Key Points
Question
In high-risk children with persistent asthma and low vitamin D levels, does vitamin D3 supplementation prolong the time to a severe asthma exacerbation?
Findings
In this randomized clinical trial that included 192 children, vitamin D3 supplementation, compared with placebo, did not significantly improve the time to a severe asthma exacerbation (adjusted hazard ratio, 1.13).
Meaning
The findings from this trial do not support the use of vitamin D3 supplementation to improve the time to a severe asthma exacerbation in children with asthma and low serum vitamin D levels.
Abstract
Importance
Severe asthma exacerbations cause significant morbidity and costs. Whether vitamin D3 supplementation reduces severe childhood asthma exacerbations is unclear.
Objective
To determine whether vitamin D3 supplementation improves the time to a severe exacerbation in children with asthma and low vitamin D levels.
Design, Setting, and Participants
The Vitamin D to Prevent Severe Asthma Exacerbations (VDKA) Study was a randomized, double-blind, placebo-controlled clinical trial of vitamin D3 supplementation to improve the time to severe exacerbations in high-risk children with asthma aged 6 to 16 years taking low-dose inhaled corticosteroids and with serum 25-hydroxyvitamin D levels less than 30 ng/mL. Participants were recruited from 7 US centers. Enrollment started in February 2016, with a goal of 400 participants; the trial was terminated early (March 2019) due to futility, and follow-up ended in September 2019.
Interventions
Participants were randomized to vitamin D3, 4000 IU/d (n = 96), or placebo (n = 96) for 48 weeks and maintained with fluticasone propionate, 176 μg/d (6-11 years old), or 220 μg/d (12-16 years old).
Main Outcomes and Measures
The primary outcome was the time to a severe asthma exacerbation. Secondary outcomes included the time to a viral-induced severe exacerbation, the proportion of participants in whom the dose of inhaled corticosteroid was reduced halfway through the trial, and the cumulative fluticasone dose during the trial.
Results
Among 192 randomized participants (mean age, 9.8 years; 77 girls [40%]), 180 (93.8%) completed the trial. A total of 36 participants (37.5%) in the vitamin D3 group and 33 (34.4%) in the placebo group had 1 or more severe exacerbations. Compared with placebo, vitamin D3 supplementation did not significantly improve the time to a severe exacerbation: the mean time to exacerbation was 240 days in the vitamin D3 group vs 253 days in the placebo group (mean group difference, −13.1 days [95% CI, −42.6 to 16.4]; adjusted hazard ratio, 1.13 [95% CI, 0.69 to 1.85]; P = .63). Vitamin D3 supplementation, compared with placebo, likewise did not significantly improve the time to a viral-induced severe exacerbation, the proportion of participants whose dose of inhaled corticosteroid was reduced, or the cumulative fluticasone dose during the trial. Serious adverse events were similar in both groups (vitamin D3 group, n = 11; placebo group, n = 9).
Conclusions and Relevance
Among children with persistent asthma and low vitamin D levels, vitamin D3 supplementation, compared with placebo, did not significantly improve the time to a severe asthma exacerbation. The findings do not support the use of vitamin D3 supplementation to prevent severe asthma exacerbations in this group of patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT02687815
This randomized trial compares the effects of vitamin D3 vs placebo on time to severe exacerbation in children with asthma and low vitamin D levels.
Introduction
As of 2018, an estimated 5.5 million children in the US had asthma, a major cause of health care utilization and costs.1 In 2018, asthma exacerbations led to more than 546 000 emergency department (ED) visits and 80 000 hospitalizations.1
Several observational studies have linked low serum 25-hydroxyvitamin D (henceforth, “vitamin D”) levels to severe asthma exacerbations, lower lung function, and reduced response to corticosteroids.2,3,4,5 Such findings may be explained by immune-modulatory and anti-inflammatory effects of vitamin D, including regulatory T-cell induction, attenuation of Th2 and Th17 responses, enhancement of IL-10 production, and inhibition of airway smooth muscle hypertrophy and collagen deposition.6,7,8,9 Vitamin D may attenuate viral-induced asthma attacks by reducing rhinovirus replication in bronchial epithelium, promoting interferon-mediated antiviral pathways and inducing production of antimicrobial peptides.10,11
A meta-analysis of clinical trials using participant-level data showed that vitamin D3 supplementation was associated with lower risk of asthma exacerbations, but the pooled estimate was not significant among children younger than 16 years old.12 More recently, a comprehensive review of vitamin D3 for the prevention of wheeze attacks found insufficient evidence to recommend vitamin D3 supplementation in children.13 Previous pediatric randomized clinical trials, however, have not focused on children with low vitamin D levels or who are at high risk for severe asthma exacerbations. Moreover, existing trials did not monitor vitamin D levels or did not show consistently improved levels after supplementation.
Children with a recent severe asthma exacerbation are at highest risk for subsequent exacerbations, independent of disease severity, treatment, or control.14 The primary hypothesis for this trial was that supplementation with vitamin D3 would improve the time to a severe exacerbation in high-risk children, aged 6 to 16 years, with vitamin D insufficiency and taking low-dose inhaled corticosteroids for persistent asthma. The secondary hypotheses were that this protective effect would result from reduced incidence of viral-induced exacerbations or enhanced response to corticosteroids.
