Highlights
-
•
We present a report on continuous EEG (cEEG) findings in COVID-19 patients.
-
•
cEEG revealed findings consistent with encephalopathy independent of IV anesthetics.
-
•
Acute symptomatic seizures were noted in two patients.
Keywords: Electroencephalography (EEG), Seizures, Critical illness, COVID-19
Abbreviations: cEEG, continuous electroencephalography; ASMs, anti-seizure medications; SLE, seizure-like event; PDR, posterior dominant rhythm; GPDs, Generalized periodic discharges; LRDA, Lateralized rhythmic delta activity
Abstract
Objective
As concerns regarding neurological manifestations in COVID-19 (coronavirus disease 2019) patients increase, limited data exists on continuous electroencephalography (cEEG) findings in these patients. We present a retrospective cohort study of cEEG monitoring in COVID-19 patients to better explore this knowledge gap.
Methods
Among 22 COVID-19 patients, 19 underwent cEEGs, and 3 underwent routine EEGs (<1 h). Demographic and clinical variables, including comorbid conditions, discharge disposition, survival and cEEG findings, were collected.
Results
cEEG was performed for evaluation of altered mental status (n = 17) or seizure-like events (n = 5). Five patients, including 2 with epilepsy, had epileptiform abnormalities on cEEG. Two patients had electrographic seizures without a prior epilepsy history. There were no acute neuroimaging findings. Periodic discharges were noted in one-third of patients and encephalopathic EEG findings were not associated with IV anesthetic use.
Conclusions
Interictal epileptiform abnormalities in the absence of prior epilepsy history were rare. However, the discovery of asymptomatic seizures in two of twenty-two patients was higher than previously reported and is therefore of concern.
Significance
cEEG monitoring in COVID-19 patients may aid in better understanding an epileptogenic potential of SARS-CoV2 infection. Nevertheless, larger studies utilizing cEEG are required to better examine acute epileptic risk in COVID-19 patients.
1. Introduction
As the COVID-19 pandemic continues, there is a need to better understand the neurological manifestations and associated complications of COVID-19. Acute, clinical, and non-clinical seizures are well-known complications in critically ill patients with sepsis and brain injury (Oddo et al., 2009, Newey et al., 2018, Punia et al., 2019). Continuous electroencephalogram (cEEG) monitoring in acutely ill patients has revealed that the acute seizures these patients face are mostly non-convulsive (Claassen et al., 2004, Rodriguez Ruiz et al., 2017).
An initial retrospective case series of 214 patients by Mao et al. described central nervous system (CNS) manifestations in 53 patients, of whom 6 patients had a cerebrovascular disease, and one patient had a clinical seizure (Mao et al., 2020). A study of 304 patients by Lu et al. showed no evidence of acute symptomatic seizures and only two patients with seizure-like symptoms in the settings of hypocalcemia and acute stress (Lu et al., 2020). As reported by Vollono et al., one COVID-19 patient was noted to have status epilepticus after being otherwise seizure-free for two years on valproic acid (Vollono et al., 2020).
EEG evaluations have also been studied in COVID-19 infected patients. Galanopolou et al. described EEG findings in 22 COVID-19 positive patients. Of these, 9 patients had sharp waves, including 8 in the frontal region. However, no electrographic seizures were recorded (Galanopoulou et al., 2020). This high percentage of epileptiform abnormalities (EAs) in a specific brain region in COVID-19 patients behooves the question if this is a potential epileptic effect from the SARS-CoV-2 infection or if this is a limitation with the EEG requisition method itself; since patients did not receive the minimum required 21-electrode clinical EEG recommended by American Clinical Neurophysiological Society ACNS) (Sinha et al., 2016).
To add to the understanding of EEG findings in COVID-19 patients, we present a cohort of 22 COVID-19 patients, of whom 19 underwent continuous EEG (cEEG) with 21-electrodes for at least 24 h, and 3 underwent routine EEG (<1 h). All patients in this cohort were recorded with a minimum of 21-electrodes. This is the largest cEEG study to date in COVID-19 patients. We determine the prevalence of EEG changes in this population, the characteristics of these EEG changes, and the EEG finding’s relationship to survival.
