Abstract
Inflammation is implicated in the development and severity of the coronavirus disease 2019 (COVID-19), as well as in the pathophysiology of diabetes. Diabetes, especially when uncontrolled, is also recognized as an important risk factor for COVID-19 morbidity and mortality. Furthermore, certain inflammatory markers [i.e. C-reactive protein (CRP), interleukin-6 (IL-6) and ferritin] were reported as strong predictors of worse outcomes in COVID-19 positive patients. The same biomarkers have been associated with poor glycemic control. Therefore, achieving euglycemia in patients with diabetes is even more important in the era of the COVID-19 pandemic.
Based on the above, it is clinically interesting to elucidate whether antidiabetic drugs may reduce inflammation, thus possibly minimizing the risk for COVID-19 development and severity. The present narrative review discusses the potential anti-inflammatory properties of certain antidiabetic drugs (i.e. metformin, pioglitazone, sitagliptin, linagliptin, vildagliptin, alogliptin, saxagliptin, liraglutide, dulaglutide, exenatide, lixisenatide, semaglutide, empagliflozin, dapagliflozin, canagliflozin), with a focus on CRP, IL-6 and ferritin.
Keywords: Metformin, Pioglitazone, Dipeptidyl peptidase 4 inhibitors, Glucagon-like peptide-1 receptor agonists, Sodium-glucose co-transporter-2 inhibitors, C-reactive protein, Interleukin 6, Ferritin
1. Introduction
Inflammation plays a key role in the development and severity of the new coronavirus disease 2019 (COVID-19).1 Indeed, severe COVID-19 pneumonia is related to systemic hyper-inflammation (or cytokine storm).2 Serum C-reactive protein (CRP), interleukin-6 (IL-6) and ferritin have been recognized as strong predictors of the COVID-19 severity and mortality.3., 4., 5. Diabetes is also considered as a significant risk factor for COVID-19 morbidity and mortality.6 Indeed, a recent meta-analysis (83 studies, n = 78,874 hospitalized patients with confirmed COVID-19) reported that pre-existing diabetes almost doubled the risk for severe/critical COVID-19 [n = 22 studies; odds ratio (OR) 2.10, 95% confidence interval (95%CI) 1.71–2.57] and almost tripled in-hospital mortality (OR 2.68, 95%CI 2.09–3.44).7 Similar results were reported in another meta-analysis (33 studies, n = 16,003 patients) for both COVID-19 severity (OR 2.75, 95%CI 2.09–3.62) and mortality (OR 1.90, 95%CI 1.37–2.64).8 Furthermore, chronic, low-grade inflammation is associated with insulin resistance and hyperglycemia,9., 10., 11. and inflammatory markers, such as CRP, IL-6 and ferritin, have been quantitatively related to HbA1c. 12., 13., 14. This highlights the importance of achieving euglycemia in patients with diabetes, especially in the era of COVID-19.
Based on the above, it is relevant to establish whether antidiabetic therapies may reduce the inflammatory reaction, thus possibly minimizing the risk for COVID-19 development and severity. The present narrative review discusses the potential anti-inflammatory properties of certain antidiabetic drugs (i.e. metformin, pioglitazone, sitagliptin, linagliptin, vildagliptin, alogliptin, saxagliptin, liraglutide, dulaglutide, exenatide, lixisenatide, semaglutide, empagliflozin, dapagliflozin, canagliflozin), based mainly on available data from clinical studies and with a focus on CRP, IL-6 and ferritin.
1.1. Metformin
Metformin mechanism of action classically involves both AMP-activated protein kinase (AMPK)-independent and AMPK-dependent pathways.15 In many studies, this drug has been shown to inhibit mitochondrial respiration as well as mitochondrial glycerophosphate dehydrogenase, leading to suppression of ATP production and hepatic gluconeogenesis, whereas AMPK activation enhances catabolic pathways generating ATP and switches off cellular processes consuming ATP.15 , 16
There is basic and clinical evidence for an anti-inflammatory action of metformin.17 Metformin was also shown to suppress the secretion of proinflammatory cytokines in hepatocytes and macrophages.18 Among 110 patients with type 2 diabetes mellitus (T2DM), those on metformin (n = 65) had significantly lower CRP levels compared with those on glibenclamide (n = 45) (5.6 vs 8.3 mg/dL, p = 0.01).19 In another study, 208 T2DM patients were randomly assigned to metformin or placebo for 24 weeks; serum CRP levels were significantly decreased from 14.4 ± 0.6 to 9.6 ± 0.2 mg/L (p < 0.001) in the metformin group, whereas they were unchanged in the placebo group (from 14.5 ± 0.6 to 15.8 ± 0.8 mg/L).20 In the Diabetes Prevention Program (DPP), among metformin-treated T2DM men (n = 363) and women (n = 710), CRP was significantly reduced at 1 year (by 7%, p = 0.006 and by 14%, p < 0.001, respectively).21 However, others have reported no effects of metformin on CRP22 or high-sensitivity CRP (hsCRP)23 in T2DM patients. Previous meta-analyses also reported that metformin significantly reduced CRP in middle-age and older adults with chronic low-grade inflammation [142 studies; 3247 patients; standardized mean difference (SMD): -0.16; 95%CI: −0.22 to −0.09, p < 0.0001; I2 = 79.9%],24 as well as in women with polycystic ovary syndrome (17 studies; 403 women; SMD: -0.86; 95%CI: −1.24 to −0.48, p < 0.0001; I2 = 84.6%).25
Data on the effects of metformin on IL-6 and ferritin are limited. Metformin was shown to inhibit IL-6 expression in mucosal cells of patients with inflammatory bowel disease.26 Furthermore, metformin suppressed IL-1ß-induced release of IL-6 in human macrophages, vascular smooth muscle and endothelial cells.27 Circulating IL-6 levels were reduced in a duration- and dose-dependent manner (i.e., at a dose of 1000 mg q.d. for 1 year) after 12 months of metformin therapy in T2DM patients (n = 112).28 Similarly, metformin (titrated up to 1500 mg q.d.) significantly lowered IL-6 (from 133 ± 68 to 114 ± 57 pg/mL, p < 0.05) at 12 months in 36 T2DM patients.29 In the same study, ferritin was also decreased (from 171 ± 23 to 164 ± 19 ng/mL, p < 0.05) following metformin treatment.29
1.2. Pioglitazone
Pioglitazone is a peroxisome proliferator-activated receptor (PPAR)-γ agonist that reduces insulin resistance by stimulating lipogenesis and suppressing lipolysis in the adipose tissue, as well as by decreasing hepatic triglycerides, visceral fat mass and activity, thus promoting peripheral insulin sensitivity.30 , 31 Furthermore, pioglitazone enhances glucose uptake by the skeletal muscle and beta-cell function.32
In animal studies, the expression of IL-6 was suppressed in human monocytes33 as well as in white adipose tissue and cardiomyocytes.34 , 35 In 34 T2DM patients, pioglitazone therapy for 6 months significantly decreased circulating CRP levels (from 1.73 ± 1.30 to 1.23 ± 0.75 μg/mL, p < 0.05); IL-6 levels were non-significantly reduced (from 1.78 ± 1.32 to 1.50 ± 0.97 pg/mL, p = ns).36 Similarly, a previous meta-analysis reported that pioglitazone significantly lowered hsCRP levels (15 trials, n = 1448 T2DM patients; SMD = −0.54, 95%CI: −0.92 to −0.16, p < 0.05; I2 = 90%) but not IL-6 (4 trials, n = 422 T2DM patients; SMD = −1.5, 95%CI: −3.08 to 0.07, p = 0.06). 37 No data exist on the impact of pioglitazone on ferritin.
