Abstract
Oligodendrocyte replacement using glial restricted precursors (GRPs) is a promising avenue for the treatment of acquired or genetic white matter disorders; however, limited long-term survival of these cells post-transplant may impede maximal recovery. Nanotherapeutic approaches can facilitate stem cell delivery while simultaneously delivering factors aimed at enhancing and nourishing stem cells en route to, and at, the target site. Hydroxyl polyamidoamine (PAMAM) dendrimer nanoparticles have been used in a variety of models to deliver therapeutics in a targeted manner to injury sites at low doses. Here, survival and migration of GRPs was assessed in a mouse model of neonatal white matter injury with different methods of dendrimer nanoparticle support. Our findings demonstrate the ability of GRPs to take up nanoparticle-drug conjugates and for these conjugates to act beyond the injury site in vivo. Compared to GRPs alone, mice receiving dendrimer-drug in parallel to GRPs, or via GRPs as the delivery vector, showed improved migration and differentiation of cells 8 weeks post-transplant. These studies demonstrate that drug-conjugated nanoparticles can enhance transplanted progenitor cell survival and migration, and suggest that combination therapies may allow engraftment without overt immunosuppression.
Table of Contents sentence:
Dendrimer-NAC improves the long-term engraftment of transplanted cells to the brain, suggesting targeted nanotherapeutic support may eliminate the need for overt immunosuppression or multiple invasive procedures in regenerative therapies.
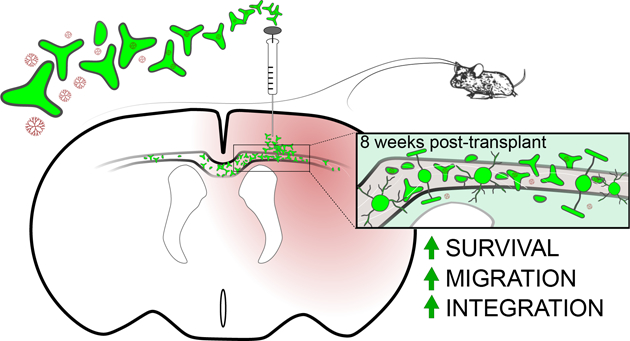
Mechanisms of therapeutic remyelination are limited by the intrinsic difficulty of cerebral white matter access for both transplant procedures and drug delivery across the blood brain barrier (BBB). Stem cells as regenerative drug delivery vehicles have been encouraging, particularly mesenchymal stem cells (MSCs), as these cells are able to cross the BBB and accumulate in sites of injury.1,2 Tripotential glial restricted precursors (GRPs) show regenerative promise especially for the replacement of brain and spinal cord oligodendrocytes, among other types of glial cells (reviewed in Srivastava et al., 2018). Although often embryonically derived, GRPs cells can be derived from multiple sources, including induced pluripotent stem cells (iPSCs), making them highly accessible, and compared to other cell types in the brain, oligodendrocytes may be relatively easy targets within the CNS for replacement.3,4 Major obstacles persist, however, such as potent allogeneic responses to transplanted cells, in particular host mediated toxicity affecting survival, migration and differentiation of the transplanted cells.
Nanoparticle aided regenerative therapies may reduce the burden of stem-cell-only approaches as numerous nanoparticle platforms offer flexibility in deliverable cargo, enabling solutions to many of the current limitations.5 Hydroxyl polyamidoamine (PAMAM) dendrimers are tree-like, multivalent, monodisperse, safe, globular macromolecules (~4 nm and neutral), which are considered one of the most flexible platforms to date. These dendrimers demonstrate a targeting and “promotional” capacity for tissue neuroprotection and/or regeneration in various animal model systems.6,7 Furthermore, dendrimers have the intrinsic capability to target neuroinflammation following systemic administration8,9 and clinical trials are planned to test hydroxyl-PAMAM dendrimer-N-acetyl cysteine (D-NAC) for a childhood orphan brain indication (clinicaltrials.gov Identifier NCT03500627).
