Chromis atripectoralis tolerated lower, but not higher, temperatures when associated with complex habitat structure compared to those under control conditions, which had no access to habitat structure. Understanding how habitat complexity influences thermal selection is critical given the rate of ocean warming and poleward expansions of tropical fishes.
Keywords: Behaviour, ocean warming, range shift, teleost fish, temperature preference, temperature threshold
Abstract
Coral reef species, like most tropical species, are sensitive to increasing environmental temperatures, with many species already living close to their thermal maxima. Ocean warming and the increasing frequency and intensity of marine heatwaves are challenging the persistence of reef-associated species through both direct physiological effects of elevated water temperatures and the degradation and loss of habitat structure following disturbance. Understanding the relative importance of habitat degradation and ocean warming in shaping species distributions is critical in predicting the likely biological effects of global warming. Using an automated shuttle box system, we investigated how habitat complexity influences the selection of thermal environments for a common coral reef damselfish, Chromis atripectoralis. In the absence of any habitat (i.e. control), C. atripectoralis avoided temperatures below 22.9 ± 0.8°C and above 31.9 ± 0.6°C, with a preferred temperature (Tpref) of 28.1 ± 0.9°C. When complex habitat was available, individual C. atripectoralis occupied temperatures down to 4.3°C lower (mean ± SE; threshold: 18.6 ± 0.7°C; Tpref: 18.9 ± 1.0°C) than control fish. Conversely, C. atripectoralis in complex habitats occupied similar upper temperatures as control fish (threshold: 31.7 ± 0.4°C; preference: 28.3 ± 0.7°C). Our results show that the availability of complex habitat can influence the selection of thermal environment by a coral reef fish, but only at temperatures below their thermal preference. The limited scope of C. atripectoralis to occupy warmer environments, even when associated with complex habitat, suggests that habitat restoration efforts in areas that continue to warm may not be effective in retaining populations of C. atripectoralis and similar species. This species may have to move to cooler (e.g. deeper or higher latitude) habitats under predicted future warming. The integration of habitat quality and thermal environment into conservation efforts will be essential to conserve of coral reef fish populations under future ocean warming scenarios.
Introduction
Changing environmental conditions, and most notably increasing temperatures, are having important direct and indirect effects on marine species (Hoegh-Guldberg & Bruno, 2010; Pecl et al., 2017; Comte & Olden 2017) and are being compounded by local anthropogenic stressors. The direct effects of increasing temperatures on an organisms physiology are driving shifts in individual behaviour (e.g. Beever et al., 2017), phenology (e.g. Nagelkerken and Munday, 2016) and species distributions (e.g. Feary et al., 2014). These shifts are especially pronounced in tropical marine ecosystems, as tropical species are generally exposed to environmental conditions that are closer to their upper thermal maxima and have fewer thermal refugia than freshwater and terrestrial ecosystems (Comte and Olden, 2017, Pinsky et al., 2019). The direct effects of increasing temperature on physiology are occurring alongside indirect effects, such as the degradation, fragmentation and/or loss of habitat (Robinson et al., 2019). For example, across tropical and temperate reef systems, climate-induced changes in environmental and biological conditions are causing massive reductions in the abundance of key habitat-forming organisms (Hughes et al., 2018b, Ling et al., 2009, Madin et al., 2018, Vergés et al., 2016, Wernberg et al., 2010). Declines in the abundance of formerly dominant habitat-forming organisms (i.e. reef-building corals and kelp forests), and corresponding declines in habitat complexity, can have a profound influence on the biodiversity and functioning of these ecosystems (Graham et al., 2006). Our ability to predict and manage populations under ongoing climate change will require a greater understanding of both the indirect and direct effects in shaping species’ distributions.