Methods
The study protocol was approved by the institutional review board (IRB) at each participating institution, and an independent data and safety monitoring board (DSMB) appointed by the National Heart, Lung, and Blood Institute monitored the study. Written parental consent was obtained for participating children, from whom written assent was also obtained.
Participants
Participants for the Vitamin D to Prevent Severe Asthma Exacerbations (Vit-D-Kids Asthma [VDKA]) Study were recruited from 7 sites across the US: Boston Children’s Hospital, Boston, Massachusetts; Washington University and St Louis Children’s Hospital, St Louis, Missouri; Cincinnati Children’s Hospital, Cincinnati, Ohio; Rainbow Babies and Children’s Hospital, Cleveland, Ohio; the University of California at San Francisco Benioff Children’s Hospital; National Jewish Health, Denver, Colorado; and University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania. The data coordinating center was based at the University of Pittsburgh Graduate School of Public Health. Based on prior observational studies on vitamin D and asthma outcomes,2,5 eligible participants were high-risk children with asthma, aged 6 to 16 years, with serum vitamin D levels less than 30 ng/mL but greater than or equal to 10 ng/mL (until July 21, 2017) or greater than or equal to 14 ng/mL (after that date, following a protocol amendment). Entry criteria included (1) physician-diagnosed asthma for at least 1 year; (2) at least 1 severe asthma exacerbation (systemic corticosteroids for at least 3 days, or a hospitalization or ED visit requiring systemic corticosteroids) in the previous year; (3) use of asthma medications (daily controller medications, or inhaled β2-agonists at least thrice per week) for at least 6 months in the previous year; (4) forced expiratory volume in the first second of expiration (FEV1) greater than or equal to 70% of predicted; and (5) either bronchodilator responsiveness (an increment in baseline FEV1 of 8% or more 15 minutes after inhalation of 180-μg albuterol) or, in those without bronchodilator response, increased airway responsiveness (a provocative concentration of methacholine at which FEV1 decreased by 20% [PC20] <8 mg/mL if not receiving inhaled corticosteroids, or <16 mg/mL if receiving inhaled corticosteroids). Exclusion criteria, listed in the full study protocol (Supplement 1), included chronic respiratory disorders other than asthma, chronic oral corticosteroid therapy, severe asthma, and inability to perform adequate spirometry. Recruitment was conducted at several locations at each study site, including EDs, pulmonary and allergy/immunology clinics, and general pediatric clinics. A study description was also posted in the study website and at IRB-approved websites (eg, Pitt+Me) to reach a broader population.
Study Design and Treatment
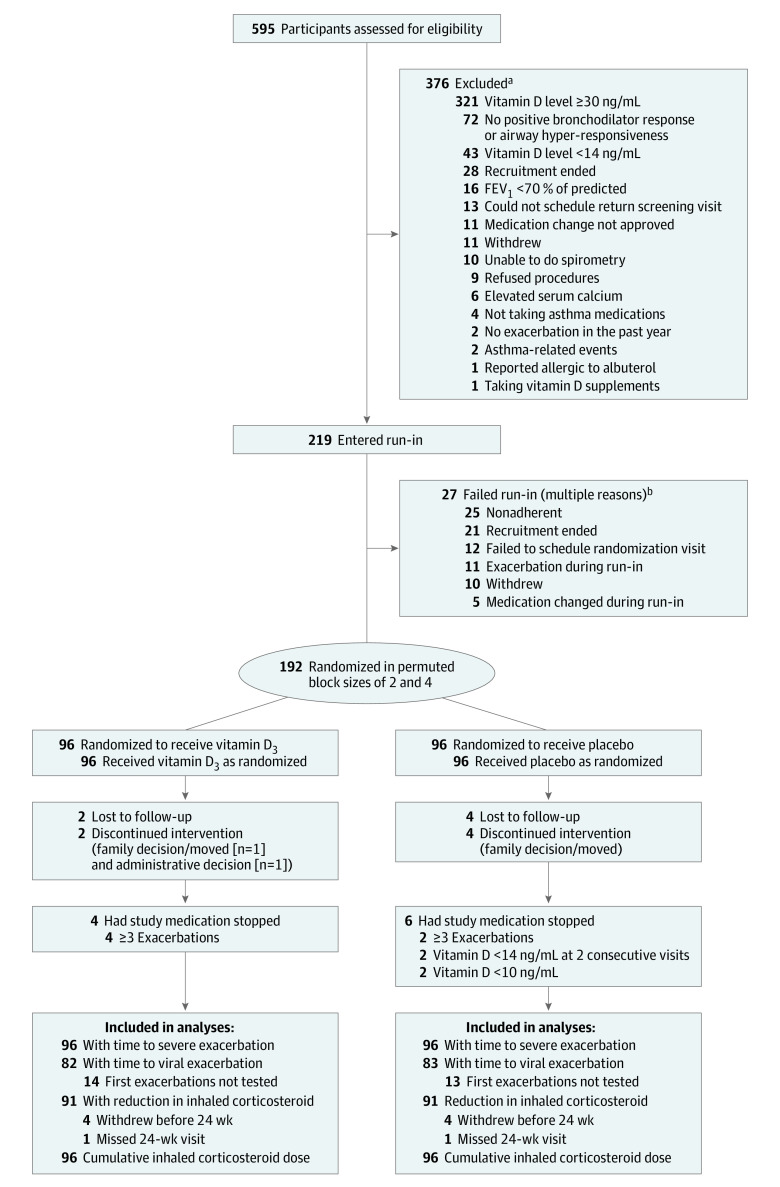
The study was a randomized, double-blind, parallel, placebo-controlled clinical trial, with each participant randomly assigned to either daily placebo capsules or daily vitamin D3, 4000 IU (Pharmavite LLC), plus inhaled fluticasone propionate (88 μg twice per day in children aged 6-11 years and 110 μg twice per day in children ≥12 years). Eligible participants were screened between February of 2016 and March of 2019 and enrolled if they met inclusion criteria. After a 4-week run-in period in which participants received placebo capsules plus inhaled fluticasone and as-needed inhaled albuterol (prior medications were discontinued), those who met inclusion criteria at entry and during run-in were randomized (Figure 1).