GRP transplantation has shown acute success in several preclinical animal models of central nervous system disease, including amyotrophic lateral sclerosis, transverse myelitis, hypomyelination, and neonatal white matter injury, yet their utility, and thus clinical translation, is limited by poor survival, migration, and functional integration long-term without significant immunosuppression.7,10–12 Here, we test the hypothesis that preconditioning GRPs with dendrimer-drug nanoparticles conjugated to an anti-inflammatory/anti-oxidant drug, N-acetyl cysteine (D-NAC), will result in an enhancement of GRP survival, migration, and differentiation. NAC was selected for its anti-inflammatory and anti-oxidant properties, reported neuroprotective effects, and its wide clinical use.13,14 Previously in this mouse model of preterm brain injury, D-NAC has been shown to reduce pro-inflammatory cytokines and improve myelination.7,8 Indeed, when combining D-NAC with GRP transplantation following preterm brain injury, we observe greater survival and integration of GRPs when co-delivered with dendrimer-drug support and further demonstrate that when GRPs are used as a nanoparticle delivery vehicle, dendrimer extravasation from transplanted GRPs takes place. These findings are important as they show transplanted cells benefit from modulation by nanomaterials, and have the capacity to simultaneously deliver factors aimed at enhancing and nourishing stem cells en route to, and at, the target site. Such methods may eliminate the need for invasive and costly combination therapies, such as immunomodulators required for transplant.
In preparation for in vivo studies, confocal imaging of dendrimer conjugated-Cy5 (D-Cy5) uptake in cultured GRPs was performed in parallel with flow cytometry studies (Figure 1A). A total of 50,000 cells were analyzed per replicate and cells were gated against non-treated GRPs seeded and harvested simultaneously (Figure 1B). These studies reveal 72.4 ± 0.74% of analyzed cells take up D-Cy5 after a 12 hour exposure (Figure 1C).
Figure 1:
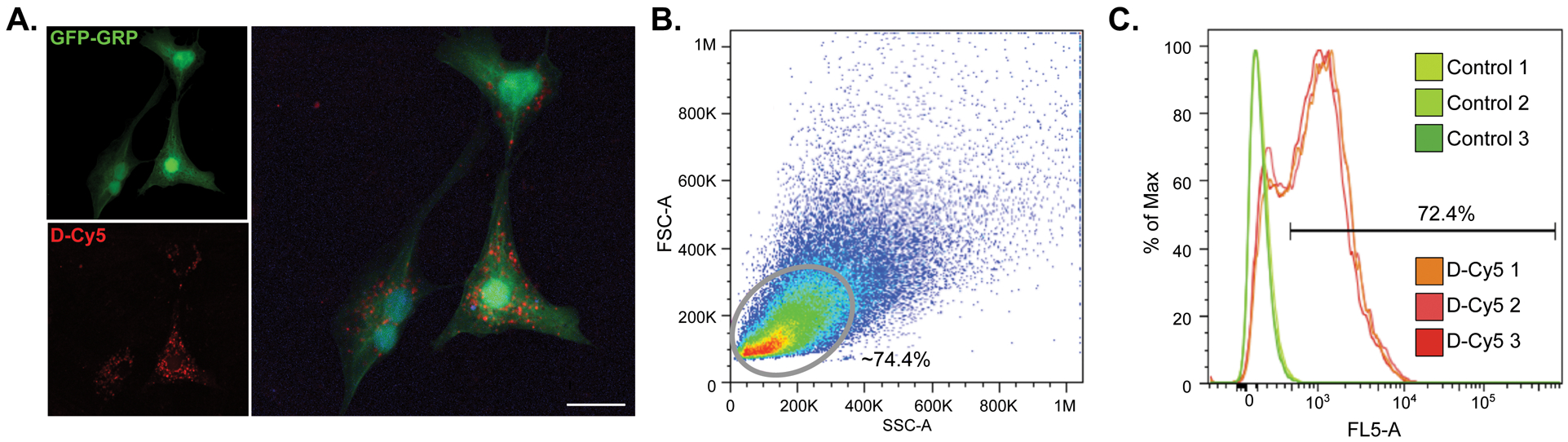
GFP-GRPs show uptake of Cy5-conjugated dendrimer (D-Cy5) after a 12 hr exposure. A) Confocal imaging of D-Cy5 exposed GRPs; scale bar equals 20 μm. B) Flow cytometry was used to confirm dendrimer uptake. Gating was conducted on GFP-expressing GRPs which reveals an average of 72.4% of D-Cy5 exposed cells across three replicates show accumulation of the conjugate (C).