1.3. Dipeptidyl peptidase-4 inhibitors (DPP4-i)
DPP4-i increase the endogenous levels of bioactive incretins, including glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP), by inhibiting their enzymatic degradation.32 As a consequence, insulin secretion is enhanced in a glucose-dependent way, whereas glucagon secretion is suppressed.32
DPP4-i may exert immune regulatory functions, that are potentially beneficial in autoimmune and inflammatory diseases, as recently reviewed.38 A recent meta-analysis (n = 1607 T2DM patients; 16 trials) reported a significant reduction in CRP levels following DPP4-i therapy compared with placebo (WMD: −0.86 mg/L; 95%CI: −1.36 to −0.36, p = 0.001; I2 = 84.4%).39
1.4. Sitagliptin
itagliptin (either 50 or 100 mg/day) has been reported to significantly decrease CRP levels in 67 newly diagnosed T2DM patients at 12 weeks (from 6.1 ± 1.0 to 3.3 ± 0.5 mg/L, p < 0.05),40 in 48 patients at 3 months (from 0.8 ± 0.1 to 0.5 ± 0.1 μg/L, p < 0.05),41 in 22 patients at 12 weeks (by 24%, p < 0.05),42 in 36 patients at 6 weeks (by 44.9%, p = 0.006)43 and in 20 T2DM patients at 6 months [from 1.60 (0.45–2.85) to 0.70 (0.35–1.25) mg/L, p < 0.01].44 Furthermore, hsCRP was significantly reduced over 7 years of follow-up in T2DM patients treated with sitagliptin (100 mg/day) in combination with either metformin (n = 201; from 2.4 ± 0.9 to 1.8 ± 0.4 mg/L, p < 0.05), pioglitazone (n = 196; from 2.5 ± 1.0 to 2.0 ± 0.6, p < 0.05) or sulfonylureas (n = 194; from 2.3 ± 0.8 to 1.8 ± 0.3 mg/L, p < 0.05).45 Sitagliptin (100 mg/day)-induced CRP decrease was also reported elsewhere.46 , 47 In contrast, the Sitagliptin Investigation on Glycemic Effects in Yokohama (SINGLE-Y) study, involving 270 T2DM patients, found that 3 months of sitagliptin (50 mg/day) did not affect hsCRP (from 0.12 ± 0.78 to 0.09 ± 0.10 mg/dL, p = ns).48 Furthermore, no significant change in hsCRP was observed in another study of 205 T2DM patients treated with sitagliptin (25, 50, or 100 mg/day) for 12 months.49 Similar results were reported in other studies using sitagliptin 50 or 100 mg/day.50., 51., 52.
IL-6 levels were non-significantly decreased at 3 months among 24 sitagliptin-treated T2DM patients (from 3.5 ± 0.6 to 2.7 ± 0.3 pg/mL, p = ns),41 whereas they were significantly lowered by sitagliptin in 22 T2DM patients at 12 weeks (by 24%, p < 0.05),42 as well as at 12 months (from 15.8 ± 6.2 to 12.8 ± 4.3 pg/mL, p = 0.03) in 31 T2DM patients.53
The impact of sitagliptin on ferritin has not been investigated. There is only one study involving 5 T2DM patients with major beta-thalassemia treated with sitagliptin; there was a trend of ferritin reduction in 4/5 patients.54
1.4.1. Linagliptin
Data on linagliptin and CRP are scarce. In one study, involving 21 T2DM patients on hemodialysis (HD), no change in CRP levels was found at 6 months after initiating linagliptin.55 Similar results were reported among 35 T2DM patients undergoing HD receiving linagliptin for 3 months56 and among 45 T2DM patients on linagliptin for 26 weeks.57 Linagliptin was found to inhibit IL-6 production in human umbilical vein endothelial cells58 and monocytes.59 No relevant human studies are available. There is only one study investigating ferritin changes after 6 months of linagliptin therapy among 25 T2DM patients on HD, reporting no effect.60
1.4.2. Vildagliptin
Among 60 T2DM patients with coronary artery disease (CAD), 12-week vildagliptin therapy reduced hsCRP by 60%.61 Similar effects were also reported in other studies.62., 63., 64., 65., 66. Neutral studies also exist.67 , 68
In animal studies, vildagliptin was shown to attenuate the isoproterenol-induced increased mRNA expression of IL-6 in cardiomyocytes.69 However, no change in IL-6 was observed following 12 weeks of vildagliptin therapy in 27 T2DM patients.70 In contrast, another study found a significant decrease in IL-6 levels in vildagliptin-treated T2DM patients at 12 weeks (from 2.47 ± 0.52 to 1.54 ± 0.16 pg/mL, p < 0.01).71 No study has investigated the impact of vildagliptin on ferritin.
1.4.3. Alogliptin
Limited data exist on the effects of alogliptin on CRP or IL-6. No significant change in hsCRP levels were observed at 24 months following alogliptin treatment in the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A) (n = 172 T2DM patients).72 In animal models, alogliptin decreased IL-6,73 , 74 as also shown in in vitro studies.75 In humans, in the SPEAD-A study, IL-6 was significantly increased [baseline = 2.1 (1.4, 2.7) ng/dL; median change 0.1 (−0.3, 0.7), p < 0.05] in alogliptin-treated T2DM patients followed for 24 months.72 There are no data on ferritin and alogliptin.
1.4.4. Saxagliptin
In diabetic mice, saxagliptin was able to reduce serum CRP.76 However, no impact on hsCRP was observed in saxagliptin-treated T2DM patients compared with placebo in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial.77 Similarly, saxagliptin did not affect the secretion of IL-6 from adipocytes in 40 non-diabetic overweight/obese patients followed for 6 weeks.78 No data on ferritin and saxagliptin exist.
1.5. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs)
GLP-1 RAs promote insulin secretion by the beta-cells and inhibit glucagon secretion by the alpha-cells of the pancreas.79 GLP-1 RAs also delay gastric emptying and suppress food intake.79 GLP-1 RAs have been reported to exert anti-inflammatory properties via several molecular mechanisms, both in vivo and in vitro 80.
1.5.1. Liraglutide
Liraglutide has been reported to exert antioxidant and anti-inflammatory effects in vitro 81 , 82 as well as in T2DM patients83 and in patients with non-ST-segment elevation myocardial infarction.84 In this context, among 52 T2DM patients receiving liraglutide at a dose of 0.6–1.2 mg/day for 12 weeks, CRP was significantly decreased (by 0.89 ± 0.59 mg/L, p = 0.018).85 Similar results were observed following liraglutide (1.2–1.8 mg/day) therapy for 24 weeks in T2DM patients.86., 87., 88., 89. Of note, liraglutide-induced weight loss was related to improvements in circulating hsCRP levels in obese patients with prediabetes or T2DM.90 In contrast, no significant changes in hsCRP were observed among 44 T2DM patients after 6 and 12 weeks of liraglutide (1.2 and 1.8 mg/day, respectively)91 as well as among 165 T2DM after 14 weeks of liraglutide (0.65–1.9 mg/day).92
Liraglutide significantly reduced both circulating and hepatic levels of IL-6 in diabetic mice93 as well as IL-6 expression in the hippocampus, suggesting protection against neuroinflammation.94 In T2DM patients, a recent meta-analysis of 13 randomized controlled trials (n = 1187; follow-up ranged from 8 to 24 weeks), found that IL-6 concentrations were significantly lower [mean difference (MD): -3.90; 95%CI: −5.03 to −2.76, p < 0.00001] in the liraglutide group (1.2 mg/day) compared with the controls in those with stage 3 diabetic nephropathy.95 Similarly, liraglutide (1.2–1.8 mg/day) for 26 weeks led to a significant reduction in circulating IL-6 levels (−22.6%; 95%CI: −38.1, −3.2, p = 0.025) among 19 type 1 DM patients.96 One study, however, reported no changes in IL-6 concentrations after 14 weeks of liraglutide (0.65–1.9 mg/day).92
Liraglutide (0.9 mg/day for 24 weeks) was shown to significantly decrease serum ferritin levels (from 158.9 ± 147.4 to 91.8 ± 69.9 ng/mL, p < 0.01) in 19 T2DM patients with NAFLD/NASH based on liver biopsy in the LEAN-J study.97 No other relevant data exist.
1.5.2. Dulaglutide
There are limited data on dulaglutide, CRP and IL-6. In one study, 755 T2DM patients were randomized to receive either dulaglutide once weekly (0.75 or 1.5 mg) or placebo; changes from baseline in hsCRP levels at 16 weeks were − 0.98, −0.08 and 0.62 mg/l for dulaglutide 1.5 mg (p < 0.001 compared with placebo), dulaglutide 0.75 mg, and placebo, respectively.98 In another smaller study, once weekly dulaglutide (0.75 mg in 8 T2DM patients and 1.5 mg in 5 patients) led to a significant reduction in serum IL-6 levels (from 1.42 ± 0.84 to 0.31 ± 0.23 pg/mL, p < 0.001) at 26 weeks.99 No study investigated the effects of dulaglutide on ferritin.
1.5.3. Exenatide
Exenatide reduced circulating markers of oxidative stress and inflammation in T2DM patients.100 , 101 In 61 T2DM patients on exenatide (5 or 10 μg twice daily) for a mean of 1.4 years, circulating CRP levels decreased from 5.1 ± 1.7 to 2.7 ± 1.2 mg/L (p < 0.001).102 Similarly, serum hsCRP was lowered by 34% (p = 0.05) in 38 T2DM patients taking exenatide (5 μg twice per day) for a mean of 26 weeks.103 In another study involving 23 T2DM patients (11 patients on placebo and 12 patients on exenatide 5 μg twice daily for 4 weeks, followed by 10 μg twice daily for 12 weeks), hsCRP was significantly reduced in the exenatide group (−37%) compared with placebo (+137%).100 Exenatide therapy for 1 year also led to lower hsCRP levels (from 1.81 ± 0.25 to 1.30 ± 0.22 mg/L, p = 0.008) in 30 T2DM patients.104
Circulating IL-6 levels fell from 3.01 ± 0.49 to 2.07 ± 0.57 pg/mL (p < 0.05) after 12 weeks of exenatide treatment (10 μg twice daily) in 12 T2DM patients.105 In another study, 18 T2DM patients received exenatide for 3 months (5 μg twice daily for the first month, followed by 10 μg for the next 2 months); serum IL-6 concentrations were reduced from 15.69 ± 10.86 ng/mL at baseline to 10.76 ± 5.15 ng/mL at 3 months (p = 0.001).106 No data on the effects of exenatide on ferritin are currently available.
