Highlights
-
•
Longus colli muscle can be a primary target for BoNT injections in dystonic anterocollis.
-
•
Both primary and secondary due to heredodegenerative disorders dystonic anterocollis may improve with administration of BoNT.
-
•
EMG navigation is sufficient to guide longus colli injections.
Keywords: Dystonia, Anterocollis, Botulinum toxin, EMG
Abstract
We review the current approaches and their feasibility to treat dystonic anterocollis by injecting longus colli muscle (LCo) with botulinum neurotoxin (BoNT) as well as present our personal experiences in this field compared with the findings from previously published studies. First, we searched the PubMed database for the publications reporting patients who received LCo injections for anterocollis; we also thoroughly examined the references included in each of the found publications. Second, we present and analyze our own experiences in injecting LCo under EMG guidance in patients with dystonic anterocollis due to heredodegenerative disorders. We found 11 publications describing administration of LCo injections for the treatment of dystonic anterocollis in a total of 28 patients with primary dystonia aged between 21 and 80 years. The mean age of our patients was 44.8 years with the mean anterocollis duration being 15 months. OnabotulinumtoxinA in a dose of up to 35 U per LCo muscle was not associated with the development of transient dysphagia. The mean percentage of patient satisfaction was 36.3%, and the mean duration of the beneficial effect was 2.5 months. All patients agreed to receive a repeat injection. We provide a set of empirically based suggestions on the current use of BoNT injections to LCo for managing anterocollis in outpatient clinics, including pretreatment work-up, injection technique, and dose range.
1. Introduction
Botulinum neurotoxin (BoNT) is considered to be a first-line therapy for cervical dystonia [1]. Additional guidance techniques (e.g., EMG, ultrasound) may improve the outcomes of treatment with BoNT [2]. However, dystonic patterns, such as anterocollis, anterocaput, and forward sagittal shift, remain challenging to manage using BoNT because it needs to be injected into deep neck muscles, which are largely responsible for these postures [3]. Anterocollis is characterized by a non-fixed anteflexion (>45°) of the cervical spine with a preserved angle between the skull base and C1 vertebra relative to the thoracic spine [4].
Routinely, in dystonic anterocollis, BoNT is frequently applied to the superficial neck flexors—namely sternocleidomastoid (SCM), levator scapulae, and scalene muscles—owing to their easy accessibility [2], [3], [5]. Although such an approach may improve the status of some patients with anterocollis [6], [7], it is mostly considered to be ineffective [8], [9], [10]. Thus, deep neck flexor—longus colli muscle (LCo)—can be viewed as an alternative target for BoNT injections in dystonic anterocollis.
The primary purpose of this report was to comprehensively analyze the current approaches and the feasibility of these approaches to treat dystonic anterocollis by injecting LCo as well as to present our personal experiences in this field compared with the findings from previously published studies.
2. Methods
2.1. Literature review
In the first part of our work, we searched the PubMed database for the publications reporting patients who received LCo injections for anterocollis. We used the keywords “antecollis” OR “anterocollis” OR “dropped head” OR “head drop” AND “botulinum” without including any restrictions in publication dates as of April 25, 2020. We also thoroughly examined the references included in each of the found publications.
2.2. Our own case series
In the second part of our study, we analyzed 4 our patients with dystonic anterocollis due to heredodegenerative disorders who were administered with LCo injections of BoNT in the period of July 2019–January 2020.
To confirm the dystonic nature of anterocollis and exclude other possible causes of a “dropped head” syndrome, we checked the strength of the neck extensors in sitting and supine positions as well as examined patients in supine, sitting, and standing positions. Variability of the posture as well as significant regression of the anterocollis in a supine position without a pillow were considered in favor of the dystonic nature of anterocollis. Serum creatine kinase (CK) levels were also tested to screen for muscle tissue involvement.
A subjective overall improvement reported in percentage (from 0 to 100%) by the patients was used for measuring the outcomes after the administration of BoNT injection for anterocollis.
