INTRODUCTION
In 1994 Cucchi et al. 7 first published a paper identifying gastrogastric fistulas (GGF) as a complication of open divided Roux-en-Y gastric bypass (RYGB). The findings showed that GGF develop regardless of the remnant division from the pouch. Some authors attribute GGF to technical failure, early postoperative leaks or even marginal ulcers. Furthermore, diagnosis is usually difficult and requires a high index of suspicion, mainly due to a lack of pathognomonic symptoms and signs 14 . As of today, there is no consensus regarding an optimal diagnostic pathway for GGF, and management is usually patient tailored 13 , 14 .
In this paper, we present a case of a lady treated at our centre with recurrent GGF, and provide an up-to-date literature review of the topic.
CASE REPORT
Woman of 42 year-old with a BMI of 44 kg/m2 underwent a previous anti-gastric anti-colic RYGB using a circular staple for the gastro-jejunostomy anastomosis (GJA) in Jaber Hospital, Kuwait. Intra-operatively the anvil had an incomplete anastomotic stapler doughnut; however, both the intra-operative methylene blue and air tests were negative. The anastomotic line was the buttressed with 2-0 absorbable sutures. Two days post-operatively the patient developed acute abdominal pain, tachycardia and fever, with a water-soluble contrast study suggesting a GJA leak. A subsequent diagnostic laparoscopy however, was unremarkable, and she was managed conservatively. Seven years later, she again presented complaining of a two month history of progressive epigastric and retrosternal chest pain. Blood investigations showed mild leucocytosis and hyper-amylasemia. Gastroscopy demonstrated bile entry to the gastric pouch, with a corresponding 6-7 mm GGF. A barium swallow confirmed GGF, with no other fistulas nor strictures. She was managed endoscopically with one endo-clip applied to GGF, and its edges were burned using argon plasma coagulation.
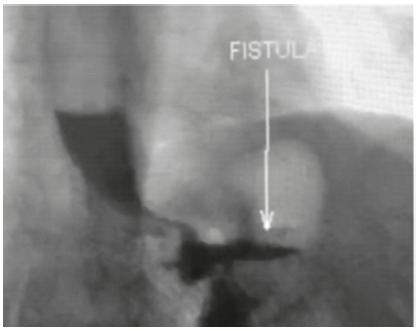
After three years she was attended again with abdominal pain and distention, associated with weight regain and vomiting. A barium swallow confirmed recurrence of the fistula (Figure 1), and gastroscopy showed a large fistulous opening measuring 15-20 mm, not feasible for endoscopic intervention.
FIGURE 1. Barium swallow showing the passage of contrast to the remnant stomach.
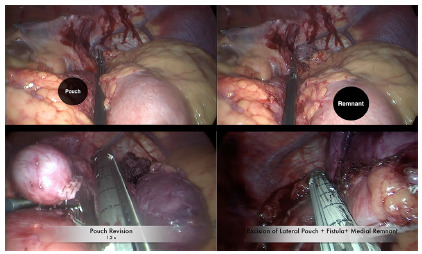
She, therefore, underwent a laparoscopic repair whereby the GJA was first taken down, followed by excision of the lateral edge of the gastric pouch and medial edge of gastric remnant (Figure 2). The GJA was then refashioned using a hand-sewn end to side technique. She had a six day hospital stay due to surgical site infection, and afterwards was discharged.
FIGURE 2. Resizing of the gastric pouch and resection of the GGF.
DISCUSSION
Incidence
Historically one in two non-divided bypasses was complicated with GGF2. However, over the past decade there has been an overall decline in the incidence. Since 2010 the incidence ranged between 0-1.18% 4 , 15 , 16 , 18 .
The true incidence of GGF is difficult to ascertain due to the fact that some patients remain asymptomatic. Nevertheless, in one study, routine upper gastrointestinal swallow performed on all RYGB patients 48 h postoperatively found the incidence to be 1.7% among 417 consecutive patients 10 .
Table 1 summarizes the incidence of GGF in published series to date.