Unilateral carotid ligation in the P5 mouse was used to induce white matter injury analogous to pre-term infant brain injury as previously described.11,15 To test the capacity for GRP-delivered dendrimer to act in vivo once transplanted, D-Cy5 was incubated with GRPs in vitro for 12 hours immediately prior to transplant into a P22 mouse exposed to P5 carotid ligation. In parallel, naïve GRPs were transplanted into P22 mice exposed to neonatal ischemia and administered D-Cy5 via intraperitoneal (i.p.) injection on P23. Tracking of GRPs in this study was facilitated by GFP expression under the ubiquitin promoter in embryos from which GRPs were derived. At 1-week post-transplant in mice receiving D-Cy5 i.p., dendrimer colocalized with GRPs located either in the injury site or in the injury penumbra (Figure 2A). Interestingly, in mice receiving D-Cy5 pre-treated GRPs, dendrimer was detected outside of GRPs both in the injection site and penumbra (Figure 2B), demonstrating movement of dendrimer from the GRP transplanted cells. In both cases, dendrimer was also detected colocalized with Iba1 stained microglia demonstrating successful stem cell delivery of viable dendrimer cargo to target tissue and resident cells. Together, independent of administration route, nanoparticle therapies are capable of accessing injury areas, making extravasated cargo available to other cell types, and may be useful for the control of injury-related inflammatory mechanisms.
Figure 2:
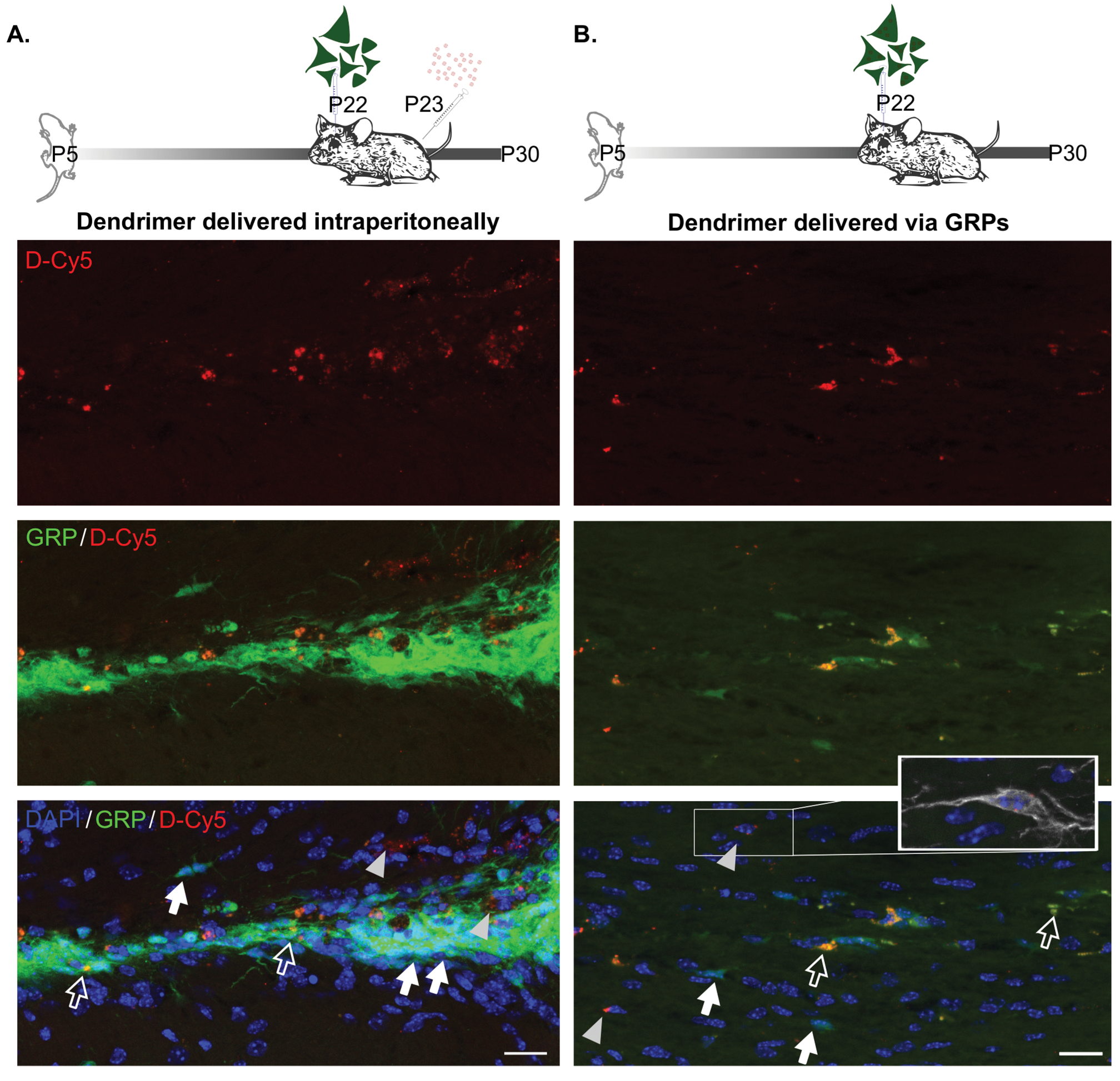