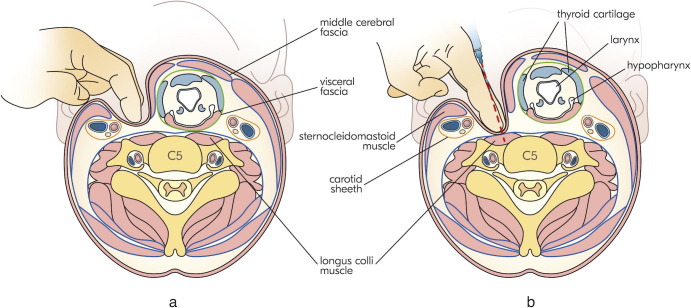
In all patients, a portable EMG device with acoustic feedback was used for guidance, along with disposable hypodermic needle electrodes of either 37 mm (26G) or 50 mm (25G). Initially, superficial neck flexors were examined with EMG for spontaneous bursts of muscle activity. The patients were then positioned supine with a flat pillow under their head and asked to turn their head to the opposite side at an approximate angle of 45°. At the level of the lower half of the thyroid cartilage (ThC) (corresponding approximately to the C5 vertebra), via the fingertips of the index and middle fingers of the left hand, the injector finds the pulse of the common carotid artery under SCM. The patients’ head is then turned back close to the midline (Fig. 1 a). Subsequently, on moderately pressing SCM with the underlying carotid sheath, the injector dives under the visceral compartment (comprising the thyroid gland, larynx, and pharynx with upper part of the esophagus) and consequently displaces the latter with the index and middle fingers in the medial direction. It is noteworthy that the abovementioned visceral compartment is relatively mobile because it is covered by the visceral fascia and moves as a whole complex. As a result of the described maneuver, the fingertips of the index and middle fingers will approach the anterior surface of the vertebral column and stand firmly on the belly of the vertical part of LCo (Fig. 1b). The EMG needle is then injected between the index and middle fingers and advanced towards the vertebral column. The needle position can often be verified by spontaneous bursts of muscle activity, but in some cases, it is necessary to ask the patients to activate the LCo by slightly flexing their neck forwards and then relaxing themselves. Normally, residual bursts of muscle activity can be appreciated even after relaxation. We also asked the patients to swallow to ensure that there was no apparent EMG activity due to the accidental hitting of the upper pharyngeal constrictors. The same approach is utilized on the other side (if necessary).
Fig. 1.
Two steps of our approach to the longus colli muscle. a — First, the injector finds the pulse of the common carotid artery under sternocleidomastoid muscle (SCM) at the level of the lower half of the thyroid cartilage. b — Second, moderately pressing the SCM with the underlying carotid sheath, the injector dives under the visceral compartment and consequently displaces the latter with the index and middle fingers in the medial direction.
In all our patients, onabotulinumtoxinA (dilution 50 U/mL) was injected with individualized doses and target muscles. No premedication or anesthesia was used. The follow-up assessments were conducted at 4 weeks and 3 months after the injection.
2.3. Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from all participants. Any anonymized data not published within the article will be shared by request from any qualified investigator.
3. Results
3.1. Literature review
We found 11 publications describing administration of LCo injections for the treatment of dystonic anterocollis (occurring either alone or as a part of complex cervical dystonia) in a total of 28 patients aged between 21 and 80 years (Supplementary Table 1). All studies were published in the period of 2004–2020.
In all patients, the major diagnosis was cervical dystonia (or Meige syndrome). Unlike our case series, none of the reported patients had dystonic anterocollis due to heredodegenerative disorders.
In all cases, various navigation techniques were used to localize LCo either alone or in combination with endoscopy (8 patients), EMG (11 patients), ultrasound (3 patients), fluoroscopy (3 patients), and computed tomography (CT) (9 patients).
