Abstract
Purpose:
The aim of this 8-year retrospective review was to determine the clinical significance of gadolinium-enhanced magnetic resonance imaging (MRI) findings in retinoblastoma patients after enucleation, particularly the presence of abnormal contrast enhancement of the transected optic nerve.
Design:
Retrospective chart review.
Subjects:
A review was done on 88 patients with retinoblastoma undergoing 90 enucleations between January 2008 and December 2015.
Methods:
These patients underwent 233 MRI scans: 90 preoperative and 143 postoperative that were included for review.
Main Outcome Measure:
The primary outcome measure assessed was abnormal MRI findings in the preoperative and postoperative MRI scans, specifically enhancement of the optic nerve and correlations between abnormal MRI findings and clinical outcomes for the 88 patients.
Results:
On the preoperative MRI, 4 optic nerves out of 90 scans showed positive enhancement. Fifty orbits had ≥1 postoperative MRI. Overall, 41 of 50 orbits (82%) of enucleated patients demonstrated postoperative contrast enhancement on MRI after enucleation, at a mean interval of 10 months after surgery. The percentage of MRI scans with optic nerve enhancement was 77% from 0 to 6 months after enucleation and 68% at >24 months after surgery. Postenucleation optic nerve enhancement did not correlate with preoperative optic nerve enhancement, chemotherapy administration, or the presence of optic nerve invasion on histopathology. No child required an orbital biopsy. None of the 88 patients were found to have subsequent orbital or metastatic disease at the last clinical follow-up visit (average, 29 months; range, 1–71).
Conclusion:
Optic nerve contrast enhancement on follow-up MRI after enucleation for retinoblastoma seems to be a common, benign radiographic finding; none of the patients in this series developed extraocular tumor relapse. The presence of postenucleation enhancement on MRI did not correlate with preoperative chemotherapy or the presence of optic nerve invasion on histopathology. Based on our findings, intervention for isolated optic nerve enhancement on MRI is not indicated in the absence of other abnormal clinical or radiographic signs. A prospective trial with a validated radiographic grading system would be helpful to clarify the MRI features to differentiate orbital recurrence from benign postoperative enhancement.
Retinoblastoma (Rb) is the most common primary intraocular malignancy in children, with an estimated incidence of 11.8 cases per million children aged 0–4 years.1 Ocular salvage rates for treated eyes have been reported to be >90% in developed countries using various treatment options such as systemic chemotherapy, intra-arterial chemotherapy, and intravitreal chemotherapy injections.2–14 However, enucleation remains the most common treatment for Rb patients worldwide and is thought to be curative in 96% to 100% of cases.15,16 Tumor relapse after enucleation is an unlikely but feared event, and may occur through hematogenous dissemination, postlaminar invasion into the optic nerve, or direct extension into the orbit through the sclera.15 High-risk pathologic features such as postlaminar optic nerve invasion, massive choroidal invasion, and scleral invasion have been correlated with an increased risk for orbital recurrence and/or distant metastasis.17–20 Orbital tumor recurrence of Rb after enucleation has an estimated incidence of 4.2%, with the majority of these patients occurring within 12 months of surgery and having evidence of concomitant systemic metastasis.15,21 Therefore, any clinical or radiographic evidence of tumor recurrence after enucleation requires immediate intervention, including tissue biopsy, systemic metastasis workup, and often multimodal therapy if the diagnosis is confirmed.
Magnetic resonance imaging (MRI) is the preferred neuroimaging modality for Rb patients given its excellent tissue resolution and the absence of potentially mutagenic radiation 0.22,23 Recent studies have shown that MRI is useful for staging Rb patients and for patients during and after treatment.22,24 At diagnosis, MRI scans have been used to assess patients for possible postlaminar optic nerve invasion and its high sensitivity for detecting this feature has been confirmed in several studies.23,25,26 Sirin et al24 recently published an analysis of MRI findings after enucleation in children with Rb, which included 3 patients with orbital masses on MRI scans who were biopsy confirmed as Rb. The authors also strongly recommended routine MRI surveillance for all Rb patients for orbital relapse for 2 years after enucleation.24 The aim of this retrospective review was to evaluate gadolinium-enhanced MRI findings of the orbit after enucleation. Our hypothesis was that enhancement of the transected end of the optic nerve on postoperative MRI scans represents a common, benign finding not associated with tumor relapse.