A) Representative images of corpus callosum in mice receiving D-Cy5 24 hr after P22 GRP transplantation. D-Cy5 is detected within GRPs (white open arrows) and outside of GRPs (gray arrowheads). GRPs without dendrimer are indicated by a white filled arrow. B) Dendrimer was similarly detected both inside of GRPs (white open arrows) and outside of GRPs (gray arrowheads), with D-Cy5 negative GRPs also present in the area (white filled arrows). Dendrimer is also detected within other cell types as indicated by inset showing Iba1+ microglial cell. Scale bars equal 20 μm.
Based on these data, we interrogated the ability of drug conjugated dendrimer (dendrimer-N-acetylcysteine; D-NAC) to enhance stem cell behavior in vivo. Of 70 mice receiving carotid ligation, 15 were excluded from analysis due to death during transplant (n = 2), death after transplant (n = 2), or severe brain injury resulting in a weight at P22 that was less than half of the litter’s mean weight and inappropriate for stereotaxic transplantation (n = 11). As a measure of GRPs’ in vivo stability, GRP survival was assessed 8 weeks post-transplant, when we previously demonstrated histopathological and functional improvements of GRP therapy after neonatal ischemia.11 At this time point, GRPs were detected in 17 of 19 mice receiving GRPs alone (LVG), 17 of 18 mice receiving D-NAC at P10 and GRPs (LDG), and all 18 mice receiving D-NAC pretreated GRPs (LVDG). Compared to ligated vehicle-treated mice receiving GRPs, there was a 47% increase in the number of GRPs detected in mice treated with D-NAC 24 