ABSTRACT
Background:
Sepsis often induces an immunosuppressive state, which is associated with high mortality rates. Immunostimulation may be beneficial for sepsis. We investigated the pharmacokinetics, pharmacodynamics, and safety of nivolumab, a human programmed death-1 immune checkpoint inhibitor approved for the treatment of several cancers.
Methods:
In this multicenter, open-label phase 1/2 study, a single 480 or 960 mg nivolumab dose was intravenously infused into Japanese patients with immunosuppressive sepsis. Doses were selected to mimic the exposure achieved with the approved dosage for cancer patients (3 mg/kg every 2 weeks [Q2W]).
Results:
Single 480 and 960 mg nivolumab doses were intravenously infused into five and eight patients, respectively. The maximum concentration after 480 mg (132 μg/mL) was similar to the predicted concentration at the end of infusion with 3 mg/kg Q2W (117 μg/mL). The concentration on Day 28 after 960 mg (33.1 μg/mL) was within the predicted trough concentration range for 3 mg/kg Q2W (90% prediction interval 19.0–163 μg/mL). Absolute lymphocyte counts and monocyte human leukocyte antigen-DR subtype expression levels appeared to increase over time. The incidences of adverse events (AEs) were 80% and 50% in the 480 mg and 960 mg groups, respectively. Drug-related AEs were observed in only one patient in the 480 mg group. No deaths related to nivolumab occurred.
Conclusions:
A single dose of 960 mg nivolumab appeared to be well tolerated and sufficient to maintain nivolumab blood concentrations. Both 480 mg and 960 mg nivolumab seemed to improve immune system indices over time.
Trial registration:
JAPIC, JapicCTI-173600.
Keywords: Anti-programmed death-1 (PD-1), clinical trial, immune checkpoint blockade, immunostimulation, immunosuppression, lymphopenia
Abbreviations: AE, adverse event, AUC, area under the curve, AUCinf, average AUC from time 0 to infinity, C14d, concentration on day 14, C28d, concentration on day 28, Ceoi, end of infusion concentration, CL, apparent total body clearance of the drug from serum, Cmax, maximum concentration, Cmin, trough concentration, CTCAE, Common Terminology Criteria for Adverse Events, FAS, full analysis set, ICU, intensive care unit, mAb, monoclonal antibody, mHLA-DR, monocyte human leukocyte antigen-DR subtype, PD-1, programmed death-1, Q2W, every 2 weeks, rTM, recombinant thrombomodulin, SD, standard deviation, SOFA, Sequential Organ Failure Assessment, T1/2, half-life, Tmax, time to maximum concentration, TSH, thyroid stimulating hormone, Vd, apparent volume of distribution, Vss, volume of distribution under steady state conditions
INTRODUCTION
Sepsis, a life-threatening syndrome characterized by organ dysfunction, is initiated by community acquired infections or those acquired in health care settings such as hospitals (1–3). Septic shock is a subset of sepsis that is associated with an increased risk of mortality due to underlying circulatory, cellular, and metabolic complications (4).
Sepsis is a major health burden that occurs in more than one million individuals annually in the US (5). Ninety-day mortality after severe sepsis has been reported to be approximately 40%, and those who survive have an increased risk of mortality and long-term morbidity (6–12). An investigation by the Japanese Society of Intensive Care Medicine on the prognosis for sepsis patients analyzed data from 266 patients registered in the Sepsis Registry of the Japanese Society of Intensive Care Medicine from October 1 to December 31, 2007 (13). This investigation reported a 28-day mortality rate of 36.4% and an in-hospital mortality rate of 37.6% in sepsis patients.
Recently, a number of clinical studies have investigated non-selective and selective anti-inflammatory drugs, as well as a range of immunotherapeutic drugs for sepsis. However, these strategies have all failed to reach clinical significance or have been stopped mid-study after failing to reduce mortality rates (14). A potential explanation for this may be found in the data on the pathophysiology of sepsis, which shows that different immunological mechanisms play different roles over the course of an infection (15, 16).
During the early stages of septic shock, there is a release of inflammatory mediators in response to infection. However, this inflammatory response is often excessive, and the body suppresses this through negative feedback mechanisms. This immunosuppression has important clinical consequences including an increased risk of secondary infection, viral reactivation, organ dysfunction, and mortality (17, 18). Considering this, anti-inflammatory therapies that target pro-inflammatory biomarkers may only be effective during the proximal phases, whereas therapies that improve immune competence could resolve the primary infection and prevent secondary infection (19, 20). Moreover, upregulation of the PD-1/PD-L1 pathway was observed in murine models of sepsis; immune function increased when this pathway was blocked, resulting in enhanced elimination of bacteria, a decrease in inflammatory cytokines, and a decrease in mortality due to the mitigation of organ damage (21–25). Upregulation of the PD-1/PD-L1 pathway was also observed in sepsis patients and was associated with an increased risk of secondary infection and death; there is also evidence that the function of innate and acquired immune cells improves when this pathway is blocked ex vivo(21, 26–29). Therefore, we investigated the pharmacokinetics, pharmacodynamics, and safety of nivolumab (Ono Pharmaceutical Co, Ltd, Osaka, Japan; and Bristol-Myers Squibb, Princeton, NJ) in patients with sepsis-induced immunosuppression.
Nivolumab is a monoclonal antibody directed against programmed death-1 (PD-1), a CD28 family receptor expressed on activated lymphocytes, that has been proven successful as an immunomodulatory drug in certain types of cancers (30–32). The 2018 Nobel Prize in physiology or medicine was awarded for the discovery of PD-1 (33–35). Nivolumab is an immune-checkpoint inhibitor that blocks inhibitory pathways, allowing T cells to become active. Therefore, nivolumab may also have the potential to improve the immunosuppression associated with sepsis. Preclinical studies to date have shown that anti-PD-1 antibodies can improve survival rates in murine models of sepsis (21, 23–25). Moreover, survival rates were high in adult patients carrying the A allele of the PD1 single-nucleotide polymorphism (rs11568821) that was suggested to disrupt its transcriptional activity (36).
