent from egg yolk in all previous proteome studies [3,5,19,20], suggesting that ovomucin is exclusively distributed in the outer layer of the CEVM. The structure-specific distribution of ovomucin is closely related to the different origins of the inner and outer layers of the CEVM. When the mature follicle is discharged from the hen ovary to the infundibulum, the CEVM comprises only the inner layer, which is secreted and formed by granulosa cells around the follicle. The outer layer of the CEVM is secreted by cells in the upper oviduct and adheres to the inner layer to form the complete CEVM [21]. The above observations suggest that the strong adhesion of CEVM ovomucin to the membrane structure might be the main adhesive force between the inner and outer layers of the CEVM. This adhesion process is likely similar to the adhesion of mucins to the digestive tract or respiratory tract. These findings suggested that CEVM might be a very valuable material for wound healing. Eggshell membrane has been developed as wound healing materials [22,23]. Compared with eggshell membrane, CEVM has potential advantages due to the adhesion and antiviral properties brought by high abundance of ovomucin.
In addition, ovomucin could provide an important innate immune barrier against various toxins and pathogens, and the sialic acid at the end of the glycans is essential in the recognition of avian influenza viruses [[24], [25], [26]]. Therefore, the high abundance of ovomucin in the CEVM provides a strong natural defense, protecting the chicken embryo (or preserving the egg yolk). Moreover, the diversity and heterogeneity of N-glycosylation sites of ovomucin may be related to the efficient recognition of influenza virus by this protein.
3.3.2. Macroglobulins
Alpha-2-macroglobulin-like protein 1 (A2ML1) was identified in the CEVM with 5 N-glycosites (N246, N782, N1034, N1191, and N1410) and a total “MS/MS count” of 181. Another 2 macroglobulin, A2ML4 (N68, N84, N330), and ovostatin (N403, N527, and N829) were also identified in the CEVM but at low abundance. According to the results of SMART analysis (http://smart.embl-heidelberg.de/), these 3 macroglobulins have the same domains (A2M-N-2, A2M, Thiol-ester-cl, and A2M-recep), but their sequence identity is only 22.5% according to Align analysis (https://www.uniprot.org/align/). Moreover, A2ML4 was identified in a previous egg yolk glycoproteome analysis, and ovostatin was identified in an egg white glycoproteome analysis [2,3]. These results suggest that these 3 proteins might be synthesized and secreted by different tissues/cells.
A2ML1, A2ML4 and ovostatin all belong to the alpha-2-macroglobulin family. Typical members of this family are glycoproteins composed of four identical subunits with high molecular weight. The primary function of alpha-2-macroglobulins is the inhibition of various proteases by encapsulating them in their cage-like molecules [27]. Alpha-2-macroglobulins are involved in innate immunity [28], tumor development [29], cell migration [30], and other processes. These observations suggest that the high abundance of A2ML1 in the CEVM might have important functions such as inhibiting proteases secreted by microorganisms to resist invasion and maintaining the balance of proteases on the CEVM to maintain the integrity of the membrane structure.
3.3.3. Apolipoprotein B and Vitellogenins
Apolipoprotein B is the precursor protein of egg yolk low-density lipoprotein, and it was previously identified as the predominant glycoprotein in egg yolk, with a total of 35 N-glycosites [3]. In the present study, apolipoprotein B was similarly the major glycoprotein in the CEVM, with a total of 13 N-glycosites and an “MS/MS count” of 154. There were 11 N-glycosites identified in both egg yolk apolipoprotein B and CEVM apolipoprotein B, and 2 sites (N1982 and N2431) were newly identified in CEVM apolipoprotein B. Among the 13 N-glycosites identified in CEVM apolipoprotein B, 10 (N275, N352, N975,N2900, N2926, N3092, N3328, N3432, N3697, and N3891) were matched to the canonical motifs of N-X-S/N-X-T and were all found to have a high “MS/MS count” (7 to 37). The remaining 3 N-glycosites (N1982, N2431 and N2718) were at low abundance and occupied the atypical motifs. Vitellogenins, the precursor proteins of egg yolk phosvitin and high-density lipoprotein, were identified in N-glycoproteomic analyses of both egg yolk and the CEVM. There were 2, 4, and 1 N-glycosites occupying CEVM vitellogenin-1, vitellogenin-2, and vitellogenin-3, respectively. All of these N-glycosites on CEVM vitellogenins were identified in the previous egg yolk glycoproteome analysis [3].