1.5.4. Lixisenatide
Lixisenatide was reported to downregulate the expression of proinflammatory cytokines, including IL-6, in human fibroblast-like synoviocytes and in primary chondrocytes.107 , 108 No human study evaluated the impact of lixisenatide on CRP, IL-6 and ferritin.
1.5.5. Semaglutide
In a 52-week weight management trial (n = 957 non-diabetic obese patients randomized to oral semaglutide 0.05–0.4 mg/day or placebo), hsCRP was reduced by up to 43% vs placebo in all semaglutide groups at week 52; these reductions were either significant or close to significance.109 However, statistical significance was lost after adjustment for change in body weight.109 In another 52-week randomized, open-label trial involving 412 T2DM patients on oral semaglutide 14 mg and 410 T2DM patients on empagliflozin 25 mg, greater decreases in CRP levels were observed at 52 weeks in the semaglutide than in the empagliflozin group (estimated treatment ratio: 0.74, 95%CI: 0.65, 0.84, p < 0.0001).110 No data exist on semaglutide, IL-6 and ferritin.
1.6. Sodium Glucose Co-Transporter-2 inhibitors (SGLT2i)
SGLT2i inhibit the renal reabsorption of glucose, thus promoting renal glucose excretion and, subsequently, lowering plasma glucose levels.111 , 112 Potential anti-inflammatory effects have been reported for SGLT2i113.
1.6.1. Empagliflozin
Among 50 T2DM patients with CAD, empagliflozin 10 mg/day significantly lowered CRP at 6 months (from 0.11 to 0.07 mg/dL, p = 0.003).114 Empagliflozin 10 mg/day for 12 months also notably reduced hsCRP by 54% (from 1.33 to 0.59 mg/L, p = 0.007) in 51 T2DM patients.115 However, in another study with 58 T2DM patients receiving empagliflozin 25 mg/day for 6 weeks, no significant changes in hsCRP levels were found (2.10 ± 1.72 vs 1.99 ± 1.19 mg/L, baseline vs 6 weeks, respectively).116
Interestingly, empagliflozin was reported to decrease markers of oxidative stress and inflammation (including IL-6) in the lungs of mice, thus suggesting a role in preventing pulmonary fibrosis,117 as well as in rat cardiomyoblasts (including reduction of IL-6 expression), thus potentially minimizing infarct size.118 Similarly, in diabetic rats empagliflozin exhibited anti-oxidant and anti-inflammatory effects in the kidneys; urinary IL-6 levels were also lowered.119 Overall, experimental data strongly support a protective role of empagliflozin against cardiac and renal inflammation.120 , 121 However, clinical evidence is lacking. There is one study reporting that empagliflozin significantly reduced circulating IL-6 levels in 32 men T2DM patients; this IL-6-lowering effect of empagliflozin was greater than that of canagliflozin.122
In a large-scale proteomics study, empagliflozin 25 mg/day led to significant decreases in circulating ferritin levels after 4 weeks of treatment.123 Furthermore, a sub-study of the EMPA-HEART CardioLink-6 randomized clinical trial, involving 82 T2DM patients with CAD, showed that 6 months of empagliflozin treatment (10 mg/day) was associated with a significant reduction in ferritin levels (mean difference − 21.83 μg/L, 95%CI: −37.96, −5.7; p = 0.008).124
1.6.2. Dapagliflozin
In animal studies, dapagliflozin was shown to exert anti-oxidant and anti-inflammatory actions in the kidney, liver and lungs.125 , 126 There is also data supporting a CRP-lowering effect of dapagliflozin in humans.127 In this context, dapagliflozin 10 mg/day combined with metformin significantly lowered CRP (from 6.2 ± 1.1to 3.1 ± 0.7 mg/L, p < 0.05) in 59 T2DM patients at 12 weeks26 as did dapagliflozin 5 mg/day after 12 weeks (from 2410 ± 2814 to 1607 ± 1960 ng/mL, p < 0.01) in 27 T2DM patients.128 In contrast, among 11 T2DM patients with non-alcoholic steatohepatitis, treatment with dapagliflozin 5 mg/day for 24 weeks led to a non-significant reduction in hsCRP.129 Furthermore, another study reported a significant increase in median hsCRP levels in 36 T2DM patients treated with dapagliflozin (10 mg/day) for 12 weeks (from 1.93 to 6.03 mg/L, p = 0.009).130
In a randomized, placebo-controlled study (n = 32 T2DM patients), 8 weeks of dapagliflozin (10 mg/day) therapy led to a significant placebo-corrected reduction in IL-6 levels (by −1.87 pg/mL, p < 0.05).131 A post-hoc analysis of another randomized, double-blind, placebo-controlled trial involving 33 T2DM patients, found that urinary IL-6 excretion was significantly decreased by 23.5% (p = 0.04).132
With regard to ferritin, there are studies reporting that dapagliflozin 5 mg/day significantly decreased ferritin levels in T2DM patients with either NASH or non-alcoholic fatty liver disease (NAFLD).129 , 133 Similarly, ferritin was significantly lowered after 12 weeks of dapagliflozin (10 mg/day) therapy in 26 obese T2DM patients.134
1.6.3. Canagliflozin
Among 100 T2DM patients treated with canagliflozin 300 mg/day for 52 weeks, there was a non-significant trend for a decrease in serum CRP levels.135 Furthermore, no change was observed in serum CRP after 12 weeks of canagliflozin 100 mg/day therapy in 12 T2DM patients.136 In contrast, hsCRP was significantly reduced from baseline at 3, 6 and 12 months of treatment (from 0.39 ± 0.07 to 0.20 ± 0.05, 0.20 ± 0.04 and 0.21 ± 0.04 ng/mL, respectively, p < 0.05) in 35 T2DM patients with chronic heart failure treated with canagliflozin 100 mg/day.137
A proteomic model found that canagliflozin 300 mg/day significantly lowered plasma IL-6 concentrations (by 26.6%, p = 0.010).138 Similar results were reported in animal studies.139 , 140 However, in human studies no significant changes in IL-6 levels have been found following treatment with canagliflozin (100 mg/day, 24 weeks).122 , 141 In contrast, canagliflozin 300 mg/day for 52 weeks was shown to significantly lower serum IL-6 (by 22%) in 100 T2DM patients.135
In a small study (9 T2DM patients), canagliflozin 100 mg/day for 12 weeks led to a significant decrease in median ferritin levels (from 72 to 55 ng/mL, p = 0.003).142 Furthermore, serum ferritin concentrations were significantly and progressively lowered from baseline to 3 and 6 months (from 184.9 to 143.8 and 117.3 ng/mL, respectively, p < 0.05) in 35 canagliflozin (100 mg/day)-treated NAFLD patients.143 Similar results were also reported in T2DM patients with biopsy-proven NASH.144 Of note, SGLT2i have been suggested for NAFLD/NASH treatment145., 146., 147. as is the case for pioglitazone and GLP-1 RAs.148 , 149
Overall, diabetes has been closely linked to inflammation.150 Furthermore, HbA1c has been positively related to hsCRP, IL-6 and ferritin levels,151., 152., 153., 154., 155. thus supporting also a role for euglycemia in reducing chronic inflammation in patients with diabetes. Apart from antidiabetic drugs, a healthy lifestyle (including diet, exercise and no smoking) can contribute to glucose and inflammation control.156 , 157 Interestingly, HbA1c level was positively associated with inflammation and hypercoagulability, as well as negatively associated with SaO2 in COVID-19 infected patients,158 thus further highlighting the importance of achieving glucose control in the COVID-19 era.