2. Methods
2.1. Study population
After institutional review board (IRB) approval, we cross-matched the Cleveland Clinic COVID-19 registry with the Cleveland Clinic EEG database (Ebase, Cleveland, OH) from April 20th, 2020 until May 20th, 2020. All hospitalized COVID-19 adults (≥18 years of age at the time of diagnosis of COVID-19) who underwent an EEG were included in the study population. Patients were excluded if they had negative COVID-19 testing or if they did not undergo EEG evaluations during their admission for COVID-19 infection. We identified 19 COVID-19 patients who underwent cEEG for at least 24 h and 3 COVID-19 patients who underwent routine EEG (20 min). All variables in this study were collected until May 20th, 2020.
2.2. Data collection
All COVID-19 patients had at least one SARS-CoV-2 positive test prior to the initiation of cEEG or during cEEG monitoring. The electronic medical record (EMR; EPIC, Verona, WI) was reviewed to extract predefined COVID-19-related clinical variables: fever, pneumonia, mechanical ventilation status, and treatment (e.g. hydroxychloroquine). Medical and neurological comorbidities, including epilepsy history and the use of anti-seizure medications (ASMs), were also extracted from the EMR. Medical comorbidities included, but were not limited to: chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), diabetes, hypertension, coronary artery disease, cancer, and immunosuppressive disease.
2.3. Continuous EEG monitoring
EEG findings were extracted from the EEG reporting database. EEG findings were classified using the ACNS terminology for cEEG (Hirsch et al., 2013). Electrographic seizures were classified based on the Salzburg criteria (Beniczky et al., 2013). We used the ILAE (International League Against Epilepsy) terminology to describe ictal semiology associated with electrographic seizures (Blume et al., 2002).
cEEG monitoring indications were collected from the history portion of the EEG requisition form and the EMR was used in cases where the indication was not clearly outlined. Indications were coded as either unexplained altered mental status (AMS) or a seizure-like event (SLE). Unexplained AMS was defined by the treating clinician in all cases, representing a change from baseline mentation not accounted for by the patient's medical condition or drugs, leading to a clinical concern for non-convulsive seizures. SLEs were also defined by the treating clinician and primarily represented motor events such as clonic or myoclonic movements. Patients with AMS after a witnessed SLE were classified into the latter category.
2.4. Clinical outcomes data collection
Whilte the main goal of this study was to report cEEG findings in a cohort of critically ill COVID-19 patients, the putative associations between survival, clinical outcomes (discharge disposition), cohort characteristics (e.g. comorbidities, age, gender) and EEG findings were also explored.
2.5. Statistical analysis
Continuous and categorical data were summarized with mean values, standard deviations, medians (continuous data), and frequencies (categorical data). Fisher’s exact test was used to compare EEG characteristics between patients who expired in the hospital and the survivors. All analyses were performed using R statistical software v3.6.3 (R Development Core Team, 2010) and all tables were created using the Table1 R package v1.2 (Rich, 2020). A Bonferroni correction was used for multiple test corrections, and the Bonferroni adjusted cutoff p-value of 0.005 was considered significant.
Table 1.
Cohort Characteristics.
| Total (N = 22) |
|
|---|---|
| Sex | |
| Female | 8 (36.4%) |
| Male | 14 (63.6%) |
| Age (years) | |
| Mean (SD) | 66.5 (11.2) |
| Race | |
| African American | 7 (31.8%) |
| Asian | 1 (4.5%) |
| White | 14 (63.6%) |
| History of non-neurological comorbidities | |
| Smoking | 10 (45.5%) |
| Chronic obstructive pulmonary disease | 2 (9.1%) |
| Asthma | 8 (36.4%) |
| Diabetes | 9 (40.9%) |
| Hypertension | 15 (68.2%) |
| Coronary artery disease | 4 (18.2%) |
| Congestive heart failure | 4 (18.2%) |
| Cancer | 7 (31.8%) |
| Immunosuppressive disease | 8 (36.4%) |
| History of neurological comorbidities | 6 (27.3%) |
| Epilepsy | 2 (9.1%) |
| Stroke | 1 (4.5%) |
| Headache | 1 (4.5%) |
| Traumatic brain injury | 1 (4.5%) |
| Spinal stenosis | 2 (9.1%) |
| COVID-19 specific characteristics | |
| Fever | 15 (68.2%) |
| Pneumonia | 6 (27.3%) |
| Mechanical ventilation required | 18 (81.8%) |
| Hydroxychloroquine given as treatment | 16 (72.7%) |
3. Results
3.1. Clinical details and outcomes
A total of 22 COVID-19 patients (8 females; 36.4%), with a mean age of 66.5 years (±11.2 years), underwent EEG monitoring. All 22 patients, except for 3, underwent cEEG monitoring. Demographics, comorbidities, and COVID-19 specific characteristics of the study population are summarized in Table 1. Eighteen patients (85.7%) received a non-contrast CT brain. Of these eighteen patients, only four had findings consistent with an acute intracranial process: one patient had an intracerebral hemorrhage, one patient had an acute ischemic stroke (confirmed on MRI), and two patients had imaging concerning for possible ischemia. None of the four patients with acute findings had EAs or electrographic seizures recorded on EEG.