As the protein components of egg yolk low-density lipoprotein and high-density lipoprotein, apolipoprotein B and vitellogenins function primarily in the binding and transport of lipids. N-glycosylation modification is likely very important for the functions of these two proteins. Covalently modified N-glycans on apolipoprotein B and vitellogenins could play directional roles in the compounding of peptide chains and lipids (lipids are hydrophobic, and N-glycans are hydrophilic). In the transmembrane transport of apolipoprotein B and vitellogenins, the extended N-glycans might play a key role in the recognition of receptors. These speculations are supported by studies on human lipoproteins. The glycosylated forms of apolipoprotein A-I (the main protein component of serum HDL) were found to increase with the time course of acute myocardial infarction, suggesting that the glycosylation of apolipoprotein A-I might be associated with this protein's cholesterol-clearance and anti-atherosclerosis effects [31]. In addition, the removal of glycan terminal sialic acid (desialylation) from LDL has been shown to accelerate the uptake and accumulation of LDL in THP-1 cells and to significantly reduce the ability of HDL to clear cholesterol esters in THP-1 cells [32].
3.3.4. Ovomucoid
Ovomucoid is the major glycoprotein in egg white and was detected at high abundance in the eggshell matrix glycoproteomic analysis [2,10]. In the CEVM, 7 N-glycosites (N34, N63, N77, N93, N99, N193, N199) of ovomucoid were identified, with a total “MS/MS count” of 138. Among the 7 identified N-glycosites of CEVM ovomucoid, 5 N-glycosites matched the canonical motifs, with high “MS/MS count” values (13 to 46), but 2 N-glycosites matched with atypical motifs (N63 and N193) and had an “MS/MS count” of only 1. Low abundance and atypical N-glycosylation were also observed in the previous egg yolk glycoproteomic analysis. This phenomenon likely contributes to the heterogeneity of N-glycosylation. Further study is needed to determine whether this low abundance, atypical N-glycosylation is an occasional erroneous modification or has a potential role in the regulation of protein structure and function.
3.3.5. HEP21
Previous investigations have shown that the HEP21 gene is expressed primarily in hen oviduct, especially the magnum, where egg white proteins are secreted [33]. Egg proteomic studies have shown that at the protein expression level, HEP21 is present in all parts of the egg, including the egg white, egg yolk, eggshell matrix, chalaza, and CEVM. As for the N-glycosylation level, N-glycosylated HEP21 has been found in all egg parts except egg white. In the present study, 3 N-glycosites of CEVM HEP21 were identified: one at N39, at high abundance and with and “MS/MS count” of 74, and 2 at N72 and N98, at low abundance and with atypical motifs. The N39 of HEP21 was also identified in the previous N-glycoproteomic analysis of egg yolk, eggshell and chalaza; but the other 2 N-glycosites have been found only on CEVM HEP21.
The function of HEP21 remains unclear, but research has shown that the expression of HEP21 mRNA is responsive to estrogen. Therefore, HEP21 is thought to play a regulatory role in the production and formation of the egg [34]. Hormone signaling and target-tissue responses to hormones need to be precise. Therefore, as a protein involved in estrogen responses, the tissue specificity of HEP21 at different levels (gene expression, protein expression and N-glycosylation) can be expected to greatly enhance the diversity of specific responses, thereby facilitating accurate response to estrogen.
3.3.6. Clusterin
Clusterin was identified in egg white and eggshell in previous N-glycoproteome analyses and in CEVM in the present study; however, it has not been identified in the N-glycoproteome of egg yolk. A total of 4 N-glycosites (N99, N141, N352, and N372) of CEVM clusterin were identified, all of which have been found to be N-glycosylated in egg white and eggshell clusterin. In the previous N-glycoproteomic analysis of egg white, heterogeneity of clusterin glycosylation was revealed by two-dimensional electrophoresis, and glycosylation was found to strongly influence the charge characteristics (isoelectric point) of clusterin [2]. The function of clusterin in chicken egg is not yet clear; however, its content has been found to be increased in the egg white of fertilized eggs after 7 d of incubation, suggesting its involvement in the development of the chicken embryo [35].
Human clusterin is considered an extracellular chaperone that can bind to misfolded proteins and then undergo degradation. A recent study revealed the mechanisms by which clusterin clears misfolded proteins: clusterin binds to heparan sulfate through electrostatic interaction, is transported into the cell through the heparan sulfate receptor on the cell surface and then is degraded in lysosome [36]. This pathway could promote the degradation of amyloid beta peptide, which explains why clusterin is associated with Alzheimer's disease [37]. It has been speculated that the diverse charge characteristics of clusterin associated with glycosylation heterogeneity are likely very important for the interaction and binding of clusterin to misfolded proteins and heparan sulfate.