1.7. Limitations of current evidence
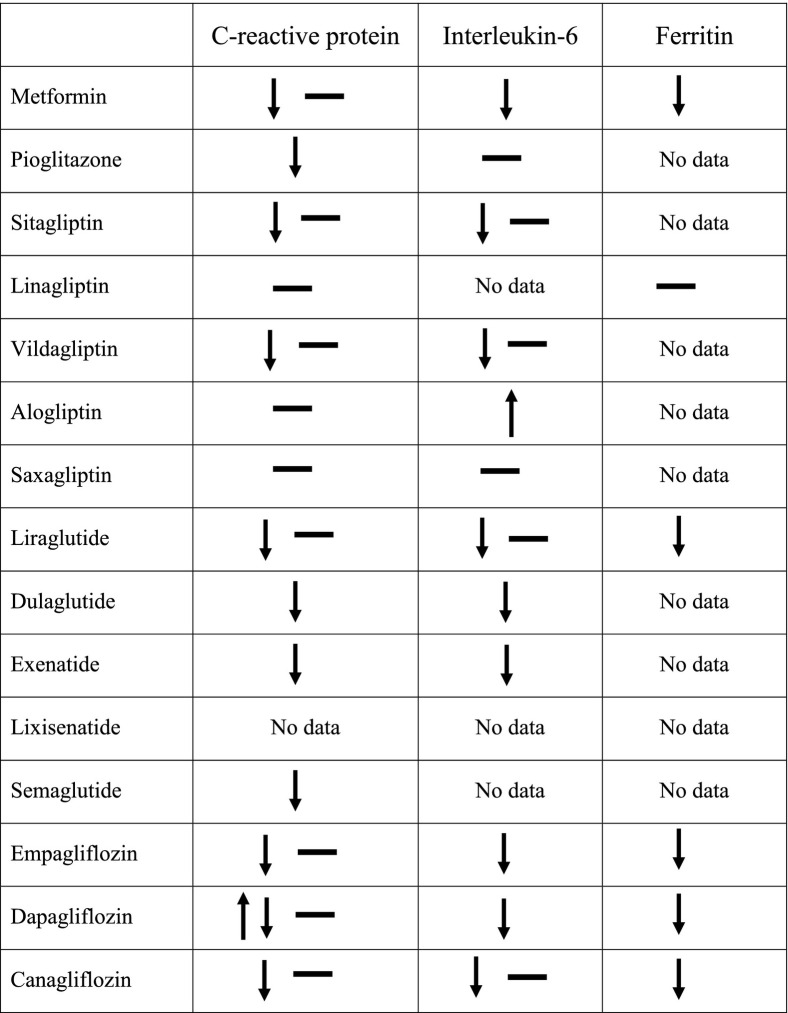
Despite the multitude of reports, the data here analyzed (summarized in Table 1 for the human studies) fall short of providing good evidence for a clinically significant anti-inflammatory effect of the most common antidiabetic classes of medication for several reasons. Firstly, studies were frequently small in size or detecting small differences. Secondly, many studies involved patients of Asian origin, without appropriate control for other ethnicities. Thirdly, studies of similar design yielded contrasting results for one or the other biomarker, or inconsistent findings across biomarkers. Finally, and perhaps more importantly, most studies did not control, or were not equipoised, for the anti-hyperglycemic effect, so that a bona fide pharmacologic effect of a given drug cannot be distinguished from a non-specific effect of improved glycemia.
Table 1.
Summary of the effects of antidiabetic drugs on C-reactive protein, interleukin-6 and ferritin in human studies.
2. Conclusions
With all these limitations, perhaps the most suggestive data are those on ferritin for the SGLT2 inhibitors, which may be related to enhanced erythropoiesis rather than tissues inflammation. In any event, large, multiethnic, equipoised trials would be required to determine whether certain antidiabetic drugs exert inherent anti-inflammatory properties above and beyond their antihyperglycemic efficacy; the latter remains decidedly useful in COVID-19 disease.
Declaration of competing interest
NK has given talks, attended conferences and participated in trials sponsored by Astra Zeneca, Bausch Health, Boehringer Ingelheim, Elpen, Mylan, NovoNordisk, Sanofi and Servier. EF has received research support by Boehringher Ingelheim/Lilly&Co., AstraZeneca and Janssen and speaker's honoraria by Boehringer Ingelheim/Lilly&Co., Sanofi and AstraZeneca.
References
- 1.Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus - a perspective. Expert Rev Clin Immunol. 2020:1–6. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020 Apr;3 doi: 10.1016/j.autrev.2020.102537. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 Apr;10 doi: 10.1515/cclm-2020-0369. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan Q., Yang K., Wang W., Jiang L., Song J. China; Intensive Care Med: 2020 Mar 3. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain A, Bhowmik B, Cristina do Vale Moreira N. COVID-19 and Diabetes: Knowledge in Progress. Diabetes Res Clin Pract. 2020 Apr 9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 7.Mantovani A., Byrne C.D., Zheng M.H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., Arora A., Sharma P., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lontchi-Yimagou E., Sobngwi E., Matsha T.E., Kengne A.P. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 10.Natali A., Toschi E., Baldeweg S., Ciociaro D., Favilla S., Saccà L., et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan A.D., Manson J.E., Rifai N., Buring J.E., Ridker P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 12.Elimam H., Abdulla A.M., Taha I.M. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13:800–804. doi: 10.1016/j.dsx.2018.11.061. [DOI] [PubMed] [Google Scholar]
- 13.King D.E., Mainous A.G. 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26:1535–1539. doi: 10.2337/diacare.26.5.1535. [DOI] [PubMed] [Google Scholar]
- 14.He Q., Dong M., Pan Q., Wang X., Guo L. Correlation between changes in inflammatory cytokines and the combination with hypertension in patients with type 2 diabetes mellitus. Minerva Endocrinol. 2019;44:252–258. doi: 10.23736/S0391-1977.18.02822-5. [DOI] [PubMed] [Google Scholar]
- 15.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madiraju A.K., Qiu Y., Perry R.J., Rahimi Y., Zhang X.M., Zhang D., et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med. 2018;24:1384–1394. doi: 10.1038/s41591-018-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets. 2015;15:196–205. doi: 10.2174/1871530315666150316124019. [DOI] [PubMed] [Google Scholar]
- 18.Cameron A.R., Morrison V.L., Levin D., Mohan M., Forteath C., Beall C., et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbar D.H. Effect of metformin and sulfonylurea on C-reactive protein level in well-controlled type 2 diabetics with metabolic syndrome. Endocrine. 2003;20:215–218. doi: 10.1385/ENDO:20:3:215. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty A., Chowdhury S., Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract. 2011;93:56–62. doi: 10.1016/j.diabres.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Haffner S., Temprosa M., Crandall J., Fowler S., Goldberg R., Horton E., et al. Barrett-Connor E; diabetes prevention program research group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H.J., Kang E.S., Kim D.J., Kim S.H., Ahn C.W., Cha B.S., et al. Effects of rosiglitazone and metformin on inflammatory markers and adipokines: decrease in interleukin-18 is an independent factor for the improvement of homeostasis model assessment-beta in type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2007;66:282–289. doi: 10.1111/j.1365-2265.2006.02723.x. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan A.D., Everett B.M., Cook N.R., Rifai N., Ridker P.M. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302:1186–1194. doi: 10.1001/jama.2009.1347. [DOI] [PubMed] [Google Scholar]
- 24.Custodero C., Mankowski R.T., Lee S.A., Chen Z., Wu S., Manini T.M., et al. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: a systematic review and meta-analysis. Ageing Res Rev. 2018;46:42–59. doi: 10.1016/j.arr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J., Zhu L., Hu K., Tang Y., Zeng X., Liu J., et al. Effects of metformin treatment on serum levels of C-reactive protein and interleukin-6 in women with polycystic ovary syndrome: a meta-analysis: a PRISMA-compliant article. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Fusco D., Dinallo V., Monteleone I., Laudisi F., Marafini I., Franzè E., et al. Metformin inhibits inflammatory signals in the gut by controlling AMPK and p38 MAP kinase activation. Clin Sci (Lond) 2018;132:1155–1168. doi: 10.1042/CS20180167. [DOI] [PubMed] [Google Scholar]
- 27.Isoda K., Young J.L., Zirlik A., MacFarlane L.A., Tsuboi N., Gerdes N., et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Liu X, Ye S. Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes. J Inflamm (Lond). 2016;13:34. [DOI] [PMC free article] [PubMed]
- 29.Mo D., Liu S., Ma H., Tian H., Yu H., Zhang X., et al. Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study. Drug Des Devel Ther. 2019;13:2769–2776. doi: 10.2147/DDDT.S208327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebovitz H.E. Thiazolidinediones: the forgotten diabetes medications. Curr Diab Rep. 2019;19:151. doi: 10.1007/s11892-019-1270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiarelli F., Di Marzio D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: current evidence and future perspectives. Vasc Health Risk Manag. 2008;4:297–304. doi: 10.2147/vhrm.s993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Upadhyay J., Polyzos S.A., Perakakis N., Thakkar B., Paschou S.A., Katsiki N., et al. Pharmacotherapy of type 2 diabetes: an update. Metabolism. 2018;78:13–42. doi: 10.1016/j.metabol.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W.Y., Schwartz E.A., Permana P.A., Reaven P.D. Pioglitazone inhibits the expression of inflammatory cytokines from both monocytes and lymphocytes in patients with impaired glucose tolerance. Arterioscler Thromb Vasc Biol. 2008;28:2312–2318. doi: 10.1161/ATVBAHA.108.175687. [DOI] [PubMed] [Google Scholar]
- 34.Mohapatra J., Sharma M., Singh S., Chatterjee A., Swain P., Balaraman R., et al. Subtherapeutic dose of pioglitazone reduces expression of inflammatory adipokines in db/db mice. Pharmacology. 2009;84:203–210. doi: 10.1159/000235996. [DOI] [PubMed] [Google Scholar]
- 35.Ye P., Yang W., Wu S.M., Sheng L. Effect of pioglitazone on the expression of inflammatory cytokines in attenuating rat cardiomyocyte hypertrophy. Methods Find Exp Clin Pharmacol. 2006;28:691–696. doi: 10.1358/mf.2006.28.10.1037500. [DOI] [PubMed] [Google Scholar]
