Key Points
Question
Does CYP2C19 genotype–guided prescription of oral P2Y12 inhibitor therapy after percutaneous coronary intervention (PCI) improve ischemic outcomes in patients with acute coronary syndromes and stable coronary artery disease?
Findings
In this randomized clinical trial that included 5302 patients undergoing PCI and included 1849 patients with CYP2C19 loss-of-function alleles in the primary analysis, genotype-guided selection of oral P2Y12 inhibitor therapy, compared with conventional therapy using clopidogrel, resulted in no significant difference in a composite end point of cardiovascular death, myocardial infarction, stroke, stent thrombosis, or severe recurrent ischemia at 12 months (4.0% vs 5.9%, respectively; hazard ratio, 0.66).
Meaning
Among patients with CYP2C19 loss-of-function alleles who underwent PCI, genotype-guided selection of an oral P2Y12 inhibitor, compared with conventional clopidogrel therapy, did not significantly reduce ischemic events based on the treatment effect that the study was powered to detect at 12 months.
Abstract
Importance
After percutaneous coronary intervention (PCI), patients with CYP2C19*2 or *3 loss-of-function (LOF) variants treated with clopidogrel have increased risk of ischemic events. Whether genotype-guided selection of oral P2Y12 inhibitor therapy improves ischemic outcomes is unknown.
Objective
To determine the effect of a genotype-guided oral P2Y12 inhibitor strategy on ischemic outcomes in CYP2C19 LOF carriers after PCI.
Design, Setting, and Participants
Open-label randomized clinical trial of 5302 patients undergoing PCI for acute coronary syndromes (ACS) or stable coronary artery disease (CAD). Patients were enrolled at 40 centers in the US, Canada, South Korea, and Mexico from May 2013 through October 2018; final date of follow-up was October 2019.
Interventions
Patients randomized to the genotype-guided group (n = 2652) underwent point-of-care genotyping. CYP2C19 LOF carriers were prescribed ticagrelor and noncarriers clopidogrel. Patients randomized to the conventional group (n = 2650) were prescribed clopidogrel and underwent genotyping after 12 months.
Main Outcomes and Measures
The primary end point was a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia at 12 months. A secondary end point was major or minor bleeding at 12 months. The primary analysis was in patients with CYP2C19 LOF variants, and secondary analysis included all randomized patients. The trial had 85% power to detect a minimum hazard ratio of 0.50.
Results
Among 5302 patients randomized (median age, 62 years; 25% women), 82% had ACS and 18% had stable CAD; 94% completed the trial. Of 1849 with CYP2C19 LOF variants, 764 of 903 (85%) assigned to genotype-guided therapy received ticagrelor, and 932 of 946 (99%) assigned to conventional therapy received clopidogrel. The primary end point occurred in 35 of 903 CYP2C19 LOF carriers (4.0%) in the genotype-guided therapy group and 54 of 946 (5.9%) in the conventional therapy group at 12 months (hazard ratio [HR], 0.66 [95% CI, 0.43-1.02]; P = .06). None of the 11 prespecified secondary end points showed significant differences, including major or minor bleeding in CYP2C19 LOF carriers in the genotype-guided group (1.9%) vs the conventional therapy group (1.6%) at 12 months (HR, 1.22 [95% CI, 0.60-2.51]; P = .58). Among all randomized patients, the primary end point occurred in 113 of 2641 (4.4%) in the genotype-guided group and 135 of 2635 (5.3%) in the conventional group (HR, 0.84 [95% CI, 0.65-1.07]; P = .16).
Conclusions and Relevance
Among CYP2C19 LOF carriers with ACS and stable CAD undergoing PCI, genotype-guided selection of an oral P2Y12 inhibitor, compared with conventional clopidogrel therapy without point-of-care genotyping, resulted in no statistically significant difference in a composite end point of cardiovascular death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia based on the prespecified analysis plan and the treatment effect that the study was powered to detect at 12 months.
Trial Registration
ClinicalTrials.gov Identifier: NCT01742117
This open-label randomized trial compares the effect of a genotype-guided oral P2Y12 inhibitor selection strategy vs conventional clopidogrel prescribing on 12-month ischemic outcomes after percutaneous coronary intervention (PCI) in CYP2C19*2/CYP2C19*3 loss-of-function allele carriers with acute coronary syndromes and stable cardiovascular disease.
Introduction
Clopidogrel is the most widely prescribed oral inhibitors of the platelet adenosine diphosphate P2Y12 receptor (P2Y12).1 A drug label “black box warning” cautions against its use in poor metabolizers of hepatic cytochrome P450 enzyme CYP2C19 because it is a prodrug and needs to be biotransformed to an active metabolite by CYP2C19.2 The most common loss-of-function (LOF) alleles, which account for most patients with reduced metabolizer status, are CYP2C19*2 and CYP2C19*3.3 Clopidogrel-treated carriers of CYP2C19 LOF alleles as compared with noncarriers have a higher incidence of ischemic events.4 Despite this association, patients are prescribed clopidogrel without knowledge of CYP2C19 genotype because of lack of prospective evidence demonstrating the clinical utility of genetic testing, ie, whether changing clopidogrel to an alternative oral P2Y12 inhibitor based on CYP2C19 LOF genotype improves clinical outcomes.2 Therefore, current guidelines do not recommend genetic testing when prescribing clopidogrel.2 TAILOR PCI was designed and conducted as a pragmatic, open-label, international, multicenter, randomized clinical trial testing the hypothesis that CYP2C19 genotype–guided use of oral P2Y12 inhibitors as compared with non–genotype-guided conventional clopidogrel therapy significantly reduces ischemic events in CYP2C19 LOF variant carriers after percutaneous coronary intervention (PCI).
Methods
Trial Design
The trial design has been published.1,5 The trial protocol (Supplement 1) and trial protocol amendments (Supplement 2) were approved by the ethics boards of participating sites. An independent National Heart, Lung, and Blood Institute–appointed data and safety monitoring board was responsible for overseeing the conduct and safety of the trial. All participants provided written informed consent.
Patients
Patients 18 years and older with acute coronary syndromes (ACS) or stable coronary artery disease (CAD) who underwent PCI with planned 12 months of dual antiplatelet therapy were eligible. A complete list of exclusion criteria is provided in eTable 1 in Supplement 3. Race/ethnicity was collected because of the genetic nature of the study, its international enrollment, and as required by the National Institutes of Health. Race/ethnicity categories were fixed prior to study initiation and were collected from the patient’s medical record. Patients were enrolled at 40 centers in the US, Canada, South Korea, and Mexico from May 29, 2013, to October 31, 2018, with follow-up completed on October 31, 2019.