TABLE1. Overview of post-Roux-en-Y gastric bypass gastrogastric fistula incidence.
| Author | Year | Total divided RYGB (open+laparoscopic ) | GFF | Incidence Rate (%) |
| Cucchi et al. | 1995 | 100 | 6 | 6 |
| Maclean et al. | 1997 | 123 | 4 | 3 |
| Corrodeguas et al. | 2005 | 1292 | 15 | 1.2 |
| Gumbs et al. | 2006 | 282 | 5 | 1.8 |
| Tucker et al. | 2007 | 1763 | 27 | 1.5 |
| Salimath et al. | 2009 | 1796 | 20 | 1.1 |
| Yao et al. | 2010 | 366 | 0 | 0 |
| Ribeiro-parenti et al. | 2017 | 1900 | 9 | 0.5 |
| Chahine et al. | 2018 | 1273 | 15 | 1.8 |
Many factors play a potential role in the formation of GGF. Technical failure due to incomplete separation of the proximal stomach has been hypothesized as a main culprit. This might be secondary to false perception of complete division, or improper intra-operative visualization. Staple line or GJA leaks are also believed to play a role. Other etiologies such as marginal ulceration causing GGF have been laid out. Much debate remains, however, as to whether GGF are a cause or a result of marginal ulceration 14 .
A number of anatomical GGF classification systems have been proposed in the literature, and are mostly based on the distance between the fistula and the GJA 4 , 15 . Chahine et al. 4 proposed a system whereby type one GGF are high and more than 1 cm away from the GJA, while type two are low and less than 1 cm away. Of note, this classification is based solely on intra-operative findings. In another series, a similar classification system was adapted by Ribeiro-parenti et al. 15 . This however is based on a combined radiological, endoscopic and intraoperative classification. Type one fistulas were termed proximal and were more than two cm from GJA, while type two were termed distal and were less than 2 cm from the GJA.
GGF are not easily recognized because of the lack of and/or ambiguity of presenting symptoms. For example, five out of seven GGF patients were found to be asymptomatic in one series 10 . Furthermore the symptoms typically mimic those of common RYGB complications making diagnosis quite a challenge. A review of literature with the common presenting symptoms and their relative frequency is summarized in Table 2 1 , 4 , 6 , 7 , 15 , 17 .
TABLE 2. Common presenting symptoms in patients with gastrogastric fistula.
| Abdominal Pain | Weight regain | Nausea | Vomiting | Reflux/heartburn | Diarrhea | Bleeding | Failure to thrive | Fever | |
| Chahine et al. | 73.3% | 80.0% | 86.6% | N/A | 40.0% | 13.3% | N/A | N/A | N/A |
| Ribeiro-Parenti et al. | 77.7% | 55.5% | N/A | 11.1% | N/A | N/A | 11.1% | N/A | N/A |
| Corcelles et al. | 72.2% | 50.0% | N/A | 50.0% | 73.0% | N/A | 5.5% | 22.0% | N/A |
| Tucker et al. | 37.0% | 33.0% | N/A | 18.5% | N/A | N/A | 11.1% | N/A | N/A |
| Cucchi et al. | 100.0% | N/A | 83.0% | 66.6% | N/A | 33.0% | N/A | N/A | 100.0% |
| Campos et al. | 51.6% | 100.0% | N/A | N/A | N/A | N/A | 9.6% | N/A | N/A |
Diagnostic tools for GGF are broadly divided into two categories: radiological and endoscopic. Radiologically, upper gastrointestinal series and computed tomography (CT) scans can serve as important tools in both diagnosis of GGF and delineation of anatomy 14 .
Today, upper gastrointestinal series remains the gold-standard radiological investigations for GGF 8 . This, however, is changing. CT recognition of GGF continues to evolve, and is playing a bigger role as more CT specific findings are defined with time. For example, in a retrospective study by Gao et al. 8 the relative attenuation ratio of contrast in the remnant stomach on CT scan was found to be 100% sensitive in GFF diagnosis.