This study, therefore, aimed to evaluate the pharmacokinetics, pharmacodynamics, safety, and tolerability of a single administration of nivolumab in Japanese patients with sepsis-induced immunosuppression.
PATIENTS AND METHODS
Patients
Patients who provided written informed consent were enrolled in the study (enrolled patients) and those patients who met the following criteria were administered the study drug (treated patients): ≥20 years of age; having a documented or suspected infection (suspected infection being treated with an ongoing antimicrobial); onset of sepsis or septic shock ≥24 h before enrollment; at least one of the three organ dysfunction criteria (hypotension [the need for treatment with any vasopressors for at least 6 h to maintain a systolic pressure ≥ 90 mm Hg or a mean arterial pressure ≥65 mm Hg], acute respiratory failure [patient required invasive mechanical ventilation for ≥24 h], or acute kidney injury [creatinine >2.0 mg/dL or urine output of <0.5 mL/kg/h for >2 h]) as set by the Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock (37); a lymphocyte count of ≤1,100/μL, which was previously reported as a poor prognosis factor within 96 h prior to the start of the study (38): and being in the intensive care unit (ICU) at the start of study treatment and having no plans to leave the ICU within 24 h based on the patient's condition.
Patients were excluded if they met the following criteria: a bodyweight of ≤50 kg or ≥150 kg; previous episode of severe sepsis or septic shock with admission to the ICU during the current hospitalization; presence of an active, known, or suspected autoimmune disease (not including type 1 diabetes, hyperthyroidism that only requires hormone replacement, or skin disorders not requiring systemic treatment); history of solid organ or bone marrow transplant; history of malignancies, human immunodeficiency virus, hepatitis C virus, or chronic hepatitis B virus infections.
Prior exposure to nivolumab or to an anti-PD-1, anti-programmed cell death ligand 1, anti- programmed cell death ligand 2, antitumor necrosis factor receptor superfamily member 9, or an anti-cytotoxic T-lymphocyte associated protein 4 antibody was prohibited as was prior exposure to granulocyte-macrophage colony-stimulating factor within 4 weeks (or five half-lives, whichever is longer) of the start of the study. Prohibited concomitant medications included immunosuppressants, except if there was an adverse event (AE) where a relationship with nivolumab could not be ruled out.
Study design and treatment
This multicenter, open-label phase 1/2 study involved a single intravenous administration of 480 mg or 960 mg nivolumab over 90 min, which was delivered by a study investigator. Using a population pharmacokinetic model from 10 clinical studies, including 187 Japanese cancer patients (ONO4538-01: solid tumor, ONO4538-02: MEL, ONO4538-05: NSCLC, ONO4538-06: NSCLC, ONO4538-08: MEL, CA209001: solid tumor, CA209003: solid tumor, CA209010: RCC, CA209063: NSCLC, and CA209037: MEL), we predicted that the maximum concentration (Cmax) from a single 480 mg administration of nivolumab would be comparable to the end of infusion concentration (Ceoi) of 3 mg/kg every 2 weeks (Q2W), which was the approved dosing regimen for patients with cancer at the time of planning. Additionally, we predicted that the trough concentration (Cmin) of 3 mg/kg Q2W nivolumab would be comparable to the concentration on Day 28 (C28d) of a single administration of 960 mg nivolumab. It was predicted that Cmax of the 960 mg nivolumab would be below the value for Ceoi after Q2W dosing with 10 mg/kg, the dose of which was well tolerated in cancer patients.
Patients were screened for 10 days and clinically evaluated with the Sequential Organ Failure Assessment (SOFA) score and assessed as to whether they required the use of a ventilator, vasopressor, or a dialysis machine (39). Patients were then administered nivolumab on Day 1 followed by a 28-day observation period. During this observation period, physical measurements, including vital signs and 12-lead electrocardiography, were performed during Week 1, on Day 14, and on the day of discharge. Additionally, general laboratory measurements were performed during Week 1, and on Days 10, 14, 21 and 28, and on the day of discharge.
Pharmacokinetics
Another primary endpoint was the measurement of nivolumab serum concentrations using a validated electrochemiluminescence assay that utilizes the Meso-Scale Discovery platform with a biotin-labeled capture antibody and a ruthenium-labeled detection antibody. After a single nivolumab administration, the Cmax, time to reach Cmax (Tmax), Cmin, area under the curve (AUC), apparent total body clearance of the drug from serum (CL), apparent volume of distribution (Vd), and half-life (T1/2) were calculated to evaluate the pharmacokinetic profile of nivolumab.
Pharmacodynamics
The secondary endpoints included analysis of absolute lymphocyte counts, which were analyzed by the validated method at each site, and monocyte human leukocyte antigen-DR subtype (mHLA-DR) expression, which was evaluated by flow cytometry. Immunosuppression references for lymphocyte counts and mHLA-DR were ≤1,100/μL and ≤8,000 monoclonal antibodies (mAb)/cell, respectively.
Safety
The primary endpoint was to assess safety outcomes including monitoring of AEs, serious AEs, and immune-related AEs, which were evaluated based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.03 (40). Physical measurements, vital signs, general laboratory tests (e.g., blood biochemistry and urinalysis), and 12-lead electrocardiography were also monitored.
Statistics
The sample size was based on the number of patients that was feasible to evaluate the safety and the pharmacokinetic profile of nivolumab and not on any statistical hypothesis. A minimum of five patients was enrolled in each treatment group, although up to 10 patients per group were allowed depending on their evaluability. The full analysis set (FAS) included all patients who were eligible for inclusion in the study and were administered nivolumab.
Summary statistics were used to calculate nivolumab pharmacokinetic and pharmacodynamic parameters. AEs were graded by CTCAE and were listed and tabulated by system organ class and preferred term for each treatment group (40).
Statistical analyses were performed using Phoenix WinNonlin Version 7.0 (Certara USA, Inc, Princeton, NJ), Autopilot Toolkit 7.0 (Certara USA, Inc, Princeton, NJ) and SAS Version 9.3 (SAS Institute, Cary, NC) for pharmacokinetic evaluation, and SAS Version 9.3 for all other analyses.