At the point of the most recent follow-up (May 20th, 2020), 6 patients (27.3%) did not survive hospitalization, and the remainder were discharged (16 patients).
3.2. cEEG findings and outcomes
Across the study population, 19 (86.4%) patients underwent cEEG, and 3 patients had routine EEGs. The summary of EEG and clinical variables of the study cohort are provided in Table 2 . Of the 19 cEEG patients, 12 (63.2%) were on IV infusions of anesthetics (fentanyl, propofol, and/or midazolam) during at least a portion of cEEG monitoring. The median duration of cEEG monitoring was 2 days (range 1–6 days). In 4 of 19 patients, cEEG was performed for evaluation of seizure-like events (SLE). These events were described as left arm clonic movements, right-face twitching (clonic), generalized tonic clonic seizures, and “eyebrow twitching with lip-smacking.” The remaining 15 patients underwent cEEG evaluation to rule out non-convulsive seizures as a cause of unexplained altered mental status AMS.
Table 2.
Cohort EEG Findings by Outcome. P values reported from Fisher’s exact test.
| Total (N = 22) |
Alive (N = 16) |
Expired in hospital (N = 6) |
P | |
|---|---|---|---|---|
| EEG indication | ||||
| Altered mental status (AMS) | 17 (77.3%) | 12 (75.0%) | 5 (83.3%) | >0.999 |
| Seizure-like event (SLE) | 5 (22.7%) | 4 (25.0%) | 1 (16.7%) | >0.999 |
| Patients on anesthesia during EEG | 14 (63.6%) | 9 (56.2%) | 5 (83.3%) | 0.351 |
| Presence of clinical seizure | 2 (9.1%) | 1 (6.2%) | 1 (16.7%) | 0.481 |
| Epileptic EEG | 5 (22.7%) | 3 (18.8%) | 2 (33.3%) | 0.585 |
| Presence of GPDs | 7 (31.8%) | 6 (37.5%) | 1 (16.7%) | 0.616 |
| Presence of PDR | 11 (50.0%) | 9 (56.2%) | 2 (33.3%) | 0.635 |
| Presence of GRDA | 11 (50.0%) | 10 (62.5%) | 1 (16.7%) | 0.149 |
| Patient started on ASMs | 6 (27.3%) | 4 (25.0%) | 2 (33.3%) | >0.999 |
| ASMs continued after discharge | 2 (9.1%) | 1 (6.2%) | 1 (16.7%) | 0.766 |
AMS = Altered Mental Status, SLE = Seizure-like event, GPD = Generalized Periodic Discharges, PDR = Posterior Dominant Rhythm, GRDA = Generalized Rhythmic Delta Activity, ASMs = Anti-seizure medications.