Endoscopy has also proven itself to be an important tool in diagnosis of GGF, but the yield is dependent on the operator’s awareness of the possibility of GGF. This awareness comes in two forms, either a pre-procedural clinical or radiological suspicion, or an intra-procedural finding such as a marginal ulcer 1 , 4 , 5 .
Many surgeons today have adapted a combined endoscopic/radiological approach, and this appears to enhance diagnostic accuracy 4 , 6 , 14 , 15 , 17 . To date, however, there is a lack of strong evidence proving the superiority of one modality over another. In one series, the sensitivity of gastroscopy was slightly superior to upper gastrointestinal series in diagnosing GGF (72.2% vs. 70% respectively), but was found to have a lower sensitivity in another study (66.6 vs. 100% respectively) 6 , 15 . Furthermore, in a third study, gastroscopy detected less GGF than CT with oral/intravenous contrast (73.3% vs. 100%) 4 .
Although once a cornerstone in GGF management, the therapeutic role of medical treatment seems to be regressing. The mainstay of medical treatment is lifestyle modification, such as smoking cession, sucralfate in the presence of marginal ulcer and pharmacotherapy using proton pump inhibitors 14 . In one series, GGF completely resolved in 18.2% of patients using conservative treatment alone, while 45.5% were symptomatically controlled 1 . It is therefore reasonable to discuss medical options with patients, and offer it to those deemed high-risk or refusing invasive interventions.
A number of endoscopic techniques are used to seal GGF including endoscopic clips, stents, and suturing 12 , 14 . Most of these interventions have yet to be proven durable intermediate-term closure techniques, and literature lacks evidence on their long-term efficacy. In one study, however, Pauli et al. 14 . achieved a relatively high success rate, with half of the patients showing promising intermediate-term results using endoscopic endo-clip. The authors also concluded that GGF size was found to be inversely related to the success rate, with GGF larger than 1 cm less likely to heal endoscopically.
Endoscopic stenting has also shown success in treating GGF post-RYGB, with reported heal rates of up to 76% 12 . This, however, is challenged with a high incidence of stent migration (30.6%), and an increased risk of perforation. All in all, endoscopy has proven itself to be very useful in the management of GGF, but future advances in endoscopic technology might prove it to be even more successful in the days to come.
To date, surgery remains the most definite treatment of GGF. Surgical techniques are variable, and there is no consensus regarding optimal surgical choice 6 . In general, surgical management falls into one of three categories: simple resection of the fistula, resection of the fistula with revision of the GJA, resection of the fistula with remnant gastrectomy +/- revision of GJA 4 , 6 , 15 .
The location of fistula can play a major role in determining the extent of surgery 4 , 15 . Surgeon preference as well as pre and intra-operative findings may also determine the need to either revise the GJA and/or perform a gastrectomy 6 . Other operative adjuncts include an omental or jejunal interposition, theoretically reducing GGF recurrence postoperatively 2 .
All in all, although it remains a rare occurrence after RYGB it is important for the bariatric surgeon today to recognize GGF as a potential complication of the procedure. Since many patients with it do not present any typical signs, a high index of suspicion should be raised when post-RYGB patients present with symptoms such as abdominal pain and weight regain. Understanding the pathogenesis can help in potentially avoiding GGF, and knowledge of the diagnostic modalities aid in swift diagnosis and subsequent tailoring of patient-specific optimal management protocols. Further research is needed to set global guidelines and reach a consensus on treatment algorithms.