Coral reefs are extremely vulnerable to climate change (Walther et al., 2002), due largely to the thermal sensitivities of the dominant habitat-forming species, reef-building corals (e.g. Baird and Marshall, 2002, Hughes et al., 2018a). The increased frequency and intensity of thermal bleaching events over the past few decades (Hughes et al., 2018a) have contributed to widespread and sustained declines in the abundance of corals and a corresponding loss of structural complexity (Bento et al., 2016, Berumen and Pratchett, 2006, Hughes et al., 2018b, Hughes et al., 2017, Loya et al., 2001, McClanahan et al., 2007, Alvarez-Filip et al., 2009). These losses of coral cover and structural complexity are having a dramatic effect on reef-associated organisms (Pratchett et al., 2008, Stella et al., 2011). Those species that rely on live corals for food and/or shelter are the most rapidly and adversely affected by declines in live coral cover (e.g. Pratchett et al., 2008, Stuart-Smith et al., 2018, Wilson et al., 2006), while species that rely on the physical structure of corals typically exhibit protracted declines as the coral skeletons erode and the physical structure is lost (Graham et al., 2006, Pratchett et al., 2011, Wilson et al., 2006).
Marine fishes, like other ectotherms, are particularly sensitive to increasing temperatures, as their rates of physiological and biochemical processes are largely determined by environmental temperature (Fry, 1947), and they generally occupy environments that are already close to their upper thermal limits (Madeira et al., 2012, Rummer et al., 2013, Vinagre et al., 2016). Moreover, tropical marine species tend to have a narrower thermal tolerance range than temperate species as they evolved in relatively constant thermal environments (Tewksbury et al., 2008), and hence exhibit smaller thermal safety margins (Pinsky et al., 2019). Indeed, many low latitude populations of tropical fishes are already living in thermal environments that are near or even above their thermal optima (Gardiner et al., 2010, Nguyen et al., 2011, Rummer et al., 2013), limiting their capacity to cope with local increases in temperatures (Collins et al., 2013, Hughes et al., 2018a, Kerr, 2011). Stuart-Smith et al. (2018) reported a restructuring of fish and invertebrate communities following the 2016 coral bleaching event on the Great Barrier Reef due to the direct effects of temperature.
Given the predicted increases in ocean temperatures with ongoing climate change and concurrent habitat degradation, a greater understanding of the preferred and threshold temperatures of coral reef fishes, and the ecological factors that may influence these temperatures, is urgently needed. Further, the relationship between habitat quality and thermal conditions will be imperative in understanding effective restoration and conservation techniques for the retention of future reef fish populations. The objective of this study was to investigate the combined effects of physical structure and thermal environment in shaping habitat choice of a common coral reef fish, Chromis atripectoralis. Specifically, this study used an automated shuttlebox system to determine how availability of a complex habitat influenced the preferred and threshold temperatures of C. atripectoralis. Given the strong positive associations between coral reef fishes and complex reef structure, we hypothesized that the ecological benefits gained through associating with complex habitat structure would allow fish to select temperatures beyond those preferred under control conditions (i.e. in the absence of complex habitat).
