To the editor
Coronavirus disease 2019 (COVID-19), which emerged in Wuhan, China in December 2019, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has become a major global public health concern [1]. Positive detection of SARS-CoV-2 RNA in nasopharyngeal swab samples, sputum samples or bronchoalveolar lavage samples by reverse transcriptase polymerase chain reaction (RT–PCR) has been used to confirm SARS-CoV-2 infection [2]. Recently, positive detection of IgM and IgG antibodies specific to SARS-CoV-2 has also been recognized as deterministic evidence for confirmed SARS-CoV-2 infection [3,4]. However, the antibody response to SARS-CoV-2 currently remains inadequately understood in COVID-19 patients. In the present study, we investigated the patterns of antibody response to SARS-CoV-2 in patients with COVID-19, aiming to better clarify the humoral immunological response during SARS-CoV-2 infection.
Patient characteristics
A total of 32 patients with a confirmed diagnosis of COVID-19 were included in our cohort. All were positive for SARS-CoV-2 according to nucleic acid testing by RT–PCR of nasopharyngeal swab, sputum, or bronchoalveolar lavage specimens. Patients exhibiting one or more of the following conditions were classified as having severe COVID-19: (a) respiratory distress (≥30 breaths/min); (b) oxygen saturation ≤93% at rest; (c) arterial partial pressure of oxygen (PaO2)/fraction of inspiration O2 (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa); (d) respiratory failure requiring mechanical ventilation; (e) septic shock development; or (f) critical organ failure requiring ICU care. Patients not meeting the above criteria were classified as having mild COVID-19.
The median age of the 32 patients was 55 years old, and 66.7% of them were male. Among the 32 patients, 18 (56.3%) were severe cases, and 14 (43.7%) were mild cases. The most common symptoms at onset of illness were fever, cough, fatigue, dyspnoea and headache. The demographic details and clinical disease severity of all patients in our cohort are shown in the supplementary materials (Table 1). Our study was approved by the Ethics Committee of Chongqing Public Health Medical Center (No. 2020-006-01), and informed consent was obtained from all subjects prior to blood sample collection.
Table 1. Demographic characteristics and antibody titre of 32 COVID-19 patients.
| Patient number | Age | Sex | severe case | Sample number | ID | day | IgG (RU/ml) | IgM (RU/ml) |
|---|---|---|---|---|---|---|---|---|
| 1 | 73 | male | YES | 2001278733 | 74827 | 4 | 0.94 | 15.57 |
| 2001312952 | 74827 | 8 | 177.44 | 176.69 | ||||
| 2002059960 | 74827 | 13 | 664.2 | 282.74 | ||||
| 2002093544 | 74827 | 17 | 731.94 | 314.94 | ||||