hours post-ligation (171.4 ± 49.8 vs 116.2 ± 27.9 SEM). When mice were administered GRPs pretreated with D-NAC, we detected a 73% increase in GRPs compared to vehicle treated mice, for an average of 202.1 ± 52.1 cells per animal, 8-weeks post-transplant (representative images of GRPs at the site of injection shown in Figure 3A). To determine if D-NAC treatment provided therapeutic benefit compared to GRPs alone, we assessed microglia in the cortex surrounding the site of injection at 8-weeks post-transplant. Microglia were measured for cell size and count within two areas of the cortex. At this long-term timepoint after injury and transplant, no difference in cell count was detected; however, cell size was increased in both mice receiving D-NAC on P10 (LDG) and via GRP transplant (LVDG; p = 0.075), suggesting these microglia to have a morphology more reflective of their resting state (ramified) than activated state (amoeboid).16
Figure 3:
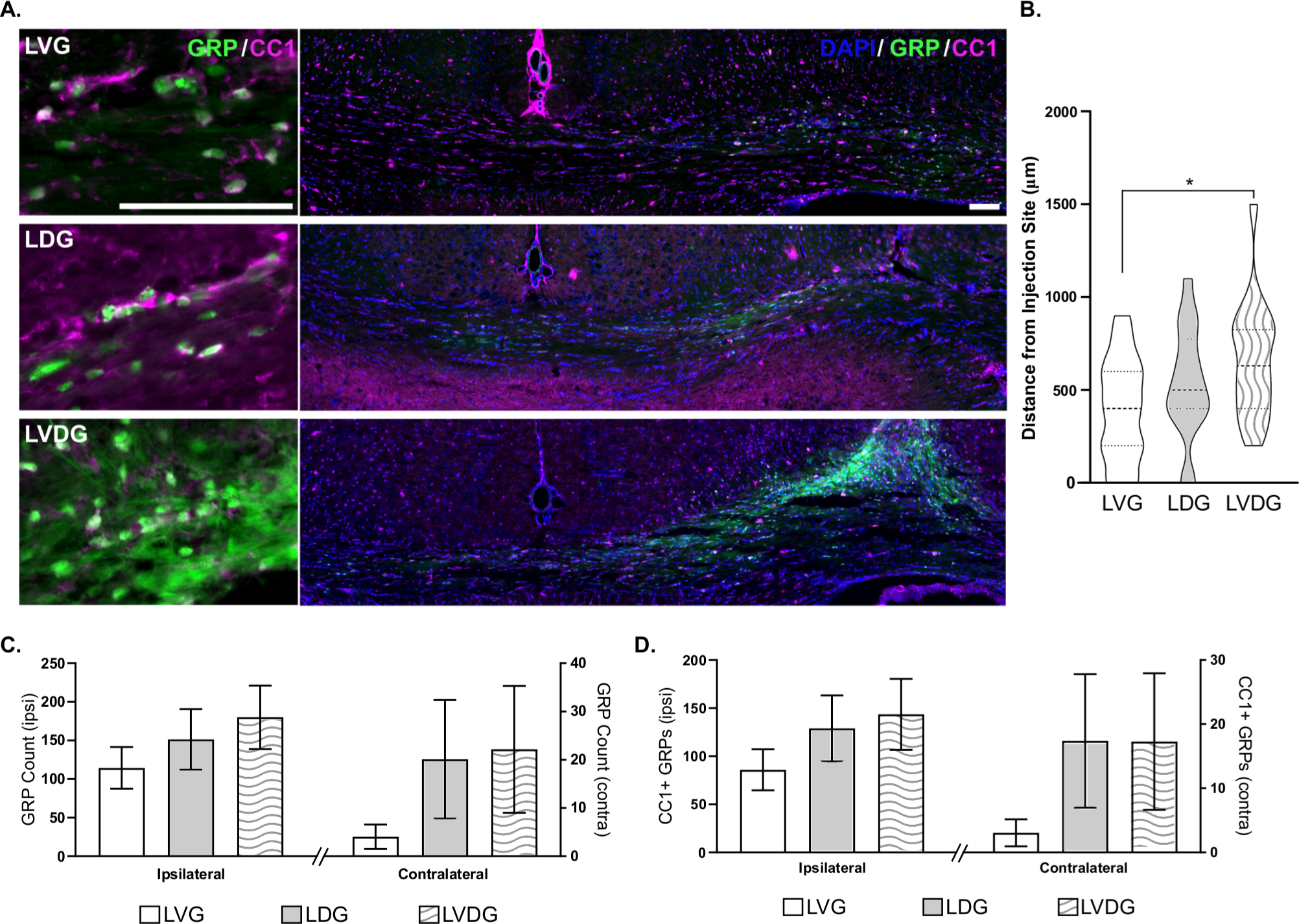
A) Representative images for GRP (green) and CC1 (magenta) colocalization and GRP distribution from injection site. Scale bars equal 100 μm. Quantification of total number of GRPs in ipsilateral and contralateral hemispheres (B), maximum A/P distance of GRP travel from injection site (C), and CC1 differentiation and migration are indicated. Error bars indicate standard error of the mean. LVG: ligated, vehicle-treated P10, and GRPs at P22; LDG: ligated, D-NAC at P10, GRPs at P22; LVDG: ligated, vehicle-treated at P10, D-NAC preconditioned GRPs at P22.
