Clinical Practice Points.
-
•
Coronavirus disease 2019 (COVID-19) has spread worldwide; however, at present, no vaccines or drugs have been approved for its treatment.
-
•
According to population-based analyses, oncologic patients might have a greater risk of developing COVID-19 and poorer outcomes compared with those without cancer.
-
•
In the present report, we have described the case of a patient with non–small-cell lung cancer undergoing chemoimmunotherapy who developed COVID-19 pneumonia.
-
•
He was treated with tocilizumab and showed significant improvement after the first dose; non–acute side effects were observed.
-
•
More data from patients undergoing chemoimmunotherapy and affected by COVID-19 are required to draw definitive conclusions.
-
•
However, the clinical outcome for our patient suggests that tocilizumab can be safely used to treat COVID-19 pneumonia in patients undergoing chemoimmunotherapy.
Introduction
Since December 2019, the outbreak of a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing infection with coronavirus disease 2019 (COVID-19) has spread worldwide.1 The number of confirmed cases had reached 16,536,862, including 654,247 deaths, as of July 27, 2020.2 The case fatality rate (number of deaths in persons who tested positive for COVID-19 divided by the total number of COVID-19 cases) has been higher for the elderly and those with underlying comorbidities, such as diabetes, chronic respiratory disease, cardiovascular disease, and cancer.3, 4, 5 In addition, increasing evidence has suggested that SARS-CoV-2 infection can induce an aberrant host immune response associated with a “cytokine storm syndrome,” characterized by a massive release of proinflammatory cytokines, including interleukin-6 (IL-6), which has been associated with a worse prognosis.6 , 7
Tocilizumab is a recombinant humanized monoclonal antibody directed against the IL-6 receptor. It has been approved by the European Medicines Agency for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, and chimeric antigen receptor T-cell–induced cytokine release syndrome.8 Additionally, tocilizumab has shown efficacy in the treatment of steroid-refractory immune-related adverse events.9 , 10
However, limited data are available for critically ill patients treated with tocilizumab for COVID-19 pneumonia. In a prospective series of 100 consecutive patients admitted to the Spedali Civili University Hospital (Brescia, Italy) with COVID-19 pneumonia and respiratory failure requiring ventilatory support, treatment with tocilizumab was associated with a clinical benefit for more than three quarters of the patients.11 The findings from other observational studies have suggested that tocilizumab might be associated with better outcomes compared with standard care for patients with severe COVID-19. However, randomized clinical trials with larger sample sizes are required confirm this hypothesis.12 In addition, scarce information is available regarding cancer patients and COVID-19.
In the present report, we have described our experience with tocilizumab treatment for a patient with stage IV lung adenocarcinoma, who had developed COVID-19 pneumonia after his first cycle of chemoimmunotherapy.
Case Report
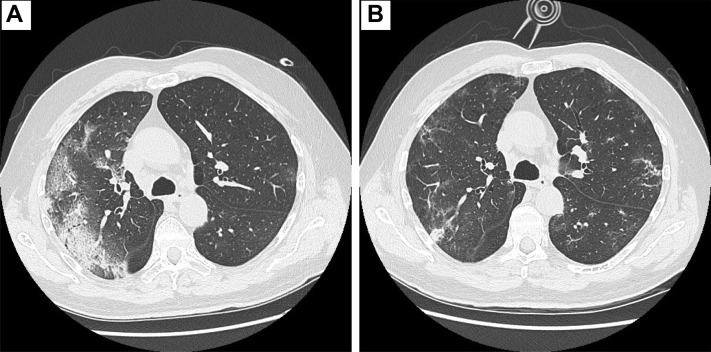
The patient was a 65-year-old man without major comorbidities. He had an Eastern Cooperative Oncology Group performance status of 1 and was a former smoker (15 pack-years). He had been diagnosed with stage IV lung adenocarcinoma (bilateral lung metastases) in February 2020. He had received a vaccination for seasonal influenza. Based on the radiologic stage and molecular profile (programmed cell death ligand 1, 2%; absence of ALK and ROS translocations, and EGFR and BRAF mutations), he started treatment with carboplatin, pemetrexed, and pembrolizumab on March 6, 2020. On March 13, 2020 (day 1 of admission), he had presented to our outpatient clinic reporting fever in the previous 24 hours and productive cough. On his initial physical examination, he was tachycardic (100 bpm) and febrile (40°C), with fine crackles heard at the right lung base. The arterial oxygen partial pressure/fractional inspired oxygen (P/F) ratio in room air was 328. Blood tests showed grade 3 leukopenia and neutropenia and elevation of C-reactive protein (CRP; Table 1 ). A chest radiograph did not demonstrate any signs of interstitial pneumonia. A nasopharyngeal swab for COVID-19 was performed. The patient was hospitalized, and treatment with granulocyte colony-stimulating factors and broad-spectrum empirical antibiotics was started. No steroids were administered. The results of reverse transcriptase polymerase chain reaction testing for C-19 nucleic acid were positive on day 2 (24 hours after admission). In accordance with hospital protocol, darunavir 800 mg/d, ritonavir 100 mg/d, and hydroxychloroquine 200 mg twice daily were prescribed. Despite ongoing therapy and oxygen supplementation with a reservoir mask at 15 L/min, his clinical condition did not improve. CRP had increased to 225 mg/L, and his IL-6 level, tested for the first time, was 101 ng/L (Table 1). The results from blood cultures and legionella and pneumococcal urinary antigen tests, performed at admission, were negative. The P/F ratio had decreased to 58 by day 7. A computed tomography (CT) scan showed areas of consolidation in the right and left lower lobes, with diffuse ground glass opacities and inter- and intralobular septal thickening (“crazy paving”; Figure 1A ). On days 8 and 9, the patient was given his first and second dose of tocilizumab (8 mg/kg weight every 12 hours) with immediate benefit seen. The P/F had increased to 100 the morning after the first dose, and in the subsequent days, the oxygen support was gradually decreased. The CRP level had decreased from 212 mg/dL to 37 mg/dL by 48 hours after the second dose (Table 1).