Different variant forms of BoNT type A with a considerably wide range of doses were used: abobotulinumtoxinA (AboB) (dose range for a single LCo, 30–250 U), onabotulinumtoxinA (OnaB) (dose range for a single LCo, 10–100 U), and incobotulinumtoxinA (IncoB) (dose range for a single LCo, 15–100 U). In many patients, the injections were not only administered to LCo but also to the other cervical muscles.
In 19 patients, no side effects have been reported. Of those, 17 patients reported a substantial improvement in their symptoms. Of the 9 patients, who experienced side effects, little or no effect was found in only 2 people.
For OnaB, side effects were frequently associated with a total dose of ≥45 U to one LCo or ≥60 U to both LCo. In case of IncoB, a total dose of 100 U to both LCo muscles caused side effects, whereas in case of AboB, a total dose of 500 U to both LCo muscles was associated with swallowing difficulties.
Dysphagia was the most common side effect. Only one of nine patients reported flexor weakness instead of swallowing difficulties. In neither of the dysphagia cases was a feeding tube required.
3.2. Our own case series
Our patient group comprised 4 patients (3 females, 1 male) with various neurological conditions (Table 1 ).
Table 1.
Clinical characteristics and outcomes of the 4 patients with anterocollis who underwent longus colli injections.
| Sex/Age | Major diagnosis | Duration of the major disease, years | Anterocollis duration, months | Possible triggers for anterocollis | Protocol of the OnaB injection | Duration of the improvement, months | Side effects | % Satisfaction | Comments | Would like to repeat |
|---|---|---|---|---|---|---|---|---|---|---|
| F/32 years | NPC, dementia | 30 | 11 | Fall | R LCo 35 U L LCo 35 U |
2 | None | 35 | Previous trial of injection included both SCMs and ASc without any improvement. Worsening of the anterocollis by the end of the day (difficult to maintain right posture) |
Yes |
| F/66 | PD | 11 | 12 | None, stable treatment | L LCo 25 × 2 U L ASc 10 U L LevSc 15 U R ASc 10 U R LevSc 15 U |
2 | 4 days following injection — swallowing difficulties for 1 week | 40 | Reduced pain in neck | Yes |
| M/63 years | PD | 8 | 5 | None, stable treatment | R LCo 40 U L LCo 40 U |
3 | 3 weeks following injection — swallowing difficulties for 1.5 months | 30 | Difficult to maintain right posture | Yes |
| F/18 years | Unspecified cerebellar degeneration | 13 | 32 | None | R LCo 30 U L LCo 30 U |
3 | None | 40 | Improved swallowing Reduced drooling |
Yes |
F, female; M, male; U, units; R, right; L, left; LCo, longus colli; OnaB, onabotulinumtoxinA; SCM, sternocleidomastoid; ASc, anterior scalene; LevSc, levator scapulae.
3.2.1. Patient 1
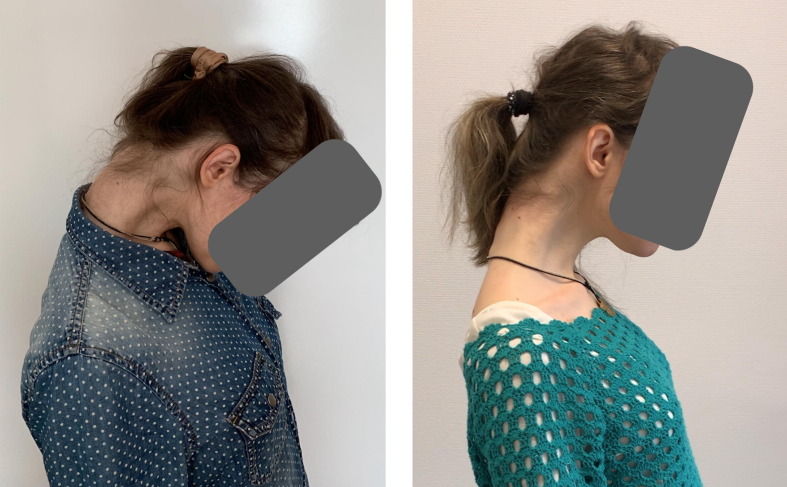
A 32-year-old woman with a 30-year history of Niemann–Pick type C disease (NPC) started developing anterocollis with a mild laterocollis to the left after a fall. According to her, she had previously felt some tension in her neck muscles; however, after a fall, she began to experience a rapid progression in the involuntary forward flexion of the neck. Abnormal neck posture substantially interfered with patient’s activities of daily living (ADL), such as personal hygiene, dressing, and functional mobility; no pre-treatment dysphagia was reported. Brain and whole spine MRI did not show any structural lesions. Serum CK was normal. Her treatment scheme (miglustat 600 mg/daily) was stable for the last 7 years. Both patient and her mother reported improvement in posture after LCo injection (Fig. 2 ); it became easier for the patient to maintain personal hygiene, dress herself, and walk. The mother assessed an overall improvement rate of 30%; however, by the end of each day, the patient’s degree of neck flexion was worse than what it was in the morning. No swallowing difficulties were reported.
