Abstract
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors represent the standard of care in patients with EGFR mutation-positive (EGFRm+) non-small cell lung cancer (NSCLC). The availability of several EGFR tyrosine kinase inhibitors approved for use in the first-line or later settings in NSCLC warrants an in-depth understanding of the pharmacological properties of, and clinical data supporting, these agents. The second-generation, irreversible ErbB-family blocker, afatinib, has been extensively studied in the context of EGFRm+ NSCLC. Results from the LUX-Lung 3 and 6 studies showed that afatinib was more active and better tolerated than chemotherapy in patients with tumors harboring EGFR mutations. Subanalysis of these trials, along with real-world data, indicates that afatinib is active in patients with certain uncommon EGFR mutations (S768I/G719X/L861Q) as well as common mutations (Del19/L858R), and in patients with active brain metastases. In LUX-Lung 7, a head-to-head phase IIb trial, afatinib improved progression-free survival and time-to-treatment failure versus the first-generation reversible EGFR tyrosine kinase inhibitor, gefitinib, albeit with a higher incidence of serious treatment-related adverse events. Nevertheless, afatinib is generally well tolerated, and adverse events are manageable through supportive care and a well-defined tolerability-guided dose adjustment scheme. In this review, we provide a detailed overview of the pharmacology, efficacy, and safety of afatinib, discuss treatment sequencing strategies following emergence of different resistance mechanisms, and shed light on the economic impact of afatinib. We also provide a comparison of afatinib with the available EGFR tyrosine kinase inhibitors and discuss its position within treatment strategies for patients with EGFRm+ NSCLC.
Keywords: Afatinib, EGFR mutation, non-small cell lung cancer, tyrosine kinase inhibitor
Introduction
Lung cancer is the most common cancer in the US, with ∼228,000 estimated new cases of lung or bronchus cancer in 2020.1 It is also the most common cause of cancer-related deaths, with more than 135,000 people estimated to die from the disease in 2020.1 Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases.2 Recent advances in tumor molecular analysis have facilitated testing for possible genetic mutations and aberrations that drive tumor growth and proliferation. Numerous oncogenic drivers have been identified in NSCLC, including mutations in the genes encoding the epidermal growth factor receptor (EGFR), KRAS, and anaplastic lymphoma kinase (ALK), among others.2–4 Identification of abnormalities in these molecular pathways has prompted the development of agents with the aim of targeting specific components of these pathways.
EGFR is a member of the ErbB family of receptor tyrosine kinases, which includes EGFR (ErbB1, HER1), HER2 (ErbB2, Neu), HER3 (ErbB3), and HER4 (ErbB4).5,6 The tyrosine kinase activity of EGFR triggers numerous cellular signaling pathways that regulate growth, proliferation, and survival.6 EGFR is a well-established molecular target in NSCLC, and mutations have been identified in 10–15% of Caucasian and up to 50% of Asian patients with adenocarcinoma, the most frequent NSCLC subtype.2,6 The most common EGFR mutations are deletions in exon 19 (Del19) and the point mutation L858R in exon 21, which together account for 85–90% of all presenting EGFR mutations.7 Numerous uncommon mutations within exons 18–21 of the EGFR gene, including S786I, G719X, and L861Q, have also been identified in NSCLC tumors.8 These activating mutations in EGFR lead to increased signaling downstream of the receptor, resulting in cellular growth and proliferation, and driving tumor development by promoting metastatic spread and resistance to apoptotic signals.6,9 The presence of EGFR mutations that drive tumorigenesis makes EGFR an attractive therapeutic target.
Advances in EGFR mutation testing
Advances in molecular screening of tumors have improved patient outcomes by allowing the development and use of targeted therapeutics. Initial tumor testing for EGFR or other mutations is routinely undertaken using direct biopsy of the tumor10,11; however, around 30% of NSCLC patients may be unable to provide a biopsy sample that is suitable for EGFR mutation analysis at diagnosis or following disease progression.11–13 In addition, tissue biopsies, particularly re-biopsies at the point of progression on initial treatment, are invasive and costly, and therefore not always feasible.10,11,14–16 Given these drawbacks, the less invasive liquid biopsy may be preferred over traditional tumor biopsy, particularly in monitoring for resistance.11
The use of liquid biopsies, which may be obtained from blood, saliva, or urine, provides a minimally invasive method for collecting cell-free circulating tumor DNA and circulating tumor cells.17,18 This approach allows for regular testing of patients at various stages of treatment, for efficacy monitoring and real-time identification of potential new mutations.15,18,19 Liquid biopsies have been tested in the context of EGFR mutation-positive (EGFRm+) NSCLC and can be used for detection of Del19 and L858R.10,14,15,20,21 EGFR mutations detected in plasma and urine have been shown to be concordant with those identified by tumor biopsy, supporting the use of liquid biopsies as a screening tool.10,14,22 In addition, use of liquid biopsies from plasma to monitor tumor progression and identify EGFR mutations associated with resistance to therapy (e.g., acquired T790M) has been documented, with such biopsies showing 60–80% sensitivity and specificity approaching 100% in detecting specific resistance mutations.15,16,21,23–25
EGFR tyrosine kinase inhibitors
EGFR-driven tumors become dependent on EGFR signaling for growth and survival, a phenomenon known as oncogene addiction.5,26 Oncogene addiction postulates that some tumors become dependent on a single oncogene for growth and survival, such that inhibition of this oncogene is sufficient to block tumor growth and induce tumor regression.26 Consequently, EGFRm+ tumors are particularly susceptible to treatments that target the EGFR pathway.9
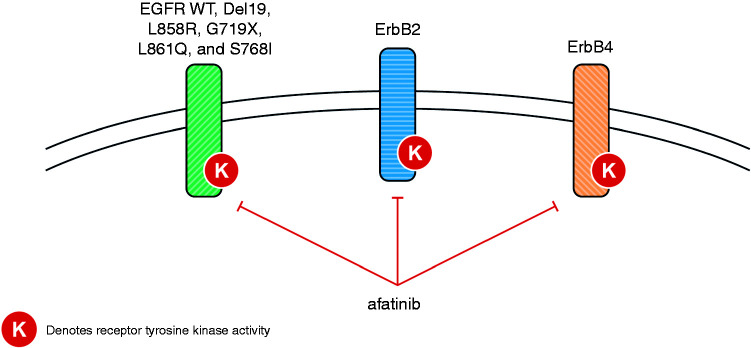
Three generations of EGFR tyrosine kinase inhibitors (TKIs) have been developed and are available for clinical use. The first-generation TKIs, gefitinib and erlotinib, are reversible inhibitors of both wild-type and common EGFR mutations,27,28 and have shown improved efficacy and tolerability compared with chemotherapy in numerous phase III trials (gefitinib: IPASS, WJTOG3405, NEJ002; erlotinib: EURTAC, OPTIMAL, ENSURE).29–34 In two head-to-head trials of gefitinib versus erlotinib (CTONG0901 and WJOG5108L), there were no significant differences between the two first-generation agents with regard to duration of progression-free survival (PFS), objective response rate (ORR), or overall survival (OS) in any line of treatment.35,36 The second-generation EGFR TKIs include afatinib and dacomitinib, which are irreversible inhibitors of wild-type and mutant EGFR, ErbB2, and ErbB4, thereby inhibiting signaling from all possible homo- or hetero-dimers of ErbB family receptors (Figure 1).27,37,38 This includes ErbB3-containing heterodimers, as the second-generation agents prevent the trans-phosphorylation of the ErbB3 receptor and subsequent signal transduction.27,37,38 When compared with chemotherapy, first-line afatinib has demonstrated significant improvements in PFS for patients with EGFRm+ NSCLC, and in OS for patients with Del19-positive disease.39,40 Compared in head-to-head trials, both afatinib and dacomitinib have shown significantly improved PFS versus gefitinib.41,42 With regard to OS in the same trials, there was no statistically significant difference with afatinib versus gefitinib, and exploratory analysis demonstrated prolonged OS with dacomitinib versus gefitinib.43,44 The third-generation TKI, osimertinib, selectively inhibits both EGFR-TKI-sensitizing and EGFR T790M resistance mutations, with lower activity against wild-type EGFR.13 The “gatekeeper” T790M mutation is a well-characterized mechanism of acquired resistance to first- and second-generation TKIs, which has been reported to occur in >60% of cases.45–47 Osimertinib has shown robust efficacy in patients who have progressed on first- or second-generation TKIs.47,48 In addition, in the recent FLAURA head-to-head trial, first-line treatment with osimertinib led to superior PFS and OS compared with a first-generation TKI (gefitinib or erlotinib).13,49
Figure 1.