Materials and Methods
The Institutional Review Board at Children’s Hospital Los Angeles approved this retrospective study. Patients eligible for this review underwent enucleation for Rb between January 2008 and December 2015 and had MRI reports available for review; subsequent histopathology reports confirmed the diagnosis of Rb in all cases and assayed for high-risk features. Gadolinium-enhanced MRI was performed routinely at diagnosis and often postoperatively as a part of our normal clinical protocol, which includes MRI evaluations every 6 months until the age of 3 for all patients with bilateral Rb or a known Rb tumor suppressor gene (RB1) mutation as screening for central nervous system disease. Primarily enucleated unilateral patients are not screened routinely unless there are significant high-risk pathologic features or clinical presentation warrants evaluation. At our institution, 6 board-certified radiologists with significant experience in pediatric neuroimaging evaluation read neuroimaging studies, including brain and imaging MRIs. Additionally, each scan has an initial radiologist evaluation, which is subsequently verified by a second, separate radiologist so that each scan is evaluated twice with consensus. Our institutional protocol for extensive optic nerve involvement on MR imaging has been described previously; we currently treat optic nerve involvement of <5 mm on MRI with primary enucleation and those with >5 mm of involvement with intensive pre-enucleation chemotherapy. No child in this study was treated with this preenucleation protocol.
All MRI reports were reviewed and the following outcome measures were assessed: (1) pretreatment contrast enhancement (and/or thickening) of the optic nerve and its sheath, (2) enhancement along the transected end of the optic nerve after enucleation, (3) evidence of other areas of abnormal enhancement or masses in the orbit (before or after treatment), and (4) evidence of trilateral Rb both before and after treatment. On histopathology, tumor extension into the optic nerve was categorized with reference to the lamina cribrosa as pre or post laminar.
Other pathologic features such as choroidal invasion and scleral invasion were also assessed. Clinical data collected from the patient medical records included unilateral or bilateral disease, international intraocular classification Rb, age at diagnosis, age at enucleation, and clinical and tumor status at the time of the last follow-up examination. MRI scans were performed according to standard neuroimaging protocols for the brain and orbit using gadolinium contrast agent in all patients with 1.5 or 3.0 Tesla MRI scanners (Siemens Health Care Sector, Erlangen, Germany). A dose of 0.1 mmol/kg body weight of gadolinium chelate was injected intravenously during the scans. All MRI examinations were performed under general anesthesia due to the age of the patients.
Statistical analysis was performed with GraphPad (La Jolla, CA). Chi-square testing was performed to evaluate for significance; P < 0.05 was considered significant.
A literature review was performed on PubMed search using terms “retinoblastoma” and “MRI” and “enucleation” from 1990 to 2016.
Results
A total of 90 enucleations were performed in 88 Rb patients between January 2008 and December 2015. Sixty eyes (67%) were designated International Classification of Retinoblastoma group D and 30 eyes (33%) were group E. Fifty-three patients (60%) had unilateral disease and 35 patients had bilateral disease (40%). The average age at diagnosis was 21.7 months (range, 0–111) and average age at enucleation 24.9 months (range, 0–127). Primary enucleation was done as treatment for intraocular Rb in 56 eyes.
There were a total of 90 preoperative MRI reports and 143 postoperative MRI reports available for review. Preoperative optic nerve enhancement on MRI was detected in 4 orbits (Table 1, Fig 1); one demonstrated concomitant thickening. On histopathology, 3 of the 4 globes with this preoperative finding demonstrated postlaminar optic nerve invasion. There were 3 other globes that did not demonstrate preoperative optic nerve enhancement on MRI but were found to have postlaminar optic nerve invasion on histopathology. No case was found to have tumor involvement to the cut end of the optic nerve. All 6 cases with postlaminar optic nerve invasion received 6 cycles of adjuvant systemic chemotherapy (carboplatin, etoposide, and vincristine). There was 1 case of trilateral RB identified on preoperative MRI scans.
Table 1.