Fig. 2.
Patient 1 before and 4 weeks after the BoNT injection to LCo at both sides.
3.2.2. Patient 2
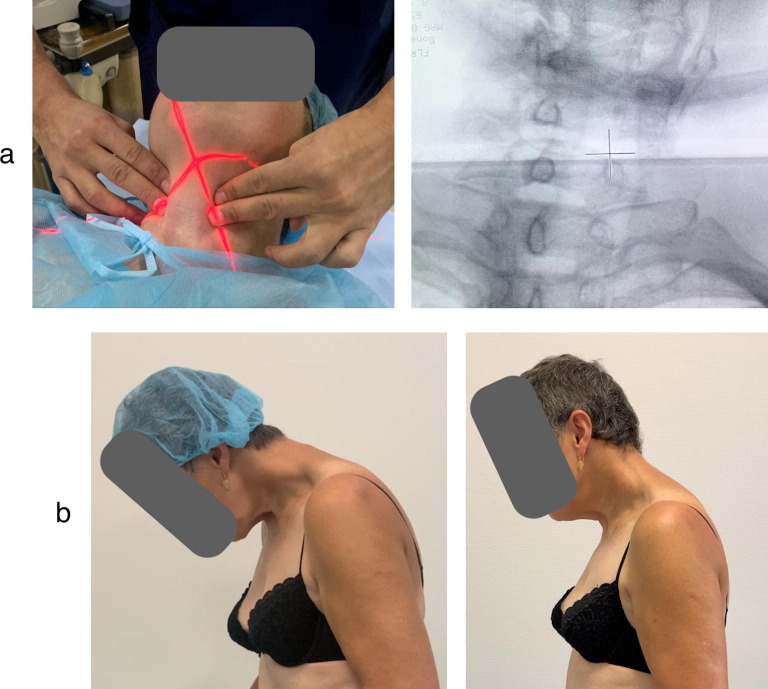
A 66-year-old woman had a 11-year history of Parkinson disease (PD) and insidious onset of anterocollis with mild laterocollis to the right during the past year. At the time of anterocollis development, her antiparkinsonian treatment regimen was stable (levodopa 800 mg/daily, pramipexol 3 mg/daily, amantadine 300 mg/daily). Due to anterocollis, dressing, mobility, and maintenance of social interactions were impaired; no pre-treatment swallowing difficulties were reported. Serum CK was normal. While administering the LCo injection, it was found that the bulk of the right LCo could not be palpated at the right side, and hence, a decision to only inject the left LCo was made; however, on the right side anterior scalene and levator scapulae, muscles were injected. Subsequently, an X-ray scan of the C-spine was taken which showed rotation of the vertebral column (Fig. 3 a) and explained why the right LCo could not be palpated. During the follow-up visit after 4 weeks, the patient reported reduced pain in anterior neck and a better ability to maintain a more upright position with her head (Fig. 3b), as well as improvement in social interactions and mobility. However, she also developed mild dysphagia, which disappeared within 1 week. Her overall rate of satisfaction with BoNT treatment was 40%.