Inhibition of ErbB family members, including WT and mutant EGFR, by afatinib. EGFR: epidermal growth factor receptor; WT: wild-type.
EGFR TKIs represent the standard of care for first-line treatment of EGFRm+ NSCLC; gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib have all been approved by the Food and Drug Administration (FDA) in this setting.50–54 The availability of multiple EGFR TKIs with distinct pharmacological, efficacy, and safety profiles raises the question of which one should be used at which stage in the treatment sequence, and also highlights the need to identify an optimal sequencing strategy for each of the TKIs, in order to achieve long-term treatment benefit and optimal survival. In this review, we will focus on the second-generation TKI, afatinib, providing an in-depth overview of its pharmacology, efficacy, and safety. We will discuss the use of afatinib in clinical settings, particularly in patients with NSCLC harboring common and uncommon EGFR mutations, patients with central nervous system (CNS) metastases, and in real-world settings. Furthermore, we will provide insight into the use of sequential first-line afatinib and second-line osimertinib.
Afatinib pharmacokinetics and interactions
Afatinib (BIBW 2992; N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide) is an adenosine triphosphate (ATP)-competitive anilinoquinazoline derivative containing a reactive acrylamide group.27 Afatinib is administered orally, at a recommended dose of 40 mg once daily, with peak plasma concentrations achieved 2–5 h post-dose.50 Administration after a high-fat meal results in an approximately 40% reduction in the area under the time–concentration curve from time 0 to infinity (414 ng · h/ml fed; 676 ng · h/ml fasted)55; consequently, afatinib should be taken on an empty stomach, at least 1 h before or 2 h after eating.50 The elimination half-life of afatinib is 37 h following repeat dosing,50,56 and steady-state plasma concentrations are achieved within 8 days of multiple dosing.50,55–57
Excretion occurs primarily via the feces (85%), with the parent compound representing 89% of the recovered dose.58 Moderate-to-severe renal impairment has been shown to have a minor influence on afatinib pharmacokinetics.59 Afatinib treatment can be considered without the need for starting dose modifications in patients with mild or moderate renal impairment, although a starting dose of 30 mg/day is recommended in patients with severe renal impairment.50 Close monitoring of patients with severe renal impairment is advised, and dose adjustment is recommended if tolerability issues arise.59 No afatinib starting dose adjustments are needed for patients with mild or moderate hepatic impairment.60 However, afatinib has not been studied in patients with severe hepatic impairment, and these patients should be closely monitored and the dose adjusted, if needed.50
Afatinib is highly soluble across the physiological pH range of 1–7.5 and is not expected to interact with acid-reducing agents.61,62 This is in contrast to gefitinib, erlotinib, and dacomitinib, which exhibit pH-dependent solubility, such that absorption and bioavailability may be decreased when co-administered with treatments that increase gastric pH (e.g., histamine H2-receptor antagonists and proton pump inhibitors).61 In vitro studies indicate that plasma protein–binding of afatinib is high (∼94%).60 However, enzyme-catalyzed metabolism of afatinib plays a negligible role in its metabolism in vivo.61 Unlike other EGFR TKIs, which undergo metabolism via cytochrome P450 (CYP) enzymes, CYP enzymes play a negligible role in the metabolism of afatinib.61 Smoking status does not impact the pharmacokinetics of afatinib,62 whereas for erlotinib, smoking reduces exposure.61,63 Moreover, afatinib does not relevantly inhibit or induce CYP enzymes, including those involved in drug metabolism.62 Therefore, the risk of interactions between afatinib and co-administered drugs that undergo CYP enzyme metabolism is thought to be minimal.62 In contrast, other EGFR TKIs interact with different CYP enzymes to varying extents, potentially impacting the metabolism of concomitant drugs.51,61
Afatinib is a substrate and inhibitor of P-glycoprotein in vitro, and concomitant use of strong P-glycoprotein inhibitors can increase exposure to afatinib.62,64 Interestingly however, concomitant administration of ritonavir, a well-known, potent inhibitor of P-glycoprotein and BCRP (another ATP binding cassette drug transporter inhibitor) has shown no relevant impact on afatinib pharmacokinetics in healthy male adults.62,64 P-glycoprotein inducers such as rifampicin can reduce afatinib exposure.50,62,64 In vitro, afatinib is both a substrate and inhibitor of BCRP,50 and gefitinib, erlotinib, and osimertinib also function as inhibitors and/or substrates of P-glycoprotein and/or BCRP to varying degrees.51,53,61,65
Afatinib efficacy and safety data from clinical trials
Afatinib versus chemotherapy: LUX-Lung 3 and LUX-Lung 6
Afatinib was approved for the first-line treatment of metastatic NSCLC harboring non-resistant EGFR mutations based primarily on the outcomes of the global phase III LUX-Lung 3 trial that compared first-line afatinib with cisplatin/pemetrexed in 345 patients with advanced EGFRm+ adenocarcinoma.39 LUX-Lung 6 was another phase III trial that compared first-line afatinib with gemcitabine/cisplatin in 364 Asian patients with EGFRm+ NSCLC.40
In both trials, which included patients with NSCLC harboring common and/or uncommon EGFR mutations, afatinib treatment resulted in improved median PFS versus chemotherapy: LUX-Lung 3 (afatinib vs cisplatin/pemetrexed): 11.1 versus 6.9 months; hazard ratio (HR), 0.58 (95% confidence interval (CI), 0.43–0.78), P = 0.001; LUX-Lung 6 (afatinib vs gemcitabine/cisplatin): 11.0 versus 5.6 months; HR, 0.28 (95% CI, 0.20–0.39), P < 0.0001.39,40 While OS analysis of the two studies, as well as both studies combined, indicated no significant OS improvement with afatinib versus chemotherapy (LUX-Lung 3/6 combined: 25.8 vs 24.5 months; HR, 0.91 (95% CI, 0.75–1.11), P = 0.37), a pre-specified subgroup analysis demonstrated that afatinib prolonged OS versus chemotherapy in patients with Del19-positive tumors, in both LUX-Lung 3 (33.3 vs 21.1 months; HR, 0.54 (95% CI, 0.36–0.79), P = 0.0015) and LUX-Lung 6 (31.4 vs 18.4 months; HR, 0.64 (95% CI, 0.44–0.94), P = 0.023).66 It appears that not all EGFR TKIs confer the same OS benefits; in a meta-analysis comparing patients with Del19-positive NSCLC who were treated with erlotinib, gefitinib, or afatinib, only afatinib was associated with a statistically significant OS benefit versus chemotherapy.67
Consistent with the results observed with other EGFR TKIs,31,32 afatinib was better tolerated than chemotherapy in both studies.39,40 The most common adverse events (AEs) were class-related: gastrointestinal (diarrhea, stomatitis) and cutaneous (rash/acne).39,40,68 The discontinuation rate for afatinib due to treatment-related AEs was low, despite patients being on treatment longer with afatinib than with chemotherapy (LUX-Lung 3: 8% versus 12%; LUX-Lung 6: 6% versus 40%, with afatinib and chemotherapy, respectively; Table 1).39,40
Table 1.