Figure 1. Selection of Study Participants.
FEV1 indicates forced expiratory volume in the first second of expiration.
aSum is greater than 376 because potential participants could fail screening more than once and could fail for more than 1 reason.
bSum is greater than 27 because participants could enter run-in more than once and could fail for more than 1 reason.
Computer-generated randomization was stratified according to study site and race/ethnicity, with treatment assignments made in random permuted block sizes of 2 and 4. The soft gelatin placebo capsules were matched in appearance to those containing vitamin D3. At the randomization visit, participants and their parents received dietary counseling, including a list of foods rich in vitamin D. After randomization, participants were followed up for 48 weeks, including a total of 6 in-person visits every 8 weeks and telephone calls in-between visits. Following randomization, participants continued taking the same dose of fluticasone until the 24-week visit, when their fluticasone dose was reduced by 50% if their asthma was well controlled and if they had FEV1 and FEV1 to forced vital capacity (FEV1/FVC) greater than or equal to 80% of predicted, were using a rescue inhaler 4 or fewer times per week, and had asthma symptoms preventing full participation in daily activities no more than once per month. Asthma control was assessed using the Asthma Control Test (ACT [score range, 5-25], for participants aged ≥12 years)15 or the Childhood ACT (C-ACT [score range, 0-27], for participants aged <12 years)16; for both tests, higher scores mean better symptom control, and more than 19 points is considered well-controlled asthma. Per protocol, study medications were discontinued in participants who had 3 or more severe asthma exacerbations (n = 6); those participants were followed up and analyzed in the group to which they were randomized.
During the trial, vitamin D levels were measured during in-person study visits at 0 (randomization), 16, 32, and 48 weeks. Study medications were discontinued in participants with a vitamin D level less than 10 ng/mL (n = 2), who were referred to a pediatric endocrinologist for evaluation and followed up in the group to which they were randomized. Participants whose vitamin D level was between 10 and 13 ng/mL (n = 10) received additional dietary counseling along with a randomly selected participant (to prevent unblinding); if their vitamin D level was less than 14 ng/mL at a subsequent visit (n = 2), study medications were discontinued and the participant was referred to a pediatric endocrinologist and followed up in the group to which they were randomized. Adherence to placebo or vitamin D capsules was assessed both through returned pill counts and electronically, using the MEMS monitor (Aardex).
Viral Polymerase Chain Reaction
Nasal blows for polymerase chain reaction (PCR) assessment of a viral infection were collected within 7 days of a severe exacerbation. Nasal mucus specimens were tested for common respiratory viruses (influenza, respiratory syncytial virus A and B, parainfluenza 1-4, bocavirus, metapneumovirus, rhinoviruses and enteroviruses, and coronavirus [HKU1, NL63, OC43, and 229E]) using the NxTAG Respiratory Pathogen Panel (Luminex). Sensitivity and specificity vary by virus but on average were approximately 95% and 99%, respectively. Rhinoviruses were typed by partial sequencing as previously described.17
Outcome Measures
The primary end point was time to a severe asthma exacerbation during the 48-week trial period. A severe asthma exacerbation was defined as the occurrence of either (1) use of systemic corticosteroids (tablets, suspension, or injection) for at least 3 days or (2) a hospitalization or ED visit because of asthma, requiring systemic corticosteroids.18
There were 3 prespecified secondary outcomes: (1) the time to a viral-induced severe asthma exacerbation, defined by having both a severe exacerbation and a positive PCR result to a panel of common respiratory viruses, (2) the ability to reduce the dose of inhaled steroids by 50% at the 24-week study visit, and (3) the cumulative dose of inhaled steroids during the study period. We also evaluated 2 exploratory outcomes: (1) the mean number of severe asthma exacerbations and (2) the mean number of viral-induced severe exacerbations.
Prespecified Power Calculations
Based on prior studies,5 the trial was designed to have a sample size of 400 participants for 88% power to detect an absolute reduction in the rate of severe asthma exacerbations of 16% (from 40% in the placebo group19 to 24% in the intervention group), assuming an overall α of .05, a 2-sided test, and a withdrawal rate of 15%. Such calculations were conservative because they did not reflect the primary time-to-event analysis.