Fig. 3.
X-ray and outcomes of the BoNT injections in patient 2. a – Red marker during the X-ray scan corresponds to the cross marker in the X-ray image of the C-spine showing rotation of the vertebral column in patient 2. b – Patient 2 before and 4 weeks after the BoNT injection to the left LCo.
3.2.3. Patient 3
A 63-year-old man with an 8-year history of PD gradually developed pure anterocollis over 6 months. His antiparkinsonian treatment regimen (levodopa 700 mg/daily, pramipexol 3 mg/daily, amantadine 300 mg/daily) was stable at the moment of anterocollis onset. ADL, such as personal hygiene, mobility, and communication skills, were impaired. No pre-treatment dysphagia was reported. Four weeks following OnaB injection to LCo at the both sides (40 U each), along with improvement in posture, mobility, and communication abilities, he also developed moderate dysphagia, which regressed within 1.5 months. In addition, the patient reported slight difficulties in maintaining an upright neck posture, even though he did not show any neck extensor weakness and serum CK was normal during initial examination. Therefore, his overall rate of satisfaction with BoNT treatment was 30%.
3.2.4. Patient 4
The final patient was an 18-year-old woman with an early-onset (starting from 5 years of age) cerebellar degeneration (genetically undetermined so far; Friedreich's ataxia was excluded; gene panel testing and whole-exome sequencing were negative). Over the last 2.5 years, she gradually began to develop pure anterocollis. The abnormal neck posture interfered with personal hygiene, eating (due to swallowing difficulties), and mobility. Serum CK was normal. Following injection of LCo (OnaB 30 U each side), she reported a significant improvement in swallowing ability, reduction in drooling, and an improvement in handling personal hygiene and posture, which allowed her to expand her physical activity and mobility (subjective improvement of 40%).
The mean age of our patients was 44.8 years with the mean anterocollis duration being 15 months. In all cases, development of the abnormal neck posture was painless, pre-treatment exam didn’t show weakness of the neck extensors, and the serum CK levels were within normal range, which along with the variability in neck posture depending on the body position (standing, walking, supine) allowed us to suggest dystonic anterocollis. The mean dose of OnaB for one LCo muscle was 38.8 U. A dose of up to 35 U per LCo muscle was not associated with the development of transient dysphagia. Dysphagia developed in the two patients (both with PD) was not severe enough for the utilization of a feeding tube and lasted from 1 week to 1.5 months.
The mean percentage of patient satisfaction was 36.3%, and the mean duration of the beneficial effect was 2.5 months. All patients agreed to receive a repeat injection; however, because of the SARS-CoV-2 pandemic repeated injections were postponed. There was no association between the anterocollis duration and patient improvement score.
4. Discussion
Despite the A and B levels of recommendations with respect to the use of BoNT in the treatment of cervical dystonia [11], the level of evidence for the currently available data on safety and effectiveness of LCo injections with BoNT for specifically treating dystonic anterocollis is very low.
Involvement of deep cervical flexors in cervical dystonia postures has been supported by empirical data, EMG, and imaging studies [12], [13], [14], [15].
4.1. Dose and side effects
Based on the published data, the suggested doses are described below.
For AboB, a dose up to 60 U on each side can be considered safe; in the study by Hefter et al. (2012), one patient received a total AboB dose of 500 U to both LCo, which resulted in swallowing difficulties. In some other published cases (Supplementary Table 1), the distribution of an overall AboB dose was not specified; hence, it is not quite clear as to how much can the dosage be increased beyond 60 U/muscle.
For IncoB, 15 U/LCo was not associated with dysphagia. Owing to controversial reports, it remains uncertain whether a dose range of 75–100 U of IncoB per LCo may cause swallowing difficulties (Supplementary Table 1).