Preoperative and Postoperative MRI Demographics
| Demographic | n |
|---|---|
| Total number of orbits | 90 |
| Total number of patients | 88 |
| Total number of MRI scans | 233 |
| Number of preoperative MRI scans | 90 |
| Preoperative optic nerve enhancement | 4 |
| Number of postoperative MRI scans | 143 |
| Orbits with postoperative MRI scans | 50 |
| Orbits that showed post operative enhancement | 41 |
| Patients treated with CEV | 38 |
| Positive optic nerve enhancement in patients treated with CEV | 20 |
| No optic nerve enhancement in patients treated with CEV | 9 |
| Postlaminar optic nerve invasion | 6 |
| Postlaminar optic nerve invasion with preoperative enhancement | 3 |
| Postlaminar optic nerve invasion without preoperative enhancement | 3 |
| Median follow-up to first MRI scans, mo (range) | 10 (2–65) |
| Average clinical follow-up, mo (range) | 29 (1–71) |
CEV = carboplatin, etoposide, and vincristine; MRI = magnetic resonance imaging.
Figure 1.
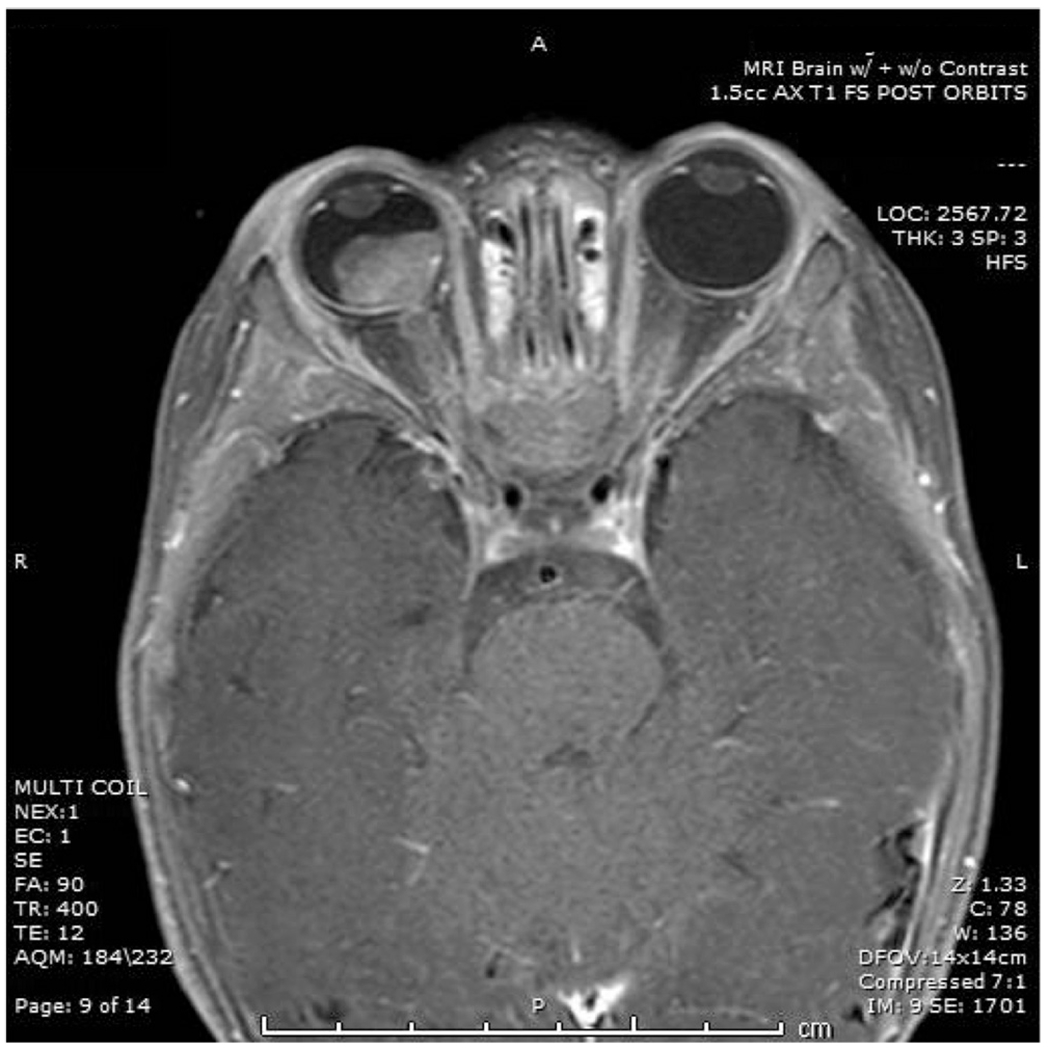
Preoperative T1 fat-suppressed post-contrast magnetic resonance imaging scans showing a retinoblastoma mass in the right globe with involvement 4.5 mm of enhancement and thickening involving the optic nerve.