Statistical Analysis
During the trial, all children were followed up in the group to which they were randomized. In the primary analysis, treatment groups were compared in a Cox proportional hazards regression model that estimated a hazard ratio (HR) for the time to a severe asthma exacerbation across the 48-week study period. The model included dropouts as censored observations and, per protocol, was stratified by site and race/ethnicity, as well as by potential confounders that were not balanced by randomization. A similar approach was used for the analysis of time to a viral-induced severe asthma exacerbation. Both asthma severity and vitamin D level may vary by race/ethnicity; information was ascertained by parental report using fixed categories for race and a separate question for Hispanic/Latino ethnicity, and analyzed using a combined variable (non-Hispanic White, non-Hispanic Black, and other). The proportionality assumption was tested visually and by fitting models with covariates and their interactions with time; none of the interactions were significant.
Linear regression was used for the analysis of the difference in mean cumulative dose of inhaled steroids between the vitamin D3 and placebo groups at the end of the trial with treatment group as the primary explanatory variable, adjusting for study site, sex, time in study (ie, follow-up time), and race/ethnicity. Logistic regression was used for the analysis of the proportion of participants achieving a reduction in inhaled corticosteroid dose at or after the 24-week visit, adjusting for site, sex, time in study, and race/ethnicity. Missing data for the primary and secondary outcomes were minimal (Figure 1) and, thus, listwise deletion was used in the analyses. Two-sided P values less than .05 were considered significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
Early Termination
After review of the second interim analysis, on March 11, 2019, the DSMB recommended that no new participants be randomized due to futility (lack of efficacy of vitamin D3 supplementation), based on the protocol threshold of less than 30% conditional power to detect the prespecified effect (a 16% reduction in severe exacerbations). At that time, there had been 26 events in 90 participants (28.9%) in the placebo group and 25 events in 91 participants (27.5%) in the vitamin D3 group. Conditional power was evaluated under 3 conditions: under the null (based on no treatment effect); under the alternative hypothesis (based on the original assumption of effect); and under the observed rates. Under all 3, conditional power was less than 30%. A close-out plan to complete follow-up of randomized participants was reviewed and approved by the National Heart, Lung, and Blood Institute; the DSMB; and the IRBs at all participating institutions in August of 2019. Follow-up of the last participant was completed in September of 2019.
Enrollment and Study Completion
After screening, a total of 219 participants entered run-in, and 192 were randomized (96 each to the vitamin D3 and placebo groups), of whom 180 (93.8%) completed the trial (92 [51%] in the vitamin D3 group and 88 [49%] in the placebo group) (Figure 1). The median duration of follow-up was 332 days. The most common reasons for study discontinuation were loss to follow-up (n = 6), family decision (n = 5), and administrative decision (n = 1) (Figure 1).
Baseline Characteristics
The trial enrolled children with a mean (SD) age of 9.8 (2.5) years, a mean (SD) ACT or C-ACT score of 21.7 (3.4), and a median of 1 (interquartile range [IQR], 1-2) severe asthma exacerbation in the prior year (Table 1). A total of 77 girls (40%) and 115 boys (60%) were enrolled, with 44 girls (46%) in the vitamin D3 group and 33 girls (34%) in the placebo group. Both groups were similar in terms of age, race/ethnicity, parental education, household smoke exposure, season of enrollment, body mass index z score, FEV1, and FEV1/FVC. The mean (SD) baseline serum vitamin D level was 22.5 (4.6) ng/mL in the vitamin D3 group and 22.8 (4.6) ng/mL in the placebo group.
Table 1. Baseline Characteristics of Randomized Participants.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Vitamin D3 (n = 96) | Placebo (n = 96) | |
| Sex | ||
| Female | 44 (46) | 33 (34) |
| Male | 52 (54) | 63 (66) |
| Racea | ||
| White | 27 (28) | 32 (33) |
| Black | 51 (53) | 49 (51) |
| Other race | 18 (19) | 15 (16) |
| Hispanic/Latino ethnicitya | 7 (7) | 6 (6) |
| Parental education, No./total No. (%) | ||
| High school or less | 21/94 (22) | 20/95 (21) |
| Some college | 22/94 (23) | 22/95 (23) |
| Completed college | 37/94 (39) | 32/95 (34) |
| Postgraduate education | 14/94 (16) | 21/95 (22) |
| Household smoke exposure, No./total No. (%) | ||
| Before age 2 y | 31/94 (33) | 32/94 (34) |
| During study | 25/93 (27) | 22/93 (24) |
| Season of enrollment | ||
| January-March | 30 (31) | 25 (26) |
| April-June | 26 (27) | 26 (27) |
| July-September | 22 (23) | 22 (23) |
| October-December | 18 (19) | 23 (24) |
| Asthma Control Test score >19b | 76 (79) | 73 (76) |
| Age at randomization, mean (SD), y | 9.9 (2.5) | 9.7 (2.5) |
| BMI z score, mean (SD)c | 0.9 (1.1) [n = 95] | 0.9 (1.3) |
| No. of severe exacerbations in the previous year, median (IQR) | 1 (1-2) [n = 88] | 1 (1-2) [n = 86] |
| Vitamin D, mean (SD), ng/mL | 22.5 (4.6) | 22.8 (4.6) |
| FEV1, mean (SD), % predictedd | 93.9 (15.8) | 90.6 (17.3) |
| FEV1/FVC, mean (SD), % predictedd | 91.5 (9.3) | 89.6 (10.1) |
| Asthma Control Test score, mean (SD)b | 22.0 (3.2) | 21.3 (3.6) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FEV1, forced expiratory volume in the first second of expiration; FVC, forced vital capacity; IQR, interquartile range.