Incidence of AEs leading to dose reduction and treatment discontinuation, and the most common afatinib-related AEs in the major afatinib clinical trials.a
| LL339 | LL640 | LL742 | LL884 | |
|---|---|---|---|---|
| Patients with dose reductions due to AEs, % | 52.2c | 28.0c | 41.9 | 26.5 |
| Patients discontinuing treatment due to afatinib-related AEs, % | 8.0 | 5.9 | 6.3 | 20.2d |
| Incidence of most common afatinib-related AEs (all grades/grade ≥3), % | ||||
| Diarrheab | 95.2/14.4 | 88.3/5.4 | 90.0/12.5 | 69.9/10.5 |
| Rash/acneb | 89.1/16.2 | 80.8/14.6 | 88.8/9.0 | 67.1/5.9 |
| Stomatitisb | 72.1/8.7 | 51.9/5.4 | 64.4/4.0 | 28.8/4.1 |
AEs: adverse events; LL: LUX-Lung.
aAll tumors in LL3 and LL6 were adenocarcinomas, 99% of tumors were of purely adenocarcinoma histology in LL7, and 96% of tumors were of purely squamous histology in LL8.
bGrouped term.
cAll dose reductions (reason not specified).
dIncludes any-cause AEs.
Afatinib versus gefitinib: LUX-Lung 7
The phase IIb LUX-Lung 7 study was the first global head-to-head comparison of a first-generation EGFR TKI (gefitinib) and a second-generation EGFR TKI (afatinib) in patients with EGFRm+ NSCLC.42 The three co-primary endpoints of the study were PFS, OS, and time-to-treatment failure (TTF). As an endpoint, TTF captures patients who are continued on treatment beyond trial-defined radiological progression in the absence of clinical deterioration. Afatinib significantly improved PFS (median 11.0 vs 10.9 months; HR, 0.73 (95% CI, 0.57‒0.95), P = 0.017) and TTF (median 13.7 vs 11.5 months; HR, 0.73 (95% CI, 0.58‒0.92), P = 0.007) versus gefitinib. PFS was also numerically longer with afatinib across most patient subgroups investigated, including EGFR mutation type (Del19 or L858R).42 Afatinib also significantly improved ORR versus gefitinib (70% vs 56%; P = 0.0083), with a longer median duration of response (10.1 months (interquartile range (IQR), 5.6–16.8) vs 8.4 months (IQR, 6.2–13.1)), and a trend towards improved OS with afatinib vs gefitinib observed (median 27.9 vs 24.5 months; HR, 0.86 (95% CI, 0.66‒1.12), P = 0.258).43
The overall frequency of grade ≥3 treatment-related AEs was higher with afatinib than with gefitinib (31.3% vs 17.6%). Grade ≥3 diarrhea, rash/acne, and stomatitis were more frequent with afatinib, while elevated alanine aminotransferase/aspartate aminotransferase was more common with gefitinib.42 Serious treatment-related AEs were reported in 11% of afatinib-treated and 4% of gefitinib-treated patients; however, discontinuations due to treatment-related AEs were similar between treatment arms (6% in each arm).42
Afatinib in patients with uncommon EGFR mutations
Uncommon mutations within exons 18–21 are present in up to 23% of EGFRm+ NSCLC tumors.69–75 While most phase III studies of EGFR TKIs in EGFRm+ NSCLC included only patients with tumors harboring common (Del19/L858R) EGFR mutations,29–34 the LUX-Lung 2, 3, and 6 studies included patients with uncommon mutations.75 In a pooled analysis of patients with uncommon mutations (n = 75 across the three studies), afatinib showed efficacy in a subgroup of patients with tumors harboring the G719X, L861Q, and S768I EGFR mutations, as well as other, rarer mutations, but not against tumors harboring T790M or exon 20 insertions.75 Median PFS (95% CI) was 13.8 months (6.8–not evaluable) for patients with tumors harboring G719X, 8.2 months (4.5–16.6) for L861Q, and 14.7 months (2.6–not evaluable) for S768I. Median OS (95% CI) was 26.9 months (16.4–not evaluable), 17.1 months (15.3–21.6), and not evaluable (3.4–not evaluable), and ORR was 78%, 56%, and 100%, for patients with NSCLC harboring these three uncommon mutations, respectively. The European Medicines Agency indicated afatinib for the first-line treatment of NSCLC with any activating EGFR mutation on first approval in 2013.76 These results from LUX-Lung 2, 3, and 6 led to the expansion of the FDA label to include patients with metastatic NSCLC harboring non-resistant mutations, including G719X, L861Q, and S768I.50
Afatinib in patients with baseline brain metastases
Brain metastases are common in patients with NSCLC, occurring in around 25% of patients during the course of their disease.77 The incidence of brain metastases is higher among patients with EGFRm+ NSCLC than in patients whose tumors have wild-type EGFR (31.4 vs 19.7% in one report, P < 0.001).77 However, patients with brain metastases have been excluded from clinical trials.41 This is of particular significance as it limits the ability to generalize the outcomes of such clinical trials to patients in the clinic. Although little is known about the brain penetrance of afatinib in humans, preclinical pharmacology studies in rats suggest low distribution to the brain.78 Nevertheless, several clinical studies with afatinib and osimertinib, including head-to-head trials with first-generation TKIs, have included patients with EGFRm+ NSCLC with CNS involvement, which has shed light on the effect of these TKIs on patients with CNS metastases.