3.3.7. Deleted in malignant brain tumors 1 protein (DMBT1)
DMBT1 was found absent from the N-glycoproteomes of egg white, egg yolk, and eggshell in previous analyses but was identified in the CEVM in the present study, with a total of 5 N-glycosites and high abundance (an “MS/MS count” of 54, accounting for 2.3% of the glycoproteins). Porcine DMBT1 has been reported to be involved in the interaction of sperm and oviduct [38], implying that DMBT1 in the CEVM might be involved in fertilization in chickens. In addition to potentially having functions related to reproduction, DMBT1 might be related to hypoxic stress. DMBT1 was identified in the egg yolks of Tibetan and lowland chickens in a comparative proteomic analysis, with significantly higher abundance in Tibetan egg yolk, with a fold-change of 1.97 [39]. Furthermore, the expression of human DMBT1 was significantly increased in the plasma of patients with acute respiratory distress syndrome relative to the plasma of healthy subjects [40]. These findings suggest a possible link between DMBT1 and COVID-19, which warrants attention given the current emergency state in response to COVID-19.
Based on bioinformatics annotations, DMBT1 is a membrane protein containing the scavenger receptor cysteine-rich (SRCR) domain, and involves in “receptor-mediated endocytosis” and “scavenger receptor activity”. It has been reported that human DMBT1 plays an important role in innate immunity because it could directly binding to a broad range of pathogens [41]. Besides, recently study showed that DMBT1 could inhibit the infiltration in the nasal cavity of eosinophils and the production of IL-4 and IL-5, then reduce the number of nasal sneezing and rubbings in allergic rhinitis mice [42]. Qiu et al. compared the egg white proteome of quail and duck and found that DMBT1 was specific in duck egg white. The specific distribution of DMBT1 in duck egg white was speculated to be related to the ducks' adaptability to the wetland habitats: more pathogen invasion challenges and therefore require stronger defenses [43]. The CEVM carries the chicken embryo and also needs strong defense against the invasion of pathogens. Therefore, the relatively high abundance of DMBT1 in CEVM should take the responsibility of innate immunity in addition to participating in fertilization and embryo development.
4. Conclusion
This study is the first large-scale mapping study of the N-glycosylation profiles of the CEVM. A total of 435 N-glycosites belonging to 208 N-glycoproteins were identified in the CEVM, revealing greater diversification in protein N-glycosylation in the CEVM than in egg white or egg yolk in chickens. Gene Ontology analysis suggested that a proteinase/inhibitor regulation system exists in the CEVM. Some subunits of ATPases, apolipoproteins and their receptors in the CEVM were identified as glycoproteins, and they likely interact and play important roles in the transmembrane transport of nutrients, especially lipids. Mucin-5B, A2ML1, apolipoprotein B, vitellogenins, ovomucoid, HEP21, clusterin and DMBT1 were the predominant N-glycoproteins identified in the CEVM. The tissue specificity of the N-glycosylation of egg proteins was revealed by comparing N-glycosylation between the CEVM and other egg parts (egg white, egg yolk, and eggshell matrix). The results provide not only a comprehensive understanding of protein N-glycosylation in chicken egg but also insights into the functions of the CEVM.
The following are the supplementary data related to this article.
The detailed identification information of chicken egg vitelline membrane N-glycoproteome.
Gene ontology enrichment analysis of the identified N-glycoproteins of chicken egg vitelline membrane.
CRediT authorship contribution statement
Jing Xiao: Investigation, Data curation, Writing - original draft. Jinqiu Wang: Data curation, Writing - original draft. Lei Cheng: Conceptualization, Funding acquisition, Writing - review & editing. Sihai Gao: Conceptualization, Writing - review & editing. Shugang Li: Resources. Ning Qiu: Methodology. Hanmei Li: Data curation. Lianxin Peng: Resources, Funding acquisition. Fang Geng: Supervision, Writing - original draft, Writing - review & editing, Funding acquisition.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgments
This research was supported by the National Key Research and Development Program of China (2018YFD0400302; 2018YFC1602101), the National Natural Science Foundation of China (31871732; 31601490), and Beijing Advanced Innovation Center for Food Nutrition and Human Health (BTBU20181021).