In our case series, OnaB dose up to 35 U per LCo was not associated with dysphagia. This is mostly in line with the previously published data, i.e., with regard to OnaB, the side effects were mostly associated with 45 U per LCo (with the exception of one patient in the study by Hefter et al. who showed good toleration of an OnaB total dose at 200 U when injected to both LCo muscles, and one patient in the study by Glass et al. who developed dysphagia after the bilateral administration of OnaB at 30 U to each LCo) (Supplementary Table 1). Nevertheless, in the view of our data and previously published reports, it can be reasonable to start with a lower dose of a BoNT for the first injection, as it was done by Allison and Odderson, who injected 10 U of the OnaB to the right LCo and 25 U — to the left LCo [16].
Dysphagia following BoNT injections is likely explained by the diffusion of the toxin to the upper pharyngeal constrictors. Given the small size of a LCo, risk of dysphagia can be minimized by a smaller — 100 U/mL — dilution of an OnaB. However, in our case series, Patient 4 reported an improvement in her swallowing function, which can be explained not only by an improvement in her neck posture but also by the probable underlying neurogenic spasm of her pharyngeal constrictors wherein the constrictor hypertonicity was decreased by BoNT diffusion. Fluoroscopic assessment of the swallowing ability wasn’t a routine pre-treatment work-up procedure in the previously published cases, nor it was in our case series. Nevertheless, based on the abovementioned, for the future LCo injections, we consider it reasonable to conduct that assessment prior to the administration of BoNT.
It is noteworthy that two of our patients (1 and 3) reported difficulties in maintaining an upright neck posture by the end of the day. In previous studies on anterocollis, it has been shown that in patients with parkinsonism, the forward flexion of their neck can not only be a result of dystonia alone but also owing to myopathy of neck extensors or myopathy alone [8]. This can hypothetically explain the abovementioned difficulties in maintaining neck posture by the end of the day; however, in neither of our patients, neck extensor weakness, elevated CK levels, or painful anterocollis onset were observed.
4.2. Effectiveness and its measure
Traditional scales for the quantitative evaluation of cervical dystonia are not considered helpful in the assessment of patients with predominant anterocollis [17].
Given that a majority of previously described patients were not rated using the subjective improvement approach, it is challenging to adequately compare our outcomes with the previous published data. However, we believe that one-third improvement relative to the baseline along with the readiness and willingness of the patients to receive repeat injection are good preliminary indicators of effectiveness. Nevertheless, our data as well as the data from previously published cases show a strong need for a unified sensitive tool to assess the severity of anterocollis as well as treatment effects.
In comparison with the previously published cases, in which all the patients had primary cervical dystonia, we reported the outcomes of BoNT injections in two patients who had anterocollis secondary to PD, 1 patient who had NPC, and 1 patient who had an unspecified cerebellar degeneration.
Our case series not only provides additional data on the use of BoNT in dystonic anterocollis but also demonstrates the potential utility of BoNT injections for dystonia occurring due to heredodegenerative neurological disorders.
From our case series, it may be speculated that the effectiveness of BoNT injections administered to LCo in cases of dystonic anterocollis of an heredodegenerative origin may be less pronounced than their effectiveness in cases of primary cervical dystonia with an anterocollis component.
4.3. Injection technique and anatomical considerations
Based on the currently published data, it appears that use of CT for navigation while injecting LCo is not practical, given that repeated injections with CT guidance involve systematic radiation exposure to the patient. Moreover, it requires certain technical facilities and potential costs, which can be a limitation for a number of institutions.
Fluoroscopy, as it has been shown in our Patient 2, may be feasible for cases where it is not possible to palpate LCo on one side but it is still necessary to administer a bilateral injection. However, in such situations, we recommend that injection should be administered only when LCo is palpable.
Ultrasound was also proposed as a guidance technique, which allows real-time visualization of the trajectory of an EMG needle, as it is impossible to verify needle positioning in the proper muscle without imaging which may influence treatment outcomes [18]. It has also been reported that the needle traversed the tissue of a thyroid gland without any consequences [16], [19]. While on one hand, it may be indeed an attractive addition to guide the procedure, particularly, in a lateral approach to LCo when the needle should be advanced between the anterior scalene muscle and the carotid sheath, which creates a risk of damaging the phrenic nerve, brachial plexus, and even the structures of the carotid sheath, on the other hand, ultrasound guidance demands more human and technical recourses as well as additional costs. From our personal experiences, having another object in the “operation field” may occasionally complicate the entire procedure, particularly, while approaching the LCo muscle through fingertips.