Acute electrographic seizures were noted in 2 (10.5%) of the 19 cEEG patients. These two patients did not have prior epilepsy and have been described in detail in a recent publication (Hepburn et al., 2020). Briefly, both were elderly men with several cardiovascular abnormalities. One of them had several episodes of left upper extremity clonic movements with worsening encephalopathy. cEEG was started and captured three left-arm clonic seizures lasting for roughly 30 s each, originating from the right centroparietal region. Levetiracetam was initiated with the subsequent cessation of clinical and electrographic seizures. In the second patient, cEEG was initiated due to right eyelid and facial twitching on admission day 5. Multiregional EEG seizures arising from the left and right fronto-temporal regions (left greater than right; Fig. 1 ) and left parieto-occipital region were captured. Most of the seizures were non-convulsive, but 4 episodes of clonic facial movements associated with fronto-temporal seizures were also noted. Seizure frequency significantly improved following levetiracetam treatment.
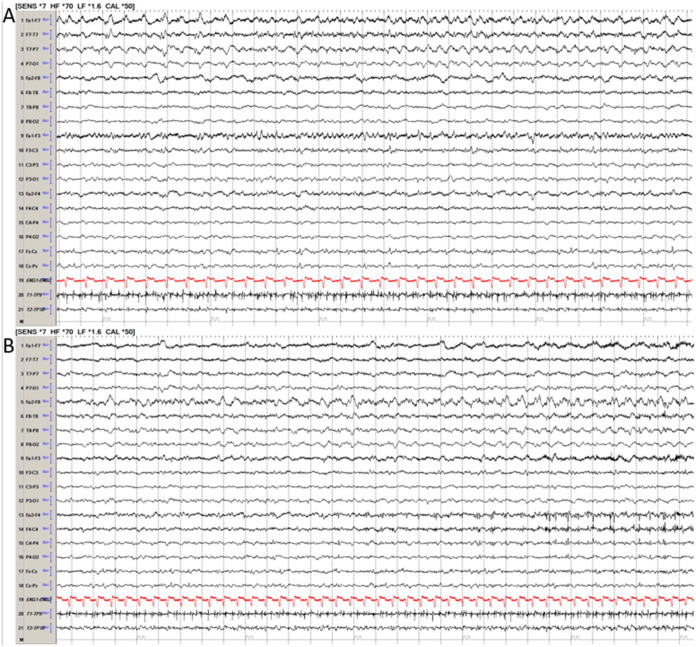
Fig. 1.
Acute electrographic seizure in a COVID-19 patient. Legend: Patient had multiregional seizure arising from left (A) and right (B) front-temporal region as well as left parieto-occipital regions (not pictured here). EEG is a bipolar 10–20 longitudinal montage.
Epileptic abnormalities were noted in 4 of 19 cEEG patients (15.7%), including the 2 with electrographic seizures from Hepburn et al. The remaining 2 patients had a history of epilepsy and were noted to have generalized polyspikes and right frontal sharp waves.
The cEEG showed a continuous (>80% of the recording) generalized polymorphic delta slowing in all patients. The posterior dominant rhythm (PDR) was absent throughout the cEEG in 8 patients (42.1%), was slow (<8 Hz) in 9 patients (47.4%), and within normal limits in the remaining patients. Six of 8 patients lacking PDR on cEEG were on IV anesthetics at some point during the cEEG monitoring. The proportion of patients on IV anesthetics was not statistically different in the patients lacking PDR compared to ones with a discernible PDR (54.5%, p = 0.633). Generalized periodic discharges (GPDs) were noted in 7 (36.8%) patients, ranging from 0.5 Hz to 1 Hz in frequency. In 5 of these patients, GPDs were of triphasic morphology, and in two, the GPDs were sharply contoured (Fig. 2 ). Although sharply contoured, given the low frequency (<1 Hz) these GPDs were not considered as definitive epileptiform (Rodriguez Ruiz et al., 2017). Ten patients (52.6%) were noted to have intermittent generalized rhythmic delta activity (GRDA), including 3 patients with sharply contoured waveforms.
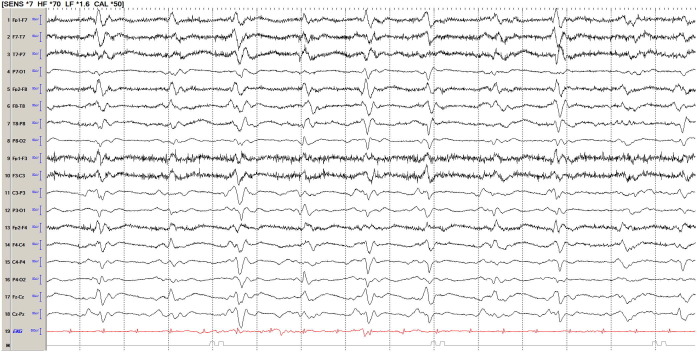
Fig. 2.