References
- 1.Mann K. Proteomic analysis of the chicken egg vitelline membrane. PROTEOMICS. 2008;8(11):2322–2332. doi: 10.1002/pmic.200800032. [DOI] [PubMed] [Google Scholar]
- 2.Geng F., Wang J.Q., Liu D.Y., Jin Y.G., Ma M.H. Identification of N-glycosites in chicken egg white proteins using an omics strategy. J. Agric. Food Chem. 2017;65(26):5357–5364. doi: 10.1021/acs.jafc.7b01706. [DOI] [PubMed] [Google Scholar]
- 3.Geng F., Xie Y.X., Wang J.Q., Majumder K., Qiu N., Ma M.H. N-glycoproteomic analysis of chicken egg yolk. J. Agric. Food Chem. 2018;66(43):11510–11516. doi: 10.1021/acs.jafc.8b04492. [DOI] [PubMed] [Google Scholar]
- 4.Meng Y.Q., Qiu N., Geng F., Huo Y.Q., Sun H.H., Keast R. Identification of the duck egg white N-glycoproteome and insight into the course of biological evolution. J. Agric. Food Chem. 2019;67(35):9950–9957. doi: 10.1021/acs.jafc.9b03059. [DOI] [PubMed] [Google Scholar]
- 5.Meng Y., Qiu N., Geng F., Keast R., Li B., Zheng X. N-glycoproteomic analysis of duck egg yolk proteins: implications for biofunctions and evolution. Int. J. Biol. Macromol. 2020;151:19–26. [Google Scholar]
- 6.Liu L., Yang R., Luo X., Dong K., Huang X., Song H., Gao H., Li S., Huang Q. Omics analysis of holoproteins and modified proteins of quail egg. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.126983. [DOI] [PubMed] [Google Scholar]
- 7.Zhu F., Qiu N., Sun H., Meng Y., Zhou Y. Integrated proteomic and N-glycoproteomic analyses of chicken egg during embryonic development. J. Agric. Food Chem. 2019;67(42):11675–11683. doi: 10.1021/acs.jafc.9b05133. [DOI] [PubMed] [Google Scholar]
- 8.Wu D., Mei S., Duan R., Geng F., Wu W.X., Li X., Cheng L., Wang C.T. How black tea pigment theaflavin dyes chicken eggs: binding affinity study of theaflavin with ovalbumin. Food Chem. 2020;303 doi: 10.1016/j.foodchem.2019.125407. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y.X., Wang J.Q., Wang Y., Wu D., Liang D.W., Ye H.L., Cai Z.X., Ma M.H., Geng F. Effects of high-intensity ultrasonic (HIU) treatment on the functional properties and assemblage structure of egg yolk. Ultrason. Sonochem. 2020;60 doi: 10.1016/j.ultsonch.2019.104767. [DOI] [PubMed] [Google Scholar]
- 10.Yang R., Geng F., Huang X., Qiu N., Li S., Teng H., Chen L., Song H., Huang Q. Integrated proteomic, phosphoproteomic and N-glycoproteomic analyses of chicken eggshell matrix. Food Chem. 2020;330 doi: 10.1016/j.foodchem.2020.127167. [DOI] [PubMed] [Google Scholar]
- 11.Wang J.Q., Xiao J., Geng F., Li X., Yu J., Zhang Y.Q., Chen Y., Liu D.Y. Metabolic and proteomic analysis of morel fruiting body (Morchella importuna) J. Food Compos. Anal. 2019;76:51–57. [Google Scholar]
- 12.Geng F., Huang Y., Huang Q., He D., Li S.G., Ma M.H. Effect of hydroxyl radical-induced oxidation on the structure and heat-induced gel properties of ovalbumin. J. Food Process. Preserv. 2018;42(6):8. [Google Scholar]
- 13.Geng F., Xie Y.X., Wang J.Q., Li S.G., Jin Y.G., Ma M.H. Large-scale purification of ovalbumin using polyethylene glycol precipitation and isoelectric precipitation. Poult. Sci. 2019;98(3):1545–1550. doi: 10.3382/ps/pey402. [DOI] [PubMed] [Google Scholar]
- 14.Gu X.D., Gao Y.L., Luo Z., Yang L., Chi F.M., Xiao J., Wang W., Geng F. In-depth mapping of the proteome of Tibetan pig tenderloin (longissimus dorsi) using offline high-pH reversed-phase fractionation and LC-MS/MS. J. Food Biochem. 2019;43(11) doi: 10.1111/jfbc.13015. [DOI] [PubMed] [Google Scholar]
- 15.Wang J.Q., Xiao J., Liu X., Geng F., Huang Q., Zhao J.L., Xiang D.B., Zhao G. Analysis of tartary buckwheat (Fagopyrum tataricum) seed proteome using offline two-dimensional liquid chromatography and tandem mass spectrometry. J. Food Biochem. 2019;43(7):9. doi: 10.1111/jfbc.12863. [DOI] [PubMed] [Google Scholar]