Study approval
Ethical approval was obtained from the relevant institutional review boards and the study adhered to the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent prior to inclusion in the study. This study was registered (JapicCTI-173600).
RESULTS
Patients
In total, 15 patients were enrolled in this study, which was conducted from July 2017 to March 2018. Two patients were excluded from the FAS: one patient did not meet both of the inclusion criteria for organ dysfunction and status of ICU admission, while the other patient met the exclusion criteria for reasons due to other sound medical, psychiatric, and/or social reasons as determined by the investigator. Therefore, 13 patients were included in the FAS, with five patients assigned to nivolumab 480 mg therapy and eight patients assigned to 960 mg therapy (Fig. 1).
Fig. 1.
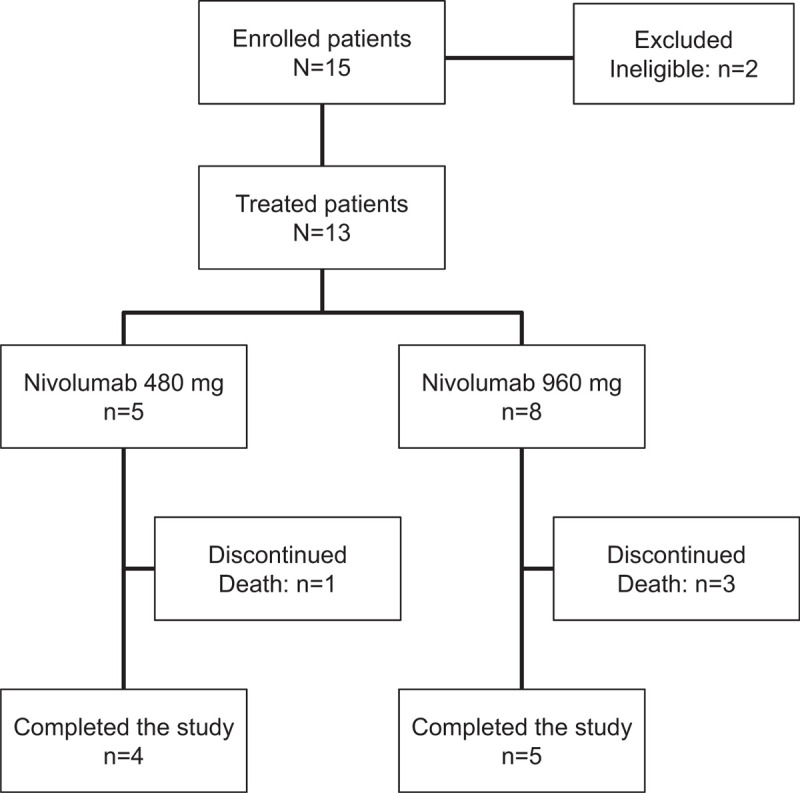
Patient disposition.
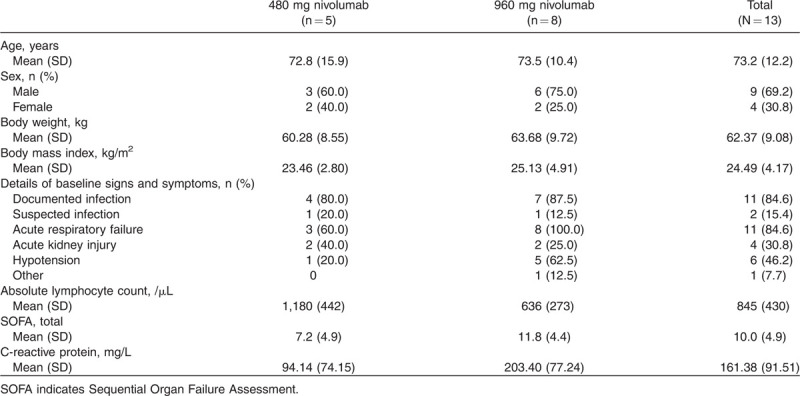
Patient baseline demographics and clinical characteristics are shown in Table 1. The absolute lymphocyte count, SOFA scores, and C-reactive protein levels appeared to be different between the 480 mg and 960 mg groups (Table 1). The mean (standard deviation [SD]) absolute lymphocyte counts were 1,180 (442)/μL and 636 (273)/μL, the mean (SD) SOFA total scores were 7.2 (4.9) and 11.8 (4.4), and the mean (SD) C-reactive protein levels were 94.14 (74.15) mg/L and 203.40 (77.24) mg/L in the 480 mg and 960 mg groups, respectively. In addition, numerical values (mean [SD]) at baseline and end of the study for differential white blood cell count, red blood cell count, platelet count, and other laboratory parameters are shown in Supplemental Digital Content 1.
Table 1.
Baseline characteristics
Pharmacokinetics
The time course of changes in nivolumab serum concentration from baseline to Day 28 for both groups is shown in Figure 2. Additionally, we have reported the individual time courses of nivolumab serum concentration for each patient (see Figure, Supplemental Digital Content 2A, which shows values in the 480 mg patient group; see Figure, Supplemental Digital Content 2B, which shows values in the 960 mg patient group).
Fig. 2.
Change in nivolumab serum concentration over time.
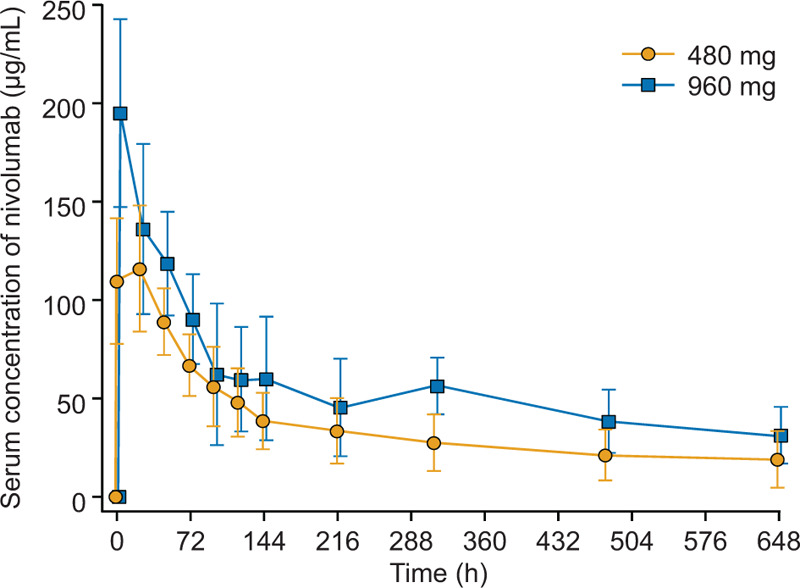
Mean (SD), SD, standard deviation, Patient numbers in the 480 mg group: baseline (n = 5), 1.5 h (n = 3), 24 to 480 h (n = 5), and 648 h (n = 4).