- 36.Park J.S., Cho M.H., Nam J.S., Yoo J.S., Ahn C.W., Cha B.S., et al. Effect of pioglitazone on serum concentrations of osteoprotegerin in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2011;164:69–74. doi: 10.1530/EJE-10-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen R., Yan J., Liu P., Wang Z. Effects of thiazolidinedione therapy on inflammatory markers of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao S., Xu Q., Yu X., Pan R., Chen Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol Ther. 2020;209:107503. doi: 10.1016/j.pharmthera.2020.107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X., Men P., Wang B., Cai G., Zhao Z. Effect of dipeptidyl-peptidase-4 inhibitors on C-reactive protein in patients with type 2 diabetes: a systematic review and meta-analysis. Lipids Health Dis. 2019;18:144. doi: 10.1186/s12944-019-1086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y., Yan D., Hao Z., Cui L., Li G. Effects of dapagliflozin and sitagliptin on insulin resistant and body fat distribution in newly diagnosed type 2 diabetic patients. Med Sci Monit. 2020;26 doi: 10.12659/MSM.921891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satoh-Asahara N., Sasaki Y., Wada H., Tochiya M., Iguchi A., Nakagawachi R., et al. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism. 2013;62:347–351. doi: 10.1016/j.metabol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Makdissi A., Ghanim H., Vora M., Green K., Abuaysheh S., Chaudhuri A., et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97:3333–3341. doi: 10.1210/jc.2012-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremblay A.J., Lamarche B., Deacon C.F., Weisnagel S.J., Couture P. Effects of sitagliptin therapy on markers of low-grade inflammation and cell adhesion molecules in patients with type 2 diabetes. Metabolism. 2014;63:1141–1148. doi: 10.1016/j.metabol.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Matsubara J., Sugiyama S., Akiyama E., Iwashita S., Kurokawa H., Ohba K., et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337–1344. doi: 10.1253/circj.cj-12-1168. [DOI] [PubMed] [Google Scholar]
- 45.Derosa G., Tritto I., Romano D., D’Angelo A., Catena G., Maffioli P. Effects of sitagliptin on lipid profile in patients with type 2 diabetes mellitus after 7 years of therapy. J Clin Pharmacol. 2019;59:1391–1399. doi: 10.1002/jcph.1431. [DOI] [PubMed] [Google Scholar]
- 46.Derosa G., Carbone A., Franzetti I., Querci F., Fogari E., Bianchi L., et al. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, β-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2012;98:51–60. doi: 10.1016/j.diabres.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Derosa G., Maffioli P., Salvadeo S.A., Ferrari I., Ragonesi P.D., Querci F., et al. Effects of sitagliptin or metformin added to pioglitazone monotherapy in poorly controlled type 2 diabetes mellitus patients. Metabolism. 2010;59:887–895. doi: 10.1016/j.metabol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Tsurutani Y, Omura M, Matsuzawa Y, Saito J, Higa M, Taniyama M, Nishikawa T; SINGLE-Y investigation group. Efficacy and Safety of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin on Atherosclerosis, β-Cell Function, and Glycemic Control in Japanese Patients with Type 2 Diabetes Mellitus Who are Treatment Naïve or Poorly Responsive to Antidiabetes Agents: A Multicenter, Prospective Observational, Uncontrolled Study. Curr Ther Res Clin Exp. 2017;84:26–31. [DOI] [PMC free article] [PubMed]
- 49.Nakamura T., Iwanaga Y., Miyaji Y., Nohara R., Ishimura T., Miyazaki S. Sitagliptin Registry Kinki Cardiologists’ Study (SIRKAS) investigators. Cardiovascular efficacy of sitagliptin in patients with diabetes at high risk of cardiovascular disease: a 12-month follow-up. Cardiovasc Diabetol. 2016;15:54. doi: 10.1186/s12933-016-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nogueira KC, Furtado M, Fukui RT, Correia MR, Dos Santos RF, Andrade JL, Rossi da Silva ME. Left ventricular diastolic function in patients with type 2 diabetes treated with a dipeptidyl peptidase-4 inhibitor- a pilot study. Diabetol Metab Syndr. 2014;6(1):103. [DOI] [PMC free article] [PubMed]
- 51.Nakamura K., Oe H., Kihara H., Shimada K., Fukuda S., Watanabe K., et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. doi: 10.1186/s12933-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S.C., Chien K.L., Wang C.H., Chen W.C., Leung C.H. Efficacy and safety of adding pioglitazone or sitagliptin to patients with type 2 diabetes insufficiently controlled with metformin and a sulfonylurea. Endocr Pract. 2013;19:980–988. doi: 10.4158/EP13148.OR. [DOI] [PubMed] [Google Scholar]
- 53.Liu X., Mei T., Chen W., Ye S. Comparison of antidiabetic medications during the treatment of atherosclerosis in T2DM patients. Mediators Inflamm. 2017;2017:5032708. doi: 10.1155/2017/5032708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zonoozi S., Barnard M., Prescott E., Jones R., Shah F.T., Tzoulis P. Effectiveness and safety of sitagliptin in patients with beta-thalassaemia major and diabetes mellitus: a case series. Mediterr J Hematol Infect Dis. 2017;9 doi: 10.4084/MJHID.2017.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura Y., Tsuji M., Hasegawa H., Kimura K., Fujita K., Inoue M., et al. Anti-inflammatory effects of linagliptin in hemodialysis patients with diabetes. Hemodial Int. 2014;18:433–442. doi: 10.1111/hdi.12127. [DOI] [PubMed] [Google Scholar]
- 56.Terawaki Y., Nomiyama T., Takahashi H., Tsutsumi Y., Murase K., Nagaishi R., et al. Efficacy of dipeptidyl peptidase-4 inhibitor linagliptin in patients with type 2 diabetes undergoing hemodialysis. Diabetol Metab Syndr. 2015;7:44. doi: 10.1186/s13098-015-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Boer S.A., Heerspink H.J.L., Juárez Orozco L.E., van Roon A.M., Kamphuisen P.W., Smit A.J., et al. Effect of linagliptin on pulse wave velocity in early type 2 diabetes: a randomized, double-blind, controlled 26-week trial (RELEASE) Diabetes Obes Metab. 2017;19:1147–1154. doi: 10.1111/dom.12925. [DOI] [PubMed] [Google Scholar]
- 58.Nakamura Y., Hasegawa H., Tsuji M., et al. Linagliptin inhibits lipopolysaccharide-stimulated interleukin-6 production, intranuclear p65 expression, and p38 mitogen-activated protein kinase phosphorylation in human umbilical vein endothelial cells. Ren Replace Ther. 2016;2:17. [Google Scholar]
- 59.Sato N., Nakamura Y., Yamadera S., Inagaki M., Kenmotsu S., Saito H., et al. Linagliptin inhibits lipopolysaccharide-induced inflammation concentration-dependently and -independently. J Inflamm Res. 2019;12:285–291. doi: 10.2147/JIR.S221761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aono M., Sato Y. Dipeptidyl peptidase 4 inhibitor linagliptin can decrease the dosage of erythropoiesis-stimulating agents in patients on hemodialysis. Ren Replace Ther. 2016;2:44. [Google Scholar]
- 61.Younis A., Eskenazi D., Goldkorn R., Leor J., Naftali-Shani N., Fisman E.Z., et al. The addition of vildagliptin to metformin prevents the elevation of interleukin 1ß in patients with type 2 diabetes and coronary artery disease: a prospective, randomized, open-label study. Cardiovasc Diabetol. 2017;16:69. doi: 10.1186/s12933-017-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayrasheva V.K., Pchelin I.Y., Dobronravov V.A., Babenko A.Y., Chefu S.G., Shatalov I.S., et al. Short-term renal and metabolic effects of low dose vildagliptin treatment added-on insulin therapy in non-proteinuric patients with type 2 diabetes: open-label randomized prospective study. Arch Endocrinol Metab. 2020 Apr;6 doi: 10.20945/2359-3997000000220. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strózik A., Stęposz A., Basiak M., Drożdż M., Okopień B. Multifactorial effects of vildagliptin added to ongoing metformin therapy in patients with type 2 diabetes mellitus. Pharmacol Rep. 2015;67:24–31. doi: 10.1016/j.pharep.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 64.Zografou I., Sampanis C., Gkaliagkousi E., Iliadis F., Papageorgiou A., Doukelis P., et al. Effect of vildagliptin on hsCRP and arterial stiffness in patients with type 2 diabetes mellitus. Hormones (Athens) 2015;14:118–125. doi: 10.14310/horm.2002.1512. [DOI] [PubMed] [Google Scholar]