| 2002127130 | 74827 | 20 | 665.58 | 236.87 | ||||
| 2 | 52 | male | YES | 2001267752 | 74833 | 7 | 39.92 | 129.6 |
| 2001290545 | 74833 | 10 | 189.55 | 194.99 | ||||
| 2002048959 | 74833 | 16 | 202.85 | 460.93 | ||||
| 2002093481 | 74833 | 21 | 246.72 | 632.64 | ||||
| 2002127090 | 74833 | 24 | 233.22 | 464 | ||||
| 3 | 70 | male | NO | 2001299883 | 74839 | 10 | 98.27 | 11.88 |
| 2001313114 | 74839 | 12 | 874.23 | 44.57 | ||||
| 2002059503 | 74839 | 17 | 1572.32 | 67.32 | ||||
| 2002093351 | 74839 | 21 | 1377.84 | 66.91 | ||||
| 2002140361 | 74839 | 26 | 1213.54 | 58.79 | ||||
| 4 | 70 | female | YES | 2001267391 | 74851 | 7 | 0.97 | 2.1 |
| 2001288808 | 74851 | 9 | 1.17 | 6.75 | ||||
| 2001312862 | 74851 | 12 | 66.3 | 25.02 | ||||
| 2002036073 | 74851 | 15 | 238.5 | 43.74 | ||||
| 2002060585 | 74851 | 18 | 275.85 | 94.55 | ||||
| 2002093998 | 74851 | 21 | 406.59 | 114.09 | ||||
| 5 | 65 | female | YES | 2001267790 | 74862 | 7 | 2.43 | 13.9 |
| 2001278746 | 74862 | 8 | 43.98 | 33.59 | ||||
| 2001299874 | 74862 | 10 | 74.37 | 109.79 | ||||
| 2001301290 | 74862 | 11 | 167.51 | 81.5 | ||||
| 2001313232 | 74862 | 12 | 299.69 | 124.98 | ||||
| 2002036823 | 74862 | 15 | 599.44 | 181.03 | ||||
| 2002072533 | 74862 | 19 | 535.16 | 132.12 | ||||
| 2002094013 | 74862 | 21 | 612.32 | 106.51 | ||||
| 2002116878 | 74862 | 23 | 596.47 | 104.57 | ||||
| 2002127664 | 74862 | 24 | 517.07 | 88.47 | ||||
| 2002139300 | 74862 | 25 | 674.04 | 115.42 | ||||
| 2002140505 | 74862 | 26 | 655.19 | 99.52 | ||||
| 6 | 38 | male | YES | 2001267654 | 74863 | 9 | 21.43 | 50.74 |
| 2001290540 | 74863 | 12 | 375.96 | 216.74 | ||||
| 2002013601 | 74863 | 15 | 585.37 | 223.08 | ||||
| 2002048134 | 74863 | 18 | 784.3 | 195.61 | ||||
| 2002072220 | 74863 | 21 | 692.54 | 188.64 | ||||
| 2002127169 | 74863 | 26 | 672.38 | 143.25 | ||||
| 7 | 60 | female | NO | 2001278322 | 74877 | 3 | 0.93 | 7.14 |
| 2001301110 | 74877 | 6 | 0.99 | 11.73 | ||||
| 2002013575 | 74877 | 8 | 2.74 | 27.07 | ||||
| 2002059329 | 74877 | 12 | 548.55 | 164.95 | ||||
| 2002093476 | 74877 | 16 | 1067.37 | 255.72 | ||||
| 8 | 38 | male | YES | 2001289774 | 74912 | 9 | 83.75 | 114.35 |
| 2001290543 | 74912 | 10 | 141.4 | 145.23 | ||||
| 2001301919 | 74912 | 11 | 190.77 | 184.79 | ||||
| 2002025775 | 74912 | 14 | 213.92 | 259.38 | ||||
| 2002048717 | 74912 | 16 | 187.13 | 344.38 | ||||
| 2002071492 | 74912 | 19 | 199.88 | 408.83 | ||||
| 2002127144 | 74912 | 24 | 221.51 | 491.04 | ||||
| 9 | 56 | female | YES | 2001290419 | 74921 | 4 | 0.93 | 5.06 |
| 2001313538 | 74921 | 6 | 2.78 | 8.81 | ||||
| 2002014606 | 74921 | 7 | 2.63 | 18.31 | ||||
| 2002061270 | 74921 | 12 | 21.88 | 329.72 | ||||