Generalized periodic discharges, sharply contoured (triphasic appearance, at times) in a COVID-19 patient. Legend: EEG is a bipolar 10–20 longitudinal montage.
Two patients with epilepsy history were already on anti-seizure medication (ASMs) at the time of admission. No changes were made to their ASMs during the admission or at discharge. Five additional patients (22.7%) were started on ASMs; levetiracetam was used in four patients, and valproic acid in one patient. Of these five patients, only two patients had epileptic EEGs in the form of EEG seizures. The other 3 patients were started on ASMs due to clinical events concerning for seizure before cEEG monitoring. One patient with acute electrographic seizures died in the hospital. Another patient with acute electrographic seizures was discharged on levetiracetam 500 mg twice a day. cEEG monitoring helped the discontinuation of ASMs (levetiracetam) in the rest of the three patients. However, after the discontinuation of levetiracetam, one of them was discharged on Valproate 500 mg at bedtime by psychiatry.
3.3. Routine EEG findings
There were 3 patients (one female) of the 22 who underwent routine EEGs. The treating team determined the EEG type (routine vs. continuous) at our institution and also made decisions as to whether to convert a routine EEG to cEEG monitoring based on patients’ clinical presentation. There were no technical or clinical limitations that prevented cEEG monitoring in these 3 patients. Of these three patients, none had a prior epilepsy history and two were receiving IV anesthesia at the time of routine EEG. Two patients were monitored for unexplained AMS, and one patient underwent EEG due to a SLE of “right-sided jerking movements.” The SLE patient had an epileptic EEG showing left hemispheric lateralized rhythmic delta activity (LRDA) up to 1.5 Hz in frequency. This patient was not started on ASMs. None of the three patients with routine EEG had PDRs.
With respect to clinical outcomes, two of the three patients expired in the hospital, and one was discharged home.
3.4. EEG findings do not show statistically significant association with survival
Among the 22 patients in the study population, there were no statistically significant differences between patients who expired in the hospital and those who remain alive with respect to the presence of epileptic EEG findings (p = 0.585), PDR (p = 0.635), GPD (0.616), or GRDA (p = 0.149) (Table 2).
4. Discussion
In this work, we present a description of EEG findings in COVID-19 infected patients. Excluding the 2 patients with a history of epilepsy, 3 of the 20 remaining patients had epileptic findings. Among them, one patient was found to have left LRDA (as epileptic as lateralized periodic discharges in critically ill patients (Gaspard et al., 2013), and two others had electrographic seizures. All cEEGs showed changes consistent with encephalopathy in the form of continuous slowing in the delta frequency range. While the contribution of IV anesthetics to cEEG findings may confound the analysis, no statistically significant difference in the rate of PDR was seen between those patients on or off of IV anesthetics. Slightly more than half of our cEEG patients were found to have GRDA, a finding known to be non-epileptic, and another one-third (36.8%) were found to have GPDs. While GPDs may be epileptic and associated with electrographic seizures, the relationship is frequency-dependent (Foreman et al., 2012, Rodriguez Ruiz et al., 2017). Two patients had sharply contoured GPDs, both with a frequency less than 1 Hz, below the 1.5 Hz threshold known to correlate with increased seizure risk (Rodriguez Ruiz et al., 2017). Galanopoulou et al. found GPDs in only 1 out of 22 (4.5%) COVID-19 patients, lower than what was observed in our study (Galanopoulou et al., 2020). Notably, this difference may have arisen due to a lower cEEG usage rate in their cohort (7 of 22 patients).