Footnotes
Financial source: none
REFERENCES
- 1.Campos J, Neto M, Martins J, Gordejuela A, Alhinho H, Pachu E, Ferraz A. Endoscopic, conservative, and surgical treatment of the gastrogastric fistula the efficacy of a stepwise approach and its long-term results. Bariatr Surg Pract Patient Care. 2015;10(2):62–67. [Google Scholar]
- 2.Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg. 1999;9(1):22–27. doi: 10.1381/096089299765553674. [DOI] [PubMed] [Google Scholar]
- 3.Carrodeguas L, Szomstein S, Soto F. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1(5):467–474. doi: 10.1016/j.soard.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Chahine E, Kassir R, Dirani M, Joumaa S, Debs T, Chouillard E. Surgical Management of Gastrogastric Fistula After Roux-en-Y Gastric Bypass 10-Year Experience. Obes Surg. 2018;28(4):939–944. doi: 10.1007/s11695-017-2949-2. [DOI] [PubMed] [Google Scholar]
- 5.Chau E, Youn H, Ren-fielding CJ, Fielding GA, Schwack BF, Kurian MS. Surgical management and outcomes of patients with marginal ulcer after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11(5):1071–1075. doi: 10.1016/j.soard.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Corcelles R, Jamal MH, Daigle CR, Rogula T, Brethauer SA, Schauer PR. Surgical management of gastrogastric fistula. Surg Obes Relat Dis. 2015;11(6):1227–1232. doi: 10.1016/j.soard.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Cucchi S, Pories W, MacDonald K, Morgan E. Gastrogastric Fistulas A Complication of Divided Gastric Bypass Surgery. Ann Surg. 1995;221(4):387–391. doi: 10.1097/00000658-199504000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao G, Nezami N, Mathur M, Balcacer P, Israel G, Spektor M. Diagnosis of gastrogastric fistula on computed tomography A quantitative approach. Abdom Radiol. 2017;43(6):1329–1333. doi: 10.1007/s00261-017-1304-3. [DOI] [PubMed] [Google Scholar]
- 9.Gumbs AA, Duffy AJ, Bell RL. Management of gastrogastric fistula after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2(2):117–121. doi: 10.1016/j.soard.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Kolakowski S, Kirkland ML, Schuricht AL. Routine postoperative upper gastrointestinal series after Roux-en-Y gastric bypass determination of whether it is necessary. Arch Surg. 2007;142(10):930–934. doi: 10.1001/archsurg.142.10.930. [DOI] [PubMed] [Google Scholar]
- 11.MacLean LD, Rhode BM, Nohr C. Stoma1ulcer after gastric bypass. J Am Cd Surg. 1997;185:1–7. doi: 10.1016/s1072-7515(01)00873-0. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki O, Bernardo WM, Brunaldi VO. Efficacy and Safety of Stents in the Treatment of Fistula After Bariatric Surgery a Systematic Review and Meta-analysis. Obes Surg. 2018;28(6):1788–1796. doi: 10.1007/s11695-018-3236-6. [DOI] [PubMed] [Google Scholar]
- 13.Palermo M, Acquafresca PA, Rogula T, Duza GE, Serra E. Late surgical complications after gastric by-pass a literature review. Arq Bras Cir Dig. 2015;28(2):139–143. doi: 10.1590/S0102-67202015000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauli EM, Beshir H, Mathew A. Gastrogastric fistulae following gastric bypass surgery-clinical recognition and treatment. Curr Gastroenterol Rep. 2014;16(9):405–405. doi: 10.1007/s11894-014-0405-1. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro-parenti L, De courville G, Daikha A, Arapis K, Chosidow D, Marmuse JP. Classification, surgical management and outcomes of patients with gastrogastric fistula after Roux-En-Y gastric bypass. Surg Obes Relat Dis. 2017;13(2):243–248. doi: 10.1016/j.soard.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Salimath J, Rosenthal RJ, Szomstein S. Laparoscopic remnant gastrectomy as a novel approach for treatment of gastrogastric fistula. Surg Endosc. 2009;23(11):2591–2595. doi: 10.1007/s00464-009-0465-8. [DOI] [PubMed] [Google Scholar]
- 17.Tucker ON, Szomstein S, Rosenthal RJ. Surgical management of gastro-gastric fistula after divided laparoscopic Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2007;11(12):1673–1679. doi: 10.1007/s11605-007-0341-6. [DOI] [PubMed] [Google Scholar]
- 18.Yao DC, Stellato TA, Schuster MM, Graf KN, Hallowell PT. Gastrogastric fistula following Roux-en-Y bypass is attributed to both surgical technique and experience. Am J Surg. 2010;199(3):382–385. doi: 10.1016/j.amjsurg.2009.09.017. [DOI] [PubMed] [Google Scholar]