Race and ethnicity were ascertained per parental report. “Other race” includes American Indian, Alaskan Native, Asian, mixed, and those reported as “other.”
Asthma Control Test (ACT)15 was for participants aged 12 years or older, and Childhood Asthma Control Test (C-ACT)16 was for participants aged 6 to younger than 12 years. The ACT is calculated based on 5 self-administered questions and has a score range of 5 to 25. The C-ACT is calculated based on 7 questions (3 answered by the parent and 4 answered with the child’s input) and has a score range of 0 to 27. For both, higher scores indicate better symptom control, and a score greater than 19 indicates well-controlled asthma symptoms.
BMI z scores calculated using US Centers for Disease Control and Prevention equations,20 which create z scores and percentiles for the child’s sex and age. A BMI z score of 0 is equivalent to the 50th percentile.
Serum Vitamin D Levels
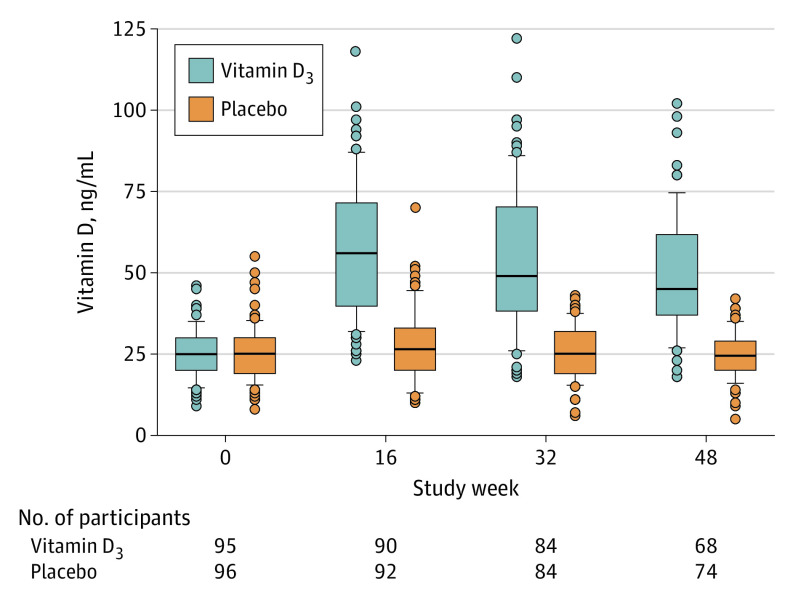
Compared with participants in the placebo group, those in the vitamin D3 group were significantly more likely to achieve a vitamin D level of 30 ng/mL or higher (94.4% in the vitamin D3 group vs 40.7% in the placebo group at 16 weeks; and 87.2% vs 30.1%, respectively, at 48 weeks; both P < .001) and to have a higher vitamin D level at all visits during the trial. In the vitamin D3 group, the mean vitamin D levels were 57.2 ng/mL (95% CI, 52.9-61.5) at 16 weeks, 53.8 ng/mL (95% CI, 48.9-58.6) at 32 weeks, and 49.4 ng/mL (95% CI, 44.9-53.9) at 48 weeks. In the placebo group, vitamin D serum levels were 27.5 ng/mL (95% CI, 25.2-29.9) at 16 weeks, 25.4 ng/mL (95% CI, 23.6-27.3) at 32 weeks, and 24.6 ng/mL (95% CI, 22.9-26.3) at 48 weeks (Figure 2), with similar levels after excluding participants who received open-label vitamin D3 supplementation due to very low levels during the trial (eTable in Supplement 2). A total of 23% and 12% of participants had vitamin D levels less than 20 ng/mL at the baseline visit and study exit visit, respectively. In the vitamin D3 group, no participants reported taking (nonstudy) vitamin D3 supplements at randomization, and 2 reported such supplementation at study exit; those numbers were 1 and 6 participants, respectively, in the placebo group. The median adherence with study medication was 85% (IQR, 72%-93%) in the vitamin D3 group and 87% (IQR, 76%-94%) in the placebo group.
Figure 2. Vitamin D Levels During Trial Period by Treatment Group.
Boxes represent median and interquartile ranges with error bars indicating the highest and lowest values within 1.5 times the interquartile range; dots represent outlying data.