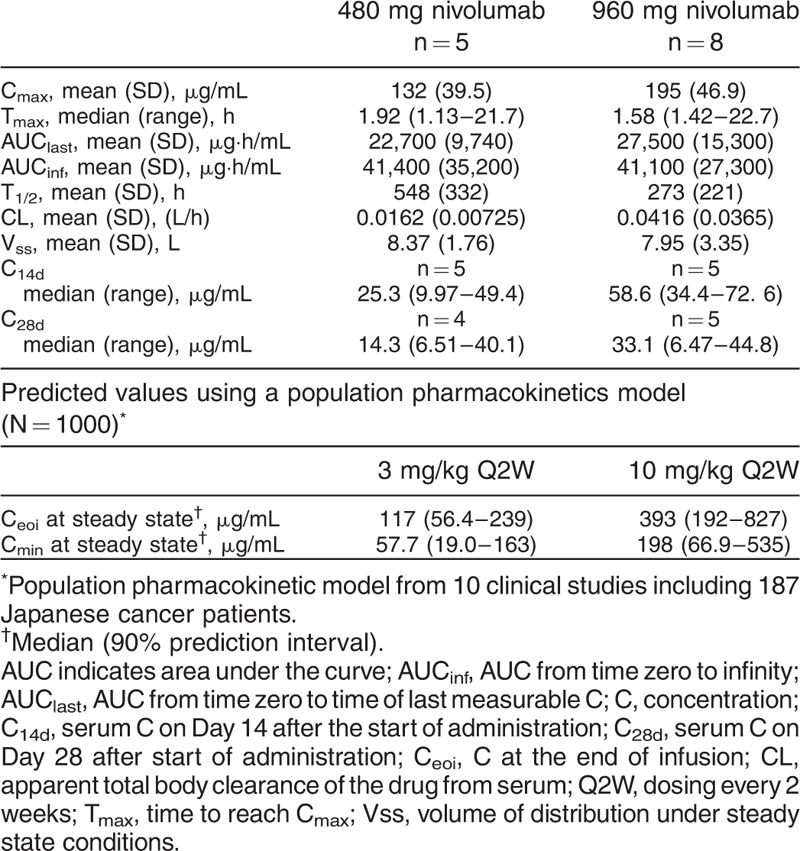
Table 2 shows the pharmacokinetic parameters of nivolumab for both treatment groups. The Cmin and the Ceoi were at a steady state after dosing Q2W with 3 mg/kg and 10 mg/kg nivolumab as predicted from data on cancer patients. The data show that the mean Cmax of 480 mg nivolumab was similar to the predicted median Ceoi after Q2W dosing with 3 mg/kg nivolumab (132 μg/mL versus 117 μg/mL, respectively). Additionally, the median values (ranges) of both the concentration on Day 14 (C14d) and C28d from the 960 mg nivolumab group (58.6 [34.4–72.6] μg/mL and 33.1 [6.47–44.8] μg/mL, respectively) were within the predicted median (90% prediction interval) Cmin after 3 mg/kg nivolumab (57.7 [19.0–163] μg/mL). Moreover, the mean Cmax of the 960 mg nivolumab group did not exceed the predicted median Ceoi after Q2W dosing with 10 mg/kg (195 μg/mL versus 393 μg/mL).
Table 2.
Pharmacokinetic parameters
Pharmacodynamics
The overall lymphocyte counts and mHLA-DR data are shown in Figure 3A and B, respectively. The mean (SD) absolute lymphocyte counts increased from 1,180 (442)/μL at baseline to 1754 (797) /μL on Day 28 in the 480 mg group and from 636 (273)/μL at baseline to 1,308 (619)/μL on Day 28 in the 960 mg group. The time course of lymphocyte counts for each patient in the 480 and 960 mg groups is shown in the supplemental content (see Figures, Supplemental Digital Content 3A and B, respectively, which show lymphocyte counts for each patient). The mean (SD) mHLA-DR increased from 5,998 (3,097) mAb/cell at baseline to 23,650 (9393) mAb/cell on Day 28 in the 480 mg group and increased from 10,665 (7208) mAb/cell at baseline to 17,181 (7566) mAb/cell on Day 28 in the 960 mg group. The time course of mHLA-DR expression for each patient in the 480 and 960 mg groups is shown in the supplemental content (see Figures, Supplemental Digital Content 4A and B, respectively, which show mHLA-DR antigen expression for each patient).
Fig. 3.
Change in (A) lymphocyte count over time and (B) mHLA-DR antigen expression over time.