In the LUX-Lung 3, 6, and 7 trials, the inclusion criteria allowed recruitment of patients with asymptomatic and stable brain metastases.39,40,42 Baseline brain metastases were present in 12%, 13%, and 16% of patients in LUX-Lung 3, 6, and 7, respectively.42,79 In a combined analysis of the LUX-Lung 3 and 6 studies, 81 patients with brain metastases at baseline (afatinib: n = 48; chemotherapy: n = 33) showed improved PFS (8.2 vs 5.4 months; HR, 0.50 (95% CI, 0.27–0.95), P = 0.0297) and ORR (combined analysis: 73% vs 24%; LUX-Lung 3: 70% vs 20%, p = 0.0058; LUX-Lung 6: 75% vs 28%, P = 0.0027) with afatinib compared with chemotherapy, while there was no improvement in OS (22.4 vs 25.0 months; HR, 1.14 (95% CI, 0.66–1.94), P = 0.6412).79 Improvements in PFS in patients with brain metastases were similar to those observed among patients without brain metastases. In LUX-Lung 7, 50 patients with brain metastases (afatinib: n = 26; gefitinib: n = 24) showed similar PFS improvement between the two treatments (afatinib vs gefitinib: 7.2 vs 7.4 months; HR, 0.76 (95% CI, 0.41–1.44)) and also compared with patients without brain metastases.42
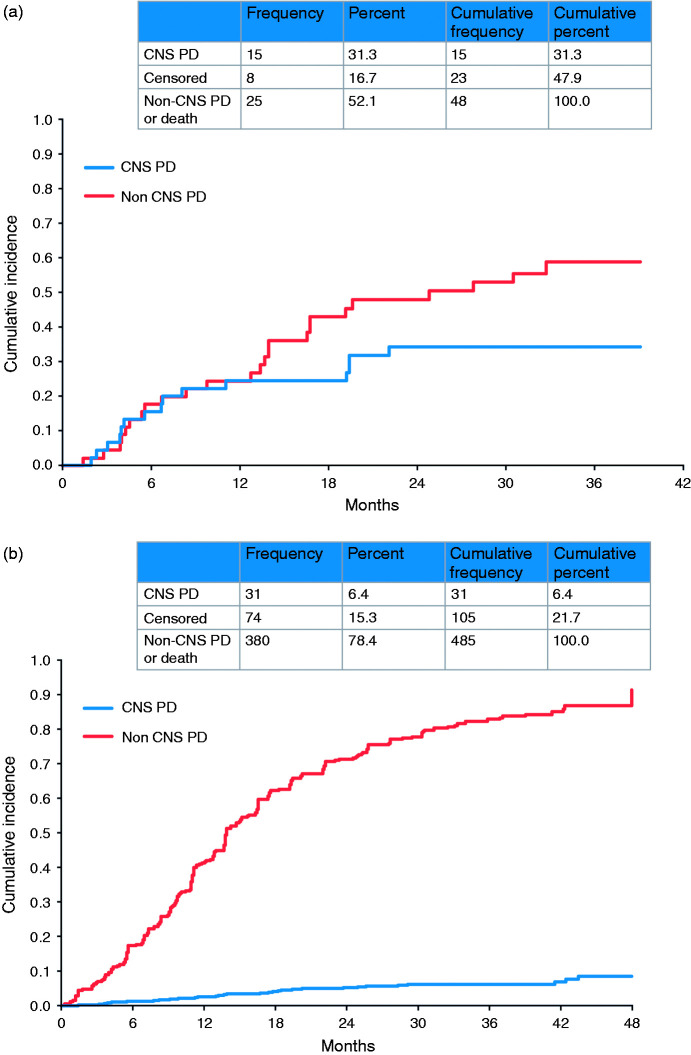
Competing risk analyses of LUX-Lung 3 and 6 showed that in patients with baseline brain metastases the cumulative incidence of CNS progression (31.3%) was lower than that of non-CNS progression (52.1%; Figure 2).28 A recent single-center, real-world study of 306 EGFRm+ NSCLC patients from Taiwan with or without baseline brain metastases assessed the impact of first-line erlotinib, gefitinib, or afatinib in treating and preventing brain metastases.80 PFS was significantly longer and OS numerically longer with afatinib (compared with erlotinib or gefitinib) in the overall patient population, although it must be noted that higher proportions of patients treated with afatinib had better Eastern Cooperative Oncology Group (ECOG) performance status and Del19 mutations. PFS and OS were similar across the three EGFR TKIs in a subgroup analysis comparing patients with an ECOG performance status of 0–1 and Del19 mutations. Another subgroup analysis, involving patients without baseline brain metastases, found that after adjusting for possible confounding factors, those in the afatinib group had a significantly lower HR for the development of subsequent brain metastases than did those in the gefitinib group. However, the three treatments showed similar efficacy in patients with baseline brain metastases.
Figure 2.
Competing risk analysis for progression in afatinib-treated patients with baseline brain metastases ((a) LUX-Lung 3 and 6) and without baseline brain metastases ((b) LUX-Lung 3, 6, and 7). CNS: central nervous system; NSCLC: non-small cell lung cancer; PD: progressive disease. Reproduced under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License (Girard et al.).30
It is also worth noting that osimertinib has also demonstrated efficacy in patients with brain metastases.13,81 Based on analysis of CNS activity in the FLAURA trial, osimertinib appears superior to first-generation TKIs in treating CNS metastases and in reducing the incidence of de novo CNS lesions.81
Afatinib in squamous cell carcinoma
While EGFR mutations are rare in lung squamous cell carcinoma (SCC) tumors, they are often associated with increased EGFR protein expression and occasionally, altered EGFR copy number.82 Certain clinical features, such as an absence of tobacco exposure have indicated a higher likelihood of a targetable mutation (including EGFR and ALK) and it is therefore reasonable to recommend testing for such alterations in non-smoker patients with lung SCC.83 Identification of increased EGFR copy number and high levels of EGFR protein expression in SCC have served as rationale to assess the impact of EGFR-targeted agents in the treatment of SCC.82 The LUX-Lung 8 study compared afatinib with erlotinib in patients with stage 3 b/4 SCC of the lung who had progressed after at least four cycles of platinum-based chemotherapy.84 Treatment with afatinib resulted in significantly longer PFS (2.6 vs 1.9 months; HR, 0.81 (95% CI, 0.69, 0.96), P = 0.0103) and improved OS (7.9 vs 6.8 months; HR, 0.81 (95% CI, 0.69, 0.95), P = 0.0077) compared with erlotinib. Biomarker analysis indicated that, among patients treated with afatinib, PFS and OS were numerically longer in patients with ErbB mutation-positive tumors than in those without mutations; this was not observed for erlotinib. Interestingly, the benefit of afatinib over erlotinib among patients with ErbB-positive tumors appeared to be driven by mutations in HER3 (ErbB3), HER4 (ErbB4), and in particular, HER2 (ErbB2).85 Based on the overall LUX-Lung 8 results, afatinib has been approved by the FDA for the treatment of metastatic, squamous NSCLC progressing after platinum-based chemotherapy.50
Management of afatinib-related AEs
The most common afatinib-related AEs across the LUX-Lung 3, 6, 7, and 8 studies were diarrhea, rash/acne, and stomatitis (Table 1).39,40,42,84 A supportive care strategy combining patient education, frequent communication, routine monitoring, early recognition, proactive management, and adherence to the recommended tolerability-guided dose adjustment schema is important to help maximize clinical benefits, optimize symptom management, and reinforce adherence, thereby limiting treatment discontinuation.86 The management of afatinib-related AEs has been well characterized, and guidelines are available that describe recommended strategies for prevention and management.68,87 For example, patients who experience diarrhea should receive medication, such as loperamide, immediately.