Postoperatively, 50 orbits underwent surveillance with a total of 143 MRI scans. The median time period to the final MRI scan post enucleation was 10 months (range, 2–65). Among the 50 orbits that had postoperative MRI scans, 40 were imaged with multiple MRI scans after enucleation and 10 orbits had a single MRI scan after enucleation.
Overall, 41 of 50 orbits (82%) showed any level of abnormal postoperative contrast enhancement on MRI scans at some point during postoperative imaging after enucleation (Table 1). Concomitant thickening was not seen. All 41 of the orbits with abnormal radiographic findings had isolated contrast enhancement of the transected portion of the optic nerve (Fig 2). There were 36 MRI scans performed <6 months from the enucleation and 27 of these scans demonstrated optic nerve enhancement, and 8 scans did not (1 scan with limited evaluation). Between 6 and 12 months after enucleation, there were 36 MRI scans performed; 25 scans demonstrated optic nerve enhancement while 10 scans did not (1 scan with limited evaluation). Between 12 and 24 months after enucleation, 44 MRI scans were performed; 38 of these demonstrated optic nerve enhancement, and 6 did not (1 limited evaluation).
Figure 2.
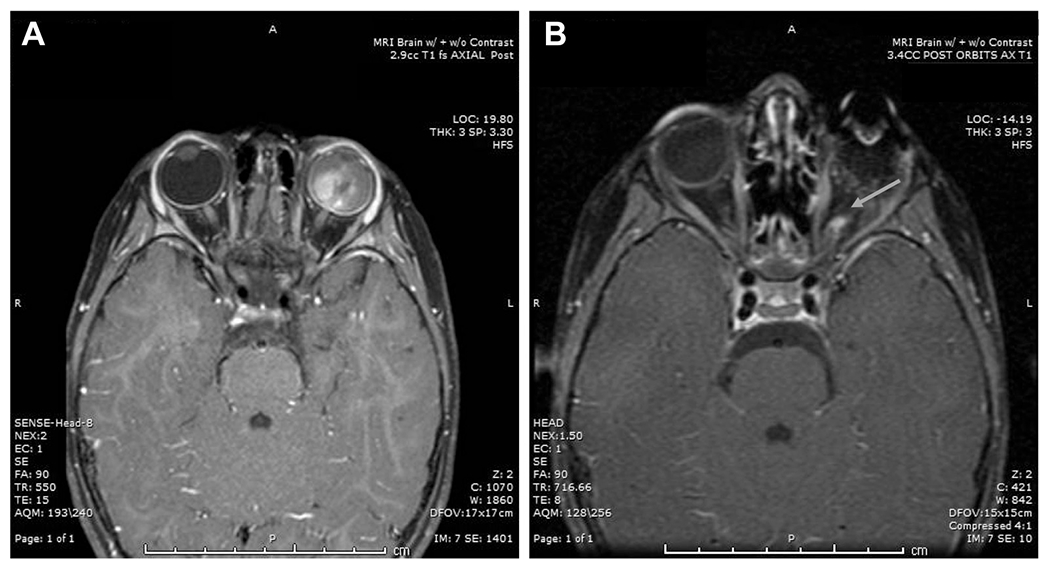
Preoperative T1 axial fat-suppressed post-contrast scan. A, Retinoblastoma is demonstrated filling the left globe without optic nerve contrast enhancement. B, Postoperative magnetic resonance imaging of the same patient demonstrates contrast enhancement along the cut end of the optic nerve of the anophthalmic socket (arrow).