- 16.Offengenden M., Fentabil M.A., Wu J. N-glycosylation of ovomucin from hen egg white. Glycoconj. J. 2011;28(3):113–123. doi: 10.1007/s10719-011-9328-3. [DOI] [PubMed] [Google Scholar]
- 17.Shan Y., Tang D., Wang R., Tu A., Yi Y., Wang X., Liu B., Zhou Y., Huang Q., Lü X. Rheological and structural properties of ovomucin from chicken eggs with different interior quality. Food Hydrocoll. 2020;100 [Google Scholar]
- 18.Liu X., Wang J., Huang Q., Cheng L., Gan R., Liu L., Wu D., Li H., Peng L., Geng F. Underlying mechanism for the differences in heat-induced gel properties between thick egg whites and thin egg whites: gel properties, structure and quantitative proteome analysis. Food Hydrocoll. 2020;106 [Google Scholar]
- 19.Liu Y.P., Qiu N., Gao D., Ma M.H. Comparative proteomic analysis of chicken, duck, and quail egg yolks. Int. J. Food Prop. 2018;21(1):1311–1321. [Google Scholar]
- 20.Arena S., Scaloni A. An extensive description of the peptidomic repertoire of the hen egg yolk plasma. J. Agric. Food Chem. 2018;66(12):3239–3255. doi: 10.1021/acs.jafc.8b01183. [DOI] [PubMed] [Google Scholar]
- 21.Kido S., Doi Y. Separation and properties of the inner and outer layers of the vitelline membrane of hen’s eggs. Poult. Sci. 1988;67(3):476–486. [Google Scholar]
- 22.Li X., Cai Z., Ahn D.U., Huang X. Development of an antibacterial nanobiomaterial for wound-care based on the absorption of AgNPs on the eggshell membrane. Colloids Surf. B: Biointerfaces. 2019;183 doi: 10.1016/j.colsurfb.2019.110449. [DOI] [PubMed] [Google Scholar]
- 23.Li X., Ma M., Ahn D.U., Huang X. Preparation and characterization of novel eggshell membrane-chitosan blend films for potential wound-care dressing: from waste to medicinal products. Int. J. Biol. Macromol. 2019;123:477–484. doi: 10.1016/j.ijbiomac.2018.10.215. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee M., Pushkaran A.C., Vasudevan A.K., Menon K.K.N., Biswas R., Mohan C.G. Understanding the adhesion mechanism of a mucin binding domain from Lactobacillus fermentum and its role in enteropathogen exclusion. Int. J. Biol. Macromol. 2017;110 doi: 10.1016/j.ijbiomac.2017.10.107. S0141813017330787. [DOI] [PubMed] [Google Scholar]
- 25.Shan Y., Xu Q., Ma M. Mg2+ binding affects the structure and activity of ovomucin. Int. J. Biol. Macromol. 2014;70:230–235. doi: 10.1016/j.ijbiomac.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 26.Xu Q., Shan Y.Y., Wang N., Liu Y.P., Zhang M.J., Ma M.H. Sialic acid involves in the interaction between ovomucin and hemagglutinin and influences the antiviral activity of ovomucin. Int. J. Biol. Macromol. 2018;119:533–539. doi: 10.1016/j.ijbiomac.2018.07.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehman A.A., Ahsan H., Khan F.H. Alpha-2-macroglobulin: a physiological guardian. J. Cell. Physiol. 2013;228(8):1665–1675. doi: 10.1002/jcp.24266. [DOI] [PubMed] [Google Scholar]
- 28.Craig-Barnes H.A., Doumouras B.S., Palaniyar N. Surfactant protein D interacts with alpha(2)-macroglobulin and increases its innate immune potential. J. Biol. Chem. 2010;285(18):13461–13470. doi: 10.1074/jbc.M110.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkridge A.M., Wysocky R.B., Petitte J.N., Anderson K.E., Mozdziak P.E., Fletcher O.J., Horowitz J.M., Muddiman D.C. Measuring the intra-individual variability of the plasma proteome in the chicken model of spontaneous ovarian adenocarcinoma. Anal. Bioanal. Chem. 2010;398(2):737–749. doi: 10.1007/s00216-010-3979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng F., Huang X., Ma M.H. Hen egg white ovomacroglobulin promotes fibroblast migration via mediating cell adhesion and cytoskeleton. J. Sci. Food Agric. 2016;96(9):3188–3194. doi: 10.1002/jsfa.7498. [DOI] [PubMed] [Google Scholar]
