ABSTRACT
For time immemorial, Chinese herbal medicines (CHMs) have been widely used in China for disease treatment and promotion of general well-being. However, in recent years, many studies have shown that mycotoxins produced by fungi could contaminate CHMs due to unfavourable pre- or post-harvest conditions, raising major concern for consumer safety. At present, there is a significant focus on developing novel mycotoxin detection methods for analysing CHMs, and numerous studies have aimed to determine which kinds of raw herbal materials are most susceptible to mycotoxin contamination. In this review, we focus on recent advances in understanding and detection of mycotoxins in domestic raw herbal materials and related products from 2000 to 2018. Aspects of mycotoxin contamination of CHMs covered in this review include common mycotoxin contaminants in CHMs, maximum mycotoxin residue limits, analytical methods for mycotoxin detection and their applications and limitations, as well as a brief discussion of the trends in ongoing research.
KEYWORDS: Chinese herbal medicine, mycotoxin, maximum residue limits, detection method, contamination
1. Introduction
Mycotoxins are secondary metabolites produced by fungi during growth that can cause pathological responses in humans and animals. Medicinal herbs are highly susceptible to toxigenic fungal infections and mycotoxin contamination that can occur at either the pre- or post-harvest stage as a result of poor growing conditions, inadequate drying, or storage in warm, humid conditions (Zhang et al. 2016). The potential for medicinal herbs and related agricultural products to have toxic effects as a result of mycotoxin contamination is attracting increasing attention worldwide (Tripathy et al. 2015; Mahfuz et al. 2018; Zhang et al. 2018c). At present, the most common mycotoxins found in Chinese herbal medicines (CHMs) are aflatoxins, ochratoxins, zearalenone, fumonisins, trichothecenes, and patulin (Zhang et al. 2015). Numerous studies have shown that these mycotoxins are highly toxic resulting in hepatotoxicity, nephrotoxicity, reproductive disorders, and immuno-suppression. These mycotoxins are also carcinogenic, teratogenic, and mutagenic making exposure to compounds of this nature a serious human health threat.
2. Aflatoxins (AFs)
In 1960, 100,000 turkeys died abruptly over the span of a few months in the UK. People later found that they had all consumed the same peanut meal that was contaminated by fungi. As a result of this occurrence, AFs were discovered and characterised (Wannop 1961). AFs are secondary metabolites that share a common difurocoumarin skeleton; they are produced by Aspergillus flavus and A. parasiticus (Shen et al. 2016). This class of compounds includes aflatoxin B1, B2, G1, G2, M1, and M2; of these, AFB1 is the most toxic and carcinogenic one. According to previous studies, the toxicity of AFB1 is 10 times greater than that of cyanide and 68 times greater than that of arsenic. In 1993, AFB1 was classified as a Class 1A carcinogen by the World Health Organisation Cancer Research Institute (Ono et al. 2001). Studies have shown that AFB1 can suppress the immune system and affect foetal development and differentiation of cells, giving this compound the ability to exert teratogenic effects. Exposure to AFB1 is also known to have damaging effects on human and animal liver tissues. In severe cases, exposure to AFB1 resulted in liver cancer and even death (Ma and Zan 2009).
2.1. The limit standards of AFs
By the end of 2003, approximately 100 countries had imposed specific limits on the levels of mycotoxins allowed in food and feed (Food and Agriculture Organization of the United Nations 2004). As depicted in Table 1, in the case of medicinal herbs and related products, the legal limit for AFB1 ranges from 2 to 10 μg∙kg−1, while the limit for other AFs ranges from 4 to 20 μg∙kg−1. Among these regulations, the European Pharmacopeia (European Pharmacopoeia Commission 2016) and the British Pharmacopeia (British Pharmacopoeia Commission 2012) have set the strictest limits (2 μg∙kg−1 for AFB1 and 4 μg∙kg−1 for total AFs), and the most commonly set limits for AFB1 and AFs are similar to those of the Chinese Pharmacopeia (Chinese Pharmacopoeia Commission 2015) and the European Union (European Union 2006), which were 5 μg∙kg−1 and 10 μg∙kg−1, respectively.
Table 1.
Limits of AFs for medicinal plants in Standards and Regulations.
| Standards and regulations | Product (Group) | AFB1 (µg∙kg−1) | Total AFs (µg∙kg−1) | Reference |
|---|---|---|---|---|
| EU | Nutmeg | (European Union 2006) | ||
| Ginger | 5 | 10 | ||
| Turmeric | ||||
| White and black pepper | ||||
| EP | Herbal drugs | 2 | 4 | (European Pharmacopoeia Commission 2016) |
| USP | Some types of raw medicinal herb materials, as well as their powder and/or dry extract | 5 | 20 | (United States Pharmacopeial Convention 2017) |
| BP | Herbal drugs | 2 | 4 | (British Pharmacopoeia Commission 2012) |
| Canada | Products containing ginseng or any substance derived from this source, Evening Primrose Oil, sugar cane, sugar beets, cottonseed | 5 | 20 | (Government of Canada, Natural and Non-prescription Health Products Directorate 2015) |
| JP | Crude drug and preparations containing crude drugs as main ingredient (crude drug preparations) | 10 | (Japanese Pharmacopoeia Commentary Editorial Committee 2016) | |
| KP | Armeniacae Semen, Arecae Semen, Cassiae Semen, Crotonis Semen, Curcumae Radix, Dolichoris Semen, Glycyrrhizae Radix et Rhizoma, Nelumbinis Semen, Myristicae Semen, Persicae Semen, Pinelliae Tuber, Polygalae Radix, Carthami Flos, Thujae Semen, Trichosanthis Semen, Zizyphi Semen | 10 | 15 | (Korean Food & Drug Administration 2012) |
| ChP | Bombyx Batryticatus, Citri Reticulatae Pericapium, Sterculiae Lychnophorae Semen, Persicae Semen, Ziziphi Spinosae Semen, Platycladi Semen, Nelumbinis Semen, Quisqualis Fructus, Arecae Semen, Hordei Fructus Germinatus, Myristicae Semen, Cassiae Semen, Polygalae Radix, Coicis Semen, Jujubae Fructus, Phenetima, Scolopendra, Hirudo, Scorpio | 5 | 10 | (Chinese Pharmacopoeia Commission 2015) |
| HKCMMS | Herbal drugs | 5 | 10 | (Department of Health, Hong Kong Special Administrative Region of the People’s Republic of China 2005) |
| Indonesia | Coconut, spices and traditional drug medicines/herbs |
20 | (Food and Agriculture Organization of the United Nations 2004) | |
| Vietnam | Nutmeg Ginger and turmeric Black and white pepper Liquorice root used for herbal tea Liquorice extract for beverage or to mix |
5 | 10 | (United States Department of Agriculture Foreign Agricultural Service 2013) |
| Germany | Any materials used in manufacture of medicinal products (including medicinal herbal products) | 2 | 4 | (World Health Organization. 2007) |
| Argentina | Herbs, herbal materials and herbal preparations used for herbal tea infusions | 5 | 20 | (World Health Organization (WHO) 2007) |
2.2. Detection methods for AFs
Thin-layer chromatography (TLC) was the first method used for detecting AFs. In correlation with the increasing demand for more precise data, the overall percentage of use of TLC for detecting AFs was reduced. However, since TLC is a straightforward approach with low-associated cost and minimal specialised equipment, it is still generally utilised in some laboratories (Li et al. 2005).
In recent years, high-performance liquid chromatography (HPLC) has become the most common method for quantifying AFs. Currently, HPLC with an FLD detector (HPLC-FLD) is the most commonly used method for detecting the presence of AFs and quantifying their levels. However, aqueous solvents are often used as the eluent in reverse-phase chromatography, and aqueous buffers will partially quench the fluorescence of AFB1 and AFG1. Therefore, a derivatisation step is usually required to stabilise and enhance detection, such as a pre-column derivatisation with trifluoroacetic acid (TFA) (Zhao et al. 2011; Li et al.2015) or a post-column derivatisation such as a chemical derivatisation with iodine or bromine (Ran et al. 2017), a photochemical derivatisation, or an electrochemical derivatisation (Zhang et al. 2005a; Zhang and Chen 2005). Compared to pre-column derivatisations, application of post-column derivatisations was reported more frequently (Zhang et al. 2018c).
HPLC-MS/MS has been increasingly used for the detection and quantification of AFs in herbal medicines (Wang et al. 2011; Liu et al. 2012; Saha et al. 2018). At the same time, HPLC-MS/MS is often used for confirmation of AF identity in order to avoid interfering signals from analogs of AFs that might be present in herbal medicines.
In addition to conventional analysis methods, immunological methods have been used for rapid detection of AFs in CHMs such as an enzyme-linked immunosorbent assay (ELISA) and a gold immunochromatographic assay (GICA). Chu et al. (Chu et al. 2015) used these approaches to successfully detect AFB1 in lotus seeds. Since the complexity matrices presented by CHMs can affect the antigen-antibody specific binding reaction, a matrix-matching curve was used to reduce the bias introduced by the matrix. For example, using AFB1-BSA and a sheep anti-mouse IgG antibody for detection and a control, respectively, Yang generated a test strip suitable for rapid detection of AFB1 in lotus seeds with a sensitivity of 2.5 ng∙mL−1 (Yang 2015).
Fluorescent immunoassays (FIAs) have recently been developed for qualitative and quantitative analysis of AFs in herbal matrices. Zhang established a fluorescence polarisation immunoassay detection method by combing AFB1 with amino fluorescein. The molecular weight and rotation speed of the AFB1 fluorescent probe will change before and after binding with an antibody. Thus, detection and quantification of AFB1 in CHMs can be accomplished by measuring the change in the fluorescence polarisation value (Zhang 2017). Based on the development of a FITC-AFB1 fluorescently labelled antibody, Yu et al. established a direct competitive fluorescent immunoassay to detect AFB1 in five CHMs (Yu et al.2015). Zhang utilised PEG-modified CdSe/CdS quantum dots (QDs) with glycine-based signal enhancement for the detection of AFB1 in medicinal herbs (Zhang et al. 2018b). This work demonstrated that a QD labelling technique could potentially serve as a novel means of performing fast trace-detection in complex herbal matrices. Due to the AFB1 self-sensitisation to fluorescence when under UV light, a label-free FIA method was developed for the specific detection of AFB1 in CHMs. Compared with traditional immunoassay approaches, this method could reduce the cost of analysis and shorten the analysis time without a complex probe labelling process (Shu 2018).
2.3. AF contaminants in CHMs
From 2000 to 2018, 2979 batches of CHMs from 66 varieties known to be easily contaminated by AFB1 were tested, as summarised in Table 2, of which 697 batches tested positive for AFB1. Levels of AFB1 ranged from 0.02 to 1268.8 μg∙kg−1. It is important to note that the percentage of the botanicals Zingiber officinale (Kuang 2000; Bao et al. 2008; Cao 2013) and S. Platycladi (Yang et al. 2004, 2005; Yang et al. 2011b; Hao et al. 2012; Hu et al. 2012; Liu et al. 2012a; Hao et al. 2013; Li 2015a; Zhu et al. 2015; Chien et al. 2018) that tested positive for AFB1 was 68% and 78%, respectively. In the case of the animal material Cantharides, the per cent positive rate for contamination with AFB1 was as high as 95%, and the lowest contaminating amount detected was 25.95 μg∙kg−1; this is more than 5 times the limit set by the EU and China (5 μg∙kg−1), indicating that these types of samples are highly susceptible to AFB1 contamination (Sun and Liu 2016). In addition, of the CHM samples that tested positive, there were 486 batches with AFB1 levels exceeding the limits set by the EU and China, accounting for 70% of the total positive samples. CHMs with a per cent positive for AFB1 of above 50% included Massa Medicata Fermentata and R. Ophiopogonis (Figure 1).
Table 2.