In addition, the availability of several different doses (20, 30, or 40 mg) of afatinib facilitates the implementation of a tolerability-guided dose adjustment strategy (described below) that can help patients to remain on afatinib long term.50 Of note, analysis of data from LUX-Lung 3 indicated that higher exposure to afatinib increased the risk of experiencing grade ≥3 toxicity or grade ≥2 diarrhea.78 Afatinib should be withheld in patients who experience any adverse reactions of grade ≥3, diarrhea of grade 2 persisting for ≥2 consecutive days while taking anti-diarrheal medication, or cutaneous reactions of grade 2 that last >7 days or are intolerable. Treatment should be resumed at a reduced dose when the adverse reaction has fully resolved, improved to grade 1, or returned to baseline. Dosing should be reduced by 10 mg decrements, to a minimum of 20 mg/day.50 Importantly, afatinib dose reductions in the LUX-Lung 3 and 6 trials (in line with the criteria described above) resulted in a major reduction in treatment-related grade ≥3 AEs (from 73.0% to 20.5% in LUX-Lung 3 and from 80.6% to 11.9% in LUX-Lung 6) without affecting therapeutic efficacy, as assessed by PFS.88 Furthermore, in a noninterventional, observational study conducted in a real-world setting, dose reductions with afatinib were shown to reduce the frequency and intensity of adverse drug reactions, without compromising treatment effectiveness.89
It has been suggested that the severity of afatinib-related skin reactions may be prophylactically reduced using a general skin care regimen combined with oral antibiotics, such as tetracyclines.90 Others recommend the regular use of emollients and protection from excessive sun exposure.68 For patients who receive prophylactic treatment but still develop skin reactions in response to EGFR TKIs, topical antibiotics, corticosteroids, and potentially antihistamines are recommended. If the patient’s skin condition does not improve, dose reduction or treatment discontinuation is recommended, along with referral to a dermatologist.68,90
Acquired resistance and treatment sequencing strategies
Despite the efficacy of EGFR TKIs in the first-line treatment of EGFRm+ NSCLC, acquired resistance to therapy occurs in the majority of patients.45 Identifying the molecular mechanisms of acquired resistance is essential in order to determine the subsequent treatment that would yield greatest benefit. The most common mechanism of resistance to first-generation EGFR TKIs is the development of the gatekeeper T790M mutation, which occurs in approximately 50–70% of patients.45,46,91 Likewise, T790M is detected in around 50–70% of patients with resistance to afatinib91,92 and is also likely to be associated with clinical resistance to dacomitinib.93A variety of other mechanisms of resistance to the first- and second-generation EGFR TKIs have been identified, including other EGFR alterations, Erb2 amplification, MET amplification, and transformation to squamous or small cell morphology.45,94,95
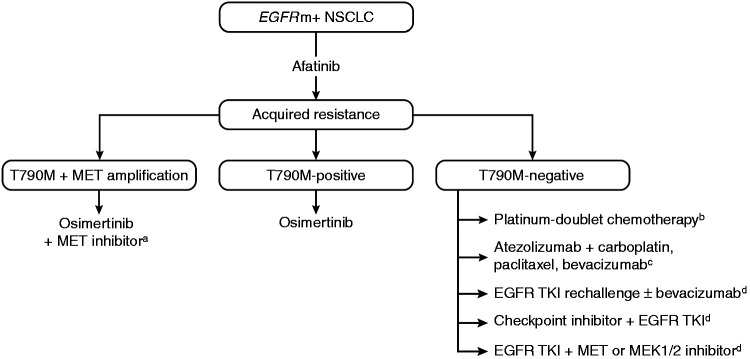
In T790M-positive tumors, following progression after first- or second-generation EGFR TKIs, second-line osimertinib has demonstrated significant efficacy compared with chemotherapy (median PFS: 10.1 vs 4.4 months; HR, 0.30 (95% CI, 0.23‒0.40), P < 0.001).48 In the LUX-Lung 7 trial, the 3-year OS rate was up to 90% in patients who received a third-generation EGFR TKI (osimertinib (10.3% of patients) or olmutinib (3.4%)) after discontinuing afatinib.43 However, given the recent positive results of the FLAURA study showing improved efficacy with first-line osimertinib compared with a first-generation TKI in patients with EGFRm+ NSCLC,13 and the subsequent approval of osimertinib in this setting,51 the most appropriate sequencing of the EGFR TKIs for optimal benefit is unclear. The second- and third-generation TKIs have shown significantly longer PFS compared with first-generation TKIs, and the third-generation TKI, osimertinib, has shown superior OS compared with first-generation TKIs.41,42,49 While the major mechanism of resistance to first- and second-generation TKIs is well defined, mechanisms of acquired resistance to osimertinib are more heterogeneous, and include EGFR C797S, acquired KRAS, and targetable gene fusions.96–98 These data suggest that the net survival benefit could potentially be optimized by reserving osimertinib for second-line use in the >60% of patients who develop T790M-mediated resistance (Figure 3). This is supported by recent data from a global, noninterventional study of patients with EGFRm+ NSCLC receiving sequential afatinib and osimertinib in a real-world setting, which showed median time to treatment failure of 28.1 months (90% CI, 26.8–30.3) and median OS of 41.3 months (90% CI, 36.8–46.3).99 In addition, MET amplification has also been shown to co-exist with the T790M mutation,95 suggesting that the combination of osimertinib with a MET inhibitor may be beneficial for such patients.100 Detractors of this sequencing strategy will note that first, many patients experience disease progression on first-line EGFR TKIs and are not able to receive second-line therapy, due to death or deterioration of performance status, among other reasons. Indeed, discontinuation of erlotinib or gefitinib in patients with acquired resistance has been reported to cause rapid progression (disease flare).101 In the FLAURA trial, 12% of patients treated with osimertinib and 17% treated with first-generation EGFR TKIs died without commencing a second-line therapy.13 Second, it is impossible to prospectively predict which patients will develop T790M and thus benefit from this sequencing strategy. One study identified de novo T790M mutations more frequently within L858R tumors than within Del19 tumors among 20 EGFR TKI-naïve patients,102 while mutation analysis of EGFR TKI-pretreated patients in the osimertinib AURA extension and AURA2 trials detected T790M mutations in a higher proportion of patients with Del19 mutations versus those with L858R.91 Patients who do not develop T790M upon progression on first- or second-generation EGFR TKIs may have been better treated with osimertinib as first-line therapy, which has shown the longest median PFS of all EGFR TKIs, at 18.9 months (assessed by investigator review) in the FLAURA trial.13
Figure 3.