Randomization and Interventions
Patients were randomized on a 1:1 ratio stratified by age group, sex, site, and CAD presentation using real-time dynamic allocation through Medidata Balance versions 2013.3.0-2018.4.1 (Medidata). Randomization took place within 72 hours after PCI and less than 24 hours in 87% of patients. The choice of stent, loading dose of oral P2Y12 inhibitors, access site, and choice of lesions to treat were at the discretion of the treating physician. Patients were randomized to either a genotype-guided therapy group using point-of-care genotyping or conventional therapy group without prospective genotyping. Point-of-care genotyping was performed using Spartan Rx (Spartan Bioscience). In the genotype-guided group, those identified as having CYP2C19*2 or *3 LOF alleles (CYP2C19 LOF carriers) were prescribed ticagrelor for maintenance therapy, and noncarriers or those with inconclusive results were prescribed clopidogrel; patients randomized to the conventional therapy group were all prescribed clopidogrel according to drug label instructions. Prasugrel was recommended as an alternative for patients who did not tolerate ticagrelor. All patients received aspirin (81 mg).
All patients provided blood samples at enrollment that were analyzed after 12 months post-PCI by laboratory-based genotyping using TaqMan (Applied Biosystems), to enable uniform comparison of CYP2C19 LOF carriers in both groups. A difference in ischemic outcomes in patients who were CYP2C19 LOF noncarriers receiving clopidogrel in the genotype-guided or conventional therapy groups was not expected. Therefore, the primary analysis was undertaken in only those patients who had CYP2C19 LOF variants identified by laboratory-based genotyping who were randomized to the genotype-guided or conventional therapy group. Patients in the conventional group could not undergo point-of-care genotyping to identify CYP2C19 LOF carriers, as they would not be able to continue clopidogrel and would have to be prescribed alternative P2Y12 therapy because of the black box warning in the drug labeling information, therefore not allowing a randomized comparison.
End Points
Study-related events were assessed at hospital discharge by the study coordinator and at 1 month, 6 months, and 12 months after PCI by telephone. If patients could not be reached by telephone after multiple attempts, the site coordinator conducted a medical record review to assess follow-up. All cardiovascular-related end points and hospitalizations were reviewed and adjudicated by an independent committee blinded to study groups and P2Y12 inhibitor received by the patient. Only study-related events confirmed by the adjudication committee to be end points were included in the analysis.
The primary outcome was the composite of cardiovascular death, myocardial infarction, stroke, definite or probable stent thrombosis, and severe recurrent ischemia at 12 months after index PCI based on standard definitions outlined in the eMethods in Supplement 3. Secondary end points were major or minor bleeding as defined by the Thrombolysis in Myocardial Infarction (TIMI) criteria6; the individual components of the primary end point; all-cause mortality; major bleeding; and bleeding end points of increasing severity defined by Bleeding Academic Research Consortium (BARC) criteria7 (class 2,3 or 5; class 3 or 5; and class 5).6 All time-to-event end points were defined with time of randomization as time zero.
Statistical Analysis
Initial sample size calculations were conducted based on assumed 12-month event rates of the primary end point of 12% in the CYP2C19 LOF carriers receiving clopidogrel and 8% in LOF carriers receiving ticagrelor8 (minimum detectable hazard ratio [HR], 0.65 at 80% power). These assumptions resulted in a required sample size of 1694 LOF carriers. To account for a potential dropout rate of 5%, enrollment of 1784 LOF carriers was planned. Assuming a prevalence of 30% of LOF carriers, a total trial enrollment of 5945 was planned. When enrollment from Korean sites was subsequently added, the total trial enrollment was reduced to 5270, assuming 1015 patients would be enrolled from Korea with a 50% prevalence of LOF carriers. When the trial had enrolled approximately 3800 patients, the Operations and Executive committees approved a reassessment of power because of the overall low event rates observed in the study. The committees approved a revision that retained the a priori sample size (5270), under the assumption of event rates of 6% and 3%, respectively (minimum detectable HR, 0.50), with 85% power. An HR of 0.50 was selected to demonstrate a clinically important absolute risk reduction in the context of the interim observed overall event rate being 4.5% and based on the effect size observed in clopidogrel-treated CYP2C19 LOF carriers in prior observational studies with low event rates.9,10,11 The proposal was additionally reviewed and approved by the data and safety monitoring board and the National Heart, Lung, and Blood Institute. The overall type I error rate was set at .05, with a plan for 3 interim analyses using stopping boundaries by Peto and Haybittle12; thus, the final significance test of the null hypothesis uses P < .0495 as its rejection region.
The primary analysis cohort included randomized patients from both groups identified as CYP2C19 LOF carriers by the laboratory-based platform. Point-of-care genotyping results were not used to determine inclusion in the primary analysis, to maintain uniformity in comparison of results between the 2 randomized groups. The “all randomized” cohort included randomized patients regardless of laboratory-based genotyping results. The per-protocol cohort included patients from the primary analysis cohort meeting all inclusion and exclusion criteria whose first dose of maintenance therapy was concordant with protocol direction. If the null hypothesis of the primary analysis was rejected, a complementary analysis of the primary end point in noncarriers was prespecified to estimate the effect of knowledge of genotype among noncarriers receiving clopidogrel. Patients were analyzed according to their randomized treatment assignment, regardless of medication received, unless otherwise noted.
Event rates at 12 months after PCI were calculated using Kaplan-Meier estimates. Patients who withdrew or who were lost to follow-up were treated as censored at the date of last contact. Patients completing follow-up through the scheduled 12-month follow-up visit were censored at 365 days after index PCI. A Cox proportional hazards model was used to estimate the HR for time to first occurrence of the primary end point, adjusted for sex, age group, and CAD presentation and with site included as a random effect. A 2-sided likelihood ratio test was used to calculate the P value. Hazard ratios are reported with 95% CIs. The same model was used to analyze time to major or minor bleeding in the primary analysis cohort as the primary adverse event analysis. The proportional hazards assumption was investigated by plotting scaled Schoenfeld residuals vs follow-up time and by testing the interaction between treatment groups and the logarithm of follow-up time. When the assumption was violated, a post hoc analysis was undertaken to estimate the treatment effect over different segments of the follow-up period. The time segments were not prespecified and were chosen based on clinical importance of these periods. A Cox proportional hazards model was used to estimate the time-specific treatment effects adjusting for the same covariates as the primary analysis, with time-dependent indicators for genotype-guided treatment. Four prespecified sensitivity analyses were conducted, including per-protocol, recurrent events, time-dependent medication, and multiple imputation for missing laboratory-based genotyping results analyses (see eMethods in Supplement 3 for details). Subgroup analyses were conducted by adding a main effect for the subgroup of interest in the primary analysis model and an interaction term between the subgroup indicator and the treatment group. The P value for the interaction term estimate was used to test for the presence of a treatment interaction between the subgroups. All hypotheses tests were 2-sided with a .05 type I error rate. Because of the potential for type 1 error due to lack of adjustment for multiple comparisons, findings from the analyses of secondary end points and the subgroup analyses should be interpreted as exploratory.