| 2002082793 | 74921 | 14 | 68.72 | 373.78 | ||||
| 2002104121 | 74921 | 16 | 127.14 | 436 | ||||
| 10 | 55 | female | NO | 2001290328 | 74929 | 9 | 194.35 | 232.74 |
| 2002013579 | 74929 | 12 | 266.34 | 233.96 | ||||
| 2002047843 | 74929 | 15 | 324.93 | 259.5 | ||||
| 2002071490 | 74929 | 18 | 350.07 | 283.65 | ||||
| 2002105698 | 74929 | 21 | 330.38 | 250.95 | ||||
| 11 | 67 | male | NO | 2001290929 | 74935 | 6 | 1.03 | 17.41 |
| 2002014497 | 74935 | 9 | 0.93 | 49.75 | ||||
| 2002025405 | 74935 | 10 | 4.37 | 115.26 | ||||
| 2002047701 | 74935 | 12 | 6.34 | 113.27 | ||||
| 2002072412 | 74935 | 15 | 199.16 | 193.81 | ||||
| 12 | 38 | male | YES | 2001290938 | 74940 | 3 | 0.93 | 0.93 |
| 2002014667 | 74940 | 6 | 0.93 | 3.91 | ||||
| 2002048327 | 74940 | 9 | 1.52 | 32.12 | ||||
| 2002083247 | 74940 | 13 | 380.16 | 144.28 | ||||
| 2002105333 | 74940 | 15 | 345.5 | 167.62 | ||||
| 2002116806 | 74940 | 16 | 438.22 | 134.65 | ||||
| 2002138679 | 74940 | 18 | 458.95 | 159.62 | ||||
| 13 | 50 | male | YES | 2001301499 | 74948 | 10 | 197.05 | 186.68 |
| 2002013584 | 74948 | 12 | 342.23 | 282.93 | ||||
| 2002047837 | 74948 | 15 | 437.01 | 354.36 | ||||
| 2002071486 | 74948 | 18 | 392.84 | 440.22 | ||||
| 2002105692 | 74948 | 21 | 352.06 | 380.02 | ||||
| 2002140348 | 74948 | 25 | 408.94 | 379.38 | ||||
| 14 | 69 | female | YES | 2001301775 | 74952 | 2 | 0.93 | 0.93 |
| 2001313338 | 74952 | 3 | 0.93 | 1.9 | ||||
| 2002037655 | 74952 | 6 | 2.37 | 17.23 | ||||
| 2002050036 | 74952 | 8 | 38.18 | 53.33 | ||||
| 2002072543 | 74952 | 10 | 105.89 | 49.7 | ||||
| 2002083134 | 74952 | 11 | 95.69 | 65.68 | ||||
| 2002093971 | 74952 | 12 | 87.47 | 61.8 | ||||
| 2002116847 | 74952 | 14 | 203.35 | 63.8 | ||||
| 2002127670 | 74952 | 15 | 217.68 | 68.85 | ||||
| 2002140610 | 74952 | 17 | 292.36 | 73.84 | ||||
| 15 | 46 | female | NO | 2001301957 | 74959 | 3 | 1.25 | 2.66 |
| 2002025001 | 74959 | 6 | 0.93 | 4.72 | ||||
| 2002037387 | 74959 | 7 | 1.59 | 9.16 | ||||
| 2002071617 | 74959 | 11 | 2.49 | 36.24 | ||||
| 2002116038 | 74959 | 15 | 40.35 | 64.59 | ||||
| 2002173413 | 74959 | 21 | 150.88 | 44.96 | ||||
| 2002239965 | 74959 | 27 | 194.04 | 32.56 | ||||
| 2002247400 | 74959 | 28 | 193.16 | 38.1 | ||||
| 16 | 47 | male | NO | 2001302006 | 74965 | 10 | 17.94 | 51.54 |
| 2002025482 | 74965 | 13 | 171.21 | 127.42 | ||||
| 2002059579 | 74965 | 16 | 333.47 | 238.26 | ||||
| 2002083197 | 74965 | 19 | 386.43 | 266.42 | ||||
| 2002140397 | 74965 | 25 | 401.4 | 275.64 | ||||
| 17 | 29 | male | NO | 2001302051 | 74966 | 2 | 0.93 | 0.93 |
| 2002037412 | 74966 | 6 | 0.93 | 3.9 | ||||
| 2002083093 | 74966 | 11 | 2.17 | 26.96 | ||||
| 2002128004 | 74966 | 15 | 2.35 | 59.39 | ||||
| 18 | 59 | male | YES | 2001302104 | 74972 | 6 | 0.93 | 6.72 |