Detection of AFs in CHM.
| AFB1 |
Afs (AFB1+ AFB2+ AFG1+ AFG2+ AFM1) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CHM | Total samples | Positive samples No(%) |
Range (µg∙kg−1) |
>EP/BP Legal limit No (%) |
>Ch.P/EU Legal limit No (%) |
Total samples | Positive samples No (%) |
Range (µg∙kg−1) |
>EP/BP Legal limit No (%) |
>Ch.P/EU Legal limit No (%) |
Reference |
| Panax Ginseng | 49 | 11(22%) | 0.02–5.81 | 5(10%) | 2(4%) | 41 | 10(24%) | 0.14–11.92 | 4(10%) | 2(5%) | (Kuang 2000; Liang and Huang 2000; Bao et al. 2008; Zheng et al. 2010b; Zhao et al. 2011; Zheng et al. 2014c; Lin et al. 2016; Chien et al. 2018) |
| Dioscorea opposita | 95 | 10(11%) | 0.03–74.84 | 5(5%) | 5(5%) | 67 | 3(4%) | 0.7–1.1 | 0 | 0 | (Kuang 2000; Liang and Huang 2000; Tang 2000; Hao et al. 2012; Wang et al. 2014b; Zheng et al. 2014c; Li 2016; Lin et al. 2016; Chien et al. 2018; Zhang et al. 2018a) |
| Rheum officinale | 8 | 5(45%) | 0.38–187.25 | 2(25%) | 2(25%) | 3 | 1(33%) | 3.3 | 0 | 0 | (Kuang 2000; Liang and Huang 2000; Tang 2000; Zhang et al. 2005; Zhao et al. 2011; Hao et al. 2012; Lin et al. 2016) |
| Atractylodes macrocephala | 16 | 4(25%) | 0.17–15 | 1(6%) | 1(6%) | 12 | 2(17%) | 0.54 | 0 | 0 | (Kuang 2000; Liang and Huang 2000; Zhang et al. 2005; Hao et al. 2012; Zheng et al. 2014c; Lin et al. 2016) |
| Pinellia ternata | 4 | 3(75%) | 0.04–1.89 | 0 | 0 | 4 | 3(75%) | 0.04–1.89 | 0 | 0 | (Kuang 2000; Liang and Huang 2000; Hu et al. 2012) |
| Salvia miltiorrhiza | 8 | 3(38%) | 1.21–3.46 | 1(13%) | 0 | 6 | 0 | 0 | 0 | 0 | (Kuang 2000; Zhang et al. 2005; Zheng et al. 2005; Yang et al. 2013) |
| Lonicera japonica | 11 | 4(36%) | 1.96–50.00 | 3(27%) | 3(27%) | 6 | 3(50%) | 0.95–203 | 2(33%) | 2(33%) | (Kuang 2000; Li and Zhuang 2000; Tang 2000; Zhang et al. 2005; Hao et al. 2012; Tan et al. 2012) |
| Fructus Crataegi | 65 | 3(5%) | 0.12–28 | 1(2%) | 1(2%) | 62 | 0 | 0 | 0 | (Kuang 2000; Zheng et al. 2005; Li et al. 2011a, Li et al. 2012; Zhang et al. 2008; Zhu et al. 2015; Chien et al. 2018) | |
| Zingiber officinale | 31 | 21(68%) | 0.03–8.88 | 9(29%) | 5(16%) | 30 | 20(67%) | 0.05–22.06 | 6(20%) | 4(13%) | (Kuang 2000; Bao et al. 2008; Cao 2013) |
| Massa Medicata Fermentata | 52 | 6(12%) | 0.38–29.38 | 6(12%) | 6(12%) | 47 | 8(17%) | 1(2%) | 1(2%) | (Li and Zhuang 2000; Li and Chen 2000; Tang 2000; Yang et al. 2004; Zhang et al. 2005; Zhu et al. 2015; Chien et al. 2018) | |
| Radix Paeoniae alba | 78 | 7(9%) | 0.78–3.44 | 3(4%) | 0 | 77 | 8(10%) | 0.47–7.82 | 1(1%) | 0 | (Li and Chen 2000; Yang et al. 2013; Li 2016; Xing et al. 2016; Chien et al. 2018; Zhang et al. 2018a) |
| Astragalus membranaceus | 228 | 11(5%) | 0.07–200 | 9(4%) | 8(4%) | 225 | 10(4%) | 0.38–64.3 | 9(4%) | 3(1%) | (Li and Chen 2000; Liang and Huang 2000; Han et al. 2012; Zheng et al. 2010b; Tan et al. 2012; Yang et al. 2013; Lin et al. 2016; Chien et al. 2018) |
| Pericarpium citri reticulatae | 181 | 19(10%) | 0.12–118.5 | 9(5%) | 5(3%) | 175 | 35(20%) | 0.03–77.55 | 6(3%) | 5(3%) | (Li and Zhuang 2000; Li and Chen 2000; Liang and Huang 2000; Tang 2000; Zhang et al. 2005; Zheng et al. 2010b; Li et al. 2011a; Yang et al. 2011a; Wang et al. 2012; Yang et al. 2013; Li et al. 2014b; Wang et al. 2014b; Wang et al. 2014c; Zhu et al. 2015; Lin et al. 2016; Chien et al. 2018) |
| Radix bupleuri | 6 | 2(33%) | 10–26.85 | 2(33%) | 2(33%) | 4 | 0 | 0 | 0 | (Li and Zhuang 2000; Li and Chen 2000; Hao et al. 2012; Yang et al. 2013) | |
| Cortex Moutan | 3 | 1(33%) | 2.5 | 1(33%) | 0 | 2 | 0 | 0 | 0 | (Li and Chen 2000; Hao et al. 2012) | |
| Radix isatidis | 5 | 3(60%) | 0.2–100 | 2(40%) | 2(40%) | 2 | 0 | 0 | 0 | (Li and Chen 2000; Liang and Huang 2000; Tang 2000; Yang et al. 2013) | |
| Scutellaria baicalensis | 5 | 2(40%) | 16.9–22.82 | 2(40%) | 2(40%) | 3 | 0 | 0 | 0 | (Li and Zhuang 2000; Li and Chen 2000; Zhang et al. 2005; Hao et al. 2012) | |
| Radix Ophiopogonis | 20 | 3(15%) | 34.12–89.96 | 3(15%) | 3(15%) | 15 | 1(7%) | 1.89 | 0 | 0 | (Li and Chen 2000; Tang 2000; Hao et al. 2012; Hu et al. 2012; Liu et al. 2012a; Yang et al. 2013) |
| Scaphium scaphigerum | 42 | 5(12%) | 1.0–8.378 | 4(10%) | 3(7%) | 40 | 3(8%) | 1–10.546 | 2(5%) | 1(3%) | (Li and Chen 2000; Yang et al. 2004; Yang et al. 2005; Zhang et al. 2005; Zheng et al. 2010b; Su et al. 2014; Wang et al. 2014c; Zhu et al. 2015; Lin et al. 2016) |
| Fructus Aurantii Immaturus | 4 | 4(100%) | 0.13–107.08 | 3(2%) | 3(2%) | (Li and Chen 2000; Liang and Huang 2000; Tang 2000) | |||||
| Poria cocos | 46 | 6(13%) | 0.05–6.12 | 2(4%) | 2(4%) | 34 | 3(9%) | 0.758–6.12 | 1(3%) | 0 | (Li and Chen 2000; Liang and Huang 2000; Zhang et al. 2005; Zhao et al. 2011; Xie 2016; Chien et al. 2018; Hu et al. 2018) |
| Polygonum cuspidatum | 3 | 1(5%) | 0.1 | 0 | 0 | 1 | 1(100%) | 6.8 | 1(100%) | 0 | (Liang and Huang 2000; Guo et al. 2012; Han et al. 2012; Lin et al. 2016) |
| Rhizoma rehmanniae | 5 | 4(33%) | 0.17–85.46 | 3(60%) | 2(40%) | 1 | 0 | 0 | 0 | (Li and Zhuang 2000; Liang and Huang 2000; Tang 2000; Han et al. 2012; Lin et al. 2016) | |
| Semen Ziziphi Spinosae | 42 | 17(40%) | 0.4–23.4 | 7(17%) | 4(10%) | 38 | 15(39%) | 0.4–25.6 | 5(13%) | 4(11%) | (Liang and Huang 2000; Zhang et al. 2008; Han et al. 2010; Zheng et al. 2010b; Zheng et al. 2010b; Li et al. 2011; Yang et al. 2011a; Hao et al. 2012; Hu et al. 2012; Yang et al. 2013; Wang et al. 2014b; Zhu et al. 2015; Lin et al. 2016; Chien et al. 2018) |
| Radix scrophulariae | 5 | 1(20%) | 0.03 | 0 | 0 | 3 | 1(33%) | 4.4 | 1(33%) | 0 | (Liang and Huang 2000; Guo et al. 2012; Hao et al. 2012; Lin et al. 2016) |
| Platycodon grandiflorum | 5 | 1(20%) | 0.16 | 0 | 0 | 3 | 0 | 0 | 0 | (Liang and Huang 2000; Zhang et al. 2005; Hao et al. 2012; Lin et al. 2016) | |
| Schisandra chinensis | 25 | 2(8%) | 0.11–0.54 | 0 | 0 | 22 | 0 | 0 | 0 | (Liang and Huang 2000; Zhang et al. 2008; Yang et al. 2013; Lin et al. 2016; Chien et al. 2018) | |
| Codonopsis pilosula | 8 | 1(13%) | 28.48 | 1(13%) | 1(13%) | 6 | 1(17%) | 471 | 1(17%) | 1(17%) | (Li and Zhuang 2000; Li and Chen 2000; Yang et al. 2004; Hao et al. 2012; Tan et al. 2012; Lin et al. 2016) |
| Polygala tenuifolia | 20 | 10(50%) | 0.01–118.1 | 9(45%) | 6(30%) | 20 | 10(50%) | 0.01–118.1 | 6(30%) | 6(30%) | (Liang and Huang 2000; Hao et al. 2012, 2013; Chien et al. 2018) |
| Radix glycyrrhizae | 80 | 25(31%) | 0.16–112.79 | 7(9%) | 7(9%) | 75 | 23(31%) | 0.027–26.11 | 4(5%) | 3(4%) | (Liang and Huang 2000; Tang 2000; Zhang et al. 2005; Wei et al. 2011; Zhao et al. 2011; Guo et al. 2012; Tan et al. 2012; Li et al. 2014b; Lin et al. 2016) |
| Semen Armeniacae Amarae | 51 | 11(22%) | 0.06–9.81 | 6(12%) | 1(2%) | 49 | 10(20%) | 0.06–11.95 | 2(4%) | 1(2%) | (Liang and Huang 2000; Yang et al. 2004; Yang et al. 2005; Zhang et al. 2005; Zheng et al. 2005; Zhang et al. 2008; Han et al. 2010; Li et al. 2011a; Han et al. 2012; Li et al. 2012; Liu et al. 2012a; Tan et al. 2012; Zheng et al. 2013; Wang et al. 2014b; Zhu et al. 2015; Zhao et al. 2016) |
| Polygonum multiflorum | 59 | 3(5%) | 0.06–6.8 | 2(3%) | 1(2%) | 57 | 22(39%) | 2.1–25 | 4(7%) | 2(4%) | (Liang and Huang 2000; Han et al. 2012; Guo et al. 2015; Li et al. 2016; Lin et al. 2016) |
| Eucommia ulmoides | 9 | 5(56%) | 0.85–61.42 | 4(44%) | 4(44%) | 5 | 1(20%) | 29.85 | 1(20%) | 1(20%) | (Tang 2000; Hao et al. 2012; Tan et al. 2012) |
| Semen Sojae Praeparatum | 6 | 2(33%) | 21.52–124.2 | 2(33%) | 2(33%) | 4 | 0 | 0 | 0 | (Li and Zhuang 2000; Tang 2000; Zhang et al. 2005; Zheng et al. 2005; Hao et al. 2012; Wang et al. 2014b) | |
| Coptis chinensis | 5 | 3(60%) | 15.71–68.5 | 3(60%) | 3(60%) | 2 | 0 | 0 | 0 | (Tang 2000; Hao et al. 2012) | |
| Angelica sinensis | 41 | 7(17%) | 0.4–121.62 | 6(15%) | 4(10%) | 37 | 2(5%) | 4.45–4.91 | 2(5%) | 0 | (Li and Zhuang 2000; Tang 2000; Zheng et al. 2010b; Hao et al. 2012; Tan et al. 2012; Yang et al. 2013; Liu et al. 2015) |
| Rhizoma Gastrodiae | 15 | 4(27%) | 0.758 | 0 | 0 | 1 | (Tang 2000; Zhang et al. 2005; Hu et al. 2012; Xie 2016) | ||||
| Semen Coicis | 208 | 25(12%) | 0.09–45.3 | 7(3%) | 5(2%) | 207 | 48(23%) | 0.10–12.4 | 5(2%) | 5(2%) | (Yang et al. 2004; Yang et al. 2005; Zhang et al. 2008; Zheng et al. 2010b; Li et al. 2011a; Zhao et al. 2011; Hao et al. 2012; Li et al. 2012; Kong et al. 2013; Wang et al. 2014b; Zheng et al. 2014b; Zhu et al. 2015; Zhao et al. 2016; Chien et al. 2018) |
| Fructus hordei germinatus | 73 | 13(18%) | 0.42–20.5 | 5(7%) | 3(4%) | 51 | 8(16%) | 0.47–9.46 | 1(2%) | (Li and Zhuang 2000; Yang et al. 2004; Yang et al. 2005; Zhang et al. 2005; Zhang et al. 2008; Wang et al. 2014b; Zheng et al. 2014c; Li 2015a; Zhu et al. 2015; Wang 2016; Wang et al. 2016; Zhang et al. 2018a) | |
| Seman Platycladi | 279 | 217(78%) | 0.25–592.0 | 214(77%) | 212(76%) | 279 | 257(92%) | 1.35–135.7 | 191(68%) | 190(68%) | (Yang et al. 2004; Yang et al. 2005; Yang et al. 2011b; Hao et al. 2012; Hu et al. 2012; Liu et al. 2012a; Hao et al. 2013; Li 2015a; Zhu et al. 2015; Chien et al. 2018) |
| Radix Gentianae Macrophyllae | 3 | 1(33%) | 34.43 | 1(33%) | 1(33%) | 3 | 2(67%) | 0.6757–34.43 | 1(33%) | 1(33%) | (Li and Zhuang 2000; Yang et al. 2004; Zhang et al. 2005) |
| Semen Persicae | 72 | 14(19%) | 0.34–35.94 | 6(8%) | 3(4%) | 72 | 14(19%) | 0.42–35.94 | 5(7%) | 1(1%) | (Li and Zhuang 2000; Yang et al. 2004; Yang et al. 2005; Han et al. 2010; Zheng et al. 2010b; Li et al. 2011a; Yang et al. 2011a; Han et al. 2012; Hao et al. 2012; Li et al. 2012; Li et al. 2014b; Wang et al. 2014b; Zheng et al. 2014a; Zhu et al. 2015; Chien et al. 2018) |
| Semen cassiae | 14 | 6(43%) | 0.55–12.9 | 5(36%) | 4(29%) | 14 | 7(50%) | 0.70–7.49 | 2(14%) | 2(14%) | (Zhang et al. 2005; Zheng et al. 2005; Guo et al. 2012; Hao et al. 2012; Liu et al. 2012a) |
| Radix sophorae flavescentis | 4 | 1(25%) | 27.6 | 1(25%) | 1(25%) | 4 | 1(25%) | 27.6 | 1(25%) | 1(25%) | (Zhang et al. 2005; Guo et al. 2012; Hao et al. 2012, 2013) |
| Radix angelicae | 14 | 3(21%) | 0.743 | 0 | 0 | 3 | (Zhang et al. 2005; Hao et al. 2012; Hu et al. 2012; Xie 2016) | ||||
| Fructus Ziziphi Jujubae | 151 | 8(5%) | 1.2–4.67 | 2(1%) | 0 | 151 | 10(7%) | 0.7–7.7 | 3(2%) | (Zheng et al. 2005; Liau et al. 2010; Zheng et al. 2010b; Li et al. 2011a; Guo et al. 2012; Liu et al. 2012a; Zhu et al. 2015; Zhao et al. 2016; Chien et al. 2018) | |
| Bombyx Batryticatus | 48 | 14(29%) | 0.23–39 | 6(13%) | 5 (10%) | 47 | 15(32%) | 0.38–42 | 6(13%) | 6(13%) | (Zheng et al. 2005; Zheng et al. 2010b; Yang et al. 2011a; Yang et al. 2011c; Li et al. 2014b; Wang et al. 2014c; Lin et al. 2016; Liu et al. 2017; Luo et al. 2018) |
| Cantharides | 21 | 20(95%) | 25.95–295.73 | 20(95%) | 20(95%) | 21 | 20(95%) | 43.47–301.87 | 20(95%) | 20(95%) | (Sun and Liu 2016) |
| Semen nelumbinis | 189 | 60(32%) | 0.21–1268.8 | 35(19%) | 34(18%) | 169 | 46(27%) | 0.21–1268.8 | 25(15%) | 23(14%) | (Li et al. 2011a, 2012; Liu et al. 2012; Liu et al. 2013; Zheng et al. 2014c; Chu et al. 2015; Wang 2016; Zhao et al. 2016; Chien et al. 2018; Zhang et al. 2018a) |
| Hirudo | 14 | 2(14%) | 1.26–3.14 | 1(7%) | 0 | 14 | 2(14%) | 1.26–3.35 | (Yang et al. 2011c; Liu et al. 2017; Luo et al. 2018) | ||
| Eupolyphaga seu Steleophaga | 36 | 17(19%) | 0.33–28.81 | 11(31%) | 8(22%) | 36 | 21(58%) | 1.1–257.55 | 14(39%) | 11(31%) | (Yang et al. 2011c; Liu et al. 2017; Sun et al. 2017; Luo et al. 2018) |
| Ligusticum wallichii | 2 | 1(50%) | 2.547 | 1(50%) | 0 | 1 | (Hao et al. 2012; Hu et al. 2012) | ||||
| Semen plantaginis | 3 | 1(33%) | 0.39 | 0 | 0 | 2 | (Zhang et al. 2008; Hao et al. 2012; Liu et al. 2012a; Chien et al. 2018; Zhang et al. 2018a) | ||||
| Palmae Areca | 34 | 17(50%) | 0.06–97.1 | 12(35%) | 10(29%) | 34 | 19(56%) | 0.21–97.1 | 8(24%) | 7(21%) | (Li et al. 2011a; Hao et al. 2012; Li et al. 2012; Hao et al. 2013; Li 2015a) |
| Rhizoma corydalis | 276 | 78(28%) | 0.21–693.4 | 77(28%) | 77(28%) | 276 | 117(42%) | 1.01–693.4 | 65(24%) | 64(23%) | (Yang et al. 2005; Hao et al. 2012; Liu et al. 2012a; Hao et al. 2013; Chien et al. 2018) |
| Rhizoma seu Radix Notopterygii | 2 | 2 | 1(50%) | 41.5 | 1(50%) | 1(50%) | (Guo et al. 2012; Hao et al. 2012) | ||||
| Semen myristicae | 15 | 6(40%) | 0.13–239.62 | 4(27%) | 3(20%) | 15 | 6(40%) | 0.13–290.8 | 3(20%) | 3(20%) | (Zhao et al. 2011; Hao et al. 2012; Liu et al. 2012a; Wang 2016) |
| Folium isatidis | 2 | 1(50%) | 1.2 | 0 | 0 | 2 | 1(50%) | 1.2 | (Hao et al. 2012) | ||
| Mangnolia officinalis | 4 | 1(25%) | 17.14 | 1(25%) | 1(25%) | 3 | 1(33%) | 1.52 | (Li and Zhuang 2000; Tan et al. 2012) | ||
| Semen juglandis | 20 | 1(5%) | 0.58 | 0 | 0 | (Guo et al. 2012; Li et al. 2014) | |||||
| Rhizoma bletillae | 11 | 1(9%) | 8 | 1(9%) | 1(9%) | 1 | 1(100%) | 8 | 1(100%) | (Guo et al. 2012; Xie 2016) | |
| Colla corii asini | 10 | 2(20%) | 1.12–2.85 | 1(10%) | 0 | 10 | 2(20%) | 2.04–2.85 | (Li et al. 2017b) | ||
| Radix Puerariae | 40 | 5(13%) | 0.751–9.9 | 3(8%) | 1(3%) | 40 | 5(13%) | 0.751–13.5 | 3(8%) | 2(5%) | (Han et al. 2012; Wang et al. 2014; Chien et al. 2018) |
| Fritillaria | 29 | 2(7%) | 1.6–10.06 | 1(3%) | 1(3%) | 29 | 2(7%) | 2.5–10.06 | 1(3%) | 1(3%) | (Han et al. 2012; Wang et al. 2013; Chien et al. 2018) |
| Radix Notoginseng | 41 | 9(22%) | 2.64–273.93 | 9(22%) | 6(15%) | 41 | 9(22%) | 2.64–267.95 | 6(15%) | 4(10%) | (Hao et al. 2012; Chen et al. 2015; Ying et al. 2018) |
| Hibiscus sabdariffa | 28 | 1(4%) | 3.11 | 1(4%) | 0 | 28 | 1(4%) | 3.11 | 0 | 0 | (Liu et al. 2018) |
Figure 1.
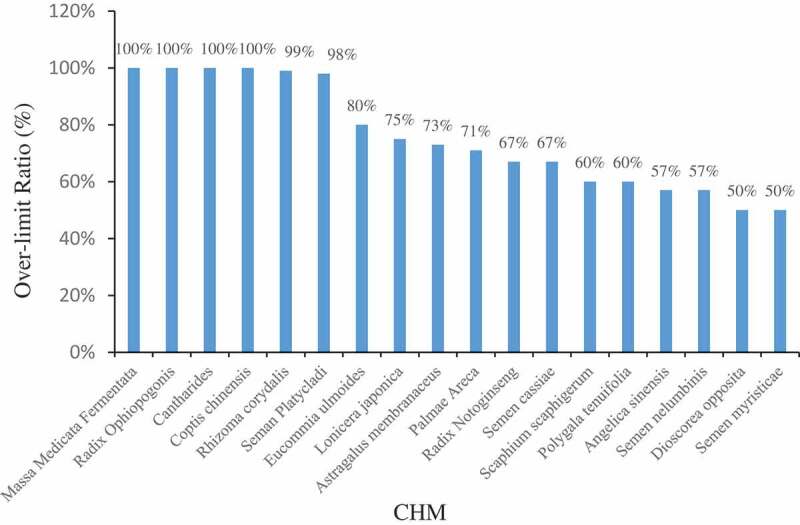
CHM with AFB1 exceeding the standard rate of 50%Note: AFB1 limit standard was 5 μg∙kg−1.
CHMs can be simultaneously contaminated by AFB1 and other AFs such as AFB2, AFG1, AFG2, and AFM1. By analysing 2734 batches of CHM samples, the simultaneous occurrence of multiple AFs (AFB1 + AFB2 + AFG1 + AFG2 + AFM1) was detected to be 30% (Figure 2). Of the positive samples, there were 378 batches that exceeded the limit set by the EU and China (10 μg∙kg−1). In the case of botanicals, the incidence of AFB1 is higher than that of other AFs. However, AFG2 is the most prevalent AF contaminant found in certain herbal materials such as Codonopsis Pilosula, with contamination level as high as 471 μg∙kg−1 (Tan et al. 2012).
Figure 2.
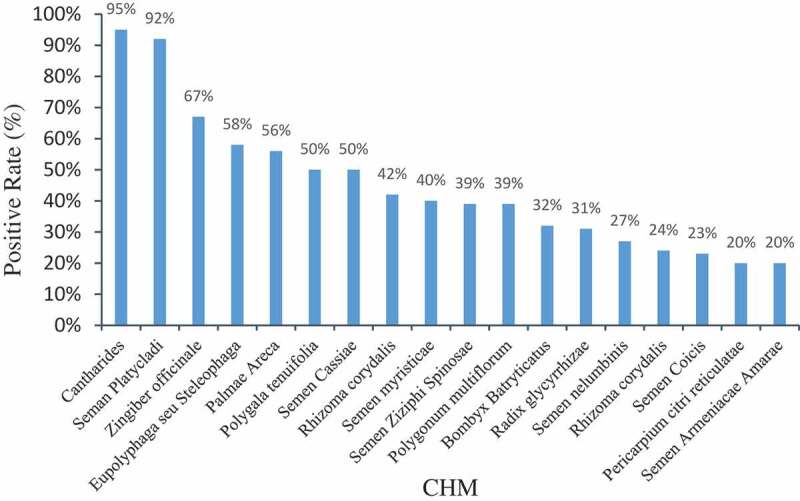
CHM with high AFs positive rate.
It is worth noting that among the 36 batches of animal material Eupolyphagaseu Steleophaga tested, 17 of 36 (47%) and 21 of 36 (58%) samples were found to be contaminated with AFB1 or multiple AFs, respectively. The occurrence rate of various AFs in different types of animal materials was not uniform. For example, AFG1 was the most commonly detected AF in the Eupolyphagaseu Steleophaga samples, with both a high occurrence rate and contamination level (Yang et al. 2011c; Liu et al. 2017; Sun et al. 2017; Luo et al. 2018), while in Cantharides, AFB1 is the most prevalent AF contaminant found (Sun and Liu 2016).
In addition, by analysing 66 types of CHMs, we found that the sample types most susceptible to AF contamination belong to different medicinal parts, including roots, rhizomes, fruits, and seeds (Figure 3). In addition, contamination of flower medicinal materials such as Lilium brownii (Zheng et al. 2014c) and Lonicera japonica (Cai et al. 2010) by AFB1 was detected at levels of 1.0 μg∙kg−1 and 50 μg∙kg−1, respectively. Lonicera japonica was easily contaminated by AFG2, and the contamination rate was 66.67%, with a highest detected contamination level of 203 μg∙kg−1 (Tan et al. 2012).
Figure 3.
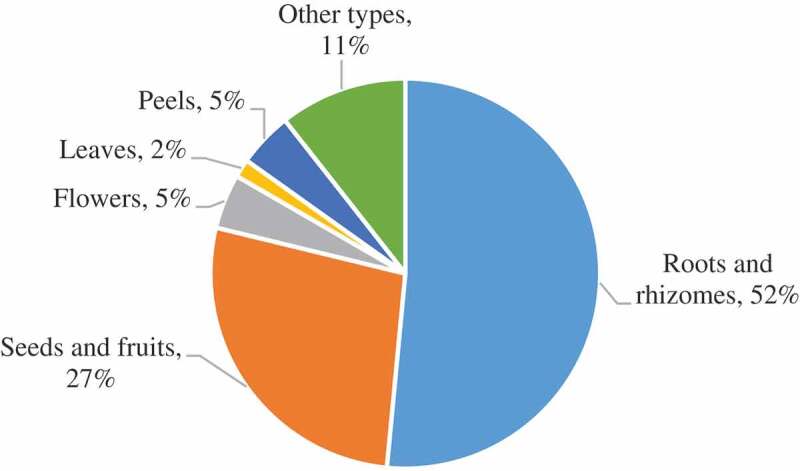
Detection of AFs in 66 CHM with different medicinal parts.
3. Ochratoxin
Ochratoxin is a type of mycotoxin mainly produced by Aspergillus ochraceus, P. verrucosum, and A. carbonarius. Ochratoxin A, ochratoxin B, ochratoxin C, and ochratoxin D are the main varieties of ochratoxins (Li and Ji 2003). Among the families of ochratoxin that have been discovered, ochratoxin A (OTA) is considered to be second after AFs in terms of prevalence and potential health hazards. OTA is carcinogenic, teratogenic, neurotoxic, and exposure can also result in hepatotoxicity and nephrotoxicity. Therefore, OTA is a mycotoxin and is classified as a Class IIB carcinogen by the International Agency for Research on Cancer (IARC) (Shu et al. 2008).
3.1. Regulatory guidelines for OTA levels
In the case of medicinal plants, the EU has official regulations on the level of OTA allowed in nutmeg, ginger, turmeric, black and white pepper, and liquorice root and its extract, with the legal limit varying from 15 μg∙kg−1 to 80 μg∙kg−1 (European Union 2006). In Vietnam, the limit for OTA levels ranges from 20 μg∙kg−1 to 80 μg∙kg−1 (United States Department of Agriculture Foreign Agricultural Service 2013) (Table 3).
Table 3.
Limit of OTA for medicinal plants in Standards and Regulations.
| Standards and regulations | Product (Group) | OTA (µg∙kg−1) |
Reference |
|---|---|---|---|
| Vietnam | Nutmeg | 30 | (United States Department of Agriculture Foreign Agricultural Service 2013) |
| Ginger and turmeric | 20 | ||
| Black and white pepper | 80 | ||
| Liquorice root used for herbal tea | |||
| Liquorice extract for beverage or to mix | |||
| EU | Nutmeg | 15 | (European Union 2006) |
| Ginger | |||
| Turmeric | |||
| White and black pepper | |||
| Liquorice root, ingredient for herbal infusion | 20 | ||
| Liquorice extract, for use in food in particular beverages and confectionary | 80 |
3.2. Detection methods for OTA
As described in previous studies, methods for detection of OTA include TLC, HPLC, ELISA, and GICA. Currently, HPLC-based methods are most commonly used for detection of OTA, with HPLC-MS/MS utilised often (Chen et al. 2011; Kuang and Qiu 2012b). In 2010, Yang et al. (2010) established the first HPLC-FLD method for detecting and quantifying OTA contamination levels in CHMs in China. Since then, HPLC-FLD has been routinely used to determine OTA levels in herbal medicines (Kuang et al. 2012a; Li 2015b). In 2010, Wu et al. also developed an HPLC with an ELSD (HPLC-ELSD) method for detection and quantification of OTA in CHMs (Wu et al. 2011a). The level of OTA present in 30 herbal medicines was determined via IAC sample purification, with a limit of detection (LOD) of 0.5 ng·g−1 and a recovery of 89.8%~94.6%.
Ultra-high performance liquid chromatography (UPLC) has also been successfully applied for analysing OTA levels in CHM (Cao et al. 2013; Yang et al. 2014b). A UPLC-based approach is more suitable for high-throughput detection of complex trace mixtures, since UPLC has the advantages of high sensitivity, high resolution, and a relatively short separation period (Zhang et al. 2018c).
Biological methods have been utilised as approaches for detection and quantification of OTA in CHMs. In 2015, Wang established a colloidal gold immunochromatographic method for rapid detection of OTA, and this approach is sensitive as low as 5 ng·mL−1 (Wang 2015). Zhou et al. developed an aptamer-based lateral flow strip relying on a competitive format that allows for rapid detection of OTA in Astragalus membranaceus (Zhou et al. 2016). After optimising some parameters, the aptamer-based assay demonstrated a visual LOD of 1 ng·mL−1. In the same year, Xiao et al. (2016) developed a rapid method for the detection of OTA in malt samples that is based on the indirect competition principle and flow microsphere technology.
3.3. OTA contaminants in CHMs
A total of 303 batches of Chinese herbal medicines (including 15 types of CHMs) were contaminated with OTA, with a per cent positive rate of 26% and a contamination range of 0.010–158.7 μg∙kg−1. Nineteen per cent of samples exceeded the EU set limit for OTA among the positive samples (Table 4). In the case of one type of CHM, OTA occurred in 4 out of 5 batches of Glycyrrhiza uralensis samples (Yang et al. 2010), and the highest contamination value was 84.4 μg∙kg−1.
Table 4.
Detection of OTA in CHM.
| OTA |
|||||
|---|---|---|---|---|---|
| CHM | Total samples | Positive samples No (%) |
Range (µg∙kg−1) |
>EU Legal limit No (%) |
Reference |
| Glycyrrhiza uralensis | 48 | 21(44%) | 0.010–94.7 | 5(10%) | (Yang et al. 2010; Wei et al. 2011; Wang et al. 2013; Wei et al. 2013) |
| Semen Armeniacae Amarum | 10 | 1(10%) | 0.7 | 0 | (Zheng et al. 2014c) |
| Semen Pruni Persicae | 10 | 1(10%) | 34.9 | 1(10%) | (Zheng et al. 2014a) |
| Semen Plantaginis | 10 | 3(30%) | 0.5–38.4 | 2(20%) | (Zheng et al. 2014c) |
| Fructus Hordei Germinatus | 32 | 4(13%) | 1.14–10.7 | 0 | (Liu et al. 2013; Zheng et al. 2014c; Wang 2016) |
| Fructus oryzae germinatus | 9 | 2(22%) | 1.7–7.9 | 0 | (Zheng et al. 2014c) |
| Radix Ginseng | 10 | 10(100%) | 0.04–5.86 | 0 | (Bao et al. 2008) |
| Zingiber Officinale Roscoe | 30 | 23(77%) | 0.02–20.66 | 3(10%) | (Bao et al. 2008; Cao 2013) |
| Astragalus Membranaceus | 3 | 3(100%) | 87.7–158.7 | 3(100%) | (Yang et al. 2010) |
| Massa Medicata Fermentata | 2 | 1(50%) | 2.4 | 0 | (Yang et al. 2010) |
| Radix Notoginseng | 33 | 1(3%) | 1.7 | 0 | (Yang et al. 2010; Chen et al. 2015) |
| Gossypium hirsutum Linn. | 1 | 1(100%) | 27.1 | 1(100%) | (Yang et al. 2010) |
| Alpinia oxyphylla | 44 | 1(2%) | 6.59 | 0 | (Zhao et al. 2017) |
| Polygonum Multiflorum | 41 | 6(15%) | 0.66–3.35 | 0 | (Li et al. 2016) |
| Radix Paeoniae alba | 20 | 1(5%) | 0.53 | 0 | (Xing et al. 2016) |
Roots, rhizomes, seeds, and the fruit of medicinal materials were susceptible to ochratoxin contamination, not unlike AF contamination in CHMs (Figure 4). The flower-based medicinal materials such as Lilium brownie (Zheng et al. 2014c) and Urena lobate (Yang et al. 2010), were found to be contaminated with OTA, and the contamination levels detected were 2.2 μg∙kg−1 and 1.7 μg∙kg−1, respectively.
Figure 4.
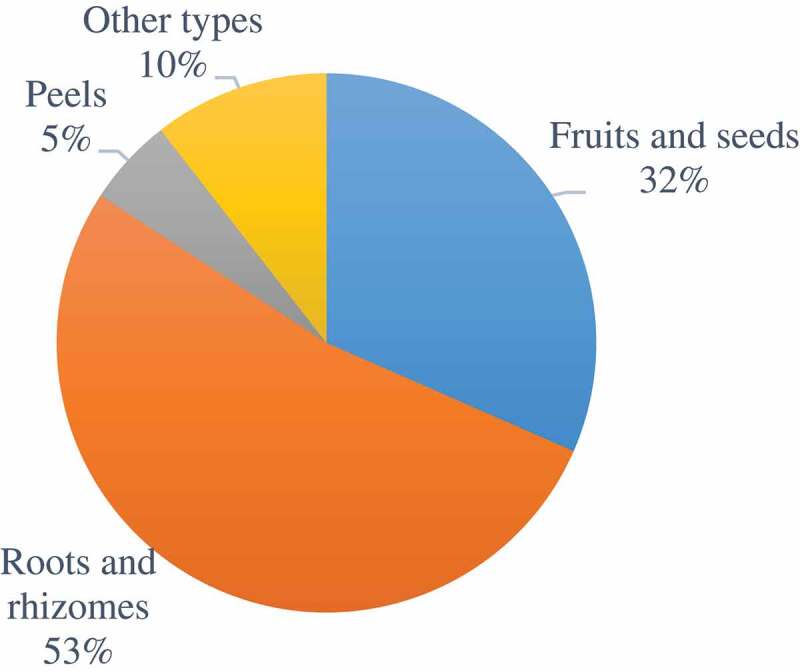
Detection of OTA in 19 CHM with different medicinal parts.
4. Zearalenone
Zearalenone (ZEN) is an oestrogen-like mycotoxin produced mainly by Fusarium graminearum and F. oxysporum. Studies have shown that ZEN is a reproductive toxin and that exposure to ZEN has teratogenic effects. At concentrations of 1 nmol∙L−1–10 nmol∙L−1, ZEN can stimulate the transcription of oestrogen receptors and affect cell division and growth (Deng and Yuan 2007). ZEN is also able to cause DNA damage, inhibit protein and DNA synthesis, and interfere with the cell cycle to block DNA replication and inhibit cell proliferation; high doses of ZEN can induce damage to the immune system as well (Jiang et al. 2011).
4.1. Detection methods of ZEN
There are relatively few studies concerning the detection of ZEN in CHMs. At present, HPLC/MS/MS method is often used to analyse ZEN levels in herbal medicines (Tan et al. 2012; Zheng et al. 2014a).
Zhang et al. (2012) detected and quantified ZEN in 107 CHM samples using an HPLC with DAD (HPLC-DAD) method. Compared with an HPLC-FLD-based approach, this method has decreased sensitivity but can provide the chromatogram of ZEN, and also obtain the spectrogram of ZEN in positive samples. HPLC-FLD cannot obtain the spectrogram of a positive sample, which gives HPLC-DAD the advantage of increased ability to avoid detection of false positives. Wu et al. (2011b) proposed an HPLC-ELSD method, which could provide a convenient and reliable alternative to commonly used HPLC-FLD methods for the rapid determination of ZEN, as it uses a relatively simple, safe, fast, and cost-effective means for sample purification.
4.2. ZEN contaminants in CHMs
A study of the prevalence of ZEN contamination in 105 different CHMs revealed that 41 of them were contaminated by ZEN (per cent positive rate was 39%), and level of ZEN contamination ranged from 0.2 to 931.07 μg∙kg−1 (Table 5). Some reports have shown that seed fruits such as S. Coicis (Yang et al. 2011c; Kong et al. 2013) and S. Persicae (Han 2011) are easily contaminated by ZEN; in the case of S. Coicis, the ZEN-positive detection rate is as high as 83%. ZEN has been detected as a contaminant in roots, rhizomes, leaves, and in the case of one study, in the cortex of the herb Juniperus procumbens (Han et al. 2012), at a level of 2.3 μg∙kg−1. A very high level of ZEN was reportedly detected in Cistanche tubulosa, but due to the small number of samples, this finding bears additional exploration (Yang et al. 2011c).
Table 5.
Detection of ZEN in CHM.
| CHM | ZEN |
Reference | ||
|---|---|---|---|---|
| Total samples | Positive samples No (%) |
Range (µg∙kg−1) |
||
| Semen Coicis | 18 | 15(83%) | 23.3–931.07 | (Yang et al. 2011c; Kong et al. 2013) |
| Alpinia oxyphylla | 44 | 2(5%) | 9.03–16.03 | (Zhao et al. 2017) |
| Radix Paeoniae alba | 27 | 13(48%) | 0.7643–4.81 | (Qin 2011; Xing et al. 2016) |
| Folium Isatidis | 5 | 2(40%) | 4.9958–20.1198 | (Qin 2011) |
| Rhizoma Corydalis | 1 | 1(100%) | 1.4 | (Yang et al. 2011c) |
| Massa Medicata Fermentata | 1 | 1(100%) | 0.2 | (Yang et al. 2011c) |
| Cistanche tubulosa | 1 | 1(100%) | 271 | (Yang et al. 2011c) |
| Semen Pruni Persicae | 2 | 2(100%) | 1.7–4.4 | (Han 2011) |
| Semen Armeniacae Amarum | 1 | 1(100%) | 2.9 | (Han 2011) |
| Polygonum Multiflorum | 2 | 1(50%) | 1.1 | (Han 2011) |
| Radix Scutellariae | 2 | 1(50%) | 2.1 | (Han 2011) |
| Lygodium japonicum | 1 | 1(100%) | 10.3 | (Han 2011) |
5. Other mycotoxins
Although AFs and OTA are the most commonly reported mycotoxin contaminants, occurrence of other mycotoxins such as fumonisins, trichothecenes, citrinin, and patulin has also been described in CHMs.
Fumonisin is a type of secondary metabolite produced by F. oxysporum and includes the A, B, C, P and FB1 derivatives. In 1993, it was classified as a Class B carcinogen by the International Agency for Research on Cancer. Fumonisin mainly damages the heart, liver, lungs, kidneys and other organs of animals, and exposure to fumonisin can result in porcine pulmonary oedema, liver damage, cardiovascular disease, and equine leukoencephalomalacia. In addition, exposure to fumonisin may cause human oesophageal cancer and neural tube defects (Yang et al. 2014a).
Trichothecenes is a class of secondary metabolites produced by different Fusarium species, such as F. graminearum and F. serrata; compounds in this group include T-2 toxin, deoxynivalenol (DON), nivalenol (NIV), diacetoxyscirpenol (DAS), and its derivatives (Yue 2009). Studies have shown that T-2 toxin is one of the most toxic mycotoxins among the type-A trichothecene mycotoxins. T-2 toxin can inhibit the synthesis of cellular proteins, DNA and RNA, trigger DNA damage via oxidative stress, induce apoptosis, alter gene expression patterns, and damage the cell membrane. T-2 toxin can also cause pathological changes in liver tissue and damage to the immune system (Zhou et al. 2011). DON, also called vomiting toxin, is highly cytotoxic, induces apoptosis, inhibits proliferation of immune cells, induces cytokine production from helper T-cells, and activates macrophages and T-cells, resulting in additional cytokine production (Huo et al. 2008).
Citrinin is a mycotoxin produced by filamentous fungi including Penicillium, Aspergillus, and Monascus. As a nephrotoxin, citrinin exposure can cause kidney disease in a variety of animals such as dogs, pigs, rats, chickens, and birds. Citrinin exposure can also induce mutations and result in deformities and tumours (Li et al. 2011b). Furthermore, the effects of citrinin can synergise with other mycotoxins (such as ochratoxin and patulin) to inflict more deleterious effects to tissues and organs (Liu and Xu 2004).
Patulin is a genotoxic compound that has been found to have broad toxicity and exposure to patulin can cause a variety of symptoms in humans and animals, including nausea, vomiting, blood in the stool, convulsions, and coma (Zhou et al. 2010). In addition, patulin exposure can result in acute and subacute poisoning. Furthermore, exposure to patulin has been reported to have cytotoxic, teratogenic, carcinogenic, and immunotoxic effects.
5.1. Detection methods for other mycotoxins
Fumonisin is currently detected using HPLC-MS/MS. In 2011, the method for simultaneously detecting fumonisin B1 (FB1) and fumonisin B2 (FB2) in 34 types of CHMs was developed by Xie et al. (2011). An immunoaffinity column was used to purify samples and the detection limit for FB1 and FB2 with this approach was 2 μg∙kg−1.
A method to detect T-2 toxin contamination in CHMs using GC with ECD (GC-ECD) was first proposed by Yue et al. (2009). In order to improve the selectivity and sensitivity of the method, sample clean-up was performed using an immunoaffinity column, and N-(heptafluoro-n-butyl) imidazole (HFBI) was then used for pre-column derivatisation. The per cent recovery of starting material ranged from 82.2% to 98.6%, and the LOD was 2.5 μg∙kg−1. Subsequently, the same authors established a method to detect DON in CHMs and related products using GC-ECD. Application of this approach showed that the per cent recovery of various CHM starting material ranged from 85.5% to 97.2%, the detection limit for DON with the method was 2.0 μg∙kg−1. This is the first report on the detection of DON contamination in CHMs and related products (Yue et al. 2010a).
In 2011, Wang et al. (2011) detected patulin in Fructus Aurantii by HPLC-MS/MS. A few years later, Zhou et al. (2015) established HPLC-DAD method for the analysis of patulin in F. Crataegi. In this study, a home-made solid-phase extraction (SPE) column was prepared using self-made poly-vinylpyrrolidone-Flory silica (PVPP-F) as sorbent for sample pre-treatment, and the detection limit of the method was 3.56–3.99 μg∙kg−1.
5.2. Other mycotoxin contaminants in CHMs
Mycotoxins such as FB, T-2 toxin, and DON have been successfully detected in CHMs (Table 6). For example, the fruit and seeds of CHMs such as S. Sterculiae Lychnophorae and S. Coicis are susceptible to fumonisin B contamination.
Table 6.
Detection of other mycotoxins.
| CHM | Detection Methods | Maximum Contamination Values of Other Toxins (μg∙kg−1) |
References | |||
|---|---|---|---|---|---|---|
| FB1 | FB2 | T-2 | DON | |||
| Semen Armeniacae Amarum | UPLC-MS/MS | 0.89 | 1.65 | (Han 2011) | ||
| Radix Paeoniae alba | UPLC-MS/MS | 0.69 | (Han 2011) | |||
| Mangnolia Officinalis | HPLC-MS/MS | 397 | 793 | (Xie et al. 2011) | ||
| Astragalus Membranaceus | HPLC-MS/MS | 158 | (Xie et al. 2011) | |||
| Radix Puerariae | HPLC-MS/MS | 1.095 | (Chen 2012) | |||
| Polygonum Multiflorum | UPLC-MS/MS | 2.57 | 1643.2 | 1.93 | (Li et al. 2016) | |
| Semen Pruni Persicae | UPLC-MS/MS HPLC-MS/MS | 82.3 | 18.9 | 803.4 | (Han 2011; Zheng et al. 2014a) | |
| Fructus Forsythiae | HPLC-MS/MS | 29.4 | 7.8 | (Ge et al. 2011) | ||
| Scutellaria Baicalensis | HPLC-MS/MS | 6.7 | 208 | (Xie et al. 2011) | ||
| Panax Notoginseng | UPLC-MS/MS | 0.258 | (Chen et al. 2015) | |||
| Semen Sterculiae Lychnophorae | HPLC-MS/MS | 125 | 2240 | (Xie et al. 2011) | ||
| Semen Coicis | HPLC-MS/MS | 562 | 167 | (Xie et al. 2011) | ||
| Lysimachia nummularia | UPLC-MS/MS | 2.50 | 1.25 | (Han 2011) | ||
| Radix Asparagi Cochinchinensis | HPLC-MS/MS | 79.4 | 173 | (Xie et al. 2011) | ||
| Radix Isatidis | HPLC-MS/MS | 23.8 | 126 | (Xie et al. 2011) | ||
| Medicinal Fermented Mass | HPLC-MS/MS | 113 | 90 | (Xie et al. 2011) | ||
| Rhizoma Dioscoreae | UFLC-MS/MS | 3.727 | (Li 2016) | |||
| Folium Isatidis | UPLC-MS/MS | 3.80 | 0.78 | (Han 2011) | ||
| Radix Salviae Miltiorrhizae | UPLC-MS/MS | 0.3 | (Han 2011) | |||
| Lonicera Japonica | UPLC-MS/MS | 0.2 | (Han 2011) | |||
| Radix Paeoniae Rubra | UPLC-MS/MS | 0.4 | (Han 2011) | |||
Xie et al. analysed 34 types of CHM samples and found 11 fumonisin-positive samples, with fumonisin concentrations ranging from 82.4 to 2349 μg∙kg−1 (Xie et al. 2011). In the same year, the contamination level of FB1 and FB2 in some CHMs was determined by Han (2011). The analysis revealed that the range of FB2 in S. Sterculiae Lychnophorae was 928–2240 μg∙kg−1, and the highest detected levels of FB1 and FB2 in S. Coicis were 562 μg∙kg−1 and 167 μg∙kg−1, respectively. Notably, it was found that FB1 and FB2 were usually detected in samples together, although the contamination levels for the two mycotoxins were rarely similar. For example, the incidence of FB2 contamination in the roots and rhizomes of Polygonum multiflorum (Li et al. 2016) was as high as 1643.2 μg∙kg−1, while FB1 was only detected at a level of 2.57 μg∙kg−1. Occurrence of T-2 and DON contaminations has been reported in several CHMs to date. Zheng et al. analysed mycotoxin content in the fruit and seeds of the CHMs S. PruniPersicae and S. Coicis. The results showed that the highest level of DON detected in S. PruniPersicae was 803.4 μg∙kg−1, but DON was not detected in the S. Coicis; T-2 was not detected in either case (Zheng et al. 2014c). The highest level of T-2 detected in the rhizomes of CHMs such as R. Paeoniae alba (Han 2011), R. Salviaemiltiorrhizae, and R. Notoginseng (Chen et al. 2015) was less than 0.7 μg∙kg−1, indicating that at least some CHMs are not easily contaminated by T-2.
6. Detection of multiple mycotoxins
There are often more than one type of mycotoxin contaminants present in CHMs. Thus, it is important to consider the possibility of and test samples for multi-mycotoxin contaminants. For example, fruit and seeds from CHMs such as S. Armeniacae Amarum (Cai et al. 2010; Han 2011; Han et al. 2011; Zheng et al. 2013; Zheng et al. 2014c; Zhao et al. 2016) S. Coicis (Cai et al. 2010; Zheng et al. 2010b; Xie et al. 2011; Kong et al. 2013; Liu et al. 2013), S. Persicae (Cai et al. 2010; Han et al. 2010; Zheng et al. 2010b; Han 2011; Han et al. 2011; Li et al. 2011a; Zheng et al. 2014a) and S. Sterculiae Lychnophorae (Cai et al. 2010; Xie et al. 2011; Su et al. 2014) are not only susceptible to AF contaminants but are also often co-contaminated with other mycotoxins such as OTA, ZEN, and FB.
Along with a variety of methods for detecting different mycotoxins, methods for simultaneous determination for various mycotoxins have been gradually developed. AFB1 and OTA contaminants in CHMs can be detected together by HPLC-FLD, with use of a composite immunoaffinity column for sample cleanup (Wei et al. 2011; Cao et al. 2013). Furthermore, simultaneous detection of DON and NIV in CHMs by HPLC-UV was first proposed by Yue et al. (2010b). The sample pre-treatment procedure used in this work abolished the derivatisation step used in the conventional approach to yield improved results. Several years later, Kong et al. (2012) developed a method for simultaneously measuring T-2 and HT-2 toxins in CHMs.
With the spread of modern MS technology, new methods for the combined detection of mycotoxins with large chemical diversity continue to be developed and applied. At present, it has been demonstrated that up to 35 different toxins can be detected from an herbal matrix in a single HPLC-MS/MS run (Han et al. 2012).
7. Conclusion
At present, mycotoxin contaminants have become some of the most prevalent hazardous substances in CHMs and a major public safety concern regarding their sale and use. In this review, we summarised some common mycotoxin contaminants found in medicinal materials and discussed methods for their detection. Mycotoxin contamination is usually heterogeneous, so screening methods for detecting these contaminants in medicinal products need to have broad coverage across, and samples should be processed carefully (Zhang et al. 2018a; Tittlemier et al. 2019). However, many existing studies of mycotoxin contamination lack detailed descriptions of how samples are selected and processed. To guarantee an accurate snapshot of any existing mycotoxin contaminants, careful considerations need to be taken with regard to sample acquisition and processing. Another complication in collecting accurate data lies in that the rapid detection methods such as ELISA and GICA are more prone to false negatives or false positives than conventional detection methods. Therefore, further validation should be performed on any significant findings that rely on rapid detection methods. In addition, the mycotoxin contamination is a known problem in Chinese herbal medicines. For example, S. Platycladi and S. Ziziphi Spinosae are prone to aflatoxin contamination, Glycyrrhiza uralensis and Zingiber Officinale Roscoe are prone to OTA contamination, and ZEN contamination is prevalent in S. Coicis and R. Paeoniae alba. In some medicines, such as S. Persicae and Polygonum multiflorum, co-occurrence of multiple mycotoxin contaminations had been detected.
Although some producers of CHMs currently have some standards in place for monitoring the levels of mycotoxins such as AFs and OTA, standardised guidelines regarding monitoring for other mycotoxins and their levels have not been established for CHMs. Therefore, it is necessary to conduct additional research to better understand which CHMs are easily contaminated by which mycotoxins. This information can then be used to establish guidelines for screening for mycotoxin contaminants and limitations on acceptable levels in CHMs.
Rapid analytical methods for mycotoxin detection are currently under development and are increasingly utilised by CHM producers. In recent years, standard biological analysis methods have been utilised for detection of mycotoxins in CHMs; such methods include the GCIA and ELISA approaches. Novel technologies such as ultrasensitive mycotoxin biosensors have been developed and utilised for mycotoxin screening in food and serum. For example, Taghdisi et al. (Taghdisi et al. 2016) developed a fluorescent aptamer sensor (aptasensor) that allowed for selective and sensitive detection of OTA in grape juice and serum. Since then, this group has proposed another accurate fluorescent sensing method for the determination of AFB1 in grape juice and human serum samples based on a hairpin structure of a G-quadruplex oligonucleotide-aptamer chimera (Taghdisi et al. 2018). A highly sensitive aptasensor utilising the fluorescence resonance energy transfer for AFM1 detection in milk samples was recently developed (Li et al. 2017a). Another group established a surface plasmon resonance (SPR) method using an SPR sensor chip for simultaneous detection of AFB1, OTA, ZEN, and DON in corn (Wei et al. 2019). However, broad application of these recently developed mycotoxin detection methods in CHMs requires further validation.
In recent years, studies on the presence of mycotoxin contaminants in CHMs have mainly focused on identifying the varieties of mycotoxins present, determining the contamination level, and refining mycotoxin detection methods. In contrast, relatively few studies have examined these mycotoxin contaminations in the context of toxigenic mechanisms, detoxification techniques, and prevention and control measures. The presence of specific mycotoxin contaminants and their relative abundance in medicinal materials is intimately related to the place of origin, processing methods, and storage conditions. Therefore, future studies should focus on investigating the occurrence of mycotoxin contamination in CHMs in various storage conditions, and findings from these studies can be used to help establish an efficient prevention strategy to minimise the presence of fungi and mycotoxin contaminants in CHMs.
Acknowledgements
This work was supported by National Key R&D Program of China (2016YFE0112900), CAMS Innovation Fund for Medical Sciences (2017-I2M-1-013).
Funding Statement
This work was supported by the National Key R&D Program of China [2016YFE0112900];CAMS Innovation Fund for Medical Sciences [2017-I2M-1-013].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bao L, Jin Y, Tian J, Zhang Y, Liang C.. 2008. Determination of total aflatoxins B1, B2, G1, G2 and ochratoxins a in ginseng and ginger by muli-toxin immunoaffinity column clean-up and liquid chromatographic quantitation. Inspection Quarantine Sci. 18(4):37–40. [Google Scholar]
- British Pharmacopoeia Commission . 2012. British Pharmacopoeia 2013 appendix XI S. London (UK):Determination of Mycotoxins in Herbal Drugs; Stationery Office. [Google Scholar]
- Cai F, Gao WW, Li HL, Chen J, Li ZZ.. 2010. Pollution status and control technology of aflatoxin in traditional Chinese medicine. China J Chin Mater. 35(19):2503–2507. [PubMed] [Google Scholar]
- Cao JL. 2013. Analysis of aflatoxins and ochratoxin A in traditional Chinese medicine, Bruceae Fructus and Zingiberis Rhizoma Recens. Chengdu: Chengdu University of TCM. [Google Scholar]
- Cao JL, Zhou SJ, Kong WJ, Yang MH, Wan L, Yang SH. 2013. Molecularly imprinted polymer-based solid phase clean-up for analysis of ochratoxin A in ginger and LC-MS/MS confirmation. Food Control. 33(2):337–343. [Google Scholar]
- Chen AJ, Huang LF, Wang LZ, Tang D, Cai F, Gao WW. 2011. Occurrence of toxigenic fungi in ochratoxin A contaminated liquorice root. Food Addit Contam. 28(8):1091–1097. [DOI] [PubMed] [Google Scholar]
- Chen L. 2012. Chromatography-mass spectrometry analysis of pesticide and mycotoxins residues in traditional Chinese medicine. Hangzhou: Zhejiang University. [Google Scholar]
- Chen Y, Chen CJ, Li J, Luan LJ, Liu XS, Wu YJ. 2015. Determination of 10 mycotoxin contaminants in Panax notoginseng by ultra performance liquid chromatography-tandem mass spectrometry. Acta Pharm Sin. 50(1):81–85. [PubMed] [Google Scholar]
- Chien MY, Yang CM, Huang CM, Chen CH. 2018. Investigation of aflatoxins contamination in herbal materia medica in a Taiwan pharmaceutical factory. J Food Drug Anal. 26(3):S1021949818300450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission . 2015. Chinese Pharmacopoeia 2015 edition. Vol. IV, Beijing (China): Chinese Medicine Science and Technology Press. [Google Scholar]
- Chu XF, Dou XW, Kong WJ, Yang MH, Zhao C, Zhao M, Ou-yang Z. 2015. Contamination level of aflatoxin B1 in lotus seeds rapid screening by indirect competitive ELISA method. China J Chinese Materia Medica. 40(4):704–709. [PubMed] [Google Scholar]
- Deng YT, Yuan H. 2007. Advances in research on toxicity mechanism of zearalenone. J Prog Vet Med. 28(2):000089–000092. [Google Scholar]
- Department of Health, Hong Kong Special Administrative Region of the People’s Republic of China . 2005. Standards for Chinese herbal medicine in Hong Kong. Vol. 1, Hong Kong: Hong Kong Chinese Medicine Press. [Google Scholar]
- European Pharmacopoeia Commission . 2016. Determination of aflatoxin B1 in herbal drugs. In: European Pharmacopoeia. 9th ed. 2.8.2018 Strasbourg (France): Council of Europe. Vol. 1, 289. [Google Scholar]
- European Union . 2006. Commission regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union. L 364:5–24. [Google Scholar]
- Food and Agriculture Organization of the United Nations . 2004. Worldwide regulations for mycotoxins in food and feed in 2003. Rome (Italy):Food and Agriculture Organization of the United Nations. [Google Scholar]
- Ge BK, Zhao KX, Wang W, Bi JB. 2011. Determination of 14 mycotoxins in Chinese herbs by liquid chromatography-tandem mass spectrometry with immunoaffinity purification. Chin J Chromatogr. 29(6):495–500. [DOI] [PubMed] [Google Scholar]
- Government of Canada, Natural and Non-prescription Health Products Directorate . 2015. Health Canada, guidance documents-legislation and guidelines-natural health products, quality of natural health products guide. Ottawa (ON, Canda): Government of Canada, Natural and Non-prescription Health Products Directorate; p. 19–20. [Google Scholar]
- Guo LM, Duan Q, Liu T. 2015. Determination of aflatoxins B1, B2, G1 and G2 in Polygonum multiflorum by HPLC. Gansu Med J. 8:618–621. [Google Scholar]
- Guo QJ, Gao YL, Wang SH. 2012. Determination of aflatoxin G2, G1, B2 and B1 in 150 Chinese herbs by HPLC. China Pharm. 15(12):1696–1698. [Google Scholar]
- Han Z. 2011. Analytical methodology and metabolic dynamics of common mycotoxins in Chinese medicinal materials. Hangzhou: Zhejiang University. [Google Scholar]
- Han Z, Ren YP, Zhou HL, Luan LJ, Cai ZX, Wu YJ. 2011. A rapid method for simultaneous determination of zearalenone, α-zearalenol, β-zearalenol, zearalanone, α-zearalanol and β-zearalanol in traditional Chinese medicines by ultra-high-performance liquid chromatography–tandem mass spectrometry. J Chromatogr B. 879(5–6):411–420. [DOI] [PubMed] [Google Scholar]
- Han Z, Ren YP, Zhu JF, Cai ZX, Chen Y, Luan LJ, Wu YJ. 2012. Multianalysis of 35 mycotoxins in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry coupled with accelerated solvent extraction. J Agric Food Chem. 60(33):8233–8247. [DOI] [PubMed] [Google Scholar]
- Han Z, Zheng YL, Luan LJ, Cai ZX, Ren YP, Wu YJ. 2010. An ultra-high-performance liquid chromatography-tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in traditional Chinese medicines. Anal Chim Acta. 664(2):165–171. [DOI] [PubMed] [Google Scholar]
- Hao AY, Zhao LY, Liu YH, Wang G, Jin HY, Bi XL, Men QM. 2012. HPLC determination of aflatoxin residues in traditional Chinese medicine Yinpian with post column photochemical derivation and fluorescence detection. Chin J Pharm Anal. 12:2203–2207. [Google Scholar]
- Hao AY, Zhao LY, Liu YH, Wang G, Jin HY, Bi XL, Men QM. 2013. False positive research on HPLC determining aflatoxin residues in Chinese herbal pieces. Chin J Pharm Anal. 3:458–464. [Google Scholar]
- Hu SR, Dou XW, Zhang L, Xie YJ, Yang SH, Yang MH. 2018. Rapid detection of aflatoxin B1 in medicinal materials of radix and rhizome by gold immunochromatographic assay. Toxicon. 150:144–150. [DOI] [PubMed] [Google Scholar]
- Hu YC, Wan L, Fan CJ, Lv W, Yang F, Ji L. 2012. Investigation on detection of aflatoxins in Chinese materia medica and pharmaceutical intermediates microbiological contaminated by immunoaffinity column clean-up combined with post-column derivatization. Chin J Exp Traditional Med Formulae. 18(10):116–119. [Google Scholar]
- Huo XH, Zhao BY, Wan XP, Guo X, Wang JJ. 2008. Advances in research on toxicity of deoxynivalenol. J Toxicol. 22(2):151–154. [Google Scholar]
- Japanese Pharmacopoeia Commentary Editorial Committee . 2016. The Japanese Pharmacopoeia 17th edition (English version), analytical methods for aflatoxins in crude drug and crude drug preparations. Tokyo (Japan): Japanese Pharmacopoeia Commentary Editorial Committee; p. 2513–2515. [Google Scholar]
- Jiang SZ, Yang WR, Yang ZB. 2011. Metabolism, toxicity and preventive measures of zearalenone. J Anim Nutr. 23(2):196–202. [Google Scholar]
- Kong WJ, Li JY, Qiu F, Wei JH, Xiao XH, Zheng Y, Yang MH. 2013. Development of a sensitive and reliable high performance liquid chromatography method with fluorescence detection for high-throughput analysis of multi-class mycotoxins in coix seed. Anal Chim Acta. 799:68–76. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Zhang XF, Shen HH, Ou-yang Z, Yang MH. 2012. Validation of a gas chromatography-electron capture detection of T-2 and HT-2 toxins in Chinese herbal medicines and related products after immunoaffinity column clean-up and pre-column derivatization. Food Chem. 132(1):574–581. [DOI] [PubMed] [Google Scholar]
- Korean Food & Drug. 2012. Korean Pharmacopoeia 10th Edition (English Version), General Tests, Processes and Apparatu; Korean Food & Drug: Chungcheongbuk-do, Korea; pp. 1673–1675. [Google Scholar]
- Kuang PL. 2000. Inspection of AFB1 quantity in Chinese traditional medicines. Chin Traditional Pat Med. 22(7):478–479. [Google Scholar]
- Kuang Y, Qiu F, Kong WJ, Yang MH. 2012a. Natural occurrence of ochratoxin A in wolfberry fruit wine marketed in China. Food Addit Contam Part B. 5(1):70–74. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Qiu F, Yang MH. 2012b. Determination of ochratoxin A in wolfberry fruit wine with high performance liquid chromatography-tandem mass spectrometry. Chin J Public Health. 28(11):1520–1522. [Google Scholar]
- Li C, Su X, Feng WH, Li RR, Liu XQ, Li PY, Wang ZM. 2016. Simultaneous determination of 12 mycotoxins in Polygoni Multiflori Radix by UPLC-ESI-MS/MS combined with modified QuEChERS. China J Chinese Materia Medica. 41(23):4368–4374. [DOI] [PubMed] [Google Scholar]
- Li FQ, Ji R. 2003. Advances in research on relationship between ochratoxin A and human health. J Hyg Res. 32(2):172–175. [PubMed] [Google Scholar]
- Li H. 2015a. Determination method of aflatoxins B1, B2, G1, G2 in Chinese medicinal herbs. Chin Pharm. 10:57–59. [Google Scholar]
- Li H. 2015b. Determination of ochratoxin A content in Chinese medicinal materials by immunoaffinity column chromatography purification-HPLC. Chin Pharm. 12:62–64. [Google Scholar]
- Li H, Yang D, Li P, Zhang Q, Zhang W, Ding X, Wu J. 2017a. Palladium nanoparticles-based fluorescence resonance energy transfer aptasensor for highly sensitive detection of aflatoxin M1 in milk. Toxins. 9(10):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JF, Jiang H, Qiu HY, Zhu-ge L. 2017b. Detection of four aflatoxins in gelatin drugs by immunoaffinity column clean-up and HPLC-MS/MS. Chin Pharm J. 52(17):1542–1546. [Google Scholar]
- Li JM, Li C, Gu LH, Jiang YQ. 2011a. Determination of aflatoxins in fruit Chinese medicinal material by HPLC with post-column derivatization. Traditional Chin Drug Res Clin Pharmacol. 22(4):461–464. [Google Scholar]
- Li JM, Li C, Gu LH, Jiang YQ. 2012. Determination of aflatoxins in fruit traditional Chinese medicines by rapid-resolution liquid chromatography tandem mass spectrometry. Chin Pharm J. 47(1):65–68. [Google Scholar]
- Li MH. 2016. Research on simultaneous detection of multi-mycotoxins in traditional Chinese medicines and their related products. Zhenjiang: Jiangsu University. [Google Scholar]
- Li PW, Yang JE, Ma L, Yang CH, Zhang W, You F. 2005. Research progress on detection technology of aflatoxin B1 of grain and oil products. Chin J Oil Prod Sci. 27(2):77–81. [Google Scholar]
- Li W, Zhuang X. 2000. Determination of aflatoxin B1 in regular Chinese medicine by ELISA method. Chin Pharm Affairs. 14(2):101–102. [Google Scholar]
- Li WG, Xu KL, Xiao R, Yin GF, Liu WW. 2015. Development of an HPLC-based method for the detection of aflatoxins in Pu-erh tea. Int J Food Prop. 18(4):842–848. [Google Scholar]
- Li XP, Zhao YG, Chen XH, Pan SD, Jin MC. 2014a. Simultaneous determination of four aflatoxins in walnut kernels by dispersive solid phase extraction-ultra fast liquid chromatography-tandem mass spectrometry. Chin J Health Inspection. 18:2647–2650. [Google Scholar]
- Li Y, Qiu F, Yang MH, Ou-yang Z. 2011b. Determination of citrinin in traditional Chinese medicine by high performance liquid chromatography-tandem mass spectrometry. Chin J Pharm Anal. 31(9):1726–1730. [Google Scholar]
- Li YP, Huang YW, Liu BB, Shang QK. 2014b. Determination of aflatoxin in traditional chinese medicinal materials by post-column photochemical derivatization-HPLC. J Mol Sci. 30(3):226–231. [Google Scholar]
- Li YS, Chen JM. 2000. Determination of aflatoxin B1 in traditional Chinese medicine by ELISA. Chin Traditional Herbal Drug. 31(8):586–587. [Google Scholar]
- Liang YQ, Huang RF. 2000. Detection of the aflatoxin B1 in Chinese meteria medica. Chin J Mod Appl Pharm. 17(3):224–226. [Google Scholar]
- Liau BC, Jong TT, Lee MR, Chang CMJ. 2010. Supercritical fluid extraction and quantification of aflatoxins in Zizyphi Fructus by liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 21(5):667–673. [DOI] [PubMed] [Google Scholar]
- Lin FF, Zheng ZY, Huang J, Chen H. 2016. Rapid determination of aflatoxin B1 content in traditional Chinese medicine pieces. Jiangsu J Traditional Chin Med. 48(12):65–66,69. [Google Scholar]
- Liu LN, Jin HY, Sun L, Ma SC, Lin RC. 2012a. Determination of aflatoxins in medicinal herbs by high-performance liquid chromatography-tandem mass spectrometry. Phytochem Anal. 23(5):469–476. [DOI] [PubMed] [Google Scholar]
- Liu LN, Li YJ, Jin HY, Ma SH. 2017. Determination of aflatoxins in animal medicines by immunoaffinity column and HPLC-FLD with photochemical derivatization fluorescence detection. Chin Traditional Herbal Drugs. 48(6):1220–1224. [Google Scholar]
- Liu QT, Kong WJ, Guo WY, Yang MH. 2015. Multi-class mycotoxins analysis in Angelica sinensis by ultra fast liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 988:175–181. [DOI] [PubMed] [Google Scholar]
- Liu RR, Xu Y. 2004. A brief introduction of citrinin and study progress of its immunoassay methods. J Hyg Res. 33(1):124. [PubMed] [Google Scholar]
- Liu SY, Qiu F, Kong WJ, Wei JH, Xiao XH, Yang MH. 2013. Development and validation of an accurate and rapid LC-ESI-MS/MS method for the simultaneous quantification of aflatoxin B1, B2, G1 and G2 in lotus seeds. Food Control. 29(1):156–161. [Google Scholar]
- Liu SY, Qiu F, Yang MH. 2012b. Determination of aflatoxins in Nelumbinis Semen by immunoaffinity column clean-up and HPLC-FLD with on-line post-column photochemical derivatization and LC-MS/MS confirmation. China J Chinese Materia Medica. 37(3):305–309. [PubMed] [Google Scholar]
- Liu XF, Ying GY, Sun CN, Yang MH, Zhang L, Zhang SS, Xing X, Li Q, Kong W. 2018. Development of an ultrasonication-assisted extraction based HPLC with a fluorescence method for sensitive determination of aflatoxins in highly acidic Hibiscus sabdariffa. Front Pharmacol. 9:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CQ, Zhang ML, Zheng RS, Cai X, Xu H, Yun XY, Qin JL. 2018. Detection of aflatoxin contamination in 6 traditional Chinese animal medicines by LC-MS/MS. Chin J Exp Traditional Med Formulae. 3:67–71. [Google Scholar]
- Ma ZK, Zan LS. 2009. Advances in studies on hazards, detection methods and biodegradation of aflatoxins. Prog Vet Med. 30(9):91–94. [Google Scholar]
- Mahfuz M, Gazi MA, Hossain M, Islam MR, Fahim SM, Ahmed T. 2018. General and advanced methods for the detection and measurement of aflatoxins and aflatoxin metabolites: a review. Toxin Rev. 1–15. [Google Scholar]
- Ono EY, Ono MA, Funo FY, Medinal AE, Oliveira TC, Kawamura O, Ueno Y, Hirooka EY. 2001. Evaluation of fumonisin-aflatoxin co-occurrence in Brazilian corn hybrids by ELISA. Food Addit Contam. 18(8):719–729. [DOI] [PubMed] [Google Scholar]
- Qin XM. 2011. Preliminary study on fungal species and contaminating mycotoxins in nine medicinal materials. Beijing: Peking Union Medical College. [Google Scholar]
- Ran C, Chen D, Ma H, Jiang Y. 2017. Graphene oxide adsorbent based dispersive solid phase extraction coupled with multi-pretreatment clean-up for analysis of trace aflatoxins in traditional proprietary Chinese medicines. J Chromatogr B. 1044–1045:120–126. [DOI] [PubMed] [Google Scholar]
- Saha A, Gajbhiye NA, Basak BB, Manivel P. 2018. High-performance liquid chromatography tandem mass spectrometry for simultaneous detection of aflatoxins B1, B2, G1 and G2 in Indian medicinal herbs using QuEChERS-based extraction procedure. Int J Environ Anal Chem. 98(7):622–643. [Google Scholar]
- Shen QS, Zhou W, Mo HZ, Hu LB. 2016. Advance in research on aflatoxin contamination control. J Food Sci. 37(9):237–243. [Google Scholar]
- Shu Q. 2018. Optical immunoassays for the detection of exogenous harmful substances in traditional Chinese medicines. Chongqing: Southwest University. [Google Scholar]
- Shu XL, Shi QS, Ouyang YS, Chen YB. 2008. Production and identification of ochratoxin. Chin J Health Lab Technol. 18(10):2183–2185. [Google Scholar]
- Su JH, Zhang C, Zhong YS, Wang MZ. 2014. Quantitative analysis of aflatoxin in Sterculiae Lychnophorae Semen by HPLC-FLD after immunoaffinity column with post-column photochemical derivatization. Chin J Exp Traditional Med Formulae. 20(15):75–78. [Google Scholar]
- Sun L, Li J, Lin WJ, Hu MJ. 2017. Detection of aflatoxins in Eupolyphaga by immunoaffinity column clean-up and high performance liquid chromatography with triple quadrupole mass spectrometry. Chin J Health Lab Tec. 22:3232–3235. [Google Scholar]
- Sun R, Liu SC. 2016. Determination of Aflatoxin B1, B2, G1, G2 in Blister Beetle by iodine derivatization HPLC-fluorescence detector and determination of LC-MS. J Liaoning Univ TCM. 4:53–56. [Google Scholar]
- Taghdisi SM, Danesh NM, Beheshti HR, Ramezani M, Abnou K. 2016. A novel fluorescent aptasensor based on gold and silica nanoparticles for the ultrasensitive detection of ochratoxin A. Nanoscale. 8(6):3439–3446. [DOI] [PubMed] [Google Scholar]
- Taghdisi SM, Danesh NM, Ramezani M, Abnous K. 2018. A new amplified fluorescent aptasensor based on hairpin structure of G-quadruplex oligonucleotide-Aptamer chimera and silica nanoparticles for sensitive detection of aflatoxin B1 in the grape juice. Food Chem. 268:342–346. [DOI] [PubMed] [Google Scholar]
- Tan J, Zheng RS, Wang WL, Xu H. 2012. Simultaneous determination of aflatoxins and zearalenone in Chinese crude drugs by high performance liquid chromatography-tandem mass spectrometry. Lishizhen Med Mater Med Res. 23(10):2469–2472. [Google Scholar]
- Tang Y. 2000. Investigation and experimental study on the content of aflatoxin B1 in Chinese patent medicines and traditional Chinese medicines. China J Chin Mater Med. [Google Scholar]
- Tittlemier SA, Cramer B, Dall’Asta C, Iha MH, Lattanzio VMT, Malone RJ, Maragos C, Solfrizzo M, Stranska-Zachariasova M, Stroka J, et al. 2019. Developments in mycotoxin analysis: an update for 2017–2018. World Mycotoxin J. 12(1):3–29. [Google Scholar]
- Tripathy V, Basak BB, Varghese TS, Saha A. 2015. Residues and contaminants in medicinal herbs-a review. Phytochem Lett. 14:67–78. [Google Scholar]
- United States Department of Agriculture Foreign Agricultural Service . 2013. Vietnam: technical regulations on Mycotoxins and heavy metals MRLs in food; gain report number VM 3070. Washington (DC, USA): United States Department of Agriculture Foreign Agricultural Service; p. 2–4. [Google Scholar]
- United States Pharmacopeial Convention . 2017. USP herbal medicines compendium. Rockville (MD, USA):United States Pharmacopeial Convention. [Google Scholar]
- Wang F, Yi Y, Yang ZN, Luo SQ, Yu ZW, Ye J. 2012. Determination of aflatoxin from Citrus reticulata Blanco and its safety evaluation. Guangdong Agric Sci. 39(3):84–86. [Google Scholar]
- Wang LZ, Wang Z, Gao WW, Chen J, Yang MH, Kuang Y, Huang LF, Chen SL. 2013. Simultaneous determination of aflatoxin B1 and ochratoxin A in licorice roots and fritillary bulbs by solid-phase extraction coupled with high-performance liquid chromatography-tandem mass spectrometry. Food Chem. 138(2–3):1048–1054. [DOI] [PubMed] [Google Scholar]
- Wang S. 2016. Research on storage specification of traditional Chinese medicines of being moldy with malt, lotus seeds and nutmeg as the models. Beijing: Peking Union Medical College. [Google Scholar]
- Wang S, Kong WJ, Yang MH. 2016. Simultaneous determination of 11 mycotoxins in malt by isotope internal standard-UHPLC-MS/MS. Acta Pharm Sin. 1:110–115. [PubMed] [Google Scholar]
- Wang SF, Cheng L, Ji S, Wang K. 2014a. Simultaneous determination of seventeen mycotoxins residues in Puerariae lobatae radix by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 98(10):201–209. [DOI] [PubMed] [Google Scholar]
- Wang SM, Zhang S, Chen J, Mao D, Ji S. 2014b. Determination of 4 aflatoxins in Chinese herbs by UHPLC-MS -/MS. Chin J Health Lab Technol. 2:190–193. [Google Scholar]
- Wang SM, Zheng R, Yu L, Chen J, Ji S, Wang K. 2011. Determination of patulin in zhike of Chinese herb by HPLC-MS/MS. Chin J Health Lab Technol. 7:1593–1594. [Google Scholar]
- Wang YL, Li WF, Liu B, Liu X, He WH. 2014c. Determination of aflatoxins in 4 kinds of traditional Chinese medicines in puer downtown areas by HPLC with post-column derivatization. Yunnan Chem Ind. 41(1):42–44. [Google Scholar]
- Wang YT. 2015. Rapid determination of ochratoxin A in traditional Chinese medicines using colloidal gold chromatographic technique. Zhenjiang: Jiangsu University. [Google Scholar]
- Wannop CC. 1961. Turkey “X” disease. Vet Rec.73:310–311. [Google Scholar]
- Wei RW, Qiu F, Kong WJ, Wei J, Yang MH, Luo Z, Qin J, Ma X. 2013. Co-occurrence of aflatoxin B1, B2, G1, G2 and ochrotoxin A in Glycyrrhiza uralensis analyzed by HPLC-MS/MS. Food Control. 32(1):216–221. [Google Scholar]
- Wei RW, Yang XL, Qiu F, Yang MH, Qin JP. 2011. Simultaneous determination of aflatoxin B1, B2, G1, G2 and ochratoxin A in Glycyrrhiza uralensis by HPLC-FLD after immunoaffinity column with online post-column photochemical derivatization. China J Chin Mater Med. 36(17):2342. [PubMed] [Google Scholar]
- Wei T, Ren P, Huang L, Ouyang Z, Wang Z, Kong X, He Q. 2019. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 300:125176. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . 2007. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva (Switzerland):WHO. [Google Scholar]
- Wu JW, Tan LJ, Zhao RH, Chen B. 2011a. Analysis of ochratoxin A in Chinese materia medica herbs by immunoaffinity column-HPLC. Chin Traditional Herbal Drugs. 42(8):1557–1559. [Google Scholar]
- Wu JW, Zhao RH, Chen B, Yang MH. 2011b. Determination of zearalenone in barley by high-performance liquid chromatography coupled with evaporative light scattering detection and natural occurrence of zearalenone in functional food. Food Chem. 126(3):1508–1511. [Google Scholar]
- Xiao CB, Liu QT, Dou XW, Yang MH, Kong WJ, Wan L. 2016. Rapid detection of ochratoxin A in malt by cytometric bead array based on indirect competition principle. Chin J Anal Chem. 44(4):625–632. [Google Scholar]
- Xie TT, Qiu F, Yang MH, Qi AD. 2011. Simultaneous determination of fumonisins B1 and B2 in traditional Chinese medicines by high-performance liquid chromatography-tandem mass spectrometry. Acta Pharm Sin. 46(7):822–827. [PubMed] [Google Scholar]
- Xie YJ. 2016. On-site visual detection of aflatoxin B1 in traditional Chinese medicines by the colloidal gold test strip. Changchun: Jilin Agricultural University. [Google Scholar]
- Xing YY, Meng W, Sun W, Li DX, Yu ZG, Tong L, Zhao YL. 2016. Simultaneous qualitative and quantitative analysis of 21 mycotoxins in Radix Paeoniae alba by ultra-high performance liquid chromatography quadrupole linear ion trap mass spectrometry and QuEChERS for sample preparation. J Chromatogr B. 1031:202–213. [DOI] [PubMed] [Google Scholar]
- Yang J, Luan GH, Liu Z. 2011a. Study on the method for determination of aflatoxin residue in traditional Chinese medicinal by LC-MS/MS. China Pharm. 14(7):929–932. [Google Scholar]
- Yang J, Luan GH, Yang RL. 2011b. Determination of aflatoxin residual quantity in PlatycladiSeed by LC-MS/MS. Chin Pharm. 20(14):35–37. [Google Scholar]
- Yang L, Wang LN, Pan JY, Xiang L, Yang MH, Logrieco AF. 2010. Determination of ochratoxin A in traditional Chinese medicinal plants by HPLC–FLD. Food Addit Contam Part A. 27(7):989–997. [DOI] [PubMed] [Google Scholar]
- Yang LM, Su JM, Lei HY, Ning LZ, Zhao ZM. 2014a. Progress in fumonisin research. Progress Vet Med. 3:97–100. [Google Scholar]
- Yang MH, Chen JM, Zhang XH. 2004. Determination of aflatoxin in Chinese herbal medicines. Beijing: Medicinal plant research and modernization of traditional Chinese medicine-proceedings of the 4th national symposium on medicinal botany and plant medicine. [Google Scholar]
- Yang MH, Chen JM, Zhang XH. 2005. Immunoaffinity column clean-up and liquid chromatography with post-column derivatization for analysis of aflatoxins in traditional Chinese medicine. Chromatographia. 62(9–10):499–504. [Google Scholar]
- Yang WW, Xiong LY, Wang RF, Liu Y, Sun QS, Lei Y, Wang Q. 2013. Determination of afl atoxin G2, G1, B2, B1 in 34 batches of Chinese herbs by HPLC associated with post column photochemical derivatization. Res Pract Chin Med. 1:43–47. [Google Scholar]
- Yang XH, Kong WJ, Hu YC, Yang MH, Huang LQ, Zhao M, Ou-yang Z. 2014b. Aptamer-affinity column clean-up coupled with ultra high performance liquid chromatography and fluorescence detection for the rapid determination of ochratoxin A in ginger powder. J Sep Sci. 37(7):853–860. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wei RW, Shen HH, Yang MH, Ou-yang Z. 2011c. Determination of aflatoxins in animal medicines by immunoaffinity column clean-up and HPLC-FLD with post-column photochemical derivatization. Chin Pharm. 20(15):4–5. [Google Scholar]
- Yang Y. 2015. Preparation based on colloidal gold immune chromatography technology rapid detection of aflatoxin B1 strip in traditional Chinese medicine research. Chengdu: Chengdu University of TCM. [Google Scholar]
- Ying GY, Liu XF, Wang HW, Wang RL, Ma XP, Guo WY, Kong WJ. 2018. An economic and green strategy for preventing Chinese medicinal materials from toxigenic fungi: couplet medicine technique. Ind Crops Prod. 124:429–434. [Google Scholar]
- Yu YY, Qiu YL, Zhang HY, Zhou YH, Wang TY. 2015. Determination of aflatoxin B1 in traditional Chinese medicines by direct competitive fluorescence immunoassay. Chemistry. 78(9):830–834. [Google Scholar]
- Yue YT. 2009. Study on detection methods of trichothecenes in traditional Chinese medicine. Zhenjiang: Jiangsu University. [Google Scholar]
- Yue YT, Zhang XF, Ou-yang Z, Gao WW, Wu J, Yang MH. 2009. Determination of T-2 toxin in traditional Chinese herbal medicines by GC-ECD. Chromatographia. 70(9–10):1495. [Google Scholar]
- Yue YT, Zhang XF, Pan JY, Ou-yang Z, Wu J, Yang MH. 2010a. Determination of deoxynivalenol in medicinal herbs and related products by GC-ECD and confirmation by GC-MS. Chromatographia. 71(5–6):533–538. [Google Scholar]
- Yue YT, Zhang XF, Yang MH, Zhen OY, Liu HB. 2010b. Simultaneous determination of deoxynivalenol and nivalenol in traditional Chinese medicine by SPE and LC. Chromatographia. 72(5–6):551–555. [Google Scholar]
- Zhang AT, Shi YB, Zhang ZL, Li S, Lu HX, Sun PJ. 2008. Determination AFB1 content of part seed and fruit traditional Chinese medicine by ELISA. J Med Res. 37(10):48–49. [Google Scholar]
- Zhang C, Dou XW, Yang MH. 2016. Toxicological and rapid detection techniques of common mycotoxins in traditional Chinese medicine. Chin J Pharmacol Toxicol. 30(12):1369–1378. [Google Scholar]
- Zhang C, Dou XW, Zhang L, Sun MF, Zhao M, Ou-yang Z, Kong DD, Antonio FL, Yang MH. 2018a. A rapid label-free fluorescent aptasensor picogreen-based strategy for aflatoxin B1 detection in traditional Chinese medicines. Toxins. 10(3):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dou XW, Cheng Z, Ying GY, Liu CM, Luo JY, Qian L, Peng L, Wang YT, Yang MH. 2018b. Facile preparation of stable PEG-functionalized quantum dots with glycine-enhanced photoluminescence and their application for screening of aflatoxin B1 in herbs. Sens Actuators B Chem. 261:188–195. [Google Scholar]
- Zhang L, Dou XW, Zhang C, Logrieco AF, Yang MH. 2018c. A review of current methods for analysis of mycotoxins in herbal medicines. Toxins. 10(2):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang F, Chen HP, Liu YP. 2015. Research status of fungal and mycotoxins pollution of Chinese medicinal materials. World Sci Technol Modernization Traditional Chin Med. 11:2381–2388. [Google Scholar]
- Zhang XF, Yang MH, Ou-yang Z. 2012. Detection of zearalenone in traditional Chinese medicinal plants by HPLC-DAD. Guizhou Agric Sci. 40(4):102–106. [Google Scholar]
- Zhang XH, Chen JM. 2005a. Determination of aflatoxin in traditional Chinese medicine by bromine derivatization method after purification of HPLC column by immunoaffinity column. China J Chinese Materia Medica. 30(3):182–184. [PubMed] [Google Scholar]
- Zhang XH, Chen JM. 2005b. HPLC analysis of aflatoxins in medicinal herb extracts by immunoaffinity column cleanup and post-column bromination. China J Chinese Mater Med. 30(3):182. [PubMed] [Google Scholar]
- Zhang XH, Liu HL, Chen JM. 2005. Immunoaffinity column cleanup with liquid chromatography using post-column bromination for aflatoxins in medicinal herbs and plant extracts. J Chromatogr Sci. 43(1):47–51. [DOI] [PubMed] [Google Scholar]
- Zhang XY. 2017. Study on new techniques for detection of aspergillus and mycotoxins in Chinese medicinal materials. Beijing: Beijing University of TCM. [Google Scholar]
- Zhao SP, Zhang D, Tan LH, Yu B, Cao WG. 2016. Analysis of aflatoxins in traditional Chinese medicines: classification of analytical method on the basis of matrix variations. Sci Rep. 6:30822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SR, Xu YX, Xu F, Gao W. 2011. High sensitive method for the detection of aflatoxins in extract from Chinese medicinal herb. J Capital Med Univ. 32(3):379–383. [Google Scholar]
- Zhao XS, Kong WJ, Wang S, Wei JH, Yang MH. 2017. Simultaneous analysis of multiple mycotoxins in Alpinia oxyphylla by UPLC-MS/MS. World Mycotoxin J. 10(1):41–51. [Google Scholar]
- Zheng R, Mao D, Wang K, Ji S. 2005. HPLC determination of aflatoxin B1, B2, G1, G2 in traditional Chinese medicine. Chin J Pharm Anal. 6:610–613. [Google Scholar]
- Zheng R, Mao D, Wang K, Ji S. 2010a. Determination of aflatoxin G2, G1, B2 and B1 in Semen Ziziphi Spinosae by HPLC with post column derivatization. Chin J Health Lab Technol. 1:36–37. [Google Scholar]
- Zheng R, Mao D, Wang SM, Wang K, Ji S. 2010b. Determination of aflatoxin G2, G1, B2, B1 in eleven kinds of Chinese herbs by HPLC. Chin J Pharm. 41(5):368–372. [Google Scholar]
- Zheng R, Mao D, Wang SM, Zhang S, Ji S. 2014a. Determination of 10 mycotoxins in Semen Pruni Persicae by LC-MS/MS. J Food Saf Qual. 3:824–832. [Google Scholar]
- Zheng RS, Xu H, Peng YX, Wang WL, Zhan RT, Chen WW. 2014b. A high throughput coupled with high performance liquid chromatography-tandem mass spectrometry method for determination of aflatoxin B1, B2, G1, G2 in 10 traditional Chinese medicines. China J Chin Mater Med. 39(2):273. [PubMed] [Google Scholar]
- Zheng RS, Xu H, Wang WL, Zhan RT, Chen WW. 2013. Determination of aflatoxin B1, B2, G1, G2 in Armeniacae Semen Amarum by high-performance liquid chromatography-tandem mass spectrometry. China J Chinese Materia Medica. 38(20):3534. [PubMed] [Google Scholar]
- Zheng RS, Xu H, Wang WL, Zhan RT, Chen WW. 2014c. Simultaneous determination of aflatoxin B1, B2, G1, G2, ochratoxin A, and sterigmatocystin in traditional Chinese medicines by LC-MS-MS. Anal Bioanal Chem. 406(13):3031–3039. [DOI] [PubMed] [Google Scholar]
- Zhou GX, Zhang HX, Hua RM. 2011. Research progress in toxicological effects and mechanism of T-2 toxin. Asian J Ecotoxicol. 6(2):121–128. [Google Scholar]
- Zhou WL, Kong WJ, Dou XW, Zhao M, Ou-yang Z, Yang MH. 2016. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J Chromatogr B Analyt Technol Biomed Life Sci. 1022:102–108. [DOI] [PubMed] [Google Scholar]
- Zhou YC, Kong WJ, Li Y, Logrieco AF, Xu J, Yang MH. 2015. A new solid-phase extraction and HPLC method for determination of patulin in apple products and hawthorn juice in China. J Sep Sci. 35(5–6):641–649. [DOI] [PubMed] [Google Scholar]
- Zhou YC, Yang MH, Xu J. 2010. Research advance on patulin. Guizhou Agric Sci. 38(2):112–116. [Google Scholar]
- Zhu D, Tan D, Xiang WY, Sun J, Li YJ, Wang AM. 2015. Determination of aflatoxins in traditional Chinese medicine by cleaning up the immunoaffinity column and HPLC with post column photochemical derivatization. J Guiyang Med Coll. 8:843–847. [Google Scholar]