All analyses were conducted with SAS software, version 9.4 (SAS Institute Inc). Additional details of the statistical analyses, including definition of the noncarrier analysis cohort, are reported in the eMethods in Supplement 3.
Results
Trial Patients
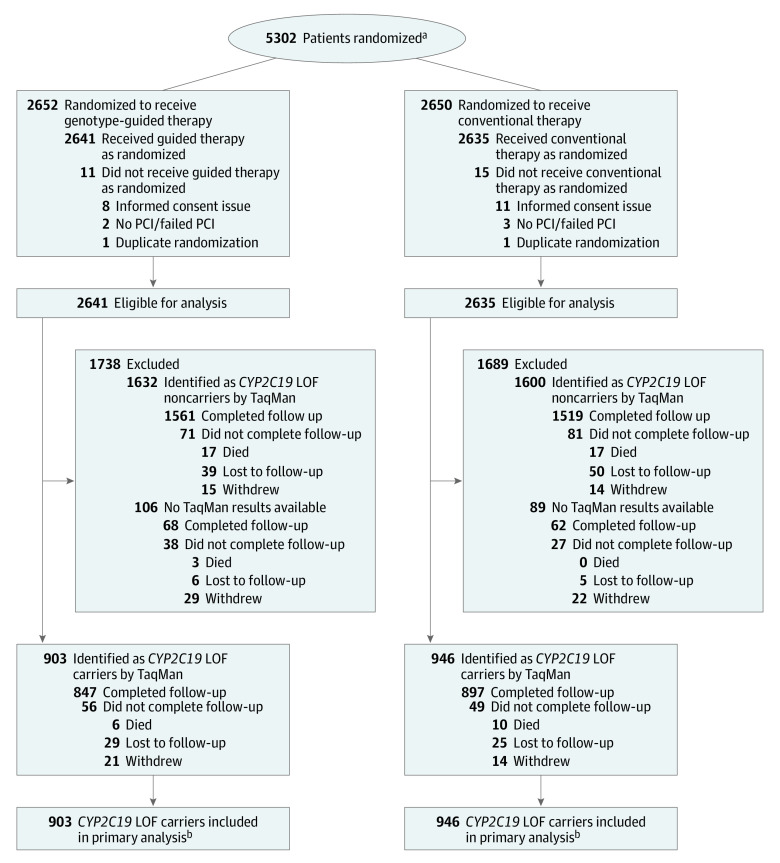
A total of 5302 patients were enrolled (Figure 1; eTable 2 in Supplement 3); of these, 26 patients were excluded from all analyses (19 improperly consented, 5 did not have PCI or failed PCI, 2 were duplicate randomizations). This resulted in inclusion of 5276 patients, with 2641 patients in the genotype-guided therapy group and 2635 in the conventional therapy group. Laboratory-based genotyping results could not be obtained for 195 patients (inadequate or unavailable DNA sample); hence, these patients were not included in the primary analysis, resulting in 903 and 946 CYP2C19 LOF carriers in the genotype-guided and conventional groups, respectively.
Figure 1. Study Flow for the TAILOR-PCI Randomized Clinical Trial.
Patients randomized in spite of the presence of exclusion criteria were still eligible for analysis. In the 5276 patients eligible for analysis, 5007 (94.9%) either completed follow-up or died during follow-up, including 1760 (95.1%) in the primary analysis cohort. The 269 who withdrew or were lost to follow-up had a mean follow-up time of 3.4 months. Of the 195 with laboratory-based genotyping results not available, the reasons were sample not received at biospecimen processing facility (n = 66), low-quality sample not suitable for analysis (n = 60), no index sample taken (n = 59), withdrew consent for use of DNA (n = 10). LOF indicates loss of function; PCI, percutaneous coronary intervention.
aPatients screened for approach but who did not provide consent were not recorded.
beTable 2 in the Supplement details the point-of-care genotyping results and the initial antiplatelet therapy according to treatment group and laboratory-based genotyping results.
Baseline patient characteristics were balanced between the 2 overall randomized groups and between the subgroups of CYP2C19 LOF carriers (Table 1) included in the primary analysis. The concordance between the point-of-care genotyping and laboratory-based genotyping was 99% (eTable 3 in Supplement 3). Point-of-care genotyping results were available within 24 hours of randomization for 99% of patients. Among the CYP2C19 LOF carriers, 85% in the genotype-guided therapy group received ticagrelor and 15% received clopidogrel as initial oral P2Y12 inhibitor therapy after randomization; 99% in the conventional therapy group received clopidogrel. The primary reasons that LOF carriers in the genotype-guided group received clopidogrel are inconclusive or unavailable point-of-care genotyping results and physician or patient preference (eTable 4 in Supplement 3). During the 12-month follow-up period, among the CYP2C19 LOF carriers the percentage of days receiving protocol oral P2Y12 therapy was 85% in the genotype-guided group and 97% in the conventional group. Reasons for switching or discontinuing assigned oral P2Y12 therapy after randomization are reported in eTable 5 in Supplement 3. Five thousand seven patients (95%) had complete follow-up or died during the study, with the remaining 269 either withdrawing before 12 months or being lost to follow-up (Figure 1). The median follow-up time was 364 days.
Table 1. Baseline Patient and Procedural Characteristics.