Primary Outcome: Time to Severe Asthma Exacerbation
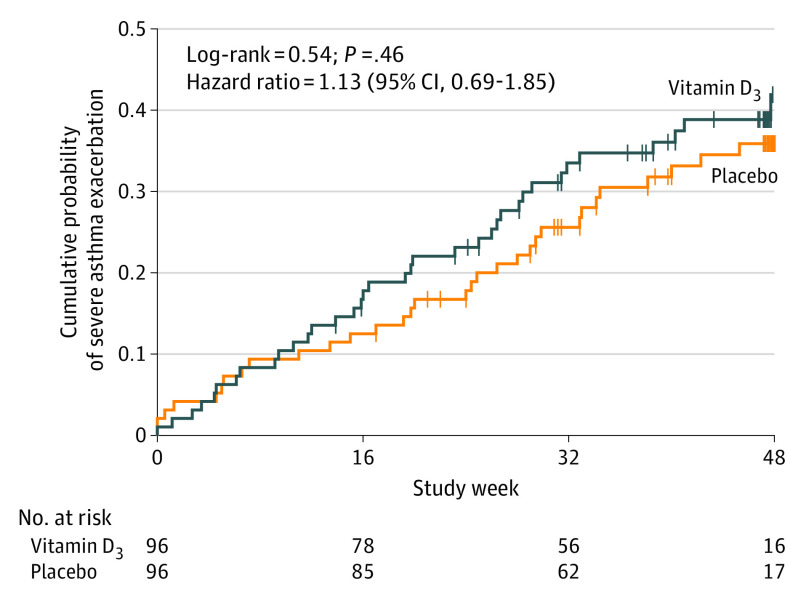
A total of 36 participants (37.5%) in the vitamin D3 group and 33 participants (34.4%) in the placebo group had at least 1 severe asthma exacerbation during the trial. Vitamin D3 supplementation did not significantly prolong the time to a severe asthma exacerbation: the mean number of days until a severe exacerbation was 240 in the vitamin D3 group and 253 in the placebo group, with a mean group difference of −13.1 days (95% CI, −42.6 to 16.4) and adjusted HR of 1.13 (95% CI, 0.69-1.85; P = .63) (Table 2 and Figure 3).
Table 2. VDKA Trial Outcomes and Serious Adverse Events for Vitamin D3 Supplementation vs Placebo.
| Measure | Vitamin D3 | Placebo | Group difference (95% CI) | Adjusted analysis | |||
|---|---|---|---|---|---|---|---|
| No. | Mean | No. | Mean | Parameter estimate (95% CI) | P value | ||
| Primary outcome | |||||||
| Days to a severe exacerbationa | 96 | 240 | 96 | 253 | −13.1 (−42.6 to 16.4) | 1.13 (0.69 to 1.85)b | .63 |
| Secondary outcomes | |||||||
| Days to viral-induced severe exacerbationc | 82 | 272 | 83 | 281 | −9.1 (−35.5 to 17.2) | 1.32 (0.63 to 2.75)b | .46 |
| Proportion of participants in whom fluticasone dose was halved, No. (%) | 91 | 28 (30.8) | 91 | 29 (31.9) | −1.1 (−14.6 to 12.4) | 0.99 (0.66 to 1.52)d | .99 |
| Cumulative fluticasone dose, mg | 96 | 59.6 | 96 | 55.2 | 4.41 (−0.99 to 9.80) | 4.40 (0.001 to 8.80)e | .049 |
| Exploratory outcomes | |||||||
| No. of severe exacerbations | 96 | 0.58f | 96 | 0.51f | 0.07 (−0.14 to 0.28) | 0.13 (−0.25 to 0.52)e | .50 |
| No. of viral-induced severe exacerbations | 86 | 0.26f | 85 | 0.22f | 0.03 (−0.11 to 0.18) | 0.18 (−0.44 to 0.81)g | .56 |
| Serious adverse eventsh | |||||||
| Participants with ≥1 serious adverse events, No. (%) | 96 | 9 (9.4) | 96 | 7 (7.3) | |||
| Total No. of serious adverse eventsi | 96 | 11 | 96 | 9 | |||
| Hospitalizations for asthma exacerbation, No. | 96 | 8 | 96 | 6 | |||
Abbreviation: VDKA, Vitamin D to Prevent Severe Asthma Exacerbations.
Defined as a hospitalization or emergency department visit for asthma requiring systemic corticosteroids, or an asthma exacerbation leading to use of systemic corticosteroids (tablets, suspension, or injection) for at least 3 days.
Hazard ratio stratified by site, sex, and race/ethnicity.
Defined as a nasal blow, collected within 7 days of the exacerbation, in which at least 1 virus was detected by polymerase chain reaction in a panel of common respiratory viruses (average, approximately 95% sensitivity and 99% specificity).
Relative risk ratio adjusted for sex, race/ethnicity, and days of follow-up in the study (model failed to converge when site included as covariate).
Parameter estimate (beta coefficient, or adjusted difference in means) from Poisson regression adjusted for study site, sex, race/ethnicity, and days of follow-up in the study.
Mean number of exacerbations per participant.
Parameter estimate (beta coefficient, or adjusted difference in means) from Poisson regression adjusted for sex, race/ethnicity, and days of follow-up in the study (model failed to converge when site included as covariate).
Serious adverse events reported as comparative frequencies alone; sample size not sufficient to allow meaningful statistical comparisons.
Serious adverse events included hospitalizations (9 in each group), eosinophilia (1), and severe neutropenia (1). There were no instances of hypercalcemia in either group.
Figure 3. Results of the Analysis of Time to a First Severe Asthma Exacerbation.
Vertical bars represent single censored events. The adjusted hazard ratio for time from randomization to a first severe exacerbation was 1.13 (95% CI, 0.69-1.85) for the vitamin D3 vs placebo treatment groups (P = .63). Models were stratified by study site, sex, and race/ethnicity. The median observation time was 274.5 days (interquartile range [IQR], 163-333) in the vitamin D3 group and 321 days (IQR, 196.5-334) in the control group.