Sporadic inter-ictal epileptiform discharges (IEDs) such as sharp waves were not seen in patients lacking a history of epilepsy. This lack of sharp waves in this study contrasts the 9 of 22 patients found to have sharp waves by Galanopoulou et al. (2020), in which all but one had frontal sharp waves. Although the duration of 8 channel-EEGs performed in the Galanopoulou et al. study is unclear, the 8 channel-EEG is typically used for screening purposes and not for continuous use. In contrast, cEEG patients in this study underwent a median duration of 2 days of monitoring with the 21-electrode system. Although the severity of illness in the two patient populations cannot be compared, the most likely explanation of this major difference in the EEG findings is the lack of a 10–20 EEG system using the minimum required 21 electrodes (Sinha et al., 2016). As acknowledged by the American Clinical Neurophysiological Society, the chances of interpretive errors increase with fewer electrodes; this is particularly the case for transient findings such as sharp waves. Another small study of 8 patients, including 5 with epilepsy history, found 3 patients with GPDs of triphasic morphology and 2 patients with electrographic seizures (one had focal epilepsy history) (Pilato et al., 2020). Otherwise, IEDs were not seen in this study as well. Overall, the findings of Pilato et al. are in line with this report.
So far, clearly documented epileptic seizures in COVID-19 patients, without pre-existing epilepsy, have been extremely rare. For example, an individual with COVID-19 RNA in their CSF was documented having a meningo-encephalitic presentation with a one-minute “generalized seizure” and multiple epileptic seizures requiring intubation (Moriguchi et al., 2020). Beyond Moriguchi et al., acute symptomatic seizures were not observed in dedicated multicenter series to assess seizure risk in more than 300 COVID-19 individuals, nor were they evident in the case series by Galanopoulou et al. from New York (Lu et al. 2020, Galanopoulou et al. 2020). As such, the two COVID-19 patients with clinical acute symptomatic seizures captured on EEG from our cohort are rare findings.
Both patients with acute symptomatic seizures in this study were elderly males with several comorbidities. In these patients, the lack of acute neuroimaging findings in both raises the suspicion of whether there is a direct or indirect contribution of COVID-19 infection in causing acute seizures. Whether the seizures are evidence neurotropism, a symptom of microthrombic events in the brain secondary to hypoxic and inflammatory processes, or multifactorial remains unclear (Montalvan et al., 2020, Oxley et al., 2020, Tan et al. 2020, Wu et al. 2020). Furthermore, while most seizures in critically ill patients are non-convulsive (Claassen et al., 2004, Rodriguez Ruiz et al., 2017), it is curious that we report convulsive, focal motor seizures in 2 patients alongside a case reported by Vollono et al. in which a COVID-19 patient with well-controlled epilepsy of over two years presented with myoclonic status epilepticus (Vollono et al., 2020).
In our cohort, just slightly less than a quarter (22.7%) of patients were started on ASMs. Of these 5 patients, cEEG helped to clarify the lack of indication for the continuation of ASM in 3 patients. In studies without cEEG monitoring, there is a higher rate of ASM usage, as in Galanopoulou et al., where more than 50% of patients were started on ASM (Galanopoulou et al., 2020).
There are several limitations of our study, including its retrospective design and small study population. Since the primary aim was to characterize the cEEG findings of COVID-19 patients, we did not include patients with clinical concerns for COVID-19 disease who tested negative. We did not find any relation of EEG findings with the clinical outcome (survival) in our study population, which is quite likely due to the small study population. Ultimately, multicenter collaborative efforts are needed to better characterize the risk of acute and long term epileptogenic potential following COVID-19 infection. Further exploration of the neurotropic capabilities of this pathogen are needed as well.
5. Conclusions
COVID-19 positive patients who were encephalopathic had a variety of epileptiform abnormalities on EEG, and a higher proportion of patients had electrographic seizures than reported in previous studies. In sharing our experience, we hope that cEEG monitoring can be utilized as a resource for medical decision-making for ASMs and to better understand this disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding for the collection of data used in the COVID-19 registry for this project and funding of authors LJ and SL was supported by NIH R01 NS097719.