| No. (%) | ||||
|---|---|---|---|---|
| LOF allele (CYP2C19 *2/*3) carriers | All randomized patients | |||
| Genotype-guided therapy (n = 903) | Conventional therapy (n = 946) | Genotype-guided therapy (n = 2641) | Conventional therapy (n = 2635) | |
| Patient characteristics | ||||
| Age, y | ||||
| Median (range) | 62 (26-95) | 62 (21-93) | 62 (26-95) | 62 (21-93) |
| <50 | 120 (13) | 123 (13) | 327 (12) | 328 (12) |
| 50-59 | 260 (29) | 280 (30) | 737 (28) | 730 (28) |
| 60-69 | 276 (31) | 310 (33) | 867 (33) | 863 (33) |
| 70-74 | 115 (13) | 104 (11) | 333 (13) | 334 (13) |
| 75-79 | 73 (8) | 80 (8) | 216 (8) | 218 (8) |
| ≥80 | 59 (7) | 49 (5) | 161 (6) | 162 (6) |
| Sex | ||||
| Men | 676 (75) | 728 (77) | 1993 (75) | 1990 (76) |
| Women | 227 (25) | 218 (23) | 648 (25) | 645 (24) |
| Race | n = 884 | n = 927 | n = 2578 | n = 2588 |
| White | 442 (50) | 462 (50) | 1750 (68) | 1754 (68) |
| East Asian | 345 (39) | 363 (39) | 595 (23) | 592 (23) |
| South Asian | 62 (7) | 66 (7) | 116 (4) | 120 (5) |
| African American | 17 (2) | 20 (2) | 57 (2) | 67 (3) |
| Othera | 18 (2) | 16 (2) | 60 (2) | 55 (2) |
| Hispanic or Latinx ethnicity | 14/884 (2) | 15/927 (2) | 78/2578 (3) | 70/2588 (3) |
| Country of enrollment | ||||
| US | 345 (38) | 380 (40) | 1359 (51) | 1358 (52) |
| South Korea | 381 (42) | 397 (42) | 654 (25) | 650 (25) |
| Canada | 168 (19) | 161 (17) | 577 (22) | 580 (22) |
| Mexico | 9 (1) | 8 (1) | 51 (2) | 47 (2) |
| BMI, median (IQR)b | 26.9 (24.3-30.9) | 27.0 (24.0-30.6) | 27.9 (24.9-31.8) | 28.1 (24.8-31.9) |
| Comorbidities | ||||
| Hypertension | 531 (59) | 575 (61) | 1636 (62) | 1667 (63) |
| Dyslipidemia | 414 (46) | 416 (44) | 1363 (52) | 1384 (53) |
| Diabetes | 253 (28) | 257 (27) | 733 (28) | 695 (26) |
| Heart failure | 107 (12) | 105 (11) | 225 (9) | 219 (8) |
| Peripheral artery disease | 20 (2) | 18 (2) | 75 (3) | 61 (2) |
| Risk factors | ||||
| Family history of CAD | 279 (31) | 303 (32) | 995 (38) | 1005 (38) |
| Cigarette use | 228 (25) | 239 (25) | 648 (25) | 637 (24) |
| History of PCI | 174 (19) | 188 (20) | 612 (23) | 612 (23) |
| History of MI (excluding index event) | 112 (12) | 111 (12) | 387 (15) | 371 (14) |
| History of CABG surgery | 53 (6) | 53 (6) | 196 (7) | 188 (7) |
| Stroke/TIA | 28 (3) | 27 (3) | 72 (3) | 76 (3) |
| CAD presentation | ||||
| Stable CAD | 127 (14) | 148 (16) | 488 (18) | 484 (18) |
| ACS: unstable angina | 336 (37) | 335 (35) | 830 (31) | 792 (30) |
| ACS: non-STEMI | 250 (28) | 263 (28) | 749 (28) | 785 (30) |
| ACS: STEMI | 190 (21) | 200 (21) | 574 (22) | 574 (22) |
| Pre-PCI LVEF, median (IQR), % | 60 (53-66) | 60 (53-67) | 60 (51-65) | 59 (52-65) |
| Kidney function, eGFR, mL/minc | ||||
| <60 | 100 (12) | 94 (11) | 243 (10) | 296 (12) |
| ≥60 | 738 (88) | 773 (89) | 2171 (90) | 2105 (88) |
| Multivessel disease | 379 (42) | 343 (36) | 1120 (43) | 1099 (42) |
| Procedural characteristics | ||||
| PCI to randomization, median (IQR), h | 4.5 (1.1-19.7) | 4.8 (1.1-20.1) | 7.0 (1.9-20.7) | 8.3 (1.9-20.6) |
| Antithrombin use | ||||
| Unfractionated heparin | 775 (86) | 828 (88) | 2255 (86) | 2262 (86) |
| Bivalirudin | 91 (10) | 96 (10) | 340 (13) | 329 (13) |
| Low-molecular-weight heparin | 38 (4) | 47 (5) | 130 (5) | 148 (6) |
| GpIIb-IIIa inhibitor use | 55 (6) | 88 (9) | 264 (10) | 270 (10) |
| Loading medication | ||||
| Clopidogrel | 606 (67) | 622 (66) | 1786 (68) | 1792 (68) |
| Ticagrelor | 219 (24) | 238 (25) | 587 (22) | 620 (24) |
| Prasugrel | 19 (2) | 23 (2) | 74 (3) | 53 (2) |
| Ticlopidine | 1 (<1) | 1 (<1) | 1 (<1) | 1 (<1) |
| Other | 4 (<1) | 2 (<1) | 8 (<1) | 5 (<1) |
| None | 52 (6) | 60 (6) | 168 (6) | 160 (6) |
| No. of stents placed, median (IQR) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) |
| Artery treated | ||||
| Left anterior descending | 456 (51) | 500 (53) | 1356 (52) | 1355 (52) |
| Right coronary | 312 (35) | 329 (35) | 935 (36) | 931 (35) |
| Left circumflex | 239 (26) | 253 (27) | 683 (26) | 727 (28) |
| Left main coronary | 32 (4) | 21 (2) | 71 (3) | 56 (2) |
| First antiplatelet after randomization | ||||
| Clopidogrel | 132 (15) | 932 (99) | 1790 (68) | 2586 (99) |
| Ticagrelor | 764 (85) | 9 (1) | 822 (31) | 35 (1) |
| Prasugrel | 4 (<1) | 2 (<1) | 9 (<1) | 3 (<1) |
| Cilostazol | 1 (<1) | 0 | 1 (<1) | 0 |
| Time from PCI to first postrandomization antiplatelet therapy, median (IQR), h | 21.7 (10.1-26.1) | 21.9 (17.7-25.0) | 21.9 (17.2-28.5) | 21.9 (17.8-27.9) |
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; GpIIb-IIIa, glycoprotein IIb/IIIa complex; IQR, interquartile range; LOF, loss of function; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack.
Other race consists of 130 patients who indicated “other” and 19 who indicated “Native American Indian or Native Alaskan.”
Calculated as weight in kilograms divided by height in meters squared.
eGFR calculated by Modification of Diet in Renal Disease equation. Equation = 186*([serum creatinine]^(–1.154))*([age, y]^(–0.203))*(0.742^[female])*(1.21^[black]).