Secondary Outcomes
Vitamin D3 supplementation did not significantly prolong the time to a first viral-induced severe exacerbation compared with placebo: the mean number of days was 272 in the vitamin D3 group and 281 in the placebo group, with a mean group difference of −9.1 days (95% CI, −35.5 to 17.2) and an adjusted HR of 1.32 (95% CI, 0.63-2.75; P = .46; Table 2; eFigure in Supplement 2). The proportion of participants whose inhaled corticosteroid dose could be reduced (halved) at the midpoint of the trial was not significantly different between the vitamin D3 (28 participants [31%]) and the placebo (29 participants [32%]) groups (group difference, −1.1% [95% CI, −14.6% to 12.4%]; adjusted relative risk ratio, 0.99 [95% CI, 0.66-1.52]; P = .99) (Table 2). Vitamin D3 supplementation, compared with placebo, did not lead to a significant reduction in the cumulative dose of fluticasone during the trial (59.6 mg vs 55.2 mg, respectively; mean group difference, 4.41 mg [95% CI, −0.99 to 9.80]; adjusted mean difference, 4.40 mg [95% CI, 0.001 to 8.80]; P = .049) (Table 2).
Exploratory Outcomes
Compared with placebo, vitamin D3 supplementation did not significantly reduce the number of severe asthma exacerbations (mean number of exacerbations, 0.58 in the vitamin D3 group vs 0.51 in the placebo group; mean group difference, 0.07 [95% CI, −0.14 to 0.28]; P = .50) or viral-induced severe exacerbations (mean number of viral-induced exacerbations, 0.26 in the vitamin D3 group vs 0.22 in the placebo group; mean group difference, 0.03 [95% CI, −0.11 to 0.18]; P = .56) (Table 2).
Adverse Events
Nine participants in the vitamin D3 group and 7 in the placebo group had a serious adverse event (Table 2). The most common serious adverse events were hospitalizations for asthma (8 in the vitamin D3 group and 6 in the placebo group). After randomization, no participant in the vitamin D3 group and 2 participants in the placebo group had medication stopped due to vitamin D levels less than 10 ng/mL. Two participants had a level less than 10 ng/mL and 2 participants had levels between 10 and 13 ng/mL at 2 visits during the trial; per protocol, all instances were referred to a pediatric endocrinologist for evaluation and management. There were no cases of hypercalcemia or vitamin D toxicity in either treatment group.
Discussion
In this randomized clinical trial of vitamin D3 added to low-dose inhaled corticosteroids in school-aged children with vitamin D insufficiency and persistent asthma at high risk for severe exacerbations, vitamin D3 supplementation did not lead to a significant improvement in the time to a severe asthma exacerbation. Moreover, vitamin D3 supplementation had no significant beneficial effects on any of the trial’s secondary end points, including time to a viral-induced severe asthma exacerbation, the proportion of participants whose inhaled corticosteroid dose was reduced, or the mean or cumulative inhaled corticosteroid dose during the trial. At an observed event rate of approximately 38% in the control group, such effect maps to an HR of about 1.93, which is not included in the 95% CI of the estimate for the primary outcome.
While observational studies have reported associations between low vitamin D levels and childhood asthma outcomes,2,3,4,5 randomized clinical trials have provided less encouraging evidence of a protective effect of vitamin D3 supplementation against morbidity from asthma.13 Moreover, both the VDAART (Vitamin D Antenatal Asthma Reduction Trial) and the COPSAC 2010 (Copenhagen Prospective Studies on Asthma in Childhood 2010 cohort) trials have recently reported no significant effect of vitamin D3 supplementation during pregnancy on asthma in the offspring by age 6 years, strongly suggesting that there is no role for such supplementation in the primary prevention of asthma.23,24,25
The results from this trial cannot be attributed to failure to achieve vitamin D sufficiency after vitamin D3 supplementation because most (>87%) of the participants in the treatment group had vitamin D levels of 30 ng/mL or greater during the trial. The placebo group had a relatively low proportion of participants in the control group achieving and sustaining vitamin D sufficiency (<40%), and participants in the vitamin D3 group had consistently higher vitamin D levels than those in the placebo group throughout the trial. Thus, there was a low risk of a false-negative result due to failure of supplementation in producing a difference in vitamin D levels between treatment groups.
Daily vitamin D3 supplementation at a dose of 4000 IU/d was well tolerated without significant adverse events, as no study participant developed vitamin D toxicity or hypercalcemia. Moreover, no study participant developed rickets, and only a small proportion of participants developed severe vitamin D deficiency (a vitamin D level <10 ng/mL) or had a vitamin D level between 10 and 13 ng/mL at 2 visits during the trial, supporting the appropriateness of the monitoring measures used in the trial. Although vitamin D3 supplementation at 4000 IU/d appears effective in achieving vitamin D sufficiency in most children aged 6 to 16 years with vitamin D levels between 14 and 29 ng/mL, study findings cannot be generalized to settings with no or limited monitoring of vitamin D levels.
Limitations
This study has several limitations. First, the rate of severe asthma exacerbations in both the vitamin D3 (37.5%) and placebo (34.4%) groups were lower than expected, and the study was not adequately powered to assess whether such a small difference was statistically significant. However, there was no evidence of a protective effect of vitamin D against severe asthma exacerbations or viral-induced severe asthma exacerbations, and vitamin D3 supplementation did not lead to a reduction in the mean or cumulative dose of inhaled corticosteroids.