Potential treatment strategies following acquired resistance to afatinib. (a) Investigational therapy100; (b) platinum-doublet chemotherapy represents the principal treatment option outside of the clinical trial setting103; (c) this combination showed considerable efficacy in EGFRm+ NSCLC but has not yet been investigated specifically in patients with EGFRm+ T790M-negative tumors109; and (d) Investigational treatment options with some activity in EGFRm+ NSCLC in early-phase studies.103,105–108EGFR: epidermal growth factor receptor; EGFRm+: EGFR mutation-positive; NSCLC: non-small cell lung cancer; TKI: tyrosine kinase inhibitor.
In contrast to T790M-positive tumors, subsequent treatment options for patients with T790M-negative acquired resistance are less well defined, reflecting the large variety of possible resistance mechanisms identified.103 Currently, the standard treatment for these tumors is platinum-doublet chemotherapy. However, several other targeted treatments and combinations are under investigation in this setting (Figure 3). In tumors with MET amplification, the use of MET inhibitors together with EGFR TKIs has shown preliminary efficacy.95,104 Other approaches include the combination of afatinib with EGFR antibodies or bevacizumab, which have shown promise but some may be associated with considerable treatment-related toxicities.105–108 In addition, novel combinations incorporating checkpoint inhibitors may be effective for patients with T790M-negative acquired resistance. Recent results from the IMPOWER 150 trial indicate that the combination of atezolizumab, bevacizumab, and chemotherapy is effective in patients with NSCLC, regardless of PD-L1 expression and EGFR or ALK genetic alteration status.109 This combination was assessed in patients with EGFRm+ NSCLC who had received prior EGFR TKI treatment, and it is possible that these findings may translate into the more specific setting of EGFRm+ NSCLC with T790M-negative acquired resistance.109
Finally, rather than switching to second-line therapy, continuation of EGFR TKI treatment beyond radiological progression may help maintain clinical benefit for some patients with acquired resistance to first-line EGFR TKIs.110 This approach may be beneficial to help avoid rapid disease progression with worsening of disease-related symptoms following treatment discontinuation (rebound tumor flare), which, as previously mentioned, can occur in some patients after discontinuation of EGFR TKIs.110 Indeed, one analysis found that 14/61 patients (23%) who discontinued erlotinib/gefitinib treatment experienced a disease flare (defined as hospitalization or death due to disease progression), with a median time to disease flare following EGFR TKI discontinuation of 8 days.101
Cost-effectiveness of afatinib
Economic assessments are essential to the healthcare decision-making process. In EGFRm+ NSCLC, EGFR TKIs have a clear benefit over platinum-based chemotherapy in terms of cost-effectiveness.111 For example, one US study showed that erlotinib and afatinib were more cost-effective than cisplatin-pemetrexed, with erlotinib holding a cost-effectiveness advantage over afatinib.112 Importantly, a budget impact analysis of first-line afatinib use in patients with EGFRm+ NSCLC in the US estimated that increasing the treatment share of afatinib in a health plan would lead to an increase in the proportion of treated patients who remained progression-free after 5 years, while having only a small impact on the health plan budget.113 In contrast, a recent analysis of the cost-effectiveness of first-line EGFR TKIs in patients with EGFRm+ NSCLC suggested that osimertinib is not a cost-effective first-line therapy compared with first- or second-generation TKIs.114 Further cost-benefit analyses of afatinib and other EGFR TKIs in patients with EGFRm+ NSCLC and SCC are needed to adequately assess the economic impact of the different treatment options and to identify the optimal sequencing strategy.
Summary and conclusions
In a little over a decade, developments in the molecular analysis of lung tumors have revealed that alterations in EGFR are major oncologic drivers in certain subtypes of lung cancer, with EGFR TKIs quickly becoming the “weapon of choice” for the treatment of patients with EGFRm+ NSCLC or SCC. Afatinib has been shown to provide significant improvements over chemotherapy and gefitinib in the first-line setting in patients with EGFRm+ NSCLC,39,40,42,43 and over erlotinib in the second-line setting in patients with SCC following chemotherapy.84 Afatinib also has proven efficacy in patients with tumors harboring certain uncommon EGFR mutations,75 and in patients with brain metastases.79 The clinical effectiveness of afatinib has been confirmed by recent results of real-world studies.89,99 In addition, afatinib-related AEs are manageable by means of tolerability-guided dose adjustments, along with the use of AE-specific treatment administered either prophylactically or as needed.68,87,89,90 The optimal EGFR TKI sequencing strategy is still under debate. While some data show that a sequential treatment strategy consisting of afatinib followed by osimertinib can provide a viable, long-term treatment option for patients who develop T790M-mediated resistance,99 this strategy would not be beneficial for the 30–50% of afatinib-treated patients who do not develop the T790M mutation.91,92 Head-to-head studies, including those of afatinib and osimertinib, are needed to adequately assess the optimal sequence of EGFR TKI therapies to achieve long-term treatment benefit. Additional studies are required to define optimal management approaches for patients with T790M-negative resistance, as are further pharmacoeconomic analyses to confirm preliminary findings that afatinib is cost-effective in the treatment of EGFRm+ NSCLC.112,113
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of the manuscript. Writing and editorial support was provided by Hashem Dbouk, PhD, of GeoMed, an Ashfield Company, part of UDG Healthcare plc, which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RDH declares research funding to his institution from Pfizer, and from AstraZeneca that supports his salary; VRA and PM declare no conflicts of interest. TB declares advisory board participation outside of the submitted work for AstraZeneca.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
R Donald Harvey https://orcid.org/0000-0002-4217-1313
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013; 82: 179–189. [DOI] [PubMed] [Google Scholar]
- 3.Garinet S, Laurent-Puig P, Blons H, et al. Current and future molecular testing in NSCLC, what can we expect from new sequencing technologies? J Clin Med 2018; 7: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shtivelman E, Hensing T, Simon GR, et al. Molecular pathways and therapeutic targets in lung cancer. Oncotarget 2014; 5: 1392–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014; 25: 282–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015; 4: 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reguart N, Remon J. Common EGFR-mutated subgroups (Del19/L858R) in advanced non-small-cell lung cancer: chasing better outcomes with tyrosine kinase inhibitors. Future Oncol 2015; 11: 1245–1257. [DOI] [PubMed] [Google Scholar]
- 8.O’Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer 2017; 109: 137–144. [DOI] [PubMed] [Google Scholar]
- 9.Lindsey S, Langhans SA. Epidermal growth factor signaling in transformed cells. Int Rev Cell Mol Biol 2015; 314: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam DC, Tam TC, Lau KM, et al. Plasma EGFR mutation detection associated with survival outcomes in advanced-stage lung cancer. Clin Lung Cancer 2015; 16: 507–513. [DOI] [PubMed] [Google Scholar]
- 11.Normanno N, Denis MG, Thress KS, et al. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget 2017; 8: 12501–12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies RSS, Smith C, Edwards G, et al. Impact of cytological sampling on EGFR mutation testing in stage III-IV lung adenocarcinoma. Lung Cancer Int 2017; 2017: 9614938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 14.Reckamp KL, Melnikova VO, Karlovich C, et al. A highly sensitive and quantitative test platform for detction of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol 2016; 11: 1690–1700. [DOI] [PubMed] [Google Scholar]