Primary End Point
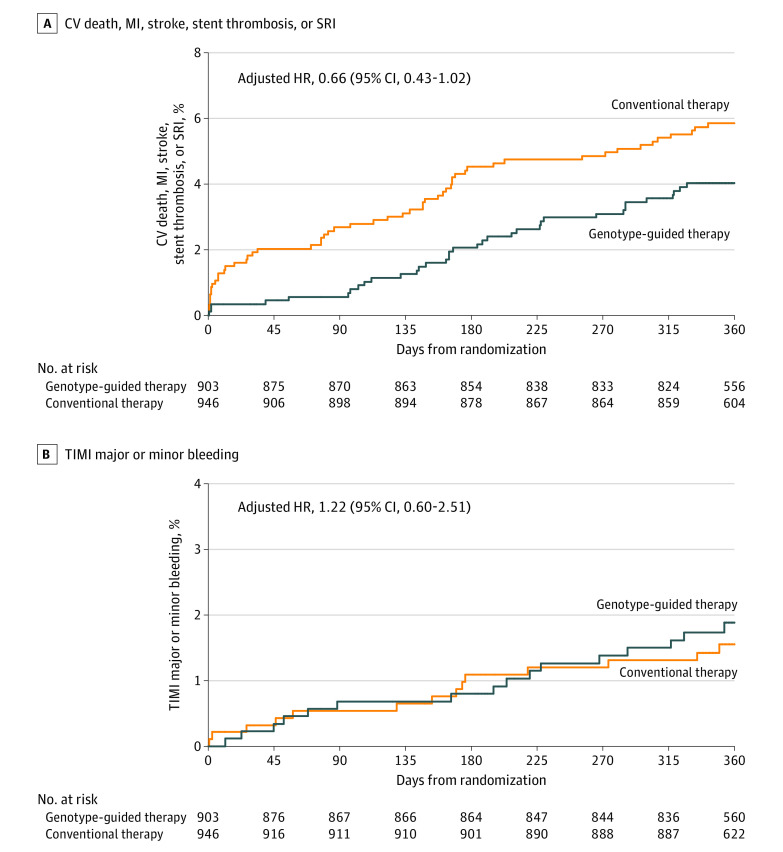
The primary end point occurred in 35 (4.0%) of the genotype-guided therapy group CYP2C19 LOF carriers vs 54 (5.9%) of the conventional therapy group CYP2C19 LOF carriers at 12 months (Table 2 and Figure 2A). The absolute difference of 1.8% (5.85% vs 4.03%) in primary outcomes between the 2 groups in the CYP2C19 LOF carriers did not meet the predetermined level of statistical significance for superiority (HR, 0.66 [95% CI, 0.43-1.02]; P = .06).
Table 2. Primary and Secondary End Points in CYP2C19 Loss-of-Function Allele Carriers.
| No. (%) | Difference in 12-mo event rates, % (95% CI)a | HR for genotype-guided therapy (95% CI)b | P valueb | ||
|---|---|---|---|---|---|
| Genotype-guided therapy (N = 903) | Conventional therapy (N = 946) | ||||
| Primary end point | |||||
| CV death, MI, stroke, severe recurrent ischemia, stent thrombosis | 35 (4.0) | 54 (5.9) | −1.8 (−3.9 to 0.1) | 0.66 (0.43 to 1.02) | .06 |
| Secondary end points | |||||
| Severe recurrent ischemia | 19 (2.2) | 29 (3.2) | −1.0 (−2.6 to 0.5) | 0.68 (0.38 to 1.22) | .19 |
| BARC bleeding | |||||
| 2,3,5c,d | 26 (3.0) | 16 (1.8) | 1.3 (−0.1 to 2.7) | 1.72 (0.92 to 3.20) | .08 |
| 3,5c,d | 17 (2.0) | 14 (1.5) | 0.5 (−0.8 to 1.8) | 1.27 (0.63 to 2.59) | .50 |
| TIMI major or minor bleeding (primary adverse events end point) | 16 (1.9) | 14 (1.6) | 0.3 (−0.9 to 1.6) | 1.22 (0.60 to 2.51) | .58 |
| Myocardial infarction | 11 (1.3) | 14 (1.5) | −0.3 (−1.3 to 0.8) | 0.82 (0.37 to 1.81) | .62 |
| Major bleeding | 11 (1.3) | 11 (1.2) | 0.1 (−1.0 to 1.1) | 1.05 (0.45 to 2.44) | .90 |
| Death from any cause | 6 (0.7) | 10 (1.1) | −0.4 (−1.2 to 0.5) | 0.56 (0.20 to 1.54) | .25 |
| CV death | 4 (0.5) | 8 (0.9) | −0.4 (−1.2 to 0.4) | 0.49 (0.15 to 1.64) | .24 |
| Stent thrombosis | 2 (0.2) | 8 (0.9) | −0.6 (−1.4 to 0.0) | 0.25 (0.05 to 1.18) | .05 |
| Minor bleeding | 5 (0.6) | 3 (0.3) | 0.2 (−0.3 to 0.9) | 2.27 (0.57 to 9.08) | .23 |
| Stroke | 2 (0.2) | 4 (0.4) | −0.2 (−0.8 to 0.3) | 0.51 (0.09 to 2.79) | .42 |
Abbreviations: BARC, Bleeding Academic Research Consortium; CV, cardiovascular; HR, hazard ratio; MI, myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Confidence intervals for differences in Kaplan-Meier rates were estimated by bootstrapping.
Hazard ratios, confidence intervals, and P values are from Cox proportional hazards regression models adjusting for age, sex, coronary artery disease presentation, and site (factors used for stratified randomization).
BARC 5 results not shown, as there are were no fatal bleeds.
BARC is a classification system for bleeding events categorizing bleeds into levels of severity, with higher numbers indicating greater severity. Class 2 are generally overt bleeds requiring medical intervention or evaluation but with minimal blood loss (<3-g/dL decrease in hemoglobin level); class 3 is generally more serious (either in amount of bleeding or location of bleed); class 4 is bleeding related to coronary artery bypass graft surgery; class 5 is fatal bleeding. More precise descriptions can be found in Mehran et al.7
Figure 2. Event Rates in the Primary Analysis Cohort.
A, Kaplan-Meier estimated event rates in the 2 treatment groups in the primary analysis cohort of CYP2C19 loss-of-function carriers for the primary end point of time to cardiovascular (CV)–related death, myocardial infarction (MI), stroke, stent thrombosis, or severe recurrent ischemia (SRI). B, Kaplan-Meier estimated event rates for the primary adverse event end point of Thrombolysis in Myocardial Infarction (TIMI) major or minor bleeding in the primary analysis cohort. The median observation time for the genotype-guided therapy group was 364 days (interquartile range, 360-365) and for the conventional therapy group was 364 days (interquartile range, 353-365). HR indicates hazard ratio.
Secondary End Points
The adverse event end point (TIMI major or minor bleeding) was observed in 30 patients in the primary analysis cohort with no significant difference between the genotype-guided therapy group (16 [1.9%]) and the conventional therapy group (14 [1.6%]) CYP2C19 LOF carriers at 12 months (HR, 1.22 [95% CI, 0.60-2.51]) (Table 2 and Figure 2B). None of the other secondary end points were significantly different between the 2 treatment groups, including the other bleeding-related end points (Table 2).
Prespecified Sensitivity Analyses of the Primary Outcome
The robustness of the primary analysis was investigated by several prespecified sensitivity analyses. An analysis allowing for multiple events per patient favored the use of genotype-guided as compared with conventional therapy in CYP2C19 LOF carriers (HR, 0.60 [95% CI, 0.41-0.89]; P = .01). Using multiple imputation analysis for patients excluded from the primary analysis because of missing laboratory-based genotyping results, the estimated HR was similar to the primary analysis results (HR, 0.68 [95% CI, 0.45-1.04]). To address the 15% lack of adherence to ticagrelor in the genotype-guided CYP2C19 LOF carriers, time-dependent variables were used to model actual medication usage over time in the CYP2C19 LOF carriers rather than treatment groups; the HR for ticagrelor vs clopidogrel was 0.69 (95% CI, 0.44-1.10).
