To the Editor: The COVID-19 pandemic turned attention to how immune-targeted therapies affect respiratory tract infections (RTIs). We reported meta-estimates of the risk of RTI associated with biologics that target interleukin (IL) 17 (odds ratio [OR], 1.56; 95% confidence interval [CI], 1.04-2.33)1 and IL-23 (OR, 1.24; 95% CI, 0.98-1.56)2 based on publicly available pivotal trial data. We now evaluate tumor necrosis factor inhibitors (TNFi) using a similar approach. TNF-α plays an important role in defense against viral infection, possibly through lysis of virus-infected cells and/or induction of an antiviral state in normal cells. In contrast, some models suggest that TNF may mediate significant tissue damage in RTIs.3 Despite extensive studies of TNF inhibitors over the past 2 decades, there are limited data on the effect of these biologics on the risk of RTIs.
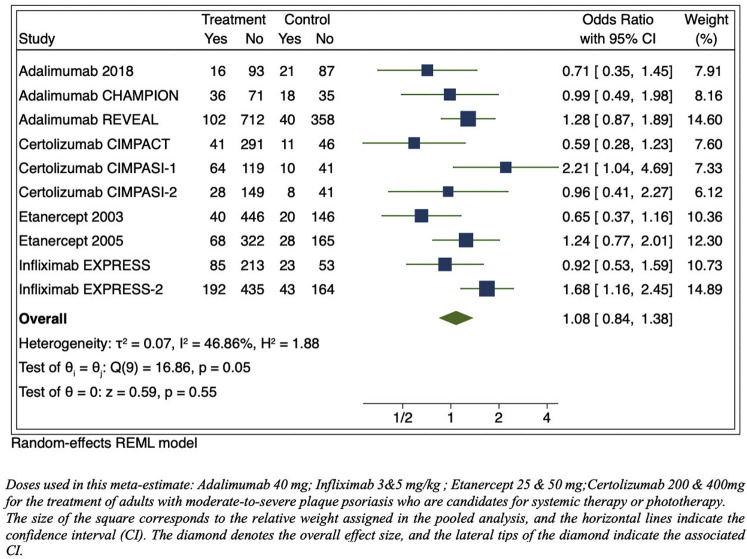
To rapidly assess the risk of RTI associated with TNFi, terms consistent with RTI were evaluated from data reported in publications of US Food and Drug Administration–approved, phase 3, placebo-controlled clinical trials listed in the prescribing information for adalimumab, infliximab, etanercept, and certolizumab. This data source was used because most trials were conducted before the initiation of clinicaltrials.gov. RTI events were summed and divided by the total number of individuals at risk in each study and compared to the placebo group by a meta-estimate. A significant increased risk of RTI was not observed in TNFi compared to placebo (OR, 1.08; 95% CI, 0.84-1.38; P = .55) (Fig 1 ). The events reported in our primary analysis used varying drug dosages. In our secondary analysis, we limited the exposure to only US Food and Drug Administration–approved dosing regimens and found similar results (OR, 1.06; 95% CI, 0.81-1.40; P = .66) (Fig 2 ). Sensitivity analyses were conducted, combining drugs with similar mechanisms of action and structure (ie, adalimumab and infliximab), which yielded similar results (OR, 1.14; 95% CI, 0.86-1.51; P = .36). We also evaluated certolizumab individually because its clinical trials occurred more recently, but the results were similar (OR, 1.18; 95% CI, 0.50-2.79; P = .70).
Fig 1.
Meta-estimate of respiratory tract infections from publications of US Food and Drug Administration–approved dosages of phase 3 pivotal trials adverse events tables (includes “upper respiratory tract infections,” “nasopharyngitis,” “rhinitis,” “rhinorrhea,” “pneumonia,” bronchitis,” “sinusitis,” “pharyngitis,” “flu syndrome,” and “cough”). CI, Confidence interval; REML, restricted maximum likelihood.
Fig 2.
Meta-estimate of respiratory tract infections from publications of US Food and Drug Administration–approved dosages of phase 3 pivotal trials adverse events tables (includes “upper respiratory tract infections,” “nasopharyngitis,” “rhinitis,” “rhinorrhea,” “pneumonia,” “bronchitis,” “sinusitis,” “pharyngitis,” “flu syndrome,” and “cough”). CI, Confidence interval; REML, restricted maximum likelihood.
In conclusion, we found no evidence of an increased risk of RTI in pivotal trials of TNFi in psoriasis. Caution is advised in comparing these results to our prior analyses of biologics targeting IL-17 and IL-23 because of statistical imprecision, different populations, and time periods studied; therefore, one should not necessarily conclude that TNFi are more or less safe than biologics targeting IL-17 and IL-23 with respect to risk of RTI and COVID-19. Furthermore, we could not estimate viral RTI specifically because objective confirmatory testing was not reported. Nevertheless, the findings are reassuring, and recent data suggest that TNFi are associated with a 60% reduction in the risk of hospitalization for patients with rheumatism infected with severe acute respiratory syndrome coronavirus 2, possibly related to a TNFi-suppressing cytokine storm.4 However, these data are derived from spontaneous case reports and should be interpreted with caution. TNFi are currently being tested in clinical trials of patients with COVID-19.5 Trial data and large-scale, prospective cohort studies are urgently needed to better define the impact of TNFi on infections with severe acute respiratory syndrome coronavirus 2 and outcomes from COVID-19 illness.
Footnotes
Funding sources: Supported in part by a grant (P30AR069589-03) from the National Institutes of Health (to Dr Gelfand).
Disclosure: Dr Syed is supported in part by a grant from Pfizer. Dr Shah is supported by a National Institutes of Health–funded T-32 postdoctoral fellowship. Dr Wan is supported in part by a grant from Pfizer. Dr Winthrop receives grants from Bristol Myers Squibb and Pfizer and is a consultant for UCB, AbbVie, Lilly, Bristol Myers Squibb, Pfizer, GlaxoSmithKline, and Roche. Dr Gelfand served as a consultant for Bristol Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Janssen Biologics, Novartis Corp, Regeneron, UCB (data safety and monitoring board), Sanofi, and Pfizer, receiving honoraria; receives research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Janssen, Novartis, Sanofi, Celgene, Ortho Dermatologics, and Pfizer; has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Company and Ortho Dermatologics; is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma; and is a deputy editor for the Journal of Investigative Dermatology, receiving honoraria from the Society for Investigative Dermatology. Dr Shin has no conflicts of interest to declare.
IRB approval status: Not applicable.
References
- 1.Wan M.T., Shin D.B., Winthrop K.L., Gelfand J.M. The risk of respiratory tract infections and symptoms in psoriasis patients treated with IL-17-pathway inhibiting biologics: a meta-estimate of pivotal trials relevant to decision-making during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:677–679. doi: 10.1016/j.jaad.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syed M.N., Shin D.B., Wan M.T., Winthrop K.L., Gelfand J.M. The risk of respiratory tract infections in psoriasis patients treated with IL-23-pathway inhibiting biologics: a meta-estimate of pivotal trials relevant to decision-making during the COVID-19 pandemic. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.06.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann M., Maini R.N., Woody J.N., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci. 2020;41:363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]