- 15.Ni J, Weng L, Liu Y, et al. Dynamic monitoring of EGFR mutations in circulating cell-free DNA for EGFR-mutant metastatic patients with lung cancer: early detection of drug resistance and prognostic significance. Oncol Lett 2017; 13: 4549–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins S, Yang JC, Ramalingam SS, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol 2017; 12: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi F. Molecular profile of liquid biopsies: next generation biomarkers to improve lung cancer treatment. Ecancermedicalscience 2015; 9: 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito A, Criscitiello C, Trapani D, et al. The emerging role of “liquid biopsies,” circulating tumor cells, and circulating cell-free tumor DNA in lung cancer diagnosis and identification of resistance mutations. Curr Oncol Rep 2017; 19: 1. [DOI] [PubMed] [Google Scholar]
- 19.Murtaza M, Dawson SJ, Tsui DW, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013; 497: 108–112. [DOI] [PubMed] [Google Scholar]
- 20.Karachaliou N, Mayo-de las Casas C, Queralt C, et al. Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol 2015; 1: 149–157. [DOI] [PubMed] [Google Scholar]
- 21.Marcq M, Vallee A, Bizieux A, et al. Detection of EGFR mutations in the plasma of patients with lung adenocarcinoma for real-time monitoring of therapeutic response to tyrosine kinase inhibitors? J Thorac Oncol 2014; 9: e49–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep 2014; 4: 6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito K, Suzuki Y, Saiki H, et al. Utility of liquid biopsy by improved PNA-LNA PCR clamp method for detecting EGFR mutation at initial diagnosis of non-small-cell lung cancer: observational study of 190 consecutive cases in clinical practice. Clin Lung Cancer 2018; 19: 181–190. [DOI] [PubMed] [Google Scholar]
- 24.Ahn M, Han JY, Tsai C, et al. Detection of EGFR mutations from plasma ctDNA in the osimertinib phase III trial (AURA3): comparison of three plasma assays. In: Presented at world conference on lung cancer 2019, Barcelona, Spain, 7–10 September 2019, abstract OA 2010. 2001.
- 25.Marchetti A, Palma JF, Felicioni L, et al. Early prediction of response to tyrosine kinase inhibitors by quantification of EGFR mutations in plasma of NSCLC patients. J Thorac Oncol 2015; 10: 1437–1443. [DOI] [PubMed] [Google Scholar]
- 26.Torti D, Trusolino L. Oncogene addiction as a foundational rationale for targeted anti-cancer therapy: promises and perils. EMBO Mol Med 2011; 3: 623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012; 343: 342–350. [DOI] [PubMed] [Google Scholar]
- 28.Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol 2018; 14: 1117–1132. [DOI] [PubMed] [Google Scholar]
- 29.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 30.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 31.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 32.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 33.Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–742. [DOI] [PubMed] [Google Scholar]
- 35.Urata Y, Katakami N, Morita S, et al. Randomized phase III study comparing gefitinib with erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. J Clin Oncol 2016; 34: 3248–3257. [DOI] [PubMed] [Google Scholar]
- 36.Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017; 116: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2009; 27: 4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzales AJ, Hook KE, Althaus IW, et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther 2008; 7: 1880–1889. [DOI] [PubMed] [Google Scholar]
- 39.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 40.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–222. [DOI] [PubMed] [Google Scholar]
- 41.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017; 18: 1454–1466. [DOI] [PubMed] [Google Scholar]
- 42.Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17: 577–589. [DOI] [PubMed] [Google Scholar]
- 43.Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017; 28: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018; 36: 2244–2250. [DOI] [PubMed] [Google Scholar]
- 45.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 2017; 35: 1288–1296. [DOI] [PubMed] [Google Scholar]
- 48.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382: 41–50. [DOI] [PubMed] [Google Scholar]
- 50.Food and Drug Administration. Gilotrif (afatinib) Highlights of Prescribing Information. 2019. www.accessdata.fda.gov/drugsatfda_docs/label/2019/201292s015lbl.pdf (accessed 26 February 2020).
- 51.Food and Drug Administration. Tagrisso (osimertinib) highlights of prescribing information. 2019. www.accessdata.fda.gov/drugsatfda_docs/label/2019/208065s013lbl.pdf (accessed 26 February 2020).
- 52.Food and Drug Administration. Tarceva (erlotinib) highlights of prescribing information. 2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf (accessed 26 February 2020).
- 53.Food and Drug Administration. Iressa (gefitinib) highlights of prescribing information. 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf (accessed 26 February 2020).
- 54.Food and Drug Administration. Vizimpro (dacomitinib) highlights of prescribing information. 2018. www.accessdata.fda.gov/drugsatfda_docs/label/2018/211288s000lbl.pdf (accessed 12 February 2019).
- 55.Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol 2010; 28: 3965–3972. [DOI] [PubMed] [Google Scholar]
- 56.Wind S, Schmid M, Erhardt J, et al. Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clin Pharmacokinet 2013; 52: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 57.Eskens FA, Mom CH, Planting AS, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 2008; 98: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stopfer P, Marzin K, Narjes H, et al. Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer Chemother Pharmacol 2012; 69: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 59.Wiebe S, Schnell D, Kulzer R, et al. Influence of renal impairment on the pharmacokinetics of afatinib: an open-label, single-dose study. Eur J Drug Metab Pharmacokinet 2017; 42: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schnell D, Buschke S, Fuchs H, et al. Pharmacokinetics of afatinib in subjects with mild or moderate hepatic impairment. Cancer Chemother Pharmacol 2014; 74: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters S, Zimmermann S, Adjei AA. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug-drug interactions. Cancer Treat Rev 2014; 40: 917–926. [DOI] [PubMed] [Google Scholar]
- 62.Wind S, Schnell D, Ebner T, et al. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet 2017; 56: 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamilton M, Wolf JL, Rusk J, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res 2006; 12: 2166–2171. [DOI] [PubMed] [Google Scholar]
- 64.Wind S, Giessmann T, Jungnik A, et al. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig 2014; 34: 173–182. [DOI] [PubMed] [Google Scholar]
- 65.Marchetti S, de Vries NA, Buckle T, et al. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol Cancer Ther 2008; 7: 2280–2287. [DOI] [PubMed] [Google Scholar]
- 66.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–151. [DOI] [PubMed] [Google Scholar]
- 67.Kuan FC, Kuo LT, Chen MC, et al. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer 2015; 113: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Califano R, Tariq N, Compton S, et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs 2015; 75: 1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014; 25: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heigener DF, Schumann C, Sebastian M, et al. Afatinib in non-small cell lung cancer harboring uncommon EGFR mutations pretreated with reversible EGFR inhibitors. Oncologist 2015; 20: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keam B, Kim DW, Park JH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol 2014; 19: 594–600. [DOI] [PubMed] [Google Scholar]
- 72.Krawczyk P, Kowalski DM, Ramlau R, et al. Comparison of the effectiveness of erlotinib, gefitinib, and afatinib for treatment of non-small cell lung cancer in patients with common and rare EGFR gene mutations. Oncol Lett 2017; 13: 4433–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuiper JL, Hashemi SM, Thunnissen E, et al. Non-classic EGFR mutations in a cohort of Dutch EGFR-mutated NSCLC patients and outcomes following EGFR-TKI treatment. Br J Cancer 2016; 115: 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen Y-C, Tseng G-C, Tu C-Y, et al. Comparing the effects of afatinib with gefitinib or erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer 2017; 110: 56–62. [DOI] [PubMed] [Google Scholar]
- 75.Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015; 16: 830–838. [DOI] [PubMed] [Google Scholar]
- 76.European Medicines Agency. Gilotrif (afatinib) summary of product characteristics. 2013. www.ema.europa.eu/en/documents/product-information/giotrif-epar-product-information_en.pdf (accessed 26 February 2020).