- 65.Derosa G., Maffioli P., Ferrari I., Mereu R., Ragonesi P.D., Querci F., et al. Effects of one year treatment of vildagliptin added to pioglitazone or glimepiride in poorly controlled type 2 diabetic patients. Horm Metab Res. 2010;42:663–669. doi: 10.1055/s-0030-1255036. [DOI] [PubMed] [Google Scholar]
- 66.Derosa G., Ragonesi P.D., Carbone A., Fogari E., Bianchi L., Bonaventura A., et al. Vildagliptin added to metformin on β-cell function after a euglycemic hyperinsulinemic and hyperglycemic clamp in type 2 diabetes patients. Diabetes Technol Ther. 2012;14:475–484. doi: 10.1089/dia.2011.0278. [DOI] [PubMed] [Google Scholar]
- 67.Choe E.Y., Cho Y., Choi Y., Yun Y., Wang H.J., Kwon O., et al. The effect of DPP-4 inhibitors on metabolic parameters in patients with type 2 diabetes. Diabetes Metab J. 2014;38:211–219. doi: 10.4093/dmj.2014.38.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim N.H., Kim D.L., Kim K.J., Kim N.H., Choi K.M., Baik S.H., et al. Effects of vildagliptin or pioglitazone on glycemic variability and oxidative stress in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a 16-week, randomised, open label, pilot study. Endocrinol Metab (Seoul) 2017;32:241–247. doi: 10.3803/EnM.2017.32.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyoshi T., Nakamura K., Yoshida M., Miura D., Oe H., Akagi S., et al. Effect of vildagliptin, a dipeptidyl peptidase 4 inhibitor, on cardiac hypertrophy induced by chronic beta-adrenergic stimulation in rats. Cardiovasc Diabetol. 2014;13:43. doi: 10.1186/1475-2840-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nomoto H., Kimachi K., Miyoshi H., Kameda H., Cho K.Y., Nakamura A., et al. Effects of 50 mg vildagliptin twice daily vs. 50 mg sitagliptin once daily on blood glucose fluctuations evaluated by long-term self-monitoring of blood glucose. Endocr J. 2017;64:417–424. doi: 10.1507/endocrj.EJ16-0546. [DOI] [PubMed] [Google Scholar]
- 71.Rizzo M.R., Barbieri M., Marfella R., Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35:2076–2082. doi: 10.2337/dc12-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, Shiraiwa T, Kosugi K, Umayahara Y, Yamamoto T, Yokoyama H, Kuribayashi N, Jinnouchi H, Gosho M, Shimomura I, Watada H; Collaborators on the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A) Trial. Alogliptin, a Dipeptidyl Peptidase 4 Inhibitor, Prevents the Progression of Carotid Atherosclerosis in Patients With Type 2 Diabetes: The Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care. 2016;39(1):139–148. [DOI] [PubMed]
- 73.Yisireyili M., Takeshita K., Hayashi M., Wu H., Uchida Y., Yamamoto K., et al. Dipeptidyl peptidase- IV inhibitor alogliptin improves stress-induced insulin resistance and prothrombotic state in a murine model. Psychoneuroendocrinology. 2016;73:186–195. doi: 10.1016/j.psyneuen.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Ta N.N., Schuyler C.A., Li Y., Lopes-Virella M.F., Huang Y. DPP-4 (CD26) inhibitor alogliptin inhibits atherosclerosis in diabetic apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2011;58:157–166. doi: 10.1097/FJC.0b013e31821e5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Q., Zhang S., Huang J., Liu K. Alogliptin inhibits IL-1β-induced inflammatory response in fibroblast-like synoviocytes. Int Immunopharmacol. 2020;83:106372. doi: 10.1016/j.intimp.2020.106372. [DOI] [PubMed] [Google Scholar]
- 76.Birnbaum Y., Bajaj M., Qian J., Ye Y. Dipeptidyl peptidase-4 inhibition by Saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res Care. 2016;4 doi: 10.1136/bmjdrc-2016-000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scirica B.M., Braunwald E., Raz I., Cavender M.A., Morrow D.A., Jarolim P., et al. Bhatt DL; SAVOR-TIMI 53 steering committee and investigators. Heart failure, saxagliptin, and diabetes mellitus:observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 78.Koska J., Osredkar T., D’Souza K., Sands M., Sinha S., Zhang W., et al. Effects of saxagliptin on adipose tissue inflammation and vascular function in overweight and obese people: a placebo-controlled study. Diabet Med. 2019;36:1399–1407. doi: 10.1111/dme.13889. [DOI] [PubMed] [Google Scholar]
- 79.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like Peptide-1. Cell Metab. 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Lee Y.S., Jun H.S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mei J., Sun J., Wu J., Zheng X. Liraglutide suppresses TNF-α-induced degradation of extracellular matrix in human chondrocytes: a therapeutic implication in osteoarthritis. Am J Transl Res. 2019;11:4800–4808. [PMC free article] [PubMed] [Google Scholar]
- 82.Shiraki A., Oyama J., Komoda H., Asaka M., Komatsu A., Sakuma M., et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 83.Zhang W.Q., Tian Y., Chen X.M., Wang L.F., Chen C.C., Qiu C.M. Liraglutide ameliorates beta-cell function, alleviates oxidative stress and inhibits low grade inflammation in young patients with new-onset type 2 diabetes. Diabetol Metab Syndr. 2018;10:91. doi: 10.1186/s13098-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen W.R., Shen X.Q., Zhang Y., Chen Y.D., Hu S.Y., Qian G., et al. Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction. Endocrine. 2016;52:516–526. doi: 10.1007/s12020-015-0798-0. [DOI] [PubMed] [Google Scholar]
- 85.Tian F, Zheng Z, Zhang D, He S, Shen J. Efficacy of liraglutide in treating type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease. Biosci Rep. 2018;38(6). [DOI] [PMC free article] [PubMed]
- 86.Liu Y., Jiang X., Chen X. Liraglutide and metformin alone or combined therapy for type 2 diabetes patients complicated with coronary artery disease. Lipids Health Dis. 2017;16:227. doi: 10.1186/s12944-017-0609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bouchi R., Nakano Y., Fukuda T., Takeuchi T., Murakami M., Minami I., et al. Reduction of visceral fat by liraglutide is associated with ameliorations of hepatic steatosis, albuminuria, and micro-inflammation in type 2 diabetic patients with insulin treatment: a randomized control trial. Endocr J. 2017;64:269–281. doi: 10.1507/endocrj.EJ16-0449. [DOI] [PubMed] [Google Scholar]
- 88.Varanasi A., Patel P., Makdissi A., Dhindsa S., Chaudhuri A., Dandona P. Clinical use of liraglutide in type 2 diabetes and its effects on cardiovascular risk factors. Endocr Pract. 2012;18:140–145. doi: 10.4158/EP11169.OR. [DOI] [PubMed] [Google Scholar]
- 89.Anholm C., Kumarathurai P., Pedersen L.R., Samkani A., Walzem R.L., Nielsen O.W., et al. Liraglutide in combination with metformin may improve the atherogenic lipid profile and decrease C-reactive protein level in statin treated obese patients with coronary artery disease and newly diagnosed type 2 diabetes: a randomized trial. Atherosclerosis. 2019;288:60–66. doi: 10.1016/j.atherosclerosis.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 90.Simeone P, Liani R, Tripaldi R, Di Castelnuovo A, Guagnano MT, Tartaro A, Bonadonna RC, Federico V, Cipollone F, Consoli A, Santilli F. Thromboxane-Dependent Platelet Activation in Obese Subjects with Prediabetes or Early Type 2 Diabetes: Effects of Liraglutide- or Lifestyle Changes-Induced Weight Loss. Nutrients. 2018;10(12). [DOI] [PMC free article] [PubMed]
- 91.Forst T., Michelson G., Ratter F., Weber M.M., Anders S., Mitry M., et al. Addition of liraglutide in patients with Type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet Med. 2012;29:1115–11158. doi: 10.1111/j.1464-5491.2012.03589.x. [DOI] [PubMed] [Google Scholar]
- 92.Courrèges J.P., Vilsbøll T., Zdravkovic M., Le-Thi T., Krarup T., Schmitz O., et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with Type 2 diabetes. Diabet Med. 2008;25:1129–1131. doi: 10.1111/j.1464-5491.2008.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou J.Y., Poudel A., Welchko R., Mekala N., Chandramani-Shivalingappa P., Rosca M.G., et al. Liraglutide improves insulin sensitivity in high fat diet induced diabetic mice through multiple pathways. Eur J Pharmacol. 2019;861:172594. doi: 10.1016/j.ejphar.2019.172594. [DOI] [PubMed] [Google Scholar]