| 2002025853 | 74972 | 9 | 0.93 | 3.06 | ||||
| 2002048964 | 74972 | 11 | 0.93 | 5.94 | ||||
| 2002071517 | 74972 | 14 | 118.25 | 24.53 | ||||
| 2002105336 | 74972 | 17 | 640.9 | 32.06 | ||||
| 2002138710 | 74972 | 20 | 940.68 | 32.43 | ||||
| 2002161517 | 74972 | 23 | 938.8 | 9.9264 | ||||
| 2002172344 | 74972 | 24 | 840.87 | 15.136 | ||||
| 2002206637 | 74972 | 27 | 936.13 | 15.8576 | ||||
| 2002212014 | 74972 | 28 | 704.64 | 15.5848 | ||||
| 19 | 29 | male | NO | 2001312176 | 74976 | 7 | 0.96 | 1.36 |
| 2002024985 | 74976 | 9 | 0.93 | 10.36 | ||||
| 2002059778 | 74976 | 12 | 1.54 | 60.06 | ||||
| 2002083023 | 74976 | 15 | 5.75 | 173.2 | ||||
| 2002127421 | 74976 | 19 | 14.51 | 264.95 | ||||
| 20 | 64 | male | NO | 2001312184 | 74977 | 3 | 0.93 | 1.98 |
| 2002024934 | 74977 | 5 | 0.93 | 29.85 | ||||
| 2002048795 | 74977 | 7 | 44.59 | 143.65 | ||||
| 2002060346 | 74977 | 9 | 357 | 283.46 | ||||
| 2002093320 | 74977 | 12 | 920.51 | 356.17 | ||||
| 2002127291 | 74977 | 15 | 914.47 | 307.65 | ||||
| 2002173315 | 74977 | 20 | 1172.36 | 107.26 | ||||
| 2002206672 | 74977 | 23 | 999.36 | 90.26 | ||||
| 2002247458 | 74977 | 27 | 641.88 | 86.3 | ||||
| 21 | 55 | male | NO | 2002014486 | 75021 | 7 | 0.96 | 7.76 |
| 2002059168 | 75021 | 11 | 1.46 | 10.33 | ||||
| 2002083123 | 75021 | 14 | 1.99 | 14.39 | ||||
| 2002138386 | 75021 | 19 | 136.11 | 107.79 | ||||
| 2002148386 | 75021 | 20 | 151.39 | 88.49 | ||||
| 2002183964 | 75021 | 24 | 168.56 | 71.72 | ||||
| 22 | 57 | female | NO | 2002048799 | 75024 | 5 | 0.94 | 0.93 |
| 2002071864 | 75024 | 8 | 1.46 | 9.03 | ||||
| 2002115816 | 75024 | 12 | 4.87 | 23.02 | ||||
| 2002128125 | 75024 | 13 | 0.93 | 8.05 | ||||
| 2002140291 | 75024 | 15 | 7.5 | 23.9 | ||||
| 2002150771 | 75024 | 16 | 6.32 | 9.6448 | ||||
| 2002162072 | 75024 | 17 | 20.44 | 11.1848 | ||||
| 2002172124 | 75024 | 18 | 46.43 | 12.5752 | ||||
| 2002185056 | 75024 | 19 | 64.48 | 13.6136 | ||||
| 2002196373 | 75024 | 20 | 95.51 | 11.6952 | ||||
| 2002206593 | 75024 | 21 | 112.44 | 15.8928 | ||||
| 2002218177 | 75024 | 22 | 98.31 | 13.0592 | ||||
| 2002229264 | 75024 | 23 | 110.88 | 12.496 | ||||
| 2002252541 | 75024 | 26 | 101.86 | 12.0296 | ||||
| 2002276138 | 75024 | 28 | 92.73 | 9.7768 | ||||
| 23 | 43 | female | YES | 2002014480 | 75025 | 3 | 0.93 | 4.83 |
| 2002061352 | 75025 | 8 | 1.03 | 4.36 | ||||
| 2002093995 | 75025 | 11 | 0.93 | 4.86 | ||||
| 2002127609 | 75025 | 14 | 21.46 | 46.51 | ||||
| 2002149871 | 75025 | 16 | 216.1 | 165.02 | ||||
| 2002183780 | 75025 | 20 | 623.16 | 98.78 | ||||
| 24 | 36 | male | YES | 2002025485 | 75054 | 4 | 0.93 | 9.72 |
| 2002037670 | 75054 | 5 | 0.93 | 26.65 | ||||
| 2002059680 | 75054 | 7 | 32.55 | 125.39 | ||||