- 77.Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015; 20: 674–679. [DOI] [PubMed] [Google Scholar]
- 78.Food and Drug Administration. FDA Drug Approval Package. Afatinib. clinical pharmacology biopharmaceutics review(s). www.accessdata.fda.gov/drugsatfda_docs/nda/2013/201292Orig1s000ClinPharmR.pdf (accessed 23 January 2019).
- 79.Schuler M, Wu YL, Hirsh V, et al. First-Line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016; 11: 380–390. [DOI] [PubMed] [Google Scholar]
- 80.Su PL, Wu YL, Chang WY, et al. Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer. Ther Adv Med Oncol 2018; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol 2018; 36: 3290–3297. [DOI] [PubMed] [Google Scholar]
- 82.Hirsh V. New developments in the treatment of advanced squamous cell lung cancer: focus on afatinib. Onco Targets Ther 2017; 10: 2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018; 142: 321–346. [DOI] [PubMed] [Google Scholar]
- 84.Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16: 897–907. [DOI] [PubMed] [Google Scholar]
- 85.Goss GD, Felip E, Cobo M, et al. Association of ERBB mutations with clinical outcomes of afatinib- or erlotinib-treated patients with lung squamous cell carcinoma: secondary analysis of the LUX-Lung 8 randomized clinical trial. JAMA Oncol 2018; 4: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Edwards RL, Andan C, Lalla RV, et al. Afatinib therapy: practical management of adverse events with an oral agent for non-small cell lung cancer treatment. Clin J Oncol Nurs 2018; 22: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti-lung cancer therapies with EGFR-TKIs. Front Oncol 2014; 4: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 2016; 27: 2103–2110. [DOI] [PubMed] [Google Scholar]
- 89.Halmos B, Tan EH, Soo RA, et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: results from a global real-world study (RealGiDo). Lung Cancer 2019; 127: 103–111. [DOI] [PubMed] [Google Scholar]
- 90.Hofheinz RD, Deplanque G, Komatsu Y, et al. Recommendations for the prophylactic management of skin reactions induced by epidermal growth factor receptor inhibitors in patients with solid tumors. Oncologist 2016; 21: 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jenkins S, Chih-Hsin Yang J, Janne PA, et al. EGFR mutation analysis for prospective patient selection in two phase II registration studies of osimertinib. J Thorac Oncol 2017; 12: 1247–1256. [DOI] [PubMed] [Google Scholar]
- 92.Wu SG, Liu YN, Tsai MF, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget 2016; 7: 12404–12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kobayashi Y, Fujino T, Nishino M, et al. EGFR T790M and C797S mutations as mechanisms of acquired resistance to dacomitinib. J Thorac Oncol 2018; 13: 727–731. [DOI] [PubMed] [Google Scholar]
- 94.van der Wekken AJ, Saber A, Hiltermann TJ, et al. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol 2016; 100: 107–116. [DOI] [PubMed] [Google Scholar]
- 95.Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018; 29: i10–i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol 2018; 4: 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. In: 2018: Presented at ESMO 2018, Munich: abst LBA2050.
- 98.Papadimitrakopoulou VA, Wu Y, Han J, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol 2018; 29: viii741. [Google Scholar]
- 99.Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol 2019; 15: 2905–2914. [DOI] [PubMed] [Google Scholar]
- 100.York ER, Varella-Garcia M, Bang TJ, et al. Tolerable and effective combination of full-dose crizotinib and osimertinib targeting MET amplification sequentially emerging after T790M positivity in EGFR-mutant non-small cell lung cancer. J Thorac Oncol 2017; 12: e85–e88. [DOI] [PubMed] [Google Scholar]
- 101.Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011; 17: 6298–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu HA, Arcila ME, Hellmann MD, et al. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol 2014; 25: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corallo S, D’Argento E, Strippoli A, et al. Treatment options for EGFR T790M-negative EGFR tyrosine kinase inhibitor-resistant non-small cell lung cancer. Target Oncol 2017; 12: 153–161. [DOI] [PubMed] [Google Scholar]
- 104.Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol 2018; 36: 2702–2709. [DOI] [PubMed] [Google Scholar]
- 105.Bennouna J. Update on afatinib-based combination regimens for the treatment of EGFR mutation-positive non-small-cell lung cancer. Future Oncol 2017; 13: 1829–1833. [DOI] [PubMed] [Google Scholar]
- 106.Hata A, Katakami N, Kaji R, et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: multicenter, single-arm, phase 2 trial (ABC Study). Cancer 2018; 124: 3830–3838. [DOI] [PubMed] [Google Scholar]
- 107.Janjigian YY, Smit EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014; 4: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee JY, Sun JM, Lim SH, et al. A phase Ib/II study of afatinib in combination with nimotuzumab in non-small cell lung cancer patients with acquired resistance to gefitinib or erlotinib. Clin Cancer Res 2016; 22: 2139–2145. [DOI] [PubMed] [Google Scholar]
- 109.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 110.Yap TA, Macklin-Doherty A, Popat S. Continuing EGFR inhibition beyond progression in advanced non-small cell lung cancer. Eur J Cancer 2017; 70: 12–21. [DOI] [PubMed] [Google Scholar]
- 111.Lange A, Prenzler A, Frank M, et al. A systematic review of the cost-effectiveness of targeted therapies for metastatic non-small cell lung cancer (NSCLC). BMC Pulm Med 2014; 14: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ting J, Tien Ho P, Xiang P, et al. Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mtation-positive non-small-cell lung cancer in the United States. Value Health 2015; 18: 774–782. [DOI] [PubMed] [Google Scholar]
- 113.Graham J, Earnshaw S, Burslem K, et al. Budget impact analysis of afatinib for first-line treatment of patients with metastatic non-small cell lung cancer with epidermal growth factor receptor Exon 19 deletions or Exon 21 substitution mutations in a U.S. health plan. J Manag Care Spec Pharm 2018; 24: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aguiar PN, Jr., Haaland B, Park W, et al. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol 2018; 4: 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]