Endoscopic guidance seems to be unreasonable for LCo in contrast to the longus capitis muscle.
Thus, despite the seeming vulnerability of the anterior neck structures while considering the LCo approach, EMG navigation remains, from our perspective, the most valuable type of assistance along with the knowledge of the underlying anatomy. This has been clearly shown in the report by Flowers et al. (2011) who introduced an EMG-guided medial two-puncture approach to LCo [20] at the level of the cricoid cartilage (which corresponds to the level of C5/C6 vertebrae) and 2–3 cm below that point. It was proposed that dividing the dose between the punctures at each side may alleviate the subsequent development of dysphagia.
Literature review and our case series suggest that the risk of dysphagia can also be largely attributed to BoNT dose. For instance, in our patients, swallowing difficulties were associated with an OnaB dose of ≥40 U to each side of LCo.
4.4. Limitations and future directions
Our study has some limitations. First, currently available PubMed publications on BoNT injections to LCo for specifically treating anterocollis lack high-level evidence data, and the published studies are heterogeneous with respect to their design and muscle approach. Second, in our case series, given the absence of neck extensor weakness, painless onset, and normal serum CK levels in our patients as observed at initial physical examination, we did not perform an EMG study to exclude mild myopathic changes. Third, at the time of manuscript preparation, because of the SARS-CoV-2 pandemic we had not repeated the administration of BoNT injections in our patients, even though all of them agreed to undergo the procedure once again. Finally, there is a lack of a reliable tool for comprehensively rating the anterocollis-predominant cervical dystonia, which would be sufficiently sensitive for clinically assessing a significant improvement after treatment.
Thus, herein, based on the published data and our case series we provide a set of empirically based suggestions on the current use of BoNT injections to LCo for managing anterocollis in outpatient clinics, including pretreatment work-up, injection technique, and dose range.
Financial disclosures
Yury Seliverstov– Received fees from Allergan for lectures on dystonia. Yury Seliverstov is also involved as an investigator in a Roche clinical trial on Huntington disease (NCT03761849).
Sergey Arestov– Reports no disclosures relevant to the manuscript.
Sergey Klyushnikov– Involved as an investigator in a Roche clinical trial on Huntington disease (NCT03761849), and in a Sanofi clinical trial on Pompe disease.
Yuliya Shpilyukova– Reports no disclosures relevant to the manuscript.
Sergey Illarioshkin– Involved as a principal investigator in a Roche clinical trial on Huntington disease (NCT03761849), and in a Sanofi clinical trial on Pompe disease.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jocn.2020.08.025.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Simpson D.M., Hallett M., Ashman E.J., Comella C.L., Green M.W., Gronseth G.S. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache. Neurology. 2016;86:1818–1826. doi: 10.1212/WNL.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagna A., Albanese A. Management of cervical dystonia with botulinum neurotoxins and EMG/ultrasound guidance. Neurol Clin Pract. 2019;9:64–73. doi: 10.1212/CPJ.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finsterer J., Revuelta G.J. Anterocollis and anterocaput. Clin Neurol Neurosurg. 2014;127:44–53. doi: 10.1016/J.CLINEURO.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Revuelta G.J. Anterocollis and camptocormia in parkinsonism: a current assessment. Curr Neurol Neurosci Rep. 2012;12:386–391. doi: 10.1007/s11910-012-0280-9. [DOI] [PubMed] [Google Scholar]