- 94.Barreto-Vianna A.R.C., Aguila M.B., Mandarim-de-Lacerda C.A. Beneficial effects of liraglutide (GLP1 analog) in the hippocampal inflammation. Metab Brain Dis. 2017;32:1735–1745. doi: 10.1007/s11011-017-0059-4. [DOI] [PubMed] [Google Scholar]
- 95.Liu W., Yu J., Tian T., Miao J., Shang W. Meta-analysis of the efficacy of liraglutide in patients with type 2 diabetes accompanied by incipient nephropathy. Exp Ther Med. 2019;18:342–351. doi: 10.3892/etm.2019.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brock C., Hansen C.S., Karmisholt J., Møller H.J., Juhl A., Farmer A.D., et al. Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol. 2019;85:2512–2523. doi: 10.1111/bcp.14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eguchi Y., Kitajima Y., Hyogo H., Takahashi H., Kojima M., Ono M., et al. Anzai K; Japan Study Group for NAFLD (JSG-NAFLD). Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J) Hepatol Res. 2015;45:269–278. doi: 10.1111/hepr.12351. [DOI] [PubMed] [Google Scholar]
- 98.Ferdinand K.C., White W.B., Calhoun D.A., Lonn E.M., Sager P.T., Brunelle R., et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension. 2014;64:731–737. doi: 10.1161/HYPERTENSIONAHA.114.03062. [DOI] [PubMed] [Google Scholar]
- 99.Li H., Xu X., Wang J., Kong X., Chen M., Jing T., et al. A randomized study to compare the effects of once-weekly dulaglutide injection and once-daily glimepiride on glucose fluctuation of type 2 diabetes mellitus patients: a 26-week follow-up. J Diabetes Res. 2019;2019:6423987. doi: 10.1155/2019/6423987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu J.D., Xu X.H., Zhu J., Ding B., Du T.X., Gao G., et al. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13:143–148. doi: 10.1089/dia.2010.0048. [DOI] [PubMed] [Google Scholar]
- 101.Mafong D.D., Henry R.R. Exenatide as a treatment for diabetes and obesity: implications for cardiovascular risk reduction. Curr Atheroscler Rep. 2008;10:55–60. doi: 10.1007/s11883-008-0009-z. [DOI] [PubMed] [Google Scholar]
- 102.Varanasi A., Chaudhuri A., Dhindsa S., Arora A., Lohano T., Vora M.R., et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192–200. doi: 10.4158/EP10199.OR. [DOI] [PubMed] [Google Scholar]
- 103.Viswanathan P., Chaudhuri A., Bhatia R., Al-Atrash F., Mohanty P., Dandona P. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract. 2007;13:444–450. doi: 10.4158/EP.13.5.444. [DOI] [PubMed] [Google Scholar]
- 104.Bunck M.C., Diamant M., Eliasson B., Cornér A., Shaginian R.M., Heine R.J., et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care. 2010;33:1734–1737. doi: 10.2337/dc09-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chaudhuri A., Ghanim H., Vora M., Sia C.L., Korzeniewski K., Dhindsa S., et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97:198–207. doi: 10.1210/jc.2011-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi L., Zhu J., Yang P., Tang X., Yu W., Pan C., et al. Comparison of exenatide and acarbose on intra-abdominal fat content in patients with obesity and type-2 diabetes: a randomized controlled trial. Obes Res Clin Pract. 2017;11:607–615. doi: 10.1016/j.orcp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Du X., Zhang H., Zhang W., Wang Q., Wang W., Ge G., et al. The protective effects of lixisenatide against inflammatory response in human rheumatoid arthritis fibroblast-like synoviocytes. Int Immunopharmacol. 2019;75:105732. doi: 10.1016/j.intimp.2019.105732. [DOI] [PubMed] [Google Scholar]
- 108.Li X., Jia F., Zhu Z., Huang L. Lixisenatide attenuates advanced glycation end products (AGEs)-induced degradation of extracellular matrix in human primary chondrocytes. Artif Cells Nanomed Biotechnol. 2019;47:1256–1264. doi: 10.1080/21691401.2019.1593996. [DOI] [PubMed] [Google Scholar]
- 109.Newsome P., Francque S., Harrison S., Ratziu V., Van Gaal L., Calanna S., et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther. 2019;50:193–203. doi: 10.1111/apt.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodbard H.W., Rosenstock J., Canani L.H., Deerochanawong C., Gumprecht J., Lindberg S.Ø., et al. Montanya E; PIONEER 2 investigators. Oral Semaglutide versus Empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272–2281. doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 111.Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27–38. doi: 10.1016/j.cmet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 112.Katsiki N., Mikhailidis D.P., Theodorakis M.J. Sodium-glucose cotransporter 2 inhibitors (SGLT2i): their role in cardiometabolic risk management. Curr Pharm Des. 2017;23:1522–1532. doi: 10.2174/1381612823666170113152742. [DOI] [PubMed] [Google Scholar]
- 113.Yaribeygi H., Katsiki N., Butler A.E., Sahebkar A. Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov Today. 2019;24:256–262. doi: 10.1016/j.drudis.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Sawada T., Uzu K., Hashimoto N., Onishi T., Takaya T., Shimane A., et al. Empagliflozin’s ameliorating effect on plasma triglycerides: association with endothelial function recovery in diabetic patients with coronary artery disease. J Atheroscler Thromb. 2019 Oct;18 doi: 10.5551/jat.50807. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hattori S. Anti-inflammatory effects of empagliflozin in patients with type 2 diabetes and insulin resistance. Diabetol Metab Syndr. 2018;10:93. doi: 10.1186/s13098-018-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bosch A., Ott C., Jung S., Striepe K., Karg M.V., Kannenkeril D., et al. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc Diabetol. 2019;18:44. doi: 10.1186/s12933-019-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kabel A.M., Estfanous R.S., Alrobaian M.M. Targeting oxidative stress, proinflammatory cytokines, apoptosis and toll like receptor 4 by empagliflozin to ameliorate bleomycin-induced lung fibrosis. Respir Physiol Neurobiol. 2020;273:103316. doi: 10.1016/j.resp.2019.103316. [DOI] [PubMed] [Google Scholar]
- 118.Andreadou I., Efentakis P., Balafas E., Togliatto G., Davos C.H., Varela A., et al. Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front Physiol. 2017;8:1077. doi: 10.3389/fphys.2017.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ashrafi Jigheh Z., Ghorbani Haghjo A., Argani H., Roshangar L., Rashtchizadeh N., Sanajou D. Nazari Soltan Ahmad S, Rashedi J, Dastmalchi S, Mesgari Abbasi M. Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran J Basic Med Sci. 2019;22:384–390. doi: 10.22038/ijbms.2019.31788.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aragón-Herrera A., Feijóo-Bandín S., Otero Santiago M., Barral L., Campos-Toimil M., Gil-Longo J., et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol. 2019;170:113677. doi: 10.1016/j.bcp.2019.113677. [DOI] [PubMed] [Google Scholar]
- 121.Yaribeygi H., Butler A.E., Atkin S.L., Katsiki N., Sahebkar A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: possible molecular pathways. J Cell Physiol. 2018;234:223–230. doi: 10.1002/jcp.26851. [DOI] [PubMed] [Google Scholar]
- 122.Tan S.A., Tan L. Empagliflozin and canagliflozin attenuate inflammatory cytokines interferon-λ, tumor necrosis factor-α, interleukin-6: possible mechanism of decreasing cardiovascular risk in diabetes mellitus. J Am Coll Cardiol. 2018;71:A1830. [Google Scholar]
- 123.Ferrannini E., Murthy A.C., Lee Y.-H., Muscelli E., Weiss S., Ostroff R.M., et al. Mechanisms of sodium-glucose cotransporter-2 inhibition: insights from large-scale proteomics. Diabetes Care. 2020 doi: 10.2337/dc20-0456. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 124.Mazer C.D., Hare G.M.T., Connelly P.W., Gilbert R.E., Shehata N., Quan A., et al. Effect of empagliflozin on erythropoietin levels, Iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141:704–707. doi: 10.1161/CIRCULATIONAHA.119.044235. [DOI] [PubMed] [Google Scholar]
- 125.Kingir Z.B., Özdemir Kural Z.N., Cam M.E., Cilingir O.T., Şekerler T., Ercan F., et al. Effects of dapagliflozin in experimental sepsis model in rats. Ulus Travma Acil Cerrahi Derg. 2019;25:213–221. doi: 10.5505/tjtes.2018.82826. [DOI] [PubMed] [Google Scholar]