- 31.Cubedo J., Padró T., Badimon L. Glycoproteome of human apolipoprotein A-I: N- and O-glycosylated forms are increased in patients with acute myocardial infarction. Transl. Res. 2014;164(3):209–222. doi: 10.1016/j.trsl.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Sukhorukov V., Gudelj I., Pučić-Baković M., Zakiev E., Orekhov A., Kontush A., Lauc G. Glycosylation of human plasma lipoproteins reveals a high level of diversity, which directly impacts their functional properties. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids. 2019;1864(5):643–653. doi: 10.1016/j.bbalip.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Nau F., Guerin-Dubiard C., Desert C., Gautron J., Bouton S., Gribonval J., Lagarrigue S. Cloning and characterization of HEP21, a new member of the uPAR/Ly6 protein superfamily predominantly expressed in hen egg white. Poult. Sci. 2003;82(2):242–250. doi: 10.1093/ps/82.2.242. [DOI] [PubMed] [Google Scholar]
- 34.Lim W., Song G. Pivotal roles for hormonally regulated expression of the HEP21 gene in the reproductive tract of chickens for oviduct development and in ovarian carcinogenesis. Domest. Anim. Endocrinol. 2014;48:136–144. doi: 10.1016/j.domaniend.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y.J., Qiu N., Ma M.H. Comparative proteomic analysis of hen egg white proteins during early phase of embryonic development by combinatorial peptide ligand library and matrix-assisted laser desorption ionization-time of flight. Poult. Sci. 2013;92(7):1897–1904. doi: 10.3382/ps.2012-02986. [DOI] [PubMed] [Google Scholar]
- 36.Itakura E., Chiba M., Murata T., Matsuura A. Heparan sulfate is a clearance receptor for aberrant extracellular proteins. J. Cell Biol. 2020;219(3) doi: 10.1083/jcb.201911126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herring S.K., Moon H.J., Rawal P., Chhibber A., Zhao L.Q. Brain clusterin protein isoforms and mitochondrial localization. eLife. 2019;8:31. doi: 10.7554/eLife.48255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teijeiro J.M., Roldán M.L., Marini P.E. Molecular identification of the sperm selection involved porcine sperm binding glycoprotein (SBG) as deleted in malignant brain tumors 1 (DMBT1) Biochimie. 2012;94(1):263–267. doi: 10.1016/j.biochi.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Sheng L., Ma M., Jin Y. Proteome-based identification of chicken egg yolk proteins associated with antioxidant activity on the Qinghai-Tibetan Plateau. Int. J. Biol. Macromol. 2020;150:1093–1103. doi: 10.1016/j.ijbiomac.2019.10.115. [DOI] [PubMed] [Google Scholar]
- 40.Ren S., Chen X., Jiang L., Zhu B., Jiang Q., Xi X. Deleted in malignant brain tumors 1 protein is a potential biomarker of acute respiratory distress syndrome induced by pneumonia. Biochem. Biophys. Res. Commun. 2016;478(3):1344–1349. doi: 10.1016/j.bbrc.2016.08.125. [DOI] [PubMed] [Google Scholar]
- 41.Ligtenberg A.J.M., Karlsson N.G., Veerman E.C.I. Deleted in malignant brain tumors-1 protein (DMBT1): a pattern recognition receptor with multiple binding sites. Int. J. Mol. Sci. 2010;11(12):5213–5234. doi: 10.3390/ijms1112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y., Tao Q., Wu J., Liu H. DMBT1 has a protective effect on allergic rhinitis. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109675. [DOI] [PubMed] [Google Scholar]
- 43.Hu S., Qiu N., Liu Y., Zhao H., Gao D., Song R., Ma M. Identification and comparative proteomic study of quail and duck egg white protein using 2-dimensional gel electrophoresis and matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry analysis. Poult. Sci. 2016;95(5):1137–1144. doi: 10.3382/ps/pew033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The detailed identification information of chicken egg vitelline membrane N-glycoproteome.
Gene ontology enrichment analysis of the identified N-glycoproteins of chicken egg vitelline membrane.