Highlights
•To date most reports on COVID-19 associated coagulopathy focused an increased activation of the plasmatic coagulation system.
•Using a novel method to assess clot lysis we observed abnormalities in fibrinolysis in COVID-19 infection.
•Our finding suggests that fibrinolysis system may contribute to the procoagulatory status in COVID-19 patients.
Keywords: Fibrinolysis, COVID-19, Coagulation
Abbreviations: M, male; y, years; SOFA, Sepsis-related organ failure assessment score; EX-Test, extrinsic test; TPA, tissue plasminogen activator; PT, prothrombin time; aPTT, activated partial thromboplastin time; PLT, platelet count; CT, cloting time; MCF, maximum clot firmness; ML, maximum clot lysis; LT, lysis time
Dear Sirs
Accumulating evidence indicates an association between 2019-nCoV pneumonia and disseminated intravascular coagulation (DIC) [1,2]. Although some controversy exists about the extent of DIC in COVID-19 [3], DIC was indicated to be a strong predictor of mortality in patients developing pneumonia with this virus [1,2,4]. The pathophysiology of infection associated-DIC is complex and multifactorial, involving interplay between cellular and plasmatic elements of the hemostatic system and components of the innate immune response to the infecting pathogen [4,5]. In this letter, we report on the viscoelastic properties of clots formed by COVID-19 patients. Using a novel method to assess clot formation a fibrinolysis shut down was observed. This finding has not been reported yet in COVID-19 infection and suggests that fibrinolysis system significantly contributes to the procoagulatory status in COVID-19 patients and might represent a therapeutic target.
There is evolving evidence that a combination of activation events initiated by exposure of the endothelium, platelets, and leukocytes to pathogen- and damage-associated molecular patterns might be responsible for the uncontrolled activation of the coagulation system in severely ill COVID-19 patients [2,4,6]. To date most reports on COVID-associated coagulopathy focused an increased activation of the plasmatic coagulation system. Due to procoagulatory nature of COVID-19, anticoagulation in therapeutic concentration is common used in ICU-patients [[6], [7], [8]]. However, we observed low response to anticoagulants in COVID-19 patients, as represented by increasing D-Dimer values despite sufficient heparin plasma concentration. Our hypothesis was that a concurrent hyperfibrinolysis might explain these findings. Hyperfibrinolysis can be investigated using viscoelastic coagulation assays such as thromboelastography or rotation thromboelastometry. Both methods have been widely used in many fields of intensive care to guide hemostatic treatment in patients with bleeding [9]. However, little is known about their use to monitor patients with disposition to thromboembolic complications. In two COVID-19 ICU patients we observed a clot lysis shut down but not hyperfibrinolysis using a new viscoelastic test system.
Case 1
A 79-year-old man with a history of arterial hypertension and alcohol abuse was admitted into the ICU due to COVID-19 induced acute respiratory distress syndrome (ARDS). Patient received unfractionated heparin (UFH) at therapeutic doses. On day 11 the patient developed acute renal insufficiency (ARI stage III) and continuous hemodialysis was initiated. In addition, the patient developed sepsis-induced liver insufficiency. Noteworthy, PCR-results from the tracheal secretion on day 22 revealed negative results for COVID-19. No relevant improvement of D-Dimer was observed despite UFH treatment (Table 1 ). Extended laboratory investigation of cellular and plasmatic coagulation system was initiated and revealed acquired storage platelet disease and factor XIII deficiency. No hyperfibrinolysis was found when citrated blood was investigated using viscoelastic coagulation assay (maximal lysis [ML] in Ex-Text: 0%, Table 1). In contrast, we observed a remarkable resistance toward recombinant tissue plasminogen activator (tPA, lysis time [LT] in TPA-test: 910 s, Table 1) and fibrinolysis shut down of the formed clot showed (ML in TPA-Text: 63%, Fig. 1 ). These findings indicate an excessive procoagulation state despite an aPTT adjusted anticoagulation with UFH. On the next day, the patients developed bilateral arterial lung emboli and the anticoagulation was switched to argatroban due to suspected heparin induced thrombocytopenia (HIT). The laboratory investigations for HIT revealed negative results in the immunoassay and the functional assay (HIPA). The clinical situation did not improve despite therapy with catecholamine, continuous dialysis and the extension of anti-infective therapy, and patients died on day 32 of ICU stay.