Subgroup and Additional Analyses
The primary outcome was also evaluated in 11 prespecified subgroups in CYP2C19 LOF carriers, with no significant subgroup interactions detected (Figure 3).
Figure 3. Subgroup Analyses of the Primary Outcome.
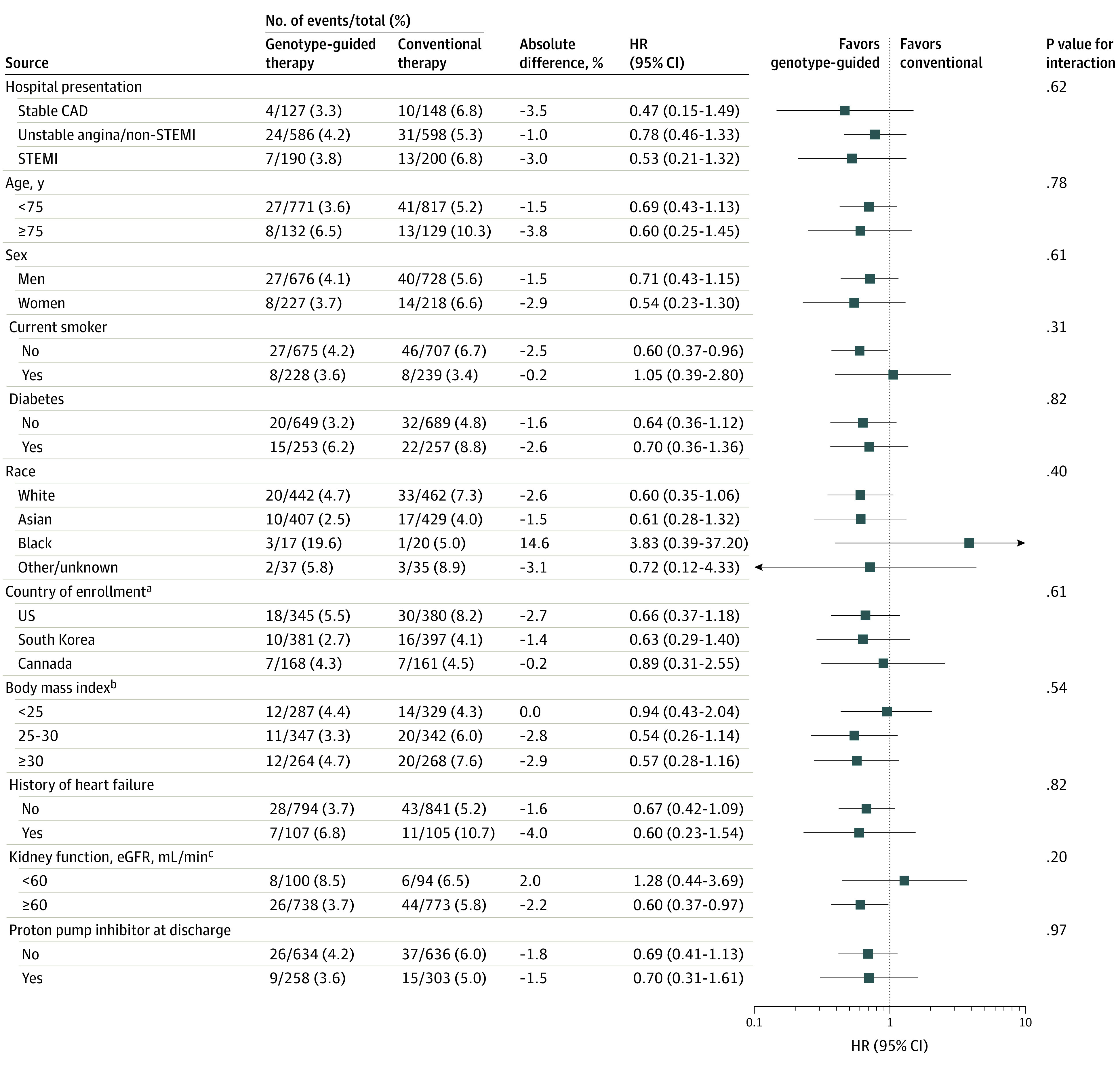
Hazard ratios and 95% CIs for the effect of genotype-guided therapy within CYP2C19 loss-of-function carriers were estimated within clinically relevant prespecified subgroups. The number of events and the sample size of each subgroup as well as the Kaplan-Meier estimated event rates at 12 months are provided according to the 2 treatment groups. P values for tests of interaction have not been adjusted for multiplicity. CAD indicates coronary artery disease; eGFR, estimated glomerular filtration rate; STEMI, ST-segment elevation myocardial infarction.
aThe hazard ratio for the Mexico subgroup is not shown, as there were no events in the genotype-guided therapy group and only 1 event in the conventional therapy group.
bCalculated as weight in kilograms divided by height in meters squared.
ceGFR calculated by Modification of Diet in Renal Disease equation.
When patients in the per-protocol cohort in the genotype-guided therapy (n = 815) and conventional therapy (n = 930) groups were analyzed, results similar to those from the primary analysis cohort were observed (HR, 0.68 [95% CI, 0.44-1.05]) (eTable 6 and eFigure 1 in Supplement 3). Results of analysis for TIMI bleeding in the per-protocol cohort were consistent with those in the primary analysis (HR, 1.29 [95% CI, 0.63-1.84]) (eTable 6 and eFigure 2 in Supplement 3). However, BARC 2,3,5 bleeding in the per-protocol genotype-guided group (3.3%) in this study was increased compared with that in the per-protocol conventional therapy group (1.7%) (HR, 1.96 [95% CI, 1.04-3.71]; P = .03) (eTable 6 in Supplement 3).
When examined in the 2 overall randomized groups (genotype-guided vs conventional therapy), the primary end point was not significantly different. There were 113 (4.4%) primary events in the 2641 genotype-guided group and 135 (5.3%) primary events in the 2635 conventional group (HR, 0.84 [95% CI, 0.65-1.07]; P = .16) (eTable 7 and eFigure 3 in Supplement 3). Similarly, in the all randomized cohort there was no significant difference in TIMI major/minor bleeding episodes in the overall genotype-guided group (1.4%) as compared with the conventional group (1.2%) (HR, 1.13 [95% CI, 0.70-1.84]) (eTable 7 and eFigure 4 in Supplement 3). The primary and secondary end point analyses in the noncarrier cohort are described in eTable 8 and eFigures 5 and 6 in Supplement 3.