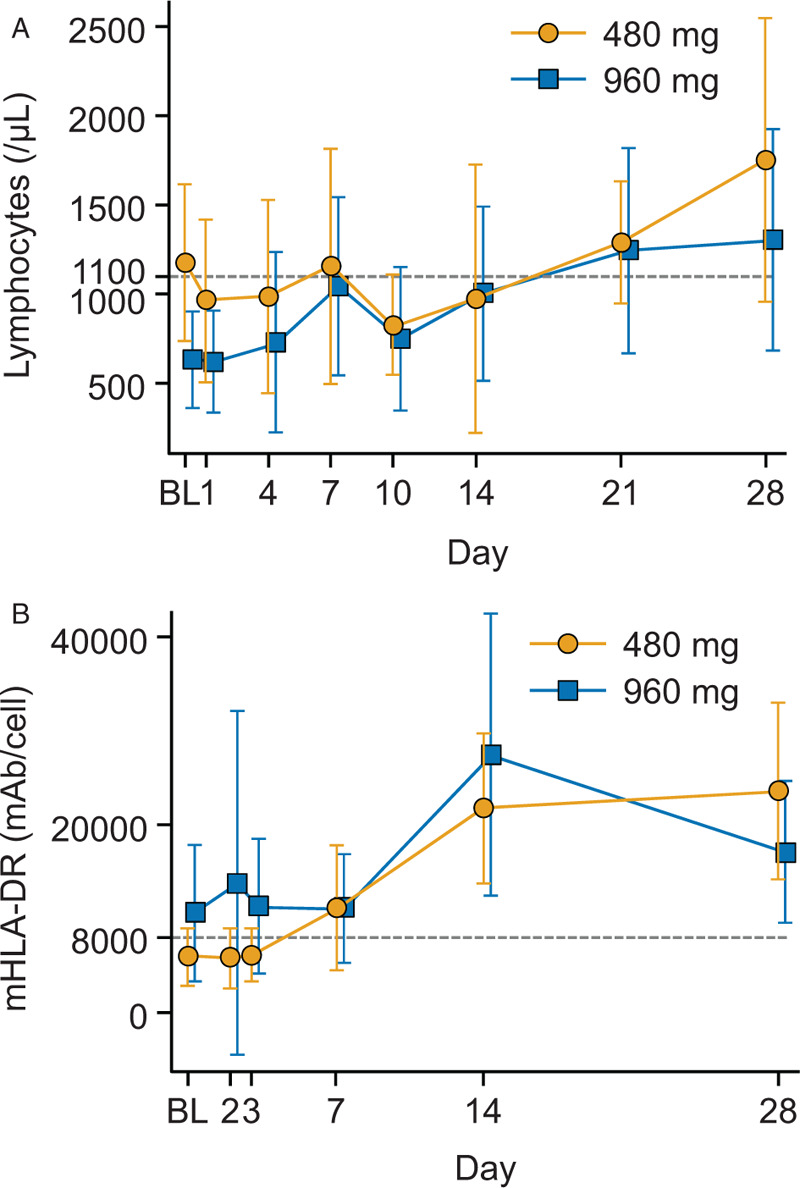
Mean (SD), BL, baseline; mAb, monoclonal antibody; mHLA-DR, monocyte human leukocyte antigen-DR subtype; SD, standard deviation. A, Patient numbers in the 480 mg group: baseline to Day 21 (n = 5) and Day 28 (n = 4). Patient numbers in the 960 mg group: baseline to Day 4 (n = 8), Day 7 to Day 14 (n = 6), Day 21 (n = 4), and Day 28 (n = 5). B, Patient numbers in the 480 mg group: baseline to Day 14 (n = 5) and Day 28 (n = 4). Patient numbers in the 960 mg group: baseline to Day 3 (n = 7), Day 7 (n = 6), and Day 14 to Day 28 (n = 5). Lymphocyte count baseline data were obtained within 48 h before the administration of nivolumab. mHLA-DR baseline data were obtained approximately 30 min before the administration of the study drug. The mHLA-DR antigen expression reference line is at 8,000 mAb/cell and the lymphocyte count reference line is at 1,100/μL.
Safety
The incidence of AEs was 80.0% (n = 4) in the 480 mg group and 50.0% (n = 4) in the 960 mg group (Table 3). There was one serious AE (multiple-organ failure) in the 480 mg group, which was judged to have no causal relationship to nivolumab. This patient was diagnosed with kidney disease and after regaining consciousness, the patient and their family refused ongoing treatment with dialysis and the patient's condition deteriorated and subsequently resulted in death due to multiple-organ failure.
Table 3.
Adverse events and adverse drug reactions
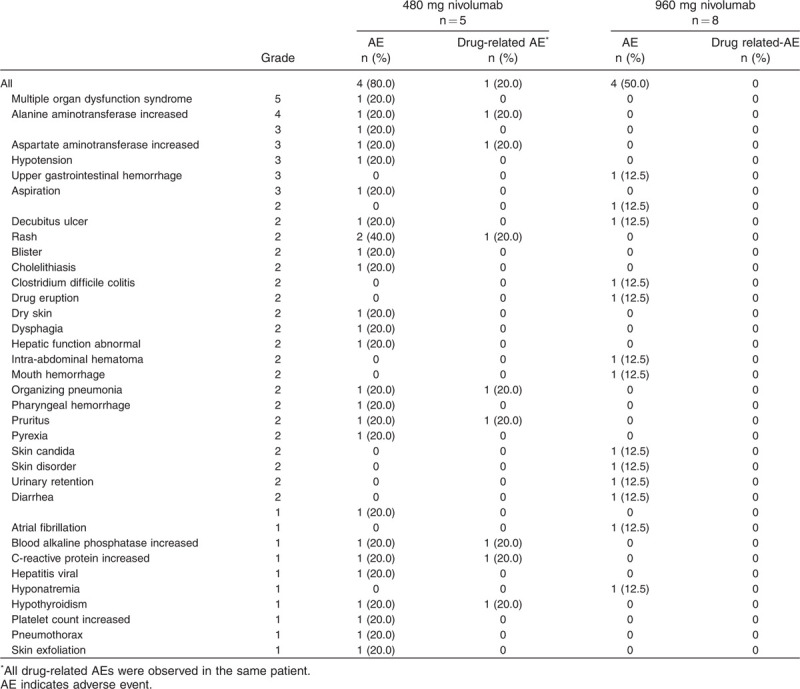
No patients withdrew from the study due to an AE. The incidences of Grade 3 or Grade 4 AEs were 60% (n = 3) and 12.5% (n = 1) in the 480 mg and 960 mg groups, respectively (Table 3). There were four deaths, one in the 480 mg group and three in the 960 mg group; however, none were judged to have a causal relationship to nivolumab (Supplemental Digital Content 5).
Multiple organ dysfunction syndrome was observed as an AE and cause of death in a single patient in the 480 mg group. All three of the patients who received 960 mg were determined to have died due to exacerbation of underlying disease.