There were 27 scans performed >24 months after enucleation and 15 showed positive enhancement of the optic nerve (Table 2). The overall percentage of scans with optic nerve enhancement for the 4 time periods is 75% (6 months), 70% (6–12 months), 86% (12–24 months), and 55% (>24 months). Therefore, there did not seem to be a decrease in the percentage of scans with optic nerve enhancement up to 24 months after enucleation. There was a decrease after 24 months; however, there were also fewer orbits undergoing imaging evaluation.
Table 2.
Timing of Postoperative MRI Scans
| Time Period (mo) | Number of MRI Scans | Positive Enhancement | Negative Enhancement | Limited Evaluation |
|---|---|---|---|---|
| 0–6 | 36 | 27 | 8 | 1 |
| 6–12 | 36 | 25 | 10 | 1 |
| 12–24 | 44 | 38 | 6 | 0 |
| >24 | 27 | 15 | 11 | 1 |
| Total number of scans | 143 |
MRI = magnetic resonance imaging.
Of the 50 orbits that underwent post operative MRI scans, 37 showed contrast enhancement of the optic nerve at the first postoperative MRI scan at a median of 6 months postoperatively (range, 0–17; Fig 3). Forty orbits underwent >1 postoperative MRI scan. At the second postoperative scan (mean, 13 months; range, 3–55) 29 of 40 orbits showed enhancement. Four orbits showed new enhancement not seen at the first postoperative MRI without associated optic nerve thickening or orbital mass. No patients developed enhancement that was not previously reported on the third MRI or thereafter. Of the patients with any level of enhancement at the second postoperative MRI, this enhancement persisted on subsequent MRI in 19 orbits, resolved in 4, and 6 orbits did not have subsequent MRI evaluation. There were no cases of orbital recurrence or systemic disease at an average follow-up of 29 months (range, 1–71). No child required an orbital biopsy.
Figure 3.
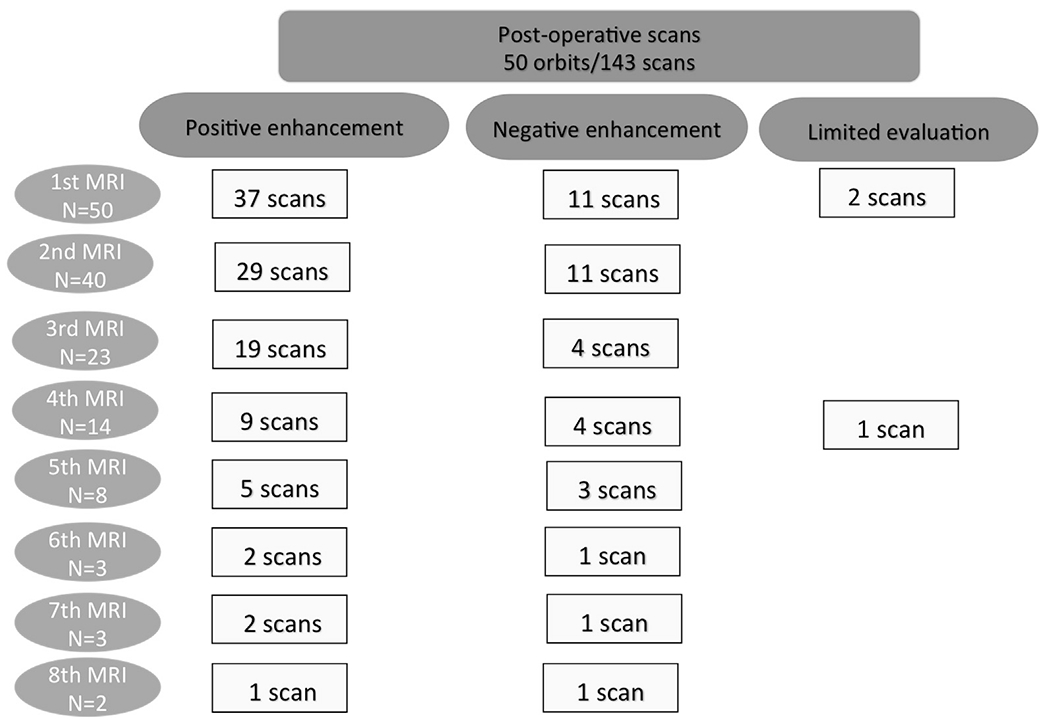
CONSORT diagram demonstrating enhancement characteristics of the optic nerve on postoperative magnetic resonance imaging (MRI) scans.