Second, there was limited statistical power to determine whether vitamin D3 supplementation reduces severe asthma exacerbations in children with vitamin D levels less than 20 ng/mL because only a modest proportion of participants had such levels. However, most children with vitamin D levels less than 30 ng/mL in the US have levels greater than 20 ng/mL, and thus the findings from this study are applicable to most children with vitamin D insufficiency in this country.26
Third, the findings cannot be extrapolated to other age groups, including preschool children, or to settings with limited ability to monitor vitamin D levels.
Conclusions
Among children with persistent asthma and low vitamin D levels, vitamin D3 supplementation, compared with placebo, did not significantly improve the time to a severe asthma exacerbation. The findings do not support the use of vitamin D3 supplementation to prevent severe asthma exacerbations in this group of patients.
Trial Protocol
eTable. Vitamin D Levels in Nonsupplemented Participants in the Placebo group
eFigure. Time to First Viral-Induced Severe Asthma Exacerbation
Data Sharing Statement
References
- 1.Centers for Disease Control and Prevention Asthma: most recent asthma data. Updated March 24, 2020. Accessed June 18, 2020. https://www.cdc.gov/asthma/most_recent_data.htm
- 2.Brehm JM, Celedón JC, Soto-Quiros ME, et al. . Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765-771. doi: 10.1164/rccm.200808-1361OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125(5):995-1000. doi: 10.1016/j.jaci.2010.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Sjoukes A, Richards D, et al. . Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184(12):1342-1349. doi: 10.1164/rccm.201107-1239OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm JM, Acosta-Pérez E, Klei L, et al. . Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186(2):140-146. doi: 10.1164/rccm.201203-0431OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112(3):585-592. doi: 10.1016/S0091-6749(03)01855-4 [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Wang L, Jia XX, Lin XX, Zhang WX. Vitamin D alleviates airway remodeling in asthma by down-regulating the activity of Wnt/β-catenin signaling pathway. Int Immunopharmacol. 2019;68:88-94. doi: 10.1016/j.intimp.2018.12.061 [DOI] [PubMed] [Google Scholar]
- 8.Heine G, Niesner U, Chang HD, et al. . 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol. 2008;38(8):2210-2218. doi: 10.1002/eji.200838216 [DOI] [PubMed] [Google Scholar]
- 9.Jeffery LE, Burke F, Mura M, et al. . 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183(9):5458-5467. doi: 10.4049/jimmunol.0803217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telcian AG, Zdrenghea MT, Edwards MR, et al. . Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017;137:93-101. doi: 10.1016/j.antiviral.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 11.Wang TT, Nestel FP, Bourdeau V, et al. . Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Correction appears in J Immunol. 2004 Nov 15;173(10):following 6489. J Immunol. 2004;173(5):2909-2912. doi: 10.4049/jimmunol.173.5.2909 [DOI] [PubMed] [Google Scholar]
- 12.Jolliffe DA, Greenberg L, Hooper RL, et al. . Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5(11):881-890. doi: 10.1016/S2213-2600(17)30306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefanidis C, Martineau AR, Nwokoro C, Griffiths CJ, Bush A. Vitamin D for secondary prevention of acute wheeze attacks in preschool and school-age children. Thorax. 2019;74(10):977-985. doi: 10.1136/thoraxjnl-2019-213278 [DOI] [PubMed] [Google Scholar]
- 14.Forno E, Celedón JC. Predicting asthma exacerbations in children. Curr Opin Pulm Med. 2012;18(1):63-69. doi: 10.1097/MCP.0b013e32834db288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan RA, Sorkness CA, Kosinski M, et al. . Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59-65. doi: 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 16.Liu AH, Zeiger R, Sorkness C, et al. . Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119(4):817-825. doi: 10.1016/j.jaci.2006.12.662 [DOI] [PubMed] [Google Scholar]
- 17.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52(7):2461-2471. doi: 10.1128/JCM.00075-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddel HK, Taylor DR, Bateman ED, et al. ; American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations . An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59-99. doi: 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 19.Covar RA, Szefler SJ, Zeiger RS, et al. ; Childhood Asthma Research and Education Network . Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol. 2008;122(4):741-747.e4. doi: 10.1016/j.jaci.2008.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Guo SS, et al. . 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1-190. [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force . Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 23.Litonjua AA, Carey VJ, Laranjo N, et al. . Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315(4):362-370. doi: 10.1001/jama.2015.18589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brustad N, Eliasen AU, Stokholm J, Bønnelykke K, Bisgaard H, Chawes BL. High-dose vitamin D supplementation during pregnancy and asthma in offspring at the age of 6 years. JAMA. 2019;321(10):1003-1005. doi: 10.1001/jama.2019.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litonjua AA, Carey VJ, Laranjo N, et al. . Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382(6):525-533. doi: 10.1056/NEJMoa1906137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansbach JM, Ginde AA, Camargo CA Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404-1410. doi: 10.1542/peds.2008-2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Vitamin D Levels in Nonsupplemented Participants in the Placebo group
eFigure. Time to First Viral-Induced Severe Asthma Exacerbation
Data Sharing Statement