Drug-related AEs were observed in one patient who received 480 mg nivolumab. The drug-related AEs and their graded progression were as follows: organizing pneumonia (Grade 1 to Grade 2) that remained unresolved (described in more detail below); Grade 1 C-reactive protein increase, which was resolved; Grade 4 alanine transaminase increase, which was partially resolved; Grade 3 aspartate transaminase increase, which was resolved; Grade 1 alkaline phosphatase increase, which was resolved; Grade 2 rash, which was resolved; Grade 2 pruritus, which was resolved; and Grade 1 hypothyroidism, which was resolved after 5 months (described in more detail below).
Further details regarding the case of organizing pneumonia are as follows: Grade 2 pyrexia was observed on Day 2, which was resolved on Day 7; Grade 1 organizing pneumonia was observed on Day 7 and increased to Grade 2 on the same day; Grade 2 rash was observed on Day 11, which was resolved on Day 21; and the case of organizing pneumonia was reported as unresolved as of Day 26. A relationship between pyrexia and nivolumab was ruled out; organizing pneumonia and rash were determined to be immune-mediated AEs and related to nivolumab because they were “Not typical as the course of sepsis.”
Hypothyroidism was observed on Day 47 and resolved on Day 200. Changes in thyroid stimulating hormone (TSH) and other laboratory findings are shown in Supplemental Digital Content 6. The incident of hypothyroidism was Grade 1, non-serious, and immune-mediated; it was determined to be related to nivolumab.
No abnormalities were observed for vital signs and electrocardiography measurements in any of the study patients.
DISCUSSION
This study investigated the pharmacokinetics, pharmacodynamics, safety, and tolerability of nivolumab, a monoclonal antibody directed against PD-1, for the treatment of sepsis. A single administration of nivolumab was well tolerated, and no special safety concerns were observed.
Nivolumab pharmacokinetics were as expected with serum concentrations within the predicted concentration ranges provided from the results of nivolumab therapy in Japanese cancer patients. When considering the median values of both C14d and C28d in the 960 mg dose group (58.6 μg/mL and 33.1 μg/mL), these were within the 90% prediction interval (19.0–163 μg/mL) of Cmin of 3 mg/kg Q2W. A single administration of 960 mg nivolumab was therefore observed to result in a sufficient serum concentration level. Furthermore, the Cmax in the 960 mg dose group did not exceed the Ceoi at steady state with 10 mg/kg Q2W, which was the drug regimen used to previously confirm tolerability in Japanese cancer patients (41, 42). However, the average AUC from time 0 to infinity (AUCinf) in the 960 mg dose group was similar to that in the 480 mg dose group. This may have been caused by over-estimation of the AUCinf in one patient from the 480 mg dose group due to the elimination phase. Additionally, there was also an underestimation in three patients from the 960 mg dose group where the drug concentration was only evaluated up to 120 h or 216 h.
It has been reported that a reduced dose of recombinant thrombomodulin (rTM) in septic disseminated intravascular coagulation patients with renal impairment did not meet the expected effective dose (43). From this, it was inferred that vascular permeability was enhanced in patients with sepsis and therefore may have had an increased clearance of rTM. In this study, the overall health condition of patients in the 960 mg group was clinically worse than that of the patients in the 480 mg group based on baseline SOFA scores and C-reactive protein data. Therefore, inflammation may have been more advanced in these patients resulting in an increase in nivolumab clearance in patients in the 960 mg dose group relative to patients in the 480 mg dose group.
Mortality was 20% (1/5) in the 480 mg group and 37.5% (3/8) in the 960 mg group. The mean (SD) baseline SOFA score was 7.2 (4.9) in the 480 mg group and 11.8 (4.4) in the 960 mg group. According to a report by Ferreira et al. (44) investigating SOFA scores and survival rates in critically ill patients, the survival rate was approximately 20% when the initial SOFA score was 6–7 and approximately 90% when it exceeded 11. According to a recent report of patients with septic shock by Hernández et al. (45), the survival rate within 28 days with a SOFA score of approximately 10 was 34.9% to 43.4%. When compared with a similar group of patients matched for SOFA scores, the mortality rate in the present study was the same or lower than that in past reports.
The pharmacodynamic analysis showed that nivolumab administration generally caused an increase in lymphocyte count and mHLA-DR expression in both treatment groups. Similarly, a case report of nivolumab administration to sepsis patients reported that both mHLA-DR and absolute lymphocyte counts tended to increase over time (46). In this report, the mean fluorescent intensity (MFI) of mHLA-DR increased from 2.88 on Day 9 (baseline) to 5.69 on Day 27, and the lymphocyte count increased from 661 on Day 0 to 1062 on Day 27. The relative increases for both mHLA-DR and lymphocyte count reported in this case study are similar to those observed in the present study. Additionally, our results are consistent with results from a phase 1b study of a different anti-PD-L1 (BMS-936559) in sepsis-associated immunopathy patients (absolute lymphocyte counts ≤1,100/μL) where mHLA-DR expression tended to increase (47). In the phase 1b study, 10, 30, 100, 300, or 900 mg of BMS-936559 was administered. Patients in the low-dose groups (10, 30, and 100 mg) experienced an increase in mHLA-DR from approximately 3,000 mAb/cell on Day 0 (baseline) to approximately 7,000 mAb/cell on Day 30. In the high-dose groups (300 and 900 mg), it increased from approximately 4,000 mAb/cell to approximately 10,000 mAb/cell. In our study, the relative increase in mHLA-DR was greater in the 480 mg group (5,998 to 23,650 mAb/cell) and similar in the 960 group (10,665 to 17,181 mAb/cell) compared to that reported in the high-dose groups of the BMS-936559 study (47). It is important to note that the PD-1 receptor has two ligands, PD-L1 and PD-L2; while nivolumab targets the PD-1 receptor, BMS-936559 (anti-PD-L1 antibody) targets PD-L1. Although there are no direct comparisons of the effects of BMS-936559 and nivolumab or of these drugs in sepsis patients, it has been reported that the efficacies of tezolizumab, an anti-PD-L1 antibody, and that of nivolumab and pembrolizumab, both anti-PD-1 antibodies, seemed to be the same in cancer patients (48–50). With respect to the reported pharmacodynamic parameters in our study, there were large variations in mHLA-DR values in the 960 mg dose group, including time points where the monocyte count was below the sensitivity threshold. This may be a sensitivity issue with the test or it may be related to the patient's state of sepsis.