- 126.Leng W., Ouyang X., Lei X., Wu M., Chen L., Wu Q., et al. The SGLT-2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE(−/−) mice. Mediators Inflamm. 2016;2016:6305735. doi: 10.1155/2016/6305735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katsiki N., Papanas N., Mikhailidis D.P. Dapagliflozin: more than just another oral glucose-lowering agent? Expert Opin Investig Drugs. 2010;19:1581–1589. doi: 10.1517/13543784.2011.539558. [DOI] [PubMed] [Google Scholar]
- 128.Okamoto A., Yokokawa H., Sanada H., Naito T. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R D. 2016;16:255–261. doi: 10.1007/s40268-016-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tobita H., Sato S., Miyake T., Ishihara S., Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled study. Curr Ther Res Clin Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zainordin NA, Hatta SFWM, Mohamed Shah FZ, Rahman TA, Ismail N, Ismail Z, Abdul Ghani R. Effects of Dapagliflozin on Endothelial Dysfunction in Type 2 Diabetes With Established Ischemic Heart Disease (EDIFIED). J Endocr Soc. 2019;4(1):bvz017. [DOI] [PMC free article] [PubMed]
- 131.Latva-Rasku A., Honka M.J., Kullberg J., Mononen N., Lehtimäki T., Saltevo J., et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care. 2019;42:931–937. doi: 10.2337/dc18-1569. [DOI] [PubMed] [Google Scholar]
- 132.Dekkers C.C.J., Petrykiv S., Laverman G.D., Cherney D.Z., Gansevoort R.T., Heerspink H.J.L. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab. 2018;20:1988–1993. doi: 10.1111/dom.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aso Y., Kato K., Sakurai S., Kishi H., Shimizu M., Jojima T., et al. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int J Clin Pract. 2019;73 doi: 10.1111/ijcp.13335. [DOI] [PubMed] [Google Scholar]
- 134.Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, Chaudhuri A, Dandona P. Dapagliflozin Suppresses Hepcidin And Increases Erythropoiesis. J Clin Endocrinol Metab. 2020;105(4). [DOI] [PubMed]
- 135.Garvey W.T., Van Gaal L., Leiter L.A., Vijapurkar U., List J., Cuddihy R., et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32–37. doi: 10.1016/j.metabol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 136.Osonoi T., Gouda M., Kubo M., Arakawa K., Hashimoto T., Abe M. Effect of canagliflozin on urinary albumin excretion in Japanese patients with type 2 diabetes mellitus and microalbuminuria: a pilot study. Diabetes Technol Ther. 2018;20:681–688. doi: 10.1089/dia.2018.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sezai A., Sekino H., Unosawa S., Taoka M., Osaka S., Tanaka M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol. 2019;18:76. doi: 10.1186/s12933-019-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heerspink H.J.L., Perco P., Mulder S., Leierer J., Hansen M.K., Heinzel A., et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–1166. doi: 10.1007/s00125-019-4859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ali B.H., Al Salam S., Al Suleimani Y., Al Za’abi M., Abdelrahman A.M., Ashique M., et al. Effects of the SGLT-2 inhibitor canagliflozin on adenine-induced chronic kidney disease in rats. Cell Physiol Biochem. 2019;52:27–39. doi: 10.33594/000000003. [DOI] [PubMed] [Google Scholar]
- 140.Abdelrahman A.M., Al Suleimani Y., Shalaby A., Ashique M., Manoj P., Nemmar A., et al. Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on cisplatin-induced nephrotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:45–53. doi: 10.1007/s00210-018-1564-7. [DOI] [PubMed] [Google Scholar]
- 141.Koike Y., Shirabe S.I., Maeda H., Yoshimoto A., Arai K., Kumakura A., et al. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;149:140–146. doi: 10.1016/j.diabres.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 142.Maruyama T., Takashima H., Oguma H., Nakamura Y., Ohno M., Utsunomiya K., et al. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther. 2019;21:713–720. doi: 10.1089/dia.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Itani T., Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes Sci Pract. 2018;4:477–482. doi: 10.1002/osp4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seko Y., Nishikawa T., Umemura A., Yamaguchi K., Moriguchi M., Yasui K., et al. Efficacy and safety of canagliflozin in type 2 diabetes mellitus patients with biopsy-proven nonalcoholic steatohepatitis classified as stage 1-3 fibrosis. Diabetes Metab Syndr Obes. 2018;11:835–843. doi: 10.2147/DMSO.S184767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Athyros V.G., Polyzos S.A., Kountouras J., et al. Non-alcoholic fatty liver disease treatment in patients with type 2 diabetes mellitus; new kids on the block. Curr Vasc Pharmacol. 2020;18:172–181. doi: 10.2174/1570161117666190405164313. [DOI] [PubMed] [Google Scholar]
- 146.Katsiki N., Perakakis N., Mantzoros C. Effects of sodium-glucose co-transporter-2 (SGLT2) inhibitors on non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: ex quo et quo vadimus? Metabolism. 2019;98:iii–ix. doi: 10.1016/j.metabol.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 147.Katsiki N., Athyros V.G., Mikhailidis D.P. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: effects of statins and antidiabetic drugs. J Diabetes Complications. 2017;31:521–522. doi: 10.1016/j.jdiacomp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 148.Ranjbar G., Mikhailidis D.P., Sahebkar A. Effects of newer antidiabetic drugs on nonalcoholic fatty liver and steatohepatitis: think out of the box! Metabolism. 2019;101:154001. doi: 10.1016/j.metabol.2019.154001. [DOI] [PubMed] [Google Scholar]
- 149.Athyros VG, Alexandrides TK, Bilianou H, Cholongitas E, Doumas M, Ganotakis ES, Goudevenos J, Elisaf MS, Germanidis G, Giouleme O, Karagiannis A, Karvounis C, Katsiki N, Kotsis V, Kountouras J, Liberopoulos E, Pitsavos C, Polyzos S, Rallidis LS, Richter D, Tsapas AG, Tselepis AD, Tsioufis K, Tziomalos K, Tzotzas T, Vasiliadis TG, Vlachopoulos C, Mikhailidis DP, Mantzoros C. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism. 2017;71:17–32. [DOI] [PubMed]
- 150.Donath M.Y., Meier D.T., Böni-Schnetzler M. Inflammation in the pathophysiology and therapy of cardiometabolic disease. Endocr Rev. 2019;40:1080–1091. doi: 10.1210/er.2019-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bahceci M., Tuzcu A., Ogun C., Canoruc N., Iltimur K., Aslan C. Is serum C-reactive protein concentration correlated with HbA1c and insulin resistance in type 2 diabetic men with or without coronary heart disease? J Endocrinol Invest. 2005;28:145–150. doi: 10.1007/BF03345357. [DOI] [PubMed] [Google Scholar]
- 152.Ahmadi-Abhari S., Kaptoge S., Luben R.N., Wareham N.J., Khaw K.T. Longitudinal association of C-reactive protein and Haemoglobin A1c over 13 years: the European Prospective Investigation into Cancer—Norfolk study. Cardiovasc Diabetol. 2015;14:61. doi: 10.1186/s12933-015-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhan Y., Tang Z., Yu J. Serum ferritin, diabetes, diabetes control, and insulin resistance. Acta Diabetol. 2014;51:991–998. doi: 10.1007/s00592-014-0656-1. [DOI] [PubMed] [Google Scholar]
- 154.Batchuluun B., Matsumata T., Batchuluun B., et al. Serum ferritin level is higher in poorly controlled patients with type 2 diabetes and people without diabetes, aged over 55 years. Diabet Med. 2014;31:419–424. doi: 10.1111/dme.12343. [DOI] [PubMed] [Google Scholar]
- 155.Al-Shukaili A., Al-Ghafri S., Al-Marhoobi S., Al-Abri S., Al-Lawati J., Al-Maskari M. Analysis of inflammatory mediators in type 2 diabetes patients. Int J Endocrinol. 2013;2013:976810. doi: 10.1155/2013/976810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Minihane A.M., Vinoy S., Russell W.R., et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pedersen B.K. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47:600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- 158.Wang Z., Du Z., Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract. 2020;164:108214. doi: 10.1016/j.diabres.2020.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]