Among the 88 patients undergoing enucleation, 50 underwent primary enucleation and 38 received systemic chemoreduction with the standard 3-drug regimen of carboplatin, etoposide, and vincristine before enucleation. Of these, 20 patients showed optic nerve enhancement on follow-up MRIs scans, 9 did not demonstrate enhancement, and 9 did not undergo further MRI scans (Table 1). There was no correlation between postoperative MRI optic nerve enhancement and preoperative chemotherapy (P = 0.1). There was a statistically significant correlation between preoperative optic nerve enhancement and postoperative optic nerve enhancement (P = 0.001). However, there was no correlation between preoperative MRI optic nerve enhancement and postlaminar optic nerve invasion (P = 0.74). Similarly, there was no statistical correlation between postoperative MRI optic nerve enhancement and postlaminar optic nerve invasion (P = 0.58).
Discussion
Magnetic resonance imaging is the most useful neuroimaging modality to assess Rb patients during the staging process and to follow patients during and after treatment. Magnetic resonance imaging findings can help to support the diagnosis of intraocular Rb, assess patients for optic nerve invasion, and evaluate the pineal gland as well as the skull base.22,23 The Rb patients in this series had routine MRI scans at the time of diagnosis as a part of disease staging to evaluate for optic nerve involvement and central nervous system disease and subsequently after enucleation for the following indications: (1) bilateral or familial patients to assess for trilateral disease, (2) unilateral patients with positive or unknown genetic status who required trilateral screening, (3) screening for patients with a clinical presentation suggestive of an orbital process, and (4) rarely as screening secondary to high-risk features on histopathology. The sensitivity and specificity for MRI scans to evaluate patients for postlaminar optic nerve invasion at diagnosis has been found to be 75% and 100%, respectively.23 However, there have been few studies assessing the role of MRI scans in assessing patients for orbital tumor recurrence after enucleation. Recently, Sirin et al24 published their analysis of MRI findings after enucleation in children with Rb and described 3 of 55 orbits that had definite features of orbital recurrence on postoperative scans. There were also 2 orbits in their series that were rated as “suspicious of tumor” that were not biopsied and subsequently treated with chemotherapy. In our clinical experience, there is commonly a management dilemma regarding Rb patients with “abnormal” orbital enhancement on MRI scans after enucleation. Pediatric oncologists and radiologists often recommend either a follow-up MRI scan (requiring anesthesia) and/or orbital biopsy in this setting to rule out the possibility of tumor recurrence.
Our retrospective analysis revealed that isolated optic nerve enhancement on MRI is a very common finding in Rb patients after enucleation in >70% of patients undergoing MRI within 12 months of enucleation. We hypothesize that the transected end of the optic nerve has granulation tissue that continues to enhance for a prolonged period. In fact, even ≥2 years after enucleation, 68% of scans demonstrated some level of enhancement. In our retrospective study, this finding did not correlate with optic nerve invasion (P = 0.58) or pretreatment chemotherapy (P = 0.1). No patient in our series required a biopsy or was found to have an orbital recurrence. The Rb population is unique in that many patients are routinely scanned with MRI after enucleation, and the age at the time of surgery is typically between 1 and 5 years. Previous MRI studies of adults after enucleation have demonstrated enhancement of porous implants due to ingrowth and vascularization, but optic nerve enhancement is not described routinely.27,28 In the recent paper by Sirin et al,24 there were 3 patients with biopsy-confirmed orbital tumor recurrence associated with abnormal MRI findings. However, all 3 of these cases showed evidence of an abnormal soft tissue mass in the orbit, in addition to the enhancement of the transected optic nerve and its sheath. Similar to our findings, their analysis also showed that approximately 70% of orbits after enucleation demonstrate “abnormal” contrast enhancement of the optic nerve and its meningeal sheath, which persists for >3 years after enucleation in 57% of orbits.24 Although further studies are needed to confirm these findings, it seems that isolated contrast enhancement of the transected optic nerve is a common, benign finding that may persist on follow-up MRI scans in Rb patients undergoing enucleation.