Migration and maturation of GRPs plays a pivotal role in the ability of these cells to integrate to host tissue and mediate functional recovery after transplantation. As many stem cells show homing behavior to injury sites, the use of stem cells as delivery vehicles for nano- or small molecule therapies remains an emergent possibility.5,17 At 8 weeks post-transplant, enhanced migration of GRPs was observed in brain tissue of mice supplemented with D-NAC 5 days post-injury (LDG) or in mice receiving D-NAC preconditioned GRPs (LVDG). The maximum distance of cells travelled in each group increased with GRP exposure to D-NAC (Figure 3B; F2,50 = 3.54; p < 0.05, post-hoc LVG vs LVDG p = 0.02), with mice receiving preconditioned GRPs showing a maximum migratory distance of 1500 μm from the injection site and a group mean of 660 μm from the injection site. Migration of these cells to the hemisphere contralateral to injury was also greater in mice exposed to D-NAC (Figure 3C). Similarly, the proportion of cells expressing CC1, a marker of mature oligodendrocytes or sometimes astrocytes, increased bilaterally with D-NAC treatment, indicating improved differentiation and maturation of GRPs (Figure 3D). Together, these data suggest that D-NAC exerts effects on the target tissue or the transplanted cells directly to enhance and promote an environment for survival and integration.
Conclusions
Stem cell preconditioning has been used in attempts to improve both the tracking of cells as well as the homing of cells to target sites.17,18 A recent clinical trial revealed no major adverse effects of human-human neural cell transplantation for remyelination in Pelizaeus-Merzbacher disease;19 however, the indication for allorecognition by two out of four recipients underscores the need for advanced and prolonged immunologic monitoring and modulation. Similarly, mesenchymal stem cell transplants for corneal epithelial stem cell deficiencies are less efficacious in the context of inflammatory disease compared to patients without immune challenges,20,21 highlighting the persistent need for immunomodulation. Finally, recent work from Li et al., (2019) demonstrates the use of co-stimulation blockade to modulate T cell activity, thereby protecting allografts in otherwise rejection-prone regions of the corpus callosum. Using this method, they further identify “biomarker” miRNAs that can be combined with other transplant methods to serve as early indicators of transplanted cell success. Results herein show nanoparticle preconditioning or co-administration can promote stem cell survival and integration into host tissue, by stimulating the survival of stem cells directly, and/or by enhancing the surrounding tissue environment to promote overall survival. Further, dendrimer treatment showed effects on microglia at 8 weeks post-transplant, which may represent the capacity to modulate the inflammatory response following an event or procedure. As dendrimer nanoparticles provide a flexible platform for multifunctional use, such that therapeutic, targeting, imaging, and tracking moieties can exist on a single compound. Using this or similar frameworks, immune modulators can be tailored to suit particular needs in addition to codelivery of factors known to affect migration potential and survival of pluripotent cells.23–25 Such custom nanotherapies together with allogeneic or autologous transplant may provide the greatest likelihood of immune tolerance, cell migration and differentiation thus eliminating the need for engineering of the delivery cell or multiple invasive procedures.
Experimental Section
Dendrimer Conjugates
D-NAC and Cy5-tagged dendrimer (D-Cy5) were synthesized and characterized using our previously published procedures.6,26
GRP Derivation and Assessment
GRPs were obtained from E13.5 C57BL/6-Tg (UBC-GFP) 30Scha/J mice following A2B5 magnetic bead sorting (Miltenyi Biotec; Bergisch Gladbach, Germany). GRPs were grown for 6–10 days in defined GRP media as previously described11,27 with a full media change every 2–3 days. 12 hr prior to transplant, media was changed on all cells and at which point D-NAC pretreated GRPs were given media with 10 μg/ml D-NAC. Cells were detached with 0.05% Trypsin, washed twice, and suspended at 100,000 cells/μl in PBS. For transplant, cell suspensions were kept on ice prior to transplantation and each batch was transplanted within an hour. For in vitro assessment, cells were incubated with 10 μg/mL D-Cy5 for 12 hr, fixed in 10% formalin, and analyzed via Leica SP8 confocal microscopy and Sony SH800S Fluorescence Assisted Cell Sorter.