- 5.Jost W.H., Tatu L. Selection of muscles for botulinum toxin injections in cervical dystonia. Mov Disord Clin Pract. 2015;2:224–226. doi: 10.1002/mdc3.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papapetropoulos S, Tuchman A, Sengun C, Russell A, Mitsi G, Singer C. Anterocollis: clinical features and treatment options. Med Sci Monit 2008;14:CR427-30. [PubMed]
- 7.Peng-Chen Z. Bilateral lower sternocleidomastoid botulinum toxin injections to address refractory anterocollis. Neurologist. 2016;21 doi: 10.1097/NRL.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 8.Savica R., Kumar N., Ahlskog J.E., Josephs K.A., Matsumoto J.Y., McKeon A. Parkinsonism and dropped head: dystonia, myopathy or both? Parkinsonism Relat Disord. 2012;18:30–34. doi: 10.1016/j.parkreldis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Oyama G., Hayashi A., Mizuno Y., Hattori N. Mechanism and treatment of dropped head syndrome associated with parkinsonism. Parkinsonism Relat Disord. 2009;15:181–186. doi: 10.1016/j.parkreldis.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 10.van de Warrenburg B.P.C., Cordivari C., Ryan A.M., Phadke R., Holton J.L., Bhatia K.P. The phenomenon of disproportionate antecollis in Parkinson’s disease and multiple system atrophy. Mov Disord. 2007;22:2325–2331. doi: 10.1002/mds.21634. [DOI] [PubMed] [Google Scholar]
- 11.Contarino M.F., Van Den Dool J., Balash Y., Bhatia K., Giladi N., Koelman J.H. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H.B., An Y.-S., Lee H.Y., Hwang J.H., Lee H.J., Jeong K.Y. Usefulness of (18)f-fluorodeoxyglucose positron emission tomography/computed tomography in management of cervical dystonia. Ann Rehabil Med. 2012;36:745–755. doi: 10.5535/arm.2012.36.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H.J., An Y.-S., Ahn Y.-W., Yim S.-Y. Threshold of clinical severity of cervical dystonia for positive (18)F-FDG PET/CT. Ann Rehabil Med. 2013;37:777–784. doi: 10.5535/arm.2013.37.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung D.H., Choi J.Y., Kim D.-H., Kim E.-S., Son Y.-I., Cho Y.-S. Localization of dystonic muscles with 18F-FDG PET/CT in idiopathic cervical dystonia. J Nucl Med. 2007;48:1790–1795. doi: 10.2967/jnumed.107.044024. [DOI] [PubMed] [Google Scholar]
- 15.Bhidayasiri R. Treatment of complex cervical dystonia with botulinum toxin: Involvement of deep-cervical muscles may contribute to suboptimal responses. Parkinsonism Relat Disord. 2011;17:S20–S24. doi: 10.1016/j.parkreldis.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Allison S.K., Odderson I.R. Ultrasound and electromyography guidance for Injection of the longus colli with botulinum toxin for the treatment of cervical dystonia. Ultrasound Q. 2016;32 doi: 10.1097/RUQ.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 17.Hicklin L.A., Marion M.-H. Endoscopic Injection of the longus capiti muscle in the treatment of dystonic head flexion. Mov Disord Clin Pract. 2020;7:293–297. doi: 10.1002/mdc3.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaymak B, Kara M, Gürçay E, Özçakar L. Sonographic guide for botulinum toxin injections of the neck muscles in cervical dystonia. Phys Med Rehabil Clin N Am 2018;29:105–23. https://doi.org/10.1016/j.pmr.2017.08.009. [DOI] [PubMed]
- 19.Tyślerowicz M, Jost WH. Injection into the longus colli muscle via the thyroid gland. Tremor Other Hyperkinet Mov (N Y) 2019;9:10.7916/tohm.v0.718. https://doi.org/10.7916/tohm.v0.718. [DOI] [PMC free article] [PubMed]
- 20.Flowers J.M., Hicklin L.A., Marion M.-H. Anterior and posterior sagittal shift in cervical dystonia: a clinical and electromyographic study, including a new EMG approach of the longus colli muscle. Mov Disord. 2011;26:2409–2414. doi: 10.1002/mds.23905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.