Orbital tumor recurrence after enucleation for intraocular Rb is a rare finding in developed countries, and almost always develops during the first year after surgery.15 A previous series written by Kim et al15 demonstrated an incidence of 4.2% when analyzing patients treated over an 80-year period at Memorial Sloan-Kettering Cancer Center (MSKCC). A more recent series from the same center showed a 7.9% risk of orbital recurrence among a smaller sample size after enucleation versus a 1.3% risk after intraarterial chemotherapy.14 The vast majority of patients with orbital recurrence have high-risk histopathologic features and are diagnosed after presenting with clinical symptoms such as a nonfitting prosthesis, proptosis, periorbital edema, or other local findings. None of the patients in the original series at MSKCC were diagnosed from a screening MRI scan.15 All 3 cases of orbital recurrence identified on MRI in the series by Sirin et al24 had postlaminar optic nerve invasion and 2 of the 3 cases underwent radiographic investigation because of problems with the prosthesis. Unlike the conclusions of Sirin et al,24 we do not believe that there is enough evidence to recommend MRI scans as a routine screening tool for children who have undergone enucleations for Rb. We did not find any cases of orbital recurrence in our series of 88 enucleated patients after 130 postoperative MRI scans were performed under general anesthesia. We do recommend that patients at risk for trilateral Rb have brain MRI scans until gene testing shows negative germinal status or the patient reaches 3 to 4 years of age. The follow-up of patients with high-risk histopathologic features after enucleation should be individualized based on the clinical course and the use of adjuvant chemotherapy per the center’s treatment protocols. For patients without high-risk histopathologic features, we do not believe that routine screening with serial neuroimaging studies under general anesthesia are indicated in this overall low-risk population and clinical presentation is likely to guide the need for radiographic evaluation for orbital relapse.
Conclusions
Isolated enhancement of the distal end of the optic nerve is a common postoperative radiographic finding after enucleation in children with Rb and can persist for >2 years after surgery. In our study, this finding was not associated with preoperative optic nerve invasion or the use of chemotherapy and did not increase the risk of orbital or systemic relapse. Therefore, we conclude that isolated enhancement of the transected end of the optic nerve likely represents a benign finding that (if stable) does not warrant aggressive investigation or management. We recommend that patients with this radiographic finding and no other abnormalities be followed clinically, similar to other asymptomatic Rb patients after enucleation. Orbital biopsy and/or further intervention are not recommended in the absence of abnormal clinical signs or other neuroimaging findings. However, given that the incidence of optic nerve enhancement is rather high and orbital recurrence is rather low, a prospective study with a validated radiographic grading system for optic nerve enhancement would be useful to clarify features on MRI that may help differentiate orbital recurrence from this otherwise benign postoperative finding.
Acknowledgments.
The authors thank Subramanian Krishnan, PhD, Medical Technical Writer, Research Accelerator Program for the Division of Ophthalmology (RAPIDO), The Vision Center, Children’s Hospital Los Angeles, for providing writing and editing assistance on our manuscript drafts.