Post Hoc Analysis of the Primary Outcome
The test of the proportional hazards assumption was significant (P = .03), suggesting that the assumption may not hold; this was further supported by graphical displays (eFigure 7 in Supplement 3). Therefore, a post hoc analysis was performed that demonstrated an estimated HR for the CYP2C19 LOF genotype-guided therapy group as compared with the conventional therapy group of 0.21 (95% CI, 0.08-0.54; P = .001) at 0 to 3 months’ follow-up, 0.78 (95% CI, 0.38-1.61; P = .50) at 3 to 6 months’ follow-up, and 1.44 (95% CI, 0.69-3.03; P = .33) at 6 to 12 months’ follow-up.
Discussion
Among CYP2C19 LOF carriers with ACS and stable CAD who underwent PCI, genotype-guided oral P2Y12 inhibitor therapy, compared with conventional clopidogrel therapy without point-of-care genotyping, resulted in no significant difference in a composite end point of cardiovascular death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia at 12 months. These results should be interpreted in the context of the treatment effect (50% reduction in ischemic events) that the study was powered to detect based on the prespecified analysis plan. This study is the first clinical trial to our knowledge to prospectively address the potential utility of genotype-guided oral P2Y12 inhibitor therapy as compared with conventional therapy.
The advent of point-of-care genotyping technology to personalize P2Y12 therapy, as shown in the RAPID GENE study, provides proof of concept to apply the technology early after PCI.13 Time-to-first-event analysis, which was the analysis of the primary end point of this trial, does not account for recurrent events that can occur during the follow-up period. Measuring the total burden of recurrent events in a study population is reflective of overall morbidity, and studying the effect of an intervention such as genotype-guided antiplatelet therapy, as was done in a prespecified analyses in this trial, on cumulative ischemic end points is important.14
As in other post-PCI trials using newer-generation drug-eluting stents, the primary event rate in this trial was much lower than the event rate assumed when the trial was initially designed, necessitating a recalculation of power. The use of ticagrelor as compared with clopidogrel without a genotyping strategy in the PLATO trial decreased ischemic events (HR, 0.84) in 18 624 patients with ACS, with an overall ischemic event rate that ranged from 9.8% to 11.7%.15 In the current study a lower HR of 0.5 was selected that was greater than that when using ticagrelor for all patients, irrespective of genotype status and based on the large effect size of the CYP2C19 genotype noted in other observational studies with low event rates.9,10,11 The trial’s primary results did not meet the predetermined level of statistical significance. The potential effect of a precision medicine approach may be more important early after PCI, as suggested in the post hoc analysis that demonstrated the potential benefit of genotype-guided oral P2Y12 inhibitor therapy in the first 3 months after PCI, and may question the 12-month duration of follow-up in this trial to demonstrate the efficacy of such an approach.
All patients in the conventional therapy group in this trial were assigned clopidogrel, specifically to provide guidance to the medical practitioner regarding the utility of genetic testing when prescribing clopidogrel. The relevance and importance of this approach is demonstrated by the use of clopidogrel in 44% to 72%16 of patients after PCI and in up to 51% to 70%17 of patients with ACS.18,19 Prescription data from the OptumLabs Data Warehouse, a large national administrative claims database that includes longitudinal health data of more than 120 million individuals enrolled in private and Medicare Advantage health plans, were analyzed to evaluate which antiplatelet agent was initiated after PCI in 2018. Clopidogrel was prescribed in 61% of patients after PCI, ticagrelor in 31%, and prasugrel in 8%.
In contrast to this trial, patients with MI in the conventional therapy group in the POPular Genetics trial received ticagrelor after PCI and were compared with patients receiving genotype-guided P2Y12 inhibitors, demonstrating noninferiority with event rates of 4.6% and 4.7%, respectively, at 12 months.20 In clinical practice the question arises whether a genotype-guided choice of P2Y12 inhibitors vs clopidogrel for all or ticagrelor for all without point-of-care genotyping is an appropriate strategy. This trial was not powered to demonstrate superiority in outcomes of the overall genotype-guided therapy group as compared with the overall group receiving clopidogrel. However, in this study, the ischemic event rate of 4.4% in the genotype-guided group was similar to the rate for ticagrelor for all groups in the POPular Genetics trial, highlighting the efficacy of a genotype-guided strategy. The TIMI bleeding rates in both groups in the current study were similar and relatively low (≤2% at 12 months after PCI). In contrast, POPular Genetics and other clinical trials have consistently reported higher bleeding rates with ticagrelor for all use compared with a CYP2C19 genotype-guided oral P2Y12 inhibitor strategy or clopidogrel for all, respectively.15,20 Consistent with these and other studies, BARC 2,3,5 bleeding in the per-protocol genotype-guided group in this study was increased compared with the conventional therapy group.
Limitations
This study has several limitations. First, the trial was underpowered to detect an effect size less than the 50% relative risk reduction used in the revised sample size calculation. Second, the pragmatic nature of the trial, which relied on provision of P2Y12 inhibitors by an individual’s health plan, may have led to some patients not receiving designated antiplatelet therapy. However, the per-protocol analysis demonstrated findings similar to those from the primary analysis. Third, the trial was open-label; however, the conventional therapy group was blinded for the primary analysis, since genotyping was performed 12 months after PCI in that group and adjudication of all events was blinded.
Conclusions
Among CYP2C19 LOF carriers with ACS and stable CAD undergoing PCI, genotype-guided selection of an oral P2Y12 inhibitor, compared with conventional clopidogrel therapy without point-of-care genotyping, resulted in no statistically significant difference in a composite end point of cardiovascular death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia based on the prespecified analysis plan and the treatment effect that the study was powered to detect at 12 months.