Based on the absolute lymphocyte counts in three of the four patients who died, it may be that delaying the administration of nivolumab in some patients is unfavorable, although more research is required to clarify this association. Each of these patients had an absolute lymphocyte count of approximately 500/μL or lower before administration of nivolumab (see Figure, Supplemental Digital Content 3, which shows a time course of lymphocyte counts for each patient). After administration of nivolumab, improvements in their lymphocyte count appeared weaker than in other patients in this study. During the early stage of sepsis and septic shock, there should be an elevation in inflammatory cytokines that usually peaks within 36 h but then decreases over the subsequent 72 h of observation (51). This absence of a surge in absolute lymphocyte counts may, therefore, be related to their cause of death.
Sepsis patients are known to have heterogeneous baseline characteristics, which may make it difficult to select candidates for nivolumab treatment based solely on immunosuppression-related selection criteria. Based on reports published to date, an absolute lymphocyte count of <1,100/μL is thought to be a prognostic factor (46, 47). Therefore, one idea is to use the absolute lymphocyte count as a rough guideline for administering nivolumab to patients with an absolute lymphocyte count <1,100/μL. It may also be appropriate to target immunosuppressed patients for whom sufficient recovery cannot be expected with other treatments, e.g., patients with multidrug-resistant bacteria. When considering the efficacy of nivolumab in clinical practice, we should exercise caution regarding directly applying data from animal experiments, such as the effect of nivolumab treatment in murine models of cecal ligation and puncture (52). There are still many limitations to animal experiments, including the fact that young rather than old mice are generally used and experiments are often only conducted using animals of one sex. Further investigation is therefore needed to understand its mechanism of action to properly assist in the selection of patients with sepsis who are likely to respond to immunological treatment.
A limitation of this study was that it included Japanese patients only, which limits the generalizability of the results to other patient populations. Given that the characteristics of sepsis patients vary widely from patient to patient, it is unlikely that patient characteristics would differ greatly between Japanese sepsis patients and sepsis patients in other countries such as the United States. In Japan, disseminated intravascular coagulation is considered the target of treatment; soluble recombinant human thrombomodulin and antithrombin III products are generally administered for septic coagulopathy (53, 54). In addition, continuous hemodiafiltration aimed at cytokine modulation (55) is also covered by health insurance for the treatment of sepsis. The effect of these treatments on survival rates has yet to be established, meaning that the effect of these treatments on survival rates in the present study is likely limited. Other limitations include the small sample size, the open-label nature of the study, and the absence of a placebo group.
Overall, this study reveals a favorable safety profile for nivolumab therapy in the treatment of Japanese patients with sepsis or septic shock. A dose of 960 mg nivolumab appeared to be well-tolerated and sufficient to maintain serum concentrations at a level comparable to steady state nivolumab at 3 mg/kg Q2W. Furthermore, administration of nivolumab appeared to improve selected markers of immunity and alleviate immunosuppression.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank all participating patients, their families, and health care professionals who made this study possible.
The authors wish to also acknowledge the following clinical study investigators: Shingo Ihara (Emergency Medical Center, Nihon University Itabashi Hospital); Naoshi Takeyama (Department of Emergency and Critical Care Medicine, Aichi Medical University Hospital); Keisuke Tomii (Department of Respiratory Medicine, Kobe City Medical Center General Hospital); Nobuaki Shime (Department of Emergency and Critical Care Medicine, Hiroshima University Hospital); Akio Kimura (Emergency Medical Center, Center Hospital of the National Center for Global Health and Medicine); Mitsuo Sakamoto (Department of Infectious Diseases, Kawasaki Municipal Hospital); Tetsuya Komuro (Department of Intensive Care Medicine, Shonan Kamakura General Hospital); Hiroshi Adachi (Department of Intensive Care Medicine, Aso Iizuka Hospital); Masahiro Harada (Department of Emergency Medicine, Kumamoto Medical Center); and Naoki Tosaka (Department of Emergency Medicine, Shizuoka General Hospital).
The authors also thank James Graham, PhD, of Edanz Medical Writing for providing medical writing support, which was funded by Ono Pharmaceutical Co, Ltd and Bristol-Myers Squibb through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Footnotes
This study was funded by Ono Pharmaceutical Co, Ltd and Bristol-Myers Squibb. Ono Pharmaceutical Co, Ltd was involved in the design of the study; collection, analysis, and interpretation of data; and in drafting the manuscript. Bristol-Myers Squibb did not have a role in the study design; data collection, analysis and interpretation; nor writing of the manuscript.
EW received personal fees and non-financial support from Ono Pharmaceutical Co, Ltd, during the conduct of the study; and grants, personal fees and non-financial support from Asahi Kasei Pharma, and personal fees from Alexion Pharma, outside the submitted work. ON received grants and personal fees from Ono Pharmaceutical Co, Ltd, during the conduct of the study; and grants from Maruishi Pharmaceutical Co, Ltd, Fuso Pharmaceutical Industries, Ltd, Asahi Kasei Pharma, and Japan Blood Products Organization, outside the submitted work. YK received personal fees from Ono Pharmaceutical Co, Ltd, during the conduct of the study; and grants and personal fees from Ono Pharmaceutical Co, Ltd, grants from Japan Blood Products Organization, and grants from Asahi Kasei Pharma, outside the submitted work. MO, TO, and TH received personal fees from Ono Pharmaceutical Co, Ltd, during the conduct of the study. SO received personal fees from Ono Pharmaceutical Co, Ltd, during the conduct of the study; and personal fees from Ono Pharmaceutical Co, Ltd, outside the submitted work.