Surgical Procedures
All animal procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University. Twelve lactating female CD-1 mice with litters of 12 postnatal day two or three (P2 or P3; day of birth defined as P1) pups were obtained from Charles River Laboratories. On P5, mouse pups were anesthetized with isoflurane (3% for induction, 1.5% for maintenance) and underwent either a sham surgery or unilateral right common carotid artery ligation. Mice recovered postoperatively in an incubator prior to being returned to the dam. On P10, all mice received either a 50 μl injection of 10 mg/kg dendrimer-N-Acetyl-Cysteine (D-NAC, i.p. in PBS) or vehicle. On P22, mice underwent stereotaxic surgery as described in Porambo et al. (2014) for the intracerebral injection of vehicle, 1 μl of 100,000 GRPs, or 1 μl of 100,000 D-NAC pretreated GRPs over a 2 minute period.
Histology
Mice were transcardially perfused 8 weeks post transplantation with PBS and 10% formalin and brains were extracted and post-fixed 24 hr. Tissue was cryoprotected with sucrose, frozen at −80 °C, and sectioned at 20 μm using a cryostat. Tissue was collected across a 10 series and any sections dropped during sectioning were noted to calculate precise displacement between sections. To identify cell populations, slides were brought to room temperature for 30 minutes, blocked (5% Normal Serum, 0.5% Triton-X; 1 hr at rt), stained with primary antibody (oligodendrocytes and microglia were stained using CC1 [1:1000; Cal BioChem] and Iba1 [1:1000; Wako], respectively) overnight at 4 °C. Sections were treated with appropriate secondaries (1:500), counterstained with Hoechst 33342 (ThermoFisher), and coverslipped. Sections were imaged on the Zeiss AxioImager M2 microscope with three 1 μm z-stack sections with image acquisition time standardized to ensure comparable images. The section containing the injection track was identified for each animal and that section and surrounding images were captured for each antibody stain. GRP counts were done manually by an investigator blinded to treatment groups. GRPs were counted on every section where identified and the distance from the injection site was calculated. Microglia assessment was performed for each animal by measuring the total area of Iba1 signal on calibrated images and dividing by the number of objects using Fiji ImageJ (v I.52h).
Acknowledgements:
The authors thank The National Institutes of Health (NINDS 5R01NS097511, NICHD U54HD079123, S10RR027445-01) for financial support of this work. We acknowledge the Department of Anesthesiology & Critical Care Medicine (ACCM) for access to their Leica SP8 confocal microscope and George McNamara for assistance. Additionally, we thank Alexa Wolfe, Irina Shats, Melissa Rosen, Karen Smith-Connor, and Kevin Liaw, for their technical assistance with this work.
Footnotes
Conflicts of interest
AF is a paid drug safety monitoring board member for Bluebird Bio, Stealth Biotherapeutics, and a paid consultant to Calico Labs. The authors have awarded and pending patents relating to the neuroinflammation targeting ability of high surface hydroxyl dendrimers (RMK, SK, AF, AS and RS). RMK and SK are co-founders and have financial interests in Ashvattha Therapeutics LLC, Orpheris Inc., and RiniSight; three start-ups translating dendrimer drug delivery platform. RS works with Ashvattha Therapeutics and has share ownership. CLN and SNT report no conflicts of interest.