References
- Beniczky S., Hirsch L.J., Kaplan P.W., Pressler R., Bauer G., Aurlien H. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54:28–29. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- Blume W.T., Lüders H.O., Mizrahi E., Tassinari C., Van Emde B.W., Engel J. Glossary of descriptive terminology for Ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2002;42(9):1212–1218. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- Claassen J., Mayer S.A., Kowalski R.G., Emerson R.G., Hirsch L.J. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743–1748. doi: 10.1212/01.WNL.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- Foreman B., Claassen J., Abou Khaled K., Jirsch J., Alschuler D.M., Wittman J. Generalized periodic discharges in the critically ill: A case-control study of 200 patients. Neurology. 2012;79(19):1951–1960. doi: 10.1212/WNL.0b013e3182735cd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Ferastraoaru V, Correa DJ, Cherian K, Duberstein S, Gursky J, et al. EEG findings in acutely ill patients investigated for SARS‐CoV2/COVID‐19: a small case series preliminary report. Epilepsia Open. Published online May 6, 2020:epi4.12399. doi:10.1002/epi4.12399. [DOI] [PMC free article] [PubMed]
- Gaspard N., Manganas L., Rampal N., Petroff O.A.C., Hirsch L.J. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically Ill patients. JAMA Neurol. 2013;70(10):1288–1295. doi: 10.1001/jamaneurol.2013.3475. [DOI] [PubMed] [Google Scholar]
- Hepburn M, Mullaguri N, George P, Hantus S, Punia V, Bhimraj A et al. Acute symptomatic seizures in critically Ill patients with COVID-19: is there an association? Neurocrit Care. Published online May 28, 2020. doi:10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed]
- Hirsch L.J., LaRoche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30(1):1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- Lu L, Xiong W, Liu D, Liu J, Yang D, Li N et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia Published online May 2, 2020:epi.16524. doi:10.1111/epi.16524 [DOI] [PMC free article] [PubMed]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol Published online April 10, 2020. doi:10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey C.R., Kinzy T.G., Punia V., Hantus S. Continuous electroencephalography in the critically Ill: clinical and continuous electroencephalography markers for targeted monitoring. J Clin Neurophysiol. 2018;35(4):325–331. doi: 10.1097/WNP.0000000000000475. [DOI] [PubMed] [Google Scholar]
- Oddo M., Carrera E., Claassen J., Mayer S.A., Hirsch L.J. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37(6):2051–2056. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilato MS, Urban A, Alkawadri R, Barot NV, Castellano JF, Rajasekran V, et al. EEG Findings in Coronavirus Disease [published online ahead of print, 2020 Jul 1]. J Clin Neurophysiol 2020. doi: 10.1097/WNP.0000000000000752. [DOI] [PubMed]
- Punia V., Fitzgerald Z., Zhang X., Huynh H., Bena J., Morrison S. Electroencephalographic biomarkers of epilepsy development in patients with acute brain injury: a matched, parallel cohort study. Ann Clin Transl Neurol. 2019;6(11):2230–2239. doi: 10.1002/acn3.50925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. A Language and Environment for Statistical Computing: Reference Index. R Foundation for Statistical Computing; 2010. Accessed May 16, 2020. http://www.polsci.wvu.edu/duval/PS603/Notes/R/fullrefman.pdf.
- Rich B. Table1: Tables of Descriptive Statistics in HTML; 2020. https://CRAN.R-project.org/package=table1.
- Rodriguez Ruiz A., Vlachy J., Lee J.W., Gilmore E.J., Ayer T., Haider H.A. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically Ill patients. JAMA Neurol. 2017;74(2):181. doi: 10.1001/jamaneurol.2016.4990. [DOI] [PubMed] [Google Scholar]
- Sinha S.R., Sullivan L., Sabau D., San-Juan D., Dombrowski K.E., Halford J.J. American clinical neurophysiology society guideline 1: minimum technical requirements for performing clinical electroencephalography. J Clin Neurophysiol. 2016;33(4):303–307. doi: 10.1097/WNP.0000000000000308. [DOI] [PubMed] [Google Scholar]
- Tan CW, Low JGH, Wong WH, Chua YY, Goh SL, Ng HJ. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am J Hematol. Published online May 4, 2020:ajh.25822. doi:10.1002/ajh.25822. [DOI] [PMC free article] [PubMed]
- Vollono C., Rollo E., Romozzi M., Frisullo G., Servidei S., Borghetti A. Focal status epilepticus as unique clinical feature of COVID-19: A case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. Published online March 2020:S0889159120303573. doi:10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed]