Abstract
Pneumonia is a common illness that continues to be the major killer of remaining to be a significant source of morbidity and mortality in the patient population. Many microorganisms cause pneumonia, and now concern is turning to the importance of the cause the new therapies for viral pneumonia. In the current study, we report the effect of andrographolide sulfonate, a water-soluble form of andrographolide (trade name: Xi-Yan-Ping Injection), on poly I: C-induced pneumonia. Andrographolide sulfonate was administrated through intraperitoneal injection to mice with poly I: C-induced pneumonia. Recruitment of airway inflammatory cells, alteration of lung histological induced by Poly I: C were significantly ameliorated by andrographolide sulfonate. The protein levels of pro-inflammatory cytokines in bronchoalveolar fluid (BALF) and serum were reduced by andrographolide sulfonate treatment. The levels of MUC5AC and MUC5B in lung tissue were also suppressed. These results reveal that andrographolide sulfate remarkably alleviated pneumonia induced by poly I:C in mice. Moreover, andrographolide sulfonate markedly inhibited the activation of nuclear factor-κB (NF-κB). Taken together, we demonstrated that andrographolide sulfonate ameliorated poly I: C-induced pneumonia in mice, suggesting the possible use of andrographolide sulfonate for virus-induced pneumonia in clinical.
Keywords: Andrographolide, Sulfonate, Poly I:C, Pneumonia, NF-κB
Abbreviations: ALI, Acute lung injury; ARDS, acute respiratory distress syndrome; BALF, bron-choalveolar fluid; NF-κB, nuclear factor-κB; TNF-α, Tumor Necrosis Factor-α; IL-6, Interleukin-6; TNBS, trinitro-benzene-sulfonic acid; H&E, hematoxylin & eosin; Andro-S, andrographolide sulfonate
1. Introduction
Virus pneumonia is a common illness that continues to be the major killer of people, such as severe acute respiratory syndrome (SARS-CoV), the Middle East respiratory syndrome coronavirus (MERS-CoV) and pathogenic SARS-coronavirus 2 (SARS-CoV-2).1, 2, 3, 4 Molecular diagnostic tests have greatly increased our understanding of the role of viruses in pneumonia, and findings indicate that the incidence of viral pneumonia has been underestimated.5 , 6 Despite many advances, current treatments fail to reduce lung injury and mortality.7 , 8 Further studies are still needed to better understand the cause and pathogenesis of respiratory virus infections, as well as search for therapeutic strategies to improve morbidity and mortality.
Andrographolide, a diterpenoid lactone, prescribed as a treatment for upper respiratory tract infection in China, has been shown to have anti-inflammatory and anti-tumorigenic activities.9, 10, 11 Our previous research found that andrographolide inhibited NLRP3 inflammasomes, as well as the Th1/Th17 response in colitis,10 inhibited NF-κB activation, down-regulated MAPK pathway or hampered the activation of the AIM2 inflammasome to ameliorate LPS or radiation induced lung injury,12 , 13 stabilized Bax protein to reverse resistance to 5-FU in colorectal cancer.11 Despite the importance of andrographolide for inflammatory diseases and that the maturation process has been elucidated, whether andrographolide contributing to respiratory virus-induced pneumonia remains unknown.
Here, we reported the therapy effect of andrographolide sulfonate on mouse pneumonia induced by poly I:C and showed that andrographolide sulfonate selectively suppressed the activation of NF-κB and the proinflammation cytokines production. In addition, we found that the andrographolide sulfonate regulated MUC5AC and MUC5B expression in the injured lungs. With these data, we proposed that andrographolide sulfonate may be a potential drug for the treatment of patients with severe pneumonia induced by respiratory virus.
2. Materials and methods
2.1. Animals
C57BL/6 mice (Male, 6–8 weeks, 18–20 g) were purchased from Jiangsu Gempharmatech co., ltd (Nanjing, China). Mice were maintained in an animal facility under standard laboratory conditions for 1 week prior to experiments, and provided water and standard chow. Animal welfare and experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006) and the related ethical regulations of Nanjing University. All efforts were made to reduce the number of animals used and to minimize animals’ suffering.
2.2. Agents
Poly I:C-HMW (tlrl-pic) was bought from Invivogen (San Diego, California). ELISA kits for TNF-α, IL-6 and IL-1β were purchased from Dakewei (Beijing, China). Anti-p-p65 (3033) was purchased from Cell Signaling Technology (Beverly, MA) were purchased from Cell Signaling Technology (Beverly, MA, USA). β-Actin (M20011) was purchased from Abmart (Shanghai, China). Anti-MUC5AC (RLN0880), anti-MUC5B (RLN0881) were purchased from Ruiying Biological company (Suzhou, China). Anti-CD3-APC, anti-CD11b-PE, anti-Gr1+-FITC antibodies were bought for eBioscience. GTVisin™ anti-mouse/anti-rabbit immunohistochemical analysis KIT was purchased from Gene Company (Shanghai, China). Andrographolide sulfonate (Andro-S, trade name: Xi-Yan-Ping Injection) was provided by Jiangxi Qingfeng Pharmaceutical Co., Ltd (Ganzhou, China). All other chemicals were obtained from Sigma–Aldrich (St. Louis, MO).
2.3. Induction of pneumonia by poly I:C
C57BL/6 were IN (Intranasal) administered 30 μg poly I:C-HMW (Invivogen, San Diego, California) in a 50 μL volume under light anesthesia. Mice were randomly divided into five groups (n = 6 per group): control group IN administered PBS, model group IN administered poly I:C. Andrographolide sulfonate (1, 3, 10 mg/kg) and Dexamethasone (0.5 mg/kg) dissolved in saline were given to mice once (i.p) immediately after poly I:C. Mice were killed 24 h later. BALF, blood plasma and tissue samples were collected. For BALF collection, Mice were perfused with ice cold PBS. The lungs were lavaged with 300 μL saline, and the resultant BALF was centrifuged to separate the cellular components from the supernatants. Total BALF cell number was counted and the BALF cells were stained with anti-mouse CD3-APC, CD11b-PE, Gr1+-FITC antibodies and composition was evaluated by FACS analysis.
2.4. Histological analysis
To examine the histological changes, lungs from animals in each group were taken and fixed by 10% formalin, embedded in paraffin, and then sectioned to reveal the maximum longitudinal view of the main intrapulmonary bronchus of the left lung lobe. Histopathologic study was made using hematoxylin & eosin (H&E)-stained lung sections. Alveolar congestion, haemorrhage, infiltration or aggregation of inflammatory cells in airspaces or vessel walls, and the thickness of the alveolar walls were assessed as reported.14
2.5. Masson’s trichrome stain
Masson’s trichrome stain was used to detect the collagen deposition. Collagen volume fraction was performed by the ImageJ software as reported.15
2.6. Cytokine analysis by ELISA
Serum was collected from blood of mice by centrifuge at 3500 g for 15 min. Serum and BALF cytokine levels were measured by specific ELISA kits from Dakawe.
2.7. Real-time PCR
Real-time PCR was performed as reported.16 The primer sequences used in this study were as follows:
m-tnf-α forward 5′-CGAGTGACAAGCCTGTAGCCC-3′
m-tnf-α reverse 5′-GTCTTTGAGATCCATGCCGTTG-3;
m-il-1β forward 5′-CTTCAGGCAGGCAGTATCACTC-3′;
m-il-1β reverse 5′-TGCAGTTGTCTAATGGGAACGT-3′;
m-il-6 forward 5′-ACAACCACGGCCTTCCCTAC-3′;
m-il-6 reverse 5′-TCTCATTTCCACGATTTCCCAG-3′;
m-muc1 forward 5′-CAGTCCTTCTGAGAGCCACC-3′
m-muc1 reverse 5′-GCAGTGTGCCAGTGCCGCCG-3′
m-muc5ac forward 5′-AGAATATCTTTCAGGACCCCTGCT-3′,
m-muc5ac reverse R 5′-ACACCAGTGCTGAGCATACTTT-3′
m-muc5b forward 5′-TCCTGCTCTGGAATATCCAAG-3′
m-muc5b reverse 5′-GCCTCGGGGAGCTTGCCTGCC-3′
m-ifn-β forward 5′-CAGCTCCAAGAAAGGACGAAC-3′
m-ifn-β reverse 5′-GGCAGTGTAACTCTTCTGCAT-3′
m-ccl5 forward 5′-CCAAGTGCTGCCGTCATTTTC-3′
m-ccl5 reverse 5′-GGCTCGCAGGGATGATTTCAA-3′
m-ccl10 forward 5′-CCAAGTGCTGCCGTCATTTTC-3′
m-ccl10 reverse 5′-GGCTCGCAGGGATGATTTCAA-3′
β-actin forward 5′-TGCTGTCCCTGTATGCCTCT-3′
β-actin reverse 5′-TTTGATGTCACGCACGATTT-3′.
2.8. Western blot
Protein from lung tissue western blotted as reported.17
2.9. Immunohistochemical analysis
Immunohistochemical analysis was performed on paraffin-embedded lung tissue sections (5 μm) as described previously.18
2.10. Statistical analysis
Data are expressed as mean ± SEM. ANOVA with post hoc two comparisons is used to evaluate the differences between various experimental and control groups. P values less than 0.05 were considered significant.
3. Results
3.1. Andrographolide sulfonate inhibited pathological changes of pneumonia by poly I:C in mice
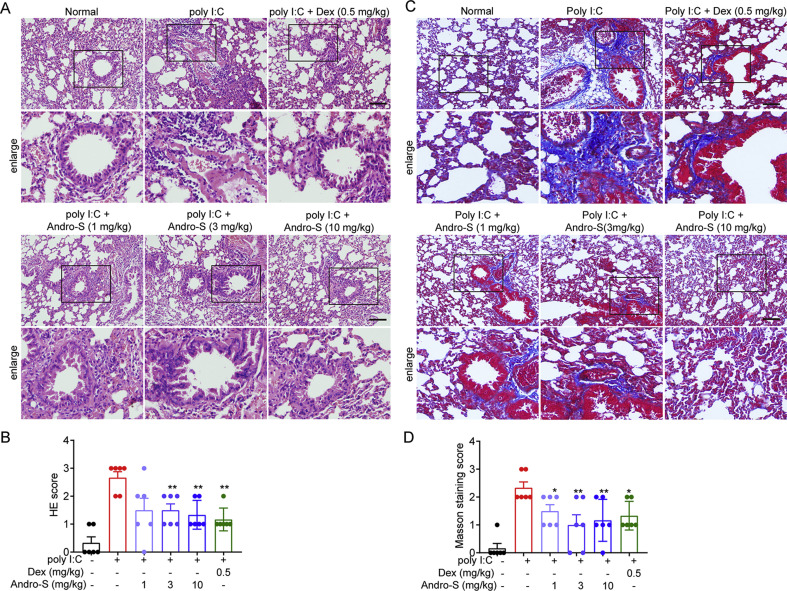
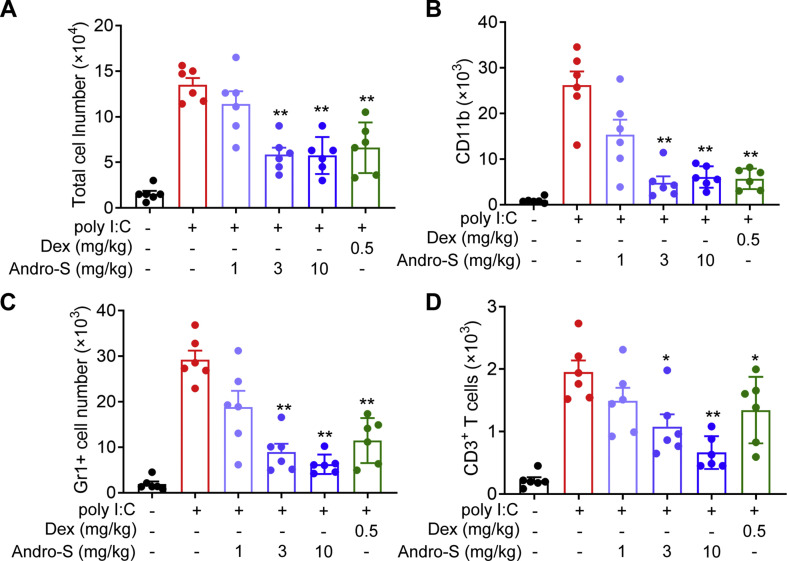
tAndrographolide sulfonate, an approved clinical medicine in China, has been identified as a crucial role in anti-inflammation.10 , 18 Thus, we wondered whether andrographolide sulfonate can improve pneumonia induced by virus. To address it, we explored a wide mouse model of poly I:C-induced pneumonia to evaluate the therapeutic effect of andrographolide sulfonate. Mice were IN administered with poly I:C and lung tissue samples were collected and sections stained with H&E were shown in Fig. 1 A and B. The representative histopathological images showed that compared with the normal mice, mice treated with poly I:C exhibited the typical lung injury associated with notable inflammatory cells infiltration, interstitial and intra-alveolar edema and patchy hemorrhage, hyaline membrane formation and some collapsed alveoli. Andrographolide sulfonate treatment significantly reduced the histopathological damage as showed by reduced H&E score (Fig. 1B). Next, we investigated whether andrographolide sulfonate could prevent the development of fibrosis after poly I:C induction, which was confirmed by Masson’s trichrome staining (Fig. 1C and D). Severe deposition of collagen in the lung tissue from the poly I:C group was observed. Notably, treatment with andrographolide sulfonate markedly reduced the deposition of collagen. Furthermore, we quantified the infiltration of inflammatory cells in BALF. The number of total infiltrated cells, T cells (CD3+), macrophages (CD11b+) and neutrophils (Gr1+) in BALF, were examined by FACS. As shown in Fig. 2 , the poly I:C stimulation-induced infiltration of the inflammatory cells was dose-dependently reduced with andrographolide sulfonate treatment. Based on these findings, it can be concluded that andrographolide sulfonate could effectively alleviate pneumonia in mice induced by poly I: C.
Fig. 1.
Andrographolide sulfonate treatment ameliorated lung damage induced by poly I:C in mice. Lung tissue from each group were taken and fixed in 4% formalin and subjected to hematoxylin/eosin (H&E) staining (A, B) and Masson staining (C, D). Scale bar 100 μm. Andro-S: andrographolide sulfonate.
Fig. 2.
Andrographolide sulfonate treatment prevented poly I:C-induced recruitment of inflammatory cells in lung tissue. Cells in BALF (bronchoalveolar lavage fluid) from each group were gathered and counted. Then they were stained with anti-CD3-APC, anti-CD11b-PE, anti-Gr1+-FITC and analyzed by FACS. Values were shown as the means ± SEM of 6 mice. ∗P < 0.05, ∗∗P < 0.01 vs. mice treated with poly I:C group. Andro-S: andrographolide sulfonate.
3.2. Andrographolide sulfonate inhibited levels of inflammatory cytokines in BALF and serum by induced of poly I:C
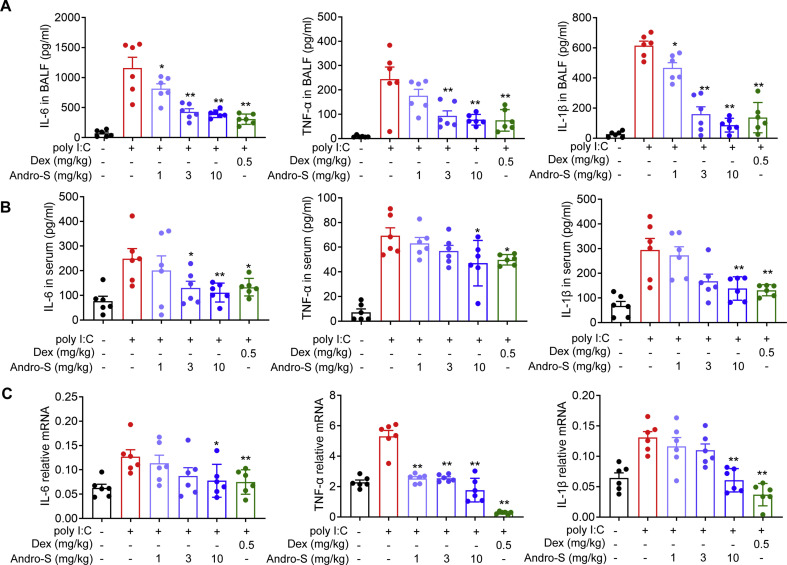
Next, the levels of inflammatory cytokines in BALF and serum was examined. As expected, the proinflammation cytokines of ploy I:C group, including IL-6, TNF-α and IL-1β, were strikingly elevated in BALF and serum, while these cytokines were markedly reduced in a dose-dependent manner when treated with andrographolide sulfonate (Fig. 3 A and B). Moreover, the dose-response effect of andrographolide sulfonate on these genes expression in mouse lungs were determined. Consistently, the production of IL-6, TNF-α, IL-1β in total lung lysates was significantly decreased in the andrographolide sulfonate-treated animals (Fig. 3C). Taken together, these data indicated that andrographolide sulfonate attenuated immune responses induced by poly I:C.
Fig. 3.
Andrographolide sulfonate treatment suppressed poly I:C-induced cytokines expression. Cytokine levels in BALF supernatant (A) and serum (B) from each group were detected by ELISA. (C) RNA of lung tissue from the mice was extracted. The mRNA expression of cytokines was examined by real-time PCR. Values were shown as the means ± SEM of 6 mice. ∗P < 0.05, ∗∗P < 0.01 vs. mice treated with poly I:C group. Andro-S: andrographolide sulfonate.
3.3. Andrographolide sulfonate inhibited mucin expressions in poly I:C induced lung
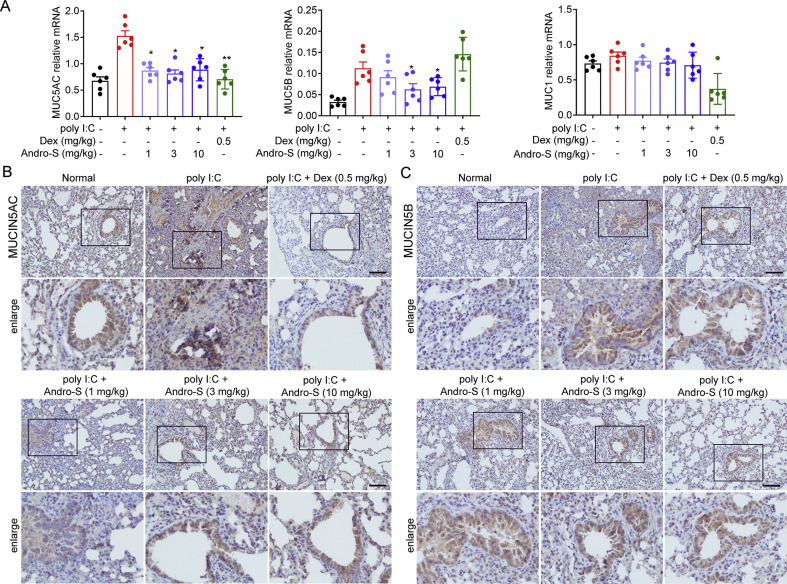
Mucus hypersecretion is a common pathological feature seen in many acute and chronic respiratory conditions.19 The two predominant mucins of the human airway are MUC5AC and MUC5B.20 MUC5AC is implicated in pulmonary diseases with mucus hypersecretion; and MUC5B is associated with familial interstitial pneumonia and idiopathic pulmonary fibrosis.21 , 22 We therefore tested whether andrographolide sulfonate could regulate the expression of mucin. We observed that, consistent with previous results, the poly I:C group exhibited increased gene and protein expression of MUC5AC and MUC5B compared to the normal group (Fig. 4 A and B). In contrast, mice treated with andrographolide sulfonate showed significant decrease of MUC5AC and MUC5B expression levels following poly I:C stimulation (Fig. 4A and B). It has been previously reported that MUC1 is a host factor known to host protects against severe pneumococcal disease.23 Unlike the dexamethasone treatment group, there was no note difference between the andrographolide sulfonate group and the poly I: C-treated group. These results indicated that andrographolide sulfonate inhibited MUC5AC and MUC5B but not MUC1 expressions induced by poly I:C.
Fig. 4.
Andrographolide sulfonate suppressed mucins expression in lung of mice with poly I:C-induced pneumonia. (A) RNA of lung tissue from the mice in each group was extracted. The mRNA expression of MUC1, MUC5AC, MUC5B were examined by real-time PCR. Values were shown as the means ± SEM of 6 mice. (B) Paraffin-embedded lung tissue sections from each group were stained for MUC5AC. Scale bar: 100 μm ∗P < 0.05, ∗∗P < 0.01 vs. mice treated with poly I:C group. Andro-S: andrographolide sulfonate.
3.4. Andrographolide sulfonate reduced ploy I:C-induced activation of NF-κB signaling
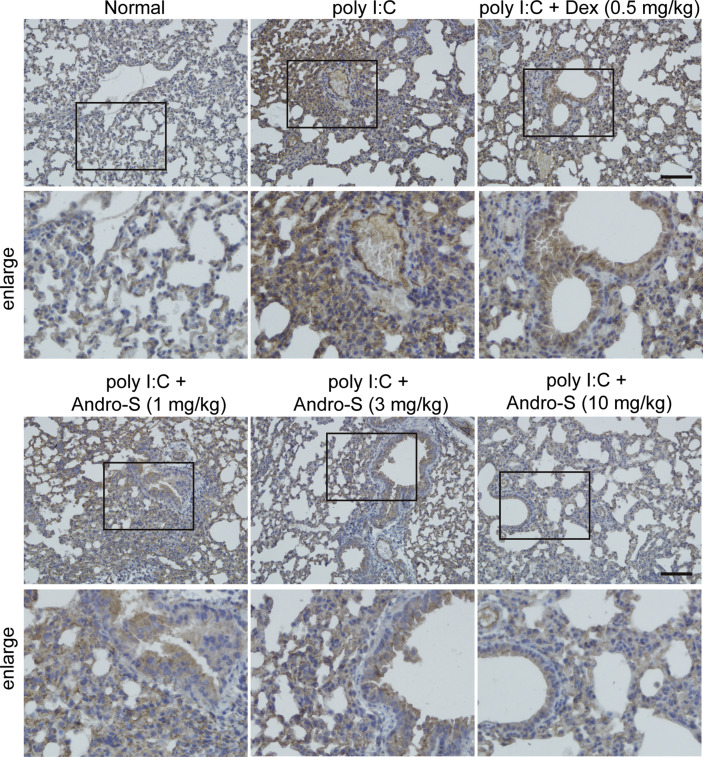
Signaling pathways that are responsive to viral infection or dsRNA involve activation of transcription factors such as NF-κB and IRF3.24 Besides, several reports described that a requirement for NF-κB in MUC5AC expression.22 , 25 We next analyzed the NF-κB activation of individual groups. As shown in Fig. 5 , poly I:C administration caused phosphorylation of p65 in the injured lung from mice with pneumonia. When treated with andrographolide sulfonate, the expression of p-p65 was significantly reduced. However, it should be noted that andrographolide sulfonate didn’t affect IRF3-mediated immune response. Q-PCR showed that the downstream of IRF3 including IFN-β, CCL5, CXCL10 remains unchanged versus poly I: C group (Fig. s1). According to these findings, andrographolide sulfonate reduced ploy I:C-induced activations of NF-κB signaling, whereas did not affect poly I:C-induced IRF3-mediated immune response.
Fig. 5.
Andrographolide sulfonate decreased activations of NF-κB in the lung of mice with poly I: C-induced pneumonia. Paraffin-embedded lung tissue sections from each group were stained for p-p65. Scale bar 100 μm. Andro-S: andrographolide sulfonate.
4. Discussion
In our study here, a mouse model of poly I:C-induced pneumonia was used to evaluate the therapeutic effect of andrographolide sulfonate. According to previous studies, we characterized that andrographolide sulfonate has anti-inflammation activity.12 , 26 , 27 As we expected, andrographolide sulfonate significantly inhibited the pneumonia model induced by poly I:C. In our research, we found andrographolide sulfonate significantly inhibited obviously the recruitment of airway inflammatory cells and the production of pro-inflammation cytokines in the mouse pneumonia model induced by poly I:C (Fig. 2, Fig. 3). Next this finding was confirmed by detecting the activation of NF-κB. The result showed that poly I:C stimulation increased NF-κB phosphorylation and pro-inflammation cytokines production. After treatment with andrographolide sulfonate, the NF-κB phosphorylation was much less than that of poly I:C stimulation, and IL-6, TNF-α and IL-1β production induced by poly I:C were abrogated (Fig. 3, Fig. 4).
Poly I:C, a synthetic analog of dsRNA associated with viral infections, that is used to exacerbate inflammation in lung injury models.28, 29, 30 Poly I:C is recognized by toll-like receptor 3 (TLR3), which lead to the activation of IRF3 and NF-κB for the induction of type I IFN and inflammatory cytokines, respectively.31, 32, 33 LPS, a major component in Gram-negative bacteria, has been used to induce pneumonia caused by bacterial.3 Recently, virus-induced pneumonia is a considerably important matter all over the world. Development of pharmacotherapy for virus-induced pneumonia is very important issue. Andrographolide sulfonate (trade name: Xi-Yan-Ping Injection), is an approved clinical medicine in China, prescribed as a treatment for upper respiratory tract infection. We first identified the importance and mechanism of andrographolide sulfonate on the therapy function of pneumonia induced by poly I:C. Also, the results of our study may provide experimental evidence for the use of andrographolide sulfonate for COVID-19 patient as several clinical trials are ongoing in China.
Intriguingly, we found that andrographolide sulfonate had little effect on IFN-β, CCL5, CXCR10 gene expression (Fig. s1), which suggesting andrographolide sulfonate has little effect on IRF3-mediated anti-virus immune response. These results suggested that andrographolide sulfonate selectively inhibited NF-κB activation to ameliorated pneumonia induced by poly I:C in mice.
Mucus hypersecretion is a common pathological feature seen in many respiratory conditions.19 MUC5AC and MUC5B are the two predominant mucins of the human airway.20 MUC5AC is implicated in pulmonary diseases with mucus hypersecretion; and MUC5B is associated with familial interstitial pneumonia and idiopathic pulmonary fibrosis.34, 35, 36 Accumulating evidence suggests that activated NF-κB signaling pathways actively engage the expression of MUC5AC and MUC5B.25 , 37 Consistent with previous results, andrographolide sulfonate suppressed the expression of MUC5AC and MUC5B. This is further explained that andrographolide sulfonate could alleviate the symptoms of pneumonia.
In conclusion, our work demonstrated that andrographolide sulfonate could inhibit pneumonia induced by poly I:C, mechanisms involving inhibition of the activation of NF-κB. Thereby, andrographolide sulfonate suppressed NF-κB-mediated inflammation cytokines production and MUC5AC and MUC5B expression. However, more in vitro studies exploring the biological activity are needed in further work. Our study not only reveals the possible use of andrographolide sulfonate on pneumonia induced by virus in clinical but also sheds light on discovering promising potential drug target.
Credit authorship contribution statement
Jian Cui: Investigation, writing. Jian Gao: Investigation, validation. Yan Li: investigation. Fan Ting: Investigation. Jiao Qu: Methodology. Yang Sun: Review & editing. Wen Liu: Funding acquisition, investigation. Wenjie Guo: Conceptualization, funding acquisition, supervision, writing – review & editing. Qiang Xu: Conceptualization, funding acquisition, writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81922067, 81730100, 81903620), Natural Science Foundation of Jiangsu Province (BK20190306), Zhejiang University special scientific research fund for COVID-19 prevention and control (2020XGZX007) and Nanjing University special scientific research fund for COVID-19 (14380134).
Footnotes
Peer review under responsibility of Japanese Pharmacological Society.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jphs.2020.08.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Keeffe S. Aspiration pneumonia. New Engl J Med. 2019;380(21):E40. doi: 10.1056/NEJMc1903636. [DOI] [PubMed] [Google Scholar]
- 3.Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100(3):1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J., Wohlford-Lenane C., Zhao J. Intranasal treatment with poly(I:C) protects aged mice from lethal respiratory virus infections. J Virol. 2012;86(21):11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu D.K.W., Pan Y., Cheng S.M.S. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drews A.L., Atmar R.L., Glezen W.P., Baxter B.D., Piedra P.A., Greenberg S.B. Dual respiratory virus infections. Clin Infect Dis. 1997;25(6):1421–1429. doi: 10.1086/516137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silversides J.A., Ferguson N.D. Clinical review: acute respiratory distress syndrome - clinical ventilator management and adjunct therapy. Crit Care. 2013;17(2):225. doi: 10.1186/cc11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav H., Thompson B.T., Gajic O. Fifty years of research in ARDS.is acute respiratory distress syndrome a preventable disease? Am J Resp Crit Care. 2017;195(6):725–736. doi: 10.1164/rccm.201609-1767CI. [DOI] [PubMed] [Google Scholar]
- 9.Gao J., Cui J., Zhong H. Andrographolide sulfonate ameliorates chronic colitis induced by TNBS in mice via decreasing inflammation and fibrosis. Int Immunopharmacol. 2020;83:106426. doi: 10.1016/j.intimp.2020.106426. [DOI] [PubMed] [Google Scholar]
- 10.Liu W., Guo W., Guo L. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int Immunopharmacol. 2014;20(2):337–345. doi: 10.1016/j.intimp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Wang W., Guo W., Li L. Andrographolide reversed 5-FU resistance in human colorectal cancer by elevating BAX expression. Biochem Pharmacol. 2016;121:8–17. doi: 10.1016/j.bcp.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Peng S., Hang N., Liu W. Andrographolide sulfonate ameliorates lipopolysaccharide-induced acute lung injury in mice by down-regulating MAPK and NF-κB pathways. Acta Pharm Sin B. 2016;6(3):205–211. doi: 10.1016/j.apsb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J., Peng S., Shan X. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 2019;10(12) doi: 10.1038/s41419-019-2195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szapiel S.V., Elson N.A., Fulmer J.D., Hunninghake G.W., Crystal R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120(4):893–899. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W., Mo X., Cui W. Nrf2 inhibits epithelial-mesenchymal transition by suppressing snail expression during pulmonary fibrosis. Sci Rep-UK. 2016;6:38646. doi: 10.1038/srep38646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W., Wu T.C., Hong D.M. Carnosic acid enhances the anti-lung cancer effect of cisplatin by inhibiting myeloid-derived suppressor cells. Chin J Nat Med. 2018;16(12):907–915. doi: 10.1016/S1875-5364(18)30132-8. [DOI] [PubMed] [Google Scholar]
- 17.Geng J., Liu J., Yuan X., Liu W., Guo W. Andrographolide triggers autophagy-mediated inflammation inhibition and attenuates chronic unpredictable mild stress (CUMS)-induced depressive-like behavior in mice. Toxicol Appl Pharm. 2019;379:114688. doi: 10.1016/j.taap.2019.114688. [DOI] [PubMed] [Google Scholar]
- 18.Guo W., Liu W., Chen G. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-κB pathways. Int Immunopharmacol. 2012;14(4):613–619. doi: 10.1016/j.intimp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Wang X., Yang X., Li Y. Lyn kinase represses mucus hypersecretion by regulating IL-13-induced endoplasmic reticulum stress in asthma. Ebiomedicine. 2017;15:137–149. doi: 10.1016/j.ebiom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahy J.V., Dickey B.F. Airway mucus function and dysfunction REPLY. New Engl J Med. 2011;364(10):978. doi: 10.1056/NEJMc1014719. [DOI] [PubMed] [Google Scholar]
- 21.Seibold M.A., Wise A.L., Speer M.C. A common MUC5B promoter polymorphism and pulmonary fibrosis. New Engl J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M., Lv Z., Zhang W. Triptolide suppresses airway goblet cell hyperplasia and Muc5ac expression via NF-κB in a murine model of asthma. Mol Immunol. 2015;64(1):99–105. doi: 10.1016/j.molimm.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Dhar P., Ng G.Z., Dunne E.M., Sutton P. Mucin 1 protects against severe Streptococcus pneumoniae infection. Virulence. 2017;8(8):1631–1642. doi: 10.1080/21505594.2017.1341021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vercammen E., Staal J., Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev. 2008;21(1):13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewson C.A., Haas J.J., Bartlett N.W. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF- B and EGFR pathways. Eur Respir J. 2010;36(6):1425–1435. doi: 10.1183/09031936.00026910. [DOI] [PubMed] [Google Scholar]
- 26.Guo W., Sun Y., Liu W. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy. 2014;10(6):972–985. doi: 10.4161/auto.28374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng J., Liu W., Gao J. Andrographolide alleviates Parkinsonism in MPTP-PD mice via targeting mitochondrial fission mediated by dynamin-related protein 1. Brit J Pharmacol. 2019;176(23):4574–4591. doi: 10.1111/bph.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris P., Sridhar S., Peng R. Double-stranded RNA induces molecular and inflammatory signatures that are directly relevant to COPD. Mucosal Immunol. 2013;6(3):474–484. doi: 10.1038/mi.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gan T., Yang Y., Hu F. TLR3 regulated poly I:C-induced neutrophil extracellular traps and acute lung injury partly through p38 MAP kinase. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.03174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang I., Harten I.A., Chang M.Y. Versican deficiency significantly reduces lung inflammatory response induced by polyinosine-polycytidylic acid stimulation. J Biol Chem. 2017;292(1):51–63. doi: 10.1074/jbc.M116.753186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y., Li W., Gao T. The severe acute respiratory syndrome coronavirus nucleocapsid inhibits type I interferon production by interfering with TRIM25-mediated RIG-I ubiquitination. J Virol. 2017;91 doi: 10.1128/JVI.02143-16. UNSP e021438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 33.Zou J., Kawai T., Tsuchida T. Poly IC triggers a cathepsin D- and IPS-1-Dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity. 2013;38(4):717–728. doi: 10.1016/j.immuni.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Mathai S.K., Humphries S., Kropski J.A. MUC5B variant is associated with visually and quantitatively detected preclinical pulmonary fibrosis. Thorax. 2019;74(12):1131–1139. doi: 10.1136/thoraxjnl-2018-212430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeppen M., McNamee E.N., Brodsky K.S. Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. 2013;6(4):762–775. doi: 10.1038/mi.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung Y., Kim S., Yuk H.J. Siraitia grosvenorii residual extract attenuates ovalbumin-induced lung inflammation by down-regulating IL-4, IL-5, IL-13, IL-17, and MUC5AC expression in mice. Phytomedicine. 2019;61:152835. doi: 10.1016/j.phymed.2019.152835. [DOI] [PubMed] [Google Scholar]
- 37.Song K.S., Yoon J., Kim K.S., Ahn D.W. c-Ets1 inhibits the interaction of NF-κB and CREB, and downregulates IL-1β-induced MUC5AC overproduction during airway inflammation. Mucosal Immunol. 2012;5(2):207–215. doi: 10.1038/mi.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.