Financial Support: Research to Prevent Blindness (www.rpbusa.org) (New York, NY; unrestricted departmental funding) and The Larry and Celia Moh Foundation (Lexington, NC). The sponsors or funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms:
- MRI
magnetic resonance imaging
- Rb
retinoblastoma
Footnotes
Financial Disclosures:
The authors have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. 2009;93:21–23. [DOI] [PubMed] [Google Scholar]
- 2.Abramson DH, Daniels AB, Marr BP, et al. Intra-arterial chemotherapy (ophthalmic artery chemosurgery) for group D retinoblastoma. PLoS One. 2016;11:e0146582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015;133:1341–1347. [DOI] [PubMed] [Google Scholar]
- 4.Francis JH, Marr BP, Brodie SE, et al. Intravitreal melphalan as salvage therapy for refractory retinal and subretinal retinoblastoma. Retin Cases Brief Rep 2016;10:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghassemi F, Shields CL, Ghadimi H, et al. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132:936–941. [DOI] [PubMed] [Google Scholar]
- 6.Munier FL, Gaillard MC, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: recent advances and perspectives. Saudi J Ophthalmol 2013;27:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–1083. [DOI] [PubMed] [Google Scholar]
- 8.Munier FL, Mosimann P, Puccinelli F, et al. First-line intraarterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20 years of treatment. Br J Ophthalmol. 2016; doi: 10.1136/bjophthalmol-2016-309298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munier FL, Soliman S, Moulin AP, et al. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96:1084–1087. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Fulco EM, Arias JD, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye (Lond) 2013;27:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields CL, Kaliki S, Al-Dahmash S, et al. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina 2013;33:2103–2109. [DOI] [PubMed] [Google Scholar]
- 12.Shields CL, Manjandavida FP, Arepalli S, et al. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132: 319–325. [DOI] [PubMed] [Google Scholar]
- 13.Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453–1460. [DOI] [PubMed] [Google Scholar]
- 14.Yannuzzi NA, Francis JH, Abramson DH. Incidence of orbital recurrence after enucleation or ophthalmic artery chemosurgery for advanced intraocular retinoblastomae–reply. JAMA Ophthalmol. 2016;134:114–115. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Kathpalia V, Dunkel IJ, et al. Orbital recurrence of retinoblastoma following enucleation. Br J Ophthalmol. 2009;93:463–467. [DOI] [PubMed] [Google Scholar]
- 16.Abramson DH, Fabius AW, Issa R, et al. Advanced unilateral retinoblastoma: the impact of ophthalmic artery chemosurgery on enucleation rate and patient survival at MSKCC. PLoS One. 2015;10:e0145436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry JL, Jubran R, Lee T, et al. Low-dose chemoreduction for infants diagnosed with retinoblastoma before 6 months of age. Ocul Oncol Pathol 2015;1:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khelfaoui F, Validire P, Auperin A, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer. 1996;77: 1206–1213. [PubMed] [Google Scholar]
- 19.Bosaleh A, Sampor C, Solernou V, et al. Outcome of children with retinoblastoma and isolated choroidal invasion. Arch Ophthalmol. 2012;130:724–729. [DOI] [PubMed] [Google Scholar]
- 20.Shields CL, Shields JA, Baez K, et al. Optic nerve invasion of retinoblastoma. Metastatic potential and clinical risk factors. Cancer. 1994;73:692–698. [DOI] [PubMed] [Google Scholar]
- 21.Hungerford J, Kingston J, Plowman N. Orbital recurrence of retinoblastoma. Ophthalmic Paediatr Genet 1987;8: 63–68. [DOI] [PubMed] [Google Scholar]
- 22.de Graaf P, Goricke S, Rodjan F, et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol 2012;42:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirin S, Schlamann M, Metz KA, et al. Diagnostic image quality of gadolinium-enhanced T1-weighted MRI with and without fat saturation in children with retinoblastoma. Pediatr Radiol 2013;43:716–724. [DOI] [PubMed] [Google Scholar]
- 24.Sirin S, de Jong MC, de Graaf P, et al. High-resolution magnetic resonance imaging can reliably detect orbital tumor recurrence after enucleation in children with retinoblastoma. Ophthalmology. 2016;123:635–645. [DOI] [PubMed] [Google Scholar]
- 25.Sirin S, Schlamann M, Metz KA, et al. High-resolution MRI using orbit surface coils for the evaluation of metastatic risk factors in 143 children with retinoblastoma: part 1: MRI vs. histopathology. Neuroradiology. 2015;57:805–814. [DOI] [PubMed] [Google Scholar]
- 26.Sirin S, Schlamann M, Metz KA, et al. High-resolution MRI using orbit surface coils for the evaluation of metastatic risk factors in 143 children with retinoblastoma: part 2: new vs. old imaging concept. Neuroradiology. 2015;57: 815–824. [DOI] [PubMed] [Google Scholar]
- 27.Spirnak JP, Nieves N, Hollsten DA, et al. Gadolinium-enhanced magnetic resonance imaging assessment of hydroxyapatite orbital implants. Am J Ophthalmol. 1995;119: 431–440. [DOI] [PubMed] [Google Scholar]
- 28.De Potter P, Duprez T, Cosnard G. Postcontrast magnetic resonance imaging assessment of porous polyethylene orbital implant (Medpor). Ophthalmology. 2000;107: 1656–1660. [DOI] [PubMed] [Google Scholar]