References
- 1.Sherman LS, Romagano MP, Williams SF and Rameshwar P, Semin. Cell Dev. Biol, 2019, 0–1. [DOI] [PubMed] [Google Scholar]
- 2.Aleynik A, Gernavage KM, Mourad YS, Sherman LS, Liu K, Gubenko YA and Rameshwar P, Clin. Transl. Med, 2014, 3, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava RK, Bulte JWM, Walczak P and Janowski M, Glia, 2018, 66, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghanekar S, Corey S, Stonesifer C, Lippert T, Diamandis Z, Sokol J and Borlongan C, Brain Circ, 2016, 2, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth CL, Fine AS and Fatemi A, Adv. Drug Deliv. Rev, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Sharma R, Sharma A, Kambhampati SP, Reddy RR, Zhang Z, Cleland JL, Kannan S, Kannan RM, Sharma A, Cleland JL, Kannan S and Zhang Z, Bioeng. Transl. Med, 2018, 3, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nance E, Porambo M, Zhang F, Mishra MK, Buelow M, Getzenberg R, Johnston M, Kannan RM, Fatemi A and Kannan S, J Control Release, 2015, 214, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R and Kannan RM, Sci Transl Med, 2012, 4, 130ra46–130ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma R, Kim S-Y, Sharma A, Zhang Z, Kambhampati SP, Kannan S and Kannan RM, Bioconjug Chem, 2017, 28, 2874–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepore AC, Donnell JO, Kim AS, Williams T, Tuteja A, Mahendra S, Kelley LL, Campanelli JT and Maragakis NJ, PLoS One, 2011, 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porambo M, W Phillips A, Marx J, Ternes K, Arauz E, Pletnikov M, Wilson MA, Rothstein JD, V Johnston M and Fatemi A, Glia, 2014, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walczak P, All AH, Rumpal N, Gorelik M, Kim H, Maybhate A, Agrawal G, Campanelli JT, Gilad AA, Kerr DA and Bulte JWM, Glia, 2011, 59, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahripour RB, Harrigan MR and V Alexandrov A, Brain Behav, 2014, 4, 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tardiolo G, Bramanti P and Mazzon E, Molecules, 2018, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatemi A, Wilson MA, Phillips AW, McMahon MT, Zhang J, Smith SA, Arauz EJ, Falahati S, Gummadavelli A, Bodagala H, Mori S and Johnston MV, J. Cereb. Blood Flow Metab, 2011, 31, 2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres-Platas SG, Comeau S, Rachalski A, Bo GD, Cruceanu C, Turecki G, Giros B and Mechawar N, J Neuroinflamm, 2014, 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labusca L, Herea DD and Mashayekhi K, World J. Stem Cells, 2018, 10, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xinaris C, Morigi M, Benedetti V, Imberti B, Fabricio AS, Squarcina E, Benigni A, Gagliardini E and Remuzzi G, Cell Transplant, 2013, 22, 423–436. [DOI] [PubMed] [Google Scholar]
- 19.Gupta N, Henry RG, Kang S-M, Strober J, Lim DA, Ryan T, Perry R, Farrell J, Ulman M, Rajalingam R, Gage A, Huhn SL, Barkovich AJ and Rowitch DH, Stem Cell Reports, 2019, 13, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Luca M, Aiuti A, Cossu G, Parmar M, Pellegrini G and Robey PG, Nat. Cell Biol, 2019, 21, 801–811. [DOI] [PubMed] [Google Scholar]
- 21.Calonge M, Perez I, Galindo S, Nieto-Miguel T, Lopez-Paniagua M, Fernandez I, Alberca M, Garcia-Sancho J, Sanchez A and Herreras J, Transl Res, 2019, 206, 18–40. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Oh BC, Chu C, Arnold A, Jablonska A, Furtmüller GJ, Qin HM, Boltze J, Magnus T, Ludewig P, Janowski M, Brandacher G and Walczak P, Brain, 2019, 142, 3456–3472. [DOI] [PubMed] [Google Scholar]
- 23.André EM, Passirani C, Seijo B, Sanchez A and Montero-Menei CN, Biomaterials, 2016, 83, 347–362. [DOI] [PubMed] [Google Scholar]
- 24.Wu K-C, Tseng C-L, Wu C-C, Kao F-C, Tu Y-K, C So E and Wang Y-K, Sci. Technol. Adv. Mater, 2013, 14, 054401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayakawa K, Haas C, Jin Y, Bouyer J, Otsuka T and Fischer I, Brain Res, 2015, 1629, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Liaw K, Sharma R, Zhang Z, Kannan S and Kannan RM, Theranostics, 2018, 8, 5529–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips AW, Falahati S, DeSilva R, Shats I, Marx JS, Arauz E, Kerr D, Rothstein JD, V Johnston M and Fatemi A, J Vis Exp, 2012, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]