Trial Protocol
Trial Protocol Amendments
e-Methods
eFigure 1. The Kaplan-Meier Estimated Event Rates for the Primary Endpoint in the Per Protocol Analysis Cohort
eFigure 2. The Kaplan-Meier Estimated Event Rates for TIMI Major or Minor Bleeding in the Per Protocol Analysis Cohort
eFigure 3. The Kaplan-Meier Estimated Event Rates for the Primary Endpoint in the All Randomized Analysis Cohort
eFigure 4. The Kaplan-Meier Estimated Event Rates for TIMI Major or Minor Bleeding in the All Randomized Analysis Cohort
eFigure 5. The Kaplan-Meier Estimated Event Rates for the Primary Endpoint in the CYP2C19*2/*3 Non- Carrier Analysis Cohort
eFigure 6. The Kaplan-Meier Estimated Event Rates for the TIMI Major or Minor Bleeding in the CYP2C19*2/*3 Non-Carrier Analysis Cohort
eFigure 7. A Plot of Scaled Schoenfeld Residuals Resulting From the Cox Model for Estimating the Effect of Genotype-Guided Therapy on the Primary Endpoint Within CYP2C19 LOF Patients
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Spartan Platform (Point of Care Genotyping) Testing Results and First Antiplatelet Medication Received Post-Randomization Tabulation Within the Terminal Groups of the CONSORT Diagram
eTable 3. Genotyping Results by Spartan (Point of Care Genotyping) and TaqMan (Laboratory Based Genotyping) Platforms
eTable 4. Reasons for Clopidogrel Assignment in CYP2C19*2 or *3 Carriers in the Genotype-Guided Therapy Group
eTable 5. Reasons for Discontinuation of Protocol Prescribed Antiplatelet Therapy
eTable 6. Primary, Secondary and Safety Endpoints in CYP2C19 LOF Patients in the Per Protocol Cohort
eTable 7. Primary, Secondary and Safety Endpoints in all Randomized Patients Eligible for Analysis
eTable 8. Primary, Secondary and Safety Endpoints in CYP2C19 LOF Non-carriers
eReference
Data Sharing Statement
References
- 1.Pereira NL, Rihal CS, So DYF, et al. Clopidogrel pharmacogenetics. Circ Cardiovasc Interv. 2019;12(4):e007811. doi: 10.1161/CIRCINTERVENTIONS.119.007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes DR Jr, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; Writing Committee Members . ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation. 2010;122(5):537-557. doi: 10.1161/CIR.0b013e3181ee08ed [DOI] [PubMed] [Google Scholar]
- 3.Pereira NL, Weinshilboum RM. Cardiovascular pharmacogenomics and individualized drug therapy. Nat Rev Cardiol. 2009;6(10):632-638. doi: 10.1038/nrcardio.2009.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mega JL, Simon T, Collet J-P, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821-1830. doi: 10.1001/jama.2010.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira NL, Sargent DJ, Farkouh ME, Rihal CS. Genotype-based clinical trials in cardiovascular disease. Nat Rev Cardiol. 2015;12(8):475-487. doi: 10.1038/nrcardio.2015.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase: clinical findings through hospital discharge. Circulation. 1987;76(1):142-154. doi: 10.1161/01.CIR.76.1.142 [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 8.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360(4):354-362. doi: 10.1056/NEJMoa0809171 [DOI] [PubMed] [Google Scholar]
- 9.Oh I-Y, Park KW, Kang S-H, et al. Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart. 2012;98(2):139-144. doi: 10.1136/hrt.2011.227272 [DOI] [PubMed] [Google Scholar]
- 10.Ono T, Kaikita K, Hokimoto S, et al. Determination of cut-off levels for on-clopidogrel platelet aggregation based on functional CYP2C19 gene variants in patients undergoing elective percutaneous coronary intervention. Thromb Res. 2011;128(6):e130-e136. doi: 10.1016/j.thromres.2011.07.028 [DOI] [PubMed] [Google Scholar]
- 11.Zou J-J, Xie H-G, Chen S-L, et al. Influence of CYP2C19 loss-of-function variants on the antiplatelet effects and cardiovascular events in clopidogrel-treated Chinese patients undergoing percutaneous coronary intervention. Eur J Clin Pharmacol. 2013;69(4):771-777. doi: 10.1007/s00228-012-1392-5 [DOI] [PubMed] [Google Scholar]
- 12.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44(526):793-797. doi: 10.1259/0007-1285-44-526-793 [DOI] [PubMed] [Google Scholar]
- 13.Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379(9827):1705-1711. doi: 10.1016/S0140-6736(12)60161-5 [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181(7):532-540. doi: 10.1093/aje/kwu289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 16.Dayoub EJ, Seigerman M, Tuteja S, et al. Trends in platelet adenosine diphosphate P2Y12 receptor inhibitor use and adherence among antiplatelet-naive patients after percutaneous coronary intervention, 2008-2016. JAMA Intern Med. 2018;178(7):943-950. doi: 10.1001/jamainternmed.2018.0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basra SS, Wang TY, Simon DN, et al. Ticagrelor use in acute myocardial infarction: insights from the National Cardiovascular Data Registry. J Am Heart Assoc. 2018;7(12):e008125. doi: 10.1161/JAHA.117.008125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karve AM, Seth M, Sharma M, et al. Contemporary use of ticagrelor in interventional practice (from Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Am J Cardiol. 2015;115(11):1502-1506. doi: 10.1016/j.amjcard.2015.02.049 [DOI] [PubMed] [Google Scholar]
- 19.Gandhi S, Zile B, Tan MK, et al. ; Canadian ACS Reflective Group . Increased uptake of guideline-recommended oral antiplatelet therapy: insights from the Canadian Acute Coronary Syndrome Reflective. Can J Cardiol. 2014;30(12):1725-1731. doi: 10.1016/j.cjca.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 20.Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381(17):1621-1631. doi: 10.1056/NEJMoa1907096 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Trial Protocol Amendments
e-Methods
eFigure 1. The Kaplan-Meier Estimated Event Rates for the Primary Endpoint in the Per Protocol Analysis Cohort
eFigure 2. The Kaplan-Meier Estimated Event Rates for TIMI Major or Minor Bleeding in the Per Protocol Analysis Cohort
eFigure 3. The Kaplan-Meier Estimated Event Rates for the Primary Endpoint in the All Randomized Analysis Cohort
eFigure 4. The Kaplan-Meier Estimated Event Rates for TIMI Major or Minor Bleeding in the All Randomized Analysis Cohort
eFigure 5. The Kaplan-Meier Estimated Event Rates for the Primary Endpoint in the CYP2C19*2/*3 Non- Carrier Analysis Cohort
eFigure 6. The Kaplan-Meier Estimated Event Rates for the TIMI Major or Minor Bleeding in the CYP2C19*2/*3 Non-Carrier Analysis Cohort
eFigure 7. A Plot of Scaled Schoenfeld Residuals Resulting From the Cox Model for Estimating the Effect of Genotype-Guided Therapy on the Primary Endpoint Within CYP2C19 LOF Patients
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Spartan Platform (Point of Care Genotyping) Testing Results and First Antiplatelet Medication Received Post-Randomization Tabulation Within the Terminal Groups of the CONSORT Diagram
eTable 3. Genotyping Results by Spartan (Point of Care Genotyping) and TaqMan (Laboratory Based Genotyping) Platforms
eTable 4. Reasons for Clopidogrel Assignment in CYP2C19*2 or *3 Carriers in the Genotype-Guided Therapy Group
eTable 5. Reasons for Discontinuation of Protocol Prescribed Antiplatelet Therapy
eTable 6. Primary, Secondary and Safety Endpoints in CYP2C19 LOF Patients in the Per Protocol Cohort
eTable 7. Primary, Secondary and Safety Endpoints in all Randomized Patients Eligible for Analysis
eTable 8. Primary, Secondary and Safety Endpoints in CYP2C19 LOF Non-carriers
eReference
Data Sharing Statement