Table 1.
Laboratory Values∗
| Laboratory Test | Reference Range | Day 1 | Day 4 | Day 7 | Day 9 | Day 11 | Day 18 |
|---|---|---|---|---|---|---|---|
| WBC count, ×103/mm3 | 3.90-10.60 | 1.61 | 1.58 | 14.81 | 5730 | 5.58 | 4.37 |
| Lymphocyte count, ×103/mm3 | 0.70-5.00 | 0.58 | 0.21 | NA | 0.65 | 0.87 | 1.02 |
| Neutrophil count, ×103/mm3 | 1.50-8.00 | 0.88 | 1.21 | NA | 4.42 | 3.75 | 2.17 |
| Platelet count, ×103/mm3 | 150-400 | 104 | 37 | 167 | 197 | 251 | 233 |
| CPR, mg/L | 0.0-5.0 | 195 | 225.16 | 212 | 131 | 37 | 2.99 |
| Ferritin, ng/mL | 21-246 | NA | NA | 7864 | NA | NA | 1439 |
| IL-6, ng/L | 0.0-7.0 | NA | 101.6 | NA | NA | NA | 139.8 |
| LDH, U/L | <248 | NA | 276 | NA | NA | 454 | NA |
Abbreviations: CPR = C-reactive protein; IL-6 = interleukin-6; LDH = lactate dehydrogenase; NA = not available; TCZ = tocilizumab; WBC = white blood cell.
First dose of TCZ on day 8 and second dose of TCZ on day 9.
Figure 1.
Computed Tomography Scans (A) Before Tocilizumab Administration and (B) After Tocilizumab Administration
A CT scan performed after administration of tocilizumab demonstrated a reduction in the ground glass opacities (Figure 1B). The lung tumor was stable. The patient was discharged with a P/F ratio of 370 (in room air) on day 13. After a second CT scan had confirmed additional improvement in the ground glass opacities, the patient resumed chemoimmunotherapy without complications.
Discussion
Short-term treatment with tocilizumab proved to be safe in our patient who had been undergoing chemoimmunotherapy for stage IV lung adenocarcinoma. According to population-based analyses, oncologic patients, especially patients with lung cancer, might have a greater risk of developing COVID-19 and having poorer outcomes compared with individuals without cancer.3 , 13, 14, 15
In the era of long-term survivors treated with immune checkpoint inhibitors (ICIs) and targeted therapies, generalizations should be avoided. The immunologic status of a patient undergoing immunotherapy will be different from that of one treated with chemotherapy or targeted therapy.
Immunotherapy can act as a double-edged sword. On the one hand, patients undergoing immunotherapy might be more immunocompetent than those receiving chemotherapy and, therefore, have a lower risk of developing COVID-19–related complications. On the other hand, ICI-related immune hyperactivation could promote the onset of a “cytokine storm syndrome.”16 The association of ICIs with chemotherapy, such as in the present case, will further complicate the scenario. Furthermore, the potential overlap of COVID-19 pneumonia and ICI-associated lung damage represents another source of concern. In both situations, a predominant role will be played by the release of cytokines, including IL-6.6 , 17 By blocking the IL-6 receptor, tocilizumab can disrupt this cascade of events and restore the functionality of the alveoli.
The present report had several limitations. First, the patient had received concomitant therapies for both febrile neutropenia and COVID-19 (antiviral agents and hydroxychloroquine). Second, monitoring of all inflammatory indexes was not performed, although CRP could be considered a good surrogate for IL-6.17 Third, the long-term outcomes are not yet available. Nevertheless, the timeline of events has suggested the potential efficacy of tocilizumab.
Conclusion
More data and longer follow-up are needed for patients with cancer who are undergoing immunotherapy (with or without chemotherapy) and become infected with COVID-19 to draw definitive conclusions. However, the clinical outcome of our patient suggests that tocilizumab can be safely used to treat COVID-19 pneumonia in patients treated with ICIs.
Disclosure
R.P. has received funds from Amgen, Astellas, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Novartis, sanofi-aventis, Roche, MSD, and Pierre Fabre for attending advisory boards or symposia and from Amgen and Pierre Fabre for funding research projects. The remaining authors declare that they have no competing interests.
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometer COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/ Available at: Accessed July 27, 2020.
- 3.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tocilizumab product information. https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_en.pdf Available at: Accessed July 27, 2020.
- 9.Horisberger A., La Rosa S., Zurcher J.-P., et al. A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J Immunother Cancer. 2018;6:156. doi: 10.1186/s40425-018-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroud C.R., Hegde A., Cherry C., et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25:551–557. doi: 10.1177/1078155217745144. [DOI] [PubMed] [Google Scholar]
- 11.Toniati P., Piva S., Cattalini M., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortegiani A., Ippolito M., Greco M., et al. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. https://doi.org/10.1016/j.pulmoe.2020.07.003 [e-pub ahead of print]. Pulmonology. accessed July 27, 2020. [DOI] [PMC free article] [PubMed]
- 13.Garassino M.C., Whisenant J.G., Huang L.-C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269–273. doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke W., Zhang L., Dai Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs) Thorac Cancer. 2020;11:835–839. doi: 10.1111/1759-7714.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]