Case 2
A 58-year-old man with no comorbidities was admitted to our ICU due to COVID-19 associated ARDS. Non-invasive ventilation did not improve the oxygenation status and veno-venous extracorporeal membrane oxygenation (vv-ECMO) was implemented on day 4. Despite therapeutic anticoagulation with UFH, microthrombembolisms were found in liver and spleen using computer tomography leading to hepatic failure. Later on, the patient developed several episodes of transfusion-demanding bleedings (epistaxis, hematoma, catheter associated bleeding). Anticoagulation was switched to argatroban due to suspected HIT. Extended laboratory investigations of cellular and plasmatic coagulation system were initiated and revealed factor XIII deficiency. The laboratory investigations for HIT revealed negative results in the immunoassay and HIPA. Interestingly, no hyperfibrinolysis was found using viscoelastic coagulation assay (ML in Ex-Text: 4%, Table 1). In contrast, the formed clot showed remarkable resistance toward tPA (LT: 610 s in TPA-Test, ML: 94%, Fig. 1 and Table 1). Anticoagulation with argatroban was continued and adjusted according to its plasma levels. On day 24 of the ICU stay, patient could be mobilized into seat and the vv-ECMO system was explanted.
The impact of the plasminogen-plasmin-system on the procoagulatory status in trauma patients is relatively well established and was found to be associated with up to 17% mortality, mainly due to multiple organ failure [10,11]. In our study, we used a tissue plasminogen activator challenge assay to unmask latent abnormalities in the plasminogen-plasmin-system in COVID-19 patients. Interestingly, in both patients, laboratory findings were clinically supported by the development of relevant thromboembolisms. Of note, the cutoff for a fibrinolysis shutdown using thromboelastography has not been defined yet and depends largely on physician expertise.
A recent report showed using rotational thromboelastometry a complete lack of spontaneous clot lysis (without addition of tPA) at 30 min in 57% of severe COVID-19 patients, which was associated with venous thromboembolic events [12]. Our work confirms this finding. However, since the pathophysiology of fibrinolysis shutdown in COVID-19 associated DIC has not been addressed yet, the molecular events need to be fully elucidated in further studies [13]. In general, upon thrombin generation fibrin polymerization occurs with considerable diversity, and the resulting viscoelastic properties are generally referred to as clot stability. Plasmin initially cleaves within the D regions of cross-linked fibrin resulting in a variety of fibrin degradation products such as D-dimer. There are a number of natural inhibitors that can attenuate plasmin activity such as α2-antiplasmin, thrombin-activatable fibrinolysis inhibitor, polyphosphate and plasminogen activator inhibitor (PAI-1). All these factors can enhance resistance to fibrinolysis [14]. D-dimers have a half life of approximately 8 h. Consequently, another potential explanation for the elevated D-dimer levels that were observed in both cases might be previous hyperfibrinolytic state prior to blood sampling. Thus, a causal correlation cannot fully confirmed between fibrinolysis shut down and thrombotic complication, especially, since in COVID-19 patients dysregulations in the fibrinolytic system may also playing a critical role in the lung parenchyma. At this point, we believe a major component is that the release of PAI-1 from activated platelets in COVID-19 patients (manuscript under review) exceeds the capacity of its cognate substrate, tPA. Platelet activation in COVID-19 patients may occur through tissue injuries (i.e. pneumonia) as well as in response to danger signaling from the viral infection. Under physiological conditions, the interaction between antifibrinolytic factors and plasmin is balanced. The precise modulatory mechanisms of the imbalanced interactions between plasmin and fibrin in COVID-19 infection should be elucidated in future studies.
Our data suggest that abnormalities in fibrinolysis system contribute to the procoagulatory status in COVID-19 patients. Of note, recent studies showed that fibrinolytic therapy can improve survival in ARDS [15] and might be beneficial in COVID-19 patients [14,16]. Although the optimal dosing regimen is still to be determined [14], these data suggest that tPA might be a promising approach awaiting effective treatments for COVID-19. Viscoelastic coagulation assays could be used to monitor the efficacy of the fibrinolytic treatment and to predict thromboembolic complications in COIVID-19 patients. Taken together, our cases show that the dysregulated hemostasis in COVID-19-associated DIC is exacerbated by an inhibition of fibrinolysis and indicate the plasminogen-plasmin-system as a potential target to prevent thromboembolic complications in COVID-19 patients.