| 2002072539 | 75054 | 9 | 179.46 | 280.55 | ||||
| 2002116856 | 75054 | 13 | 316.22 | 292.07 | ||||
| 2002140612 | 75054 | 16 | 569.92 | 184.53 | ||||
| 25 | 42 | male | YES | 2002025637 | 75062 | 6 | 0.93 | 4.86 |
| 2002059116 | 75062 | 9 | 0.93 | 4.6 | ||||
| 2002072520 | 75062 | 11 | 0.93 | 13.72 | ||||
| 2002093817 | 75062 | 13 | 10.87 | 56.42 | ||||
| 2002127491 | 75062 | 16 | 173.85 | 186.24 | ||||
| 26 | 64 | male | NO | 2002025704 | 75063 | 3 | 0.93 | 2.42 |
| 2002036781 | 75063 | 4 | 0.93 | 0.93 | ||||
| 2002059307 | 75063 | 6 | 0.93 | 7.35 | ||||
| 2002093337 | 75063 | 10 | 0.93 | 15.56 | ||||
| 2002149720 | 75063 | 15 | 1 | 9.37 | ||||
| 27 | 51 | male | YES | 2002025573 | 75064 | 5 | 1.18 | 5.86 |
| 2002050025 | 75064 | 8 | 1 | 6.89 | ||||
| 2002083137 | 75064 | 11 | 0.94 | 40.17 | ||||
| 2002127652 | 75064 | 15 | 151.37 | 214.94 | ||||
| 2002151216 | 75064 | 18 | 235.79 | 117.63 | ||||
| 2002207684 | 75064 | 23 | 200.61 | 98.39 | ||||
| 2002256162 | 75064 | 28 | 268.33 | 64.29 | ||||
| 28 | 51 | male | NO | 2001313503 | 75067 | 7 | 3.95 | 23.07 |
| 2002025616 | 75067 | 9 | 1.57 | 29.21 | ||||
| 2002059132 | 75067 | 12 | 64.59 | 85.86 | ||||
| 2002104233 | 75067 | 17 | 489.38 | 112.11 | ||||
| 2002149377 | 75067 | 21 | 158.84 | 155.96 | ||||
| 29 | 63 | female | NO | 2002025748 | 75070 | 5 | 0.93 | 0.93 |
| 2002059563 | 75070 | 8 | 0.93 | 4.74 | ||||
| 2002071702 | 75070 | 10 | 1.69 | 39.62 | ||||
| 2002093416 | 75070 | 12 | 25.16 | 100.78 | ||||
| 2002140375 | 75070 | 17 | 653.13 | 282.11 | ||||
| 30 | 77 | male | YES | 2002036834 | 75091 | 7 | 1.74 | 6.88 |
| 2002059179 | 75091 | 9 | 0.93 | 13.38 | ||||
| 2002061322 | 75091 | 10 | 1.07 | 14.8 | ||||
| 2002082791 | 75091 | 12 | 2.52 | 32.63 | ||||
| 2002104575 | 75091 | 14 | 4.07 | 47.88 | ||||
| 2002138576 | 75091 | 17 | 23.38 | 95.77 | ||||
| 31 | 57 | male | YES | 2002048509 | 75131 | 4 | 0.93 | 2.46 |
| 2002072082 | 75131 | 7 | 2.45 | 15.9 | ||||
| 2002093308 | 75131 | 9 | 1.41 | 8.07 | ||||
| 2002105687 | 75131 | 10 | 2.48 | 21.97 | ||||
| 2002116802 | 75131 | 11 | 3.23 | 29.59 | ||||
| 2002127853 | 75131 | 12 | 9.63 | 26.04 | ||||
| 2002138971 | 75131 | 13 | 15.94 | 35.01 | ||||
| 2002150964 | 75131 | 15 | 22.64 | 21.24 | ||||
| 2002184149 | 75131 | 18 | 50.6 | 30.84 | ||||
| 2002229495 | 75131 | 22 | 167.98 | 57.87 | ||||
| 2002240640 | 75131 | 24 | 213.22 | 53.94 | ||||
| 32 | 68 | male | YES | 2002048842 | 75138 | 4 | 2.41 | 4.26 |
| 2002061329 | 75138 | 6 | 0.93 | 10.52 | ||||
| 2002083162 | 75138 | 8 | 3.28 | 23.33 | ||||
| 2002093836 | 75138 | 9 | 4.19 | 34.83 | ||||
| 2002127457 | 75138 | 12 | 39.23 | 69.07 | ||||
| 2002161407 | 75138 | 16 | 126.36 | 43.64 | ||||
| 2002195400 | 75138 | 19 | 209.24 | 39.59 | ||||
| 2002230157 | 75138 | 23 | 157.06 | 28.29 | ||||
| 2002288414 | 75138 | 28 | 229.09 | 8.66 |
Detection of antibodies against SARS-CoV-2
In total, 217 blood specimens were obtained from 32 patients (6.8 blood specimens per patient on average; supplementary materials). A quantum dot immunofluorescence assay was used to semi-quantitatively detect IgM and IgG antibodies. The anti-SARS-CoV-2 IgG and IgM kits were manufactured by Chongqing Xinsaiya Biotechnology Company in Chongqing, China. Assays were performed according to the manufacturer’s detailed instructions. Briefly, serum collected from patients was incubated at 56°C for 30 min, and then, 80 μl of the diluted serum was added to the well dented on the test chip and was incubated at room temperature for 10 min. During the process, IgM or IgG antibodies in the serum sample reacted with quantum dot nanocrystal-conjugated secondary antibodies and purified recombinant SARS-CoV-2 spike (S) protein, respectively, which were both coated on a cellulose nitrate membrane. Subsequently, the immunofluorescence signal strength of the sample was analysed by a quantum dot fluorescence detector, which emitted a wavelength of 610 nm and excited a wavelength of 365 nm. The quantitative results were expressed in relative vitality units (RU/ml) according to the calibration curve. A value ≥10 RU/mL was considered to be a positive result. All serum samples were tested in triplicate, and the average of all three relative vitality units was used as the final test result.
Patterns of anti-SARS-CoV-2 IgG and IgM antibodies
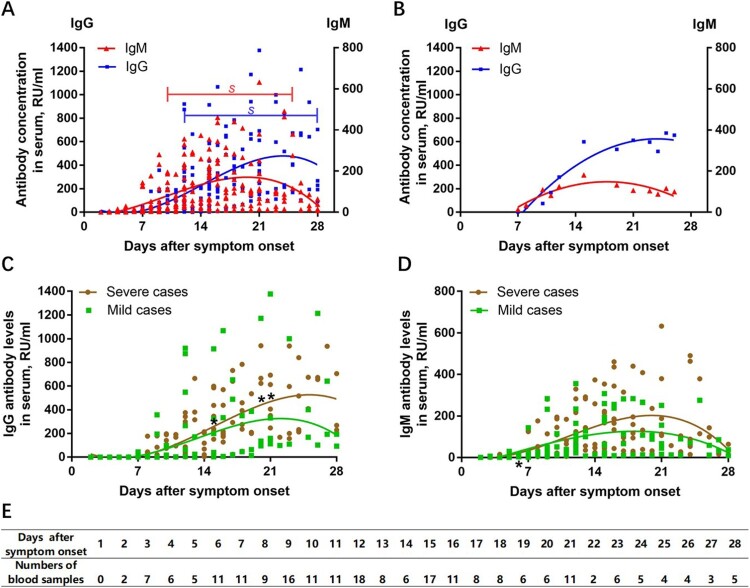
As shown in Figure 1, anti-SARS-CoV-2 S-specific IgG and IgM antibodies were not detectable in the very early days of infection (from day 0 to day 3). Anti-SARS-CoV-2 S-specific IgM antibodies were detectable from day 4 onward; the IgM antibody titres increased over time, peaking at approximately day 20, and then began to decline. The positivity rate of IgM antibody was only 60%, with a marked reduction in antibody levels 4 weeks after onset of illness. Anti-SARS-CoV-2 S-specific IgG antibodies were identifiable from day 7 onwards, peaking at approximately day 25, as shown in Figure 1(A). Serum IgG antibodies were still maintained at a high level after 4 weeks of infection. Figure 1(B) shows a typical IgG and IgM antibody response in a 65-year-old woman with COVID-19 (supplementary materials, Table 1). It is widely accepted that the IgM antibody response provides early-stage defence during viral infections prior to the development of the class-switched, high-affinity IgG response for long-term immunity and immunological memory [5].
Figure 1.
Patterns of anti-SARS-CoV-2 S antibody response in patients with COVID-19. (A) IgG and IgM antibody response patterns in serum samples of all 32 patients with confirmed COVID-19; s, a significant difference by non-parametric repeated measures ANOVA; (B) IgG and IgM antibody response in a 65-year-old woman with severe COVID-19; (C) The difference in anti-SARS-CoV-2 IgG antibody response between severe cases and mild cases; (D) The difference in anti-SARS-CoV-2 IgM antibody response between severe cases and mild cases. (E) Numbers of blood samples collected during the study period. For statistical analyses, the Mann-Whitney U test was performed for continuous variables. Statistical testing could not be performed on days 22, 24, 25 and 27 due to the very small number of available samples. *p < 0.05 was considered statistically significant.
Comparison of antibody response between mild cases and severe cases
We further compared the difference in antibody detectability between mild cases and severe cases of COVID-19. As shown in Figure 1(C), serum IgG antibody levels were not significantly correlated with clinical severity in the early stage of infection. However, the difference in IgG antibody levels between mild cases and severe cases from day 15 onward was found to be statistically significant (day 15 (N = 17), day 20 (N = 6) and day 21 (N = 11), all p < 0.05). Severe cases of COVID-19 tended to have a more vigorous IgG response against SARS-CoV-2 compared with mild cases. Notably, some patients with mild disease had a robust IgG antibody response from 9 days after symptom onset, while a few mild cases did not generate adequate IgG antibodies (approximately 21.43%). Our results also showed that mild cases tended to develop faster peak anti-SARS-CoV-2 S-specific IgM responses (approximately 17 days after symptom onset) compared with severe cases (approximately 21 days after symptom onset). It is also worth noting that IgM antibodies disappeared 4 weeks after symptom onset both in mild cases and severe cases (Figure 1(D)).
In summary, we observed that (1) the IgM antibody response to SARS-CoV-2 occurred earlier and peaked earlier than the IgG antibody response; (2) the IgM antibody response began to decline at week 3 of the illness, while the IgG antibody response persisted and was maintained in patients with COVID-19; and (3) severe cases of COVID-19 tended to have a more vigorous response in both IgG and IgM antibodies to COVID-19 illness. Our findings may be of significance in interpreting anti-SARS-CoV-2 antibody test results and in understanding humoral immune response patterns for SARS-CoV-2 infection in current and potential future COVID-19 outbreak scenarios. Importantly, the timing of IgM and IgG antibody occurrence in patients varies greatly, and this variation in timing may be associated with age as well as comorbidity [6]. More care needs to be taken when using levels of anti-SARS-CoV-2 antibodies to make a clinical diagnosis of COVID-19 or determine discharge criteria.
Funding Statement
This work was supported by the Chongqing Special Research Project for Novel Coronavirus Pneumonia Prevention and Control under Grants (No. cstc2020jscx-fyzxX0005; cstc2020jscx-fyzxX0024).
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Feb 18;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.China. NHCotPsRo . National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of novel coronavirus pneumonia (the seventh trial edition) Avialable from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 4.Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020 Dec;9(1):386–389. doi: 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Chen X, Xu A.. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med. 2003 Jul 31;349(5):508–509. doi: 10.1056/NEJM200307313490520 [DOI] [PubMed] [Google Scholar]
- 6.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 Mar 23;20(5):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]