The authors report no conflicts of interest.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315 (8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 369 (9):840–851, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13 (12):862–874, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M. Sepsis Definitions Task Force: developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315 (8):775–787, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 41 (5):1167–1174, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Carlet J. 2002 Brussels Roundtable Participants: surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med 29 (3):368–377, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304 (16):1787–1794, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 38 (5):1276–1283, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost and risk factors of readmissions among severe sepsis survivors. Crit Care Med 43 (4):738–746, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones TK, Fuchs BD, Small DS, Halpern SD, Hanish A, Baillie C, Umscheid CA, Kerlin MP, Gaieski DF, Mikkelsen ME. Severe sepsis is associated with high rates of hospital readmission. Am J Respir Crit Care Med 189:A2190, 2014. [Google Scholar]
- 11.Ortego A, Gaieski DF, Fuchs BD, Jones T, Halpern SD, Small DS, Sante SC, Drumheller B, Christie JD, Mikkelsen ME. Hospital-based acute care use in survivors of septic shock. Crit Care Med 43 (4):729–737, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 313 (10):1055–1057, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Japanese Society of Intensive Care Medicine, Committee of Sepsis Registry: 2007 JSICM Sepsis 1st Registry: Management of severe sepsis and septic shock in Japan. J Jpn Soc Intensive Care Med 20 (2):329–334, 2013. [Google Scholar]
- 14.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 15 (5):581–614, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med 14 (1–2):64–78, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13 (3):260–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto GP, Sossdorf M, Claus RA, Rödel J, Menge K, Reinhart K, Bauer M, Riedemann NC. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 15 (4):R183, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kethireddy S, Kumar A. Mortality due to septic shock following early, appropriate antibiotic therapy: can we do better? Crit Care Med 40 (7):2228–2229, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med 15 (5):496–497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe E, Thampy LK, Hotchkiss RS. Immunoadjuvant therapy in sepsis: novel strategies for immunosuppressive sepsis coming down the pike. Acute Med Surg 5 (4):309–315, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 106 (15):6303–6308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Chen Y, Chung CS, Yuan Z, Monaghan SF, Wang F, Ayala A. Identification of B7-H1 as a novel mediator of the innate immune/proinflammatory response as well as a possible myeloid cell prognostic biomarker in sepsis. J Immunol 192 (3):1091–1099, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care 14 (6):R220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol 88 (2):233–240, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care 17 (3):R85, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306 (23):2594–2605, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Chéron A, Allaouchiche B, Gueyffier F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 15 (2):R99, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, Deng X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care 15 (1):R70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang K, Svabek C, Vazquez-Guillamet C, Sato B, Rasche D, Wilson S, Robbins P, Ulbrandt N, Suzich J, Green J, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care 18 (1):R3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33 (18):2004–2012, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16 (3):257–265, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA Approval Summary: Nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 21 (5):634–642, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth MJ, Teng MW. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunol 7 (10):e1041, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11 (11):3887–3895, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 99 (19):12293–12297, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansur A, Hinz J, Hillebrecht B, Bergmann I, Popov AF, Ghadimi M, Bauer M, Beissbarth T, Mihm S. Ninety-day survival rate of patients with sepsis relates to programmed cell death 1 genetic polymorphism rs11568821. J Investig Med 62 (3):638–643, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41 (2):580–637, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 42 (5):383–391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22 (7):707–710, 1996. [DOI] [PubMed] [Google Scholar]
- 40.National Cancer Institute Common Terminology Criteria for Adverse Events v4. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed April 16, 2017.
- 41.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 366 (26):2443–2454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal S, Waxman I, Lambert A, Roy A, Darbenzio R. Evaluation of the potential for QTc prolongation in patients with solid tumors receiving nivolumab. Cancer Chemother Pharmacol 77 (3):635–641, 2016. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe E, Yamazaki S, Setoguchi D, Sadahiro T, Tateishi Y, Suzuki T, Ishii I, Oda S. Pharmacokinetics of standard- and reduced-dose recombinant human soluble thrombomodulin in patients with septic disseminated intravascular coagulation during continuous hemodiafiltration. Front Med (Lausanne) 4:15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286 (14):1754–1758, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: The ANDROMEDA-SHOCK randomized clinical trial. JAMA 321 (7):654–664, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimaldi D, Pradier O, Hotchkiss RS, Vincent JL. Nivolumab plus interferon-γ in the treatment of intractable mucormycosis. Lancet Infect Dis 17 (1):18, 2017. [DOI] [PubMed] [Google Scholar]
- 47.Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, Martin GS, Coopersmith CM, Brakenridge S, Mayr FB, et al. Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559). Crit Care Med 47 (5):632–642, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passiglia F, Galvano A, Rizzo S, Incorvaia L, Listì A, Bazan V, Russo A. Looking for the best immune-checkpoint inhibitor in pre-treated NSCLC patients: an indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int J Cancer 142 (6):1277–1284, 2018. [DOI] [PubMed] [Google Scholar]
- 49.Tan PS, Aguiar P, Jr, Haaland B, Lopes G. Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non-small cell lung cancer: a systematic review and network meta-analysis of 3024 participants. Lung Cancer 115:84–88, 2018. [DOI] [PubMed] [Google Scholar]
- 50.Armoiry X, Tsertsvadze A, Connock M, Royle P, Melendez-Torres GJ, Souquet PJ, Clarke A. Comparative efficacy and safety of licensed treatments for previously treated non-small cell lung cancer: a systematic review and network meta-analysis. PLoS One 13 (7):e0199575, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivers EP, Jaehne AK, Nguyen HB, Papamatheakis DG, Singer D, Yang JJ, Brown S, Klausner H. Early biomarker activity in severe sepsis and septic shock and a contemporary review of immunotherapy trials: not a time to give up, but to give it earlier. Shock 39 (2):127–137, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R, et al. Abandon the mouse research ship? Not just yet!. Shock 41 (6):463–475, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, Pettilä V, Wittebole X, Meziani F, Mercier E, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA 321 (20):1993–2002, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Pénzes I, Kübler A, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 286 (15):1869–1878, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Shiga H, Hirasawa H, Nishida O, Oda S, Nakamura M, Mashiko K, Matsuda K, Kitamura N, Kikuchi Y, Fuke N. Continuous hemodiafiltration with a cytokine-adsorbing hemofilter in patients with septic shock: a preliminary report. Blood Purif 38 (3–4):211–218, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