Table 1.
Clinical and laboratory data on study’s patients.
| Patient ID | Gender/Age | Comorbidities | SOFA | Coagulation parameters [normal range] |
Thromboelastographic analysis [normal range] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT [70–100%] | aPTT [21–36 s] | Fibrinogen [170–410 mg/dl] | D-Dimer [>0.5 μg/ml] | PLT [150–350 Ts d/μl] | Ex-Test |
TPA-Test |
||||||||
| CT [38–65 s] | MCF [53–68 mm] | ML [2–12%] | CT [31–57 s] | LT [100–300 s] | ML [94–100%] | |||||||||
| #1 | m/79y | arterial hypertension, alcohol abuse | 14 | 74 | 60 | >900 | 6.2 | 75 | 80 | 67 | 0 | 53 | 910 | 63 |
| #2 | m/58y | none | 6 | 46 | 47 | 534 | 1.4 | 280 | 221 | 69 | 4 | 224 | 610 | 97 |
Fig. 1.
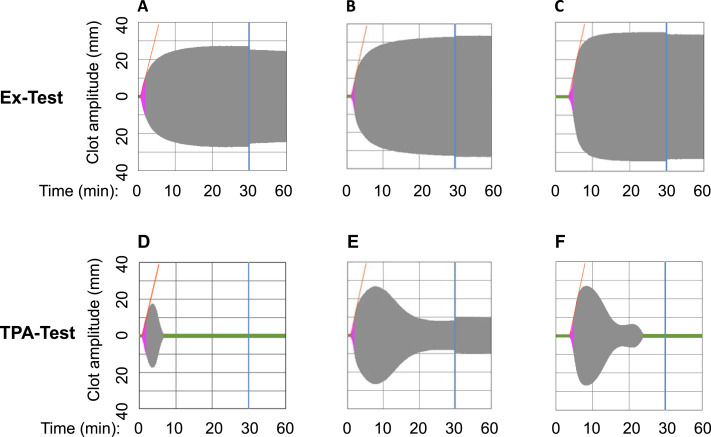
Thromboelastographic analysis of COVID-patients #1 and #2.
Compared to control (A), blood samples from COVID-19 patients #1 and #2 showed prolonged clotting time in Ex-Test (activation of the extrinsic coagulation pathway) due to the anticoagulation with heparin (B) and argatroban (C), respectively. Induction of fibrinolysis by tissue plasmin activator (TPA-test) caused an immediate resolution of the formed clot in control (D). In contrast, remarkable resistance and no complete clot lysis was observed in patient # 1 (E), while a delay of approximately 5 min was observed till complete clot lysis was obtained in patient # 2 (F).
Author’s contribution
T.B. and P.R. were responsible for the treatment of the patient. S.H. performed the experiments and collected the data; T.B., S.H., P.L. and P.R. analyzed the data, interpreted the results and wrote the manuscript. All authors read and approved the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was supported by grants from the German Research Foundation to TB (BA5158/4). The authors thank Dr Karina Althaus and Prof. Rupert Handgretinger for helpful discussion.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemostasis. 2020;18(4):786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gando S., Levi M., Toh C.H. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037. doi: 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wikkelso A., Wetterslev J., Moller A.M., Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst. Rev. 2016;8:CD007871. doi: 10.1002/14651858.CD007871.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorlinger K., Dirkmann D., Solomon C., Hanke A.A. Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability. Br. J. Anaesth. 2013;110(2):222–230. doi: 10.1093/bja/aes374. [DOI] [PubMed] [Google Scholar]
- 11.Moore E.E., Moore H.B., Gonzalez E. Postinjury fibrinolysis shutdown: rationale for selective tranexamic acid. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S65–S69. doi: 10.1097/TA.0000000000000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright F.L., Vogler T.O., Moore E.E. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J. Am. Coll. Surg. 2020;231(2):193–203 e1. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J., Hajizadeh N., Moore E.E. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemostasis. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C., Ma Y., Su Z. Meta-analysis of preclinical studies of fibrinolytic therapy for acute lung injury. Front. Immunol. 2018;9:1898. doi: 10.3389/fimmu.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whyte C.S., Morrow G.B., Mitchell J.L., Chowdary P., Mutch N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemostasis. 2020;18(7):1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]