Materials and methods
Animal husbandry
The black-axil chromis (C. atripectoralis, Pomacentridae) was selected as the model species as they are common across a wide range of latitudes on Indo-Pacific reefs (32°N-32°S, from the Ryukyu Islands, Japan to Northern Australia; Froese and Pauly, 2019). Chromis atripectoralis are relatively small bodied (maximum total length, TL: 12 cm) and closely associate with complex coral structures (e.g. branching Acropora and Pocillopora corals, see Pratchett et al., 2012), making them an ideal species for examining the impact of habitat complexity on thermal preference. Chromis atripectoralis were collected from Pioneer Bay, Orpheus Island, Queensland, Australia (18.6161° S, 146.4972° E, annual temperature range: 21–29°C; AIMS 2020) using small barrier nets and hand nets in May and June 2017. Following collection, fish were held at the Orpheus Island Research Station with fresh flow-through seawater for 48 h and then transported in bags filled with seawater with supplemental aeration delivered using a portable air pump and air stone, to the Marine Aquaculture Research Facilities Unit (MARFU) at James Cook University, Townsville, Queensland, Australia. Transport lasted ~3 h, and no mortalities were recorded during this time. Forty-five similar-sized C. atripectoralis (mean ± SEM; TL: 5.91 ± 0.16 cm; mass: 11.28 ± 0.74 g) were randomly selected and held in 100 L of aquaria, with a maximum of 10 fish per aquarium. All aquaria were equipped with supplemental aeration and were continuously supplied with filtered seawater maintained at 26 ± 1°C. Fish were fed commercial pellets twice daily and held under a 12:12-h photoperiod. Fish were habituated to laboratory conditions for 2 weeks after which they were each tagged with subcutaneous coloured elastomer (Northwest Marine Technology, Washington, USA) in the dorsal musculature for individual identification and allowed to recover for a minimum of 2 weeks prior to experimentation. The research project was conducted in compliance with the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care of Use and Animals for Scientific Purposes, 7th Edition, 2004, and the Qld Animal Care and Protection Act, 2001, and received animal ethics approval from the JCU Animal Ethics Committee Approval Number A2089.
Preferred and threshold temperatures
To establish the effect of habitat complexity on preference temperature, a modified shuttlebox design was used, in which structurally complex habitat (branching coral skeleton) was added to the centre of one chamber and a structurally simple habitat (coral rubble) of equal volume (~900 cm3) was added to the other chamber. In brief, the shuttlebox system is a two-chamber choice system, in which a temperature differential of 1.5°C is maintained between the chambers by water flowing in a clockwise direction in one chamber and counter-clockwise direction in the other chamber (Schurmann et al., 1991). The two chambers (diameter = 35 cm, water depth = 20 cm, volume = 19.2 L) are connected by a 5 cm wide opening allowing the fish to freely move between chambers. A camera (SONY® HDR-XR100E) linked to a custom programme (Laboratories Technology Corp., Andover MA) controlled the rate of temperature change in each chamber by activating or deactivating the appropriate pumps based on the position of the fish. A PC video frame grabber (USB 2.0 DVD maker®) transmitted a video signal to a positioning software (LoliTrack, Loligo Systems®, Tjele, Denmark) which continuously tracked the position of the fish. If the fish was in the warmer of the two chambers, the temperature of the entire system would increase at 6°C h−1. If the fish was in the cooler chamber, the temperature of the entire system would decrease at 6°C h−1. By moving between the chambers, the fish could actively control the temperature of their environment. An intact skeleton of the branching coral Acropora nasuta (~15-cm diameter, ~15-cm height) was used as the ‘complex’ habitat. Rubble (~15-cm diameter, ~5-cm height) was created by breaking up an A. nasuta skeleton of similar size. These pieces of coral rubble were then placed on a flat terracotta plate and used as the ‘simple’ habitat. The skeleton branching coral was used instead of live coral, as we aimed to establish the effects of structural complexity, independent of the health and condition of the coral habitat. The A. nasuta skeleton allowed fish to occupy space under, above and between coral branches, while the rubble structure was of similar volume but provided fish with limited refugia. To avoid potential problems of tracking fish within the complex structure, we placed a ‘mask’ over the habitat structures. If a fish entered the mask, the LabTech software would use the previous position of the fish until the fish moved outside of the mask. The preferred and threshold temperatures of C. atripectoralis were determined: (i) in the absence of any habitat in either chamber, i.e. ‘control’, (ii) with the complex structure in the ‘warmer’ chamber and rubble structure in the ‘cooler’ chamber and (iii) with the complex structure in the ‘cooler’ chamber and rubble structure was placed into the ‘warmer’ chamber. Fifteen C. Atripectoralis were used for each treatment, with a different individual being used for each trial (total n = 45).
Individual fish were haphazardly selected and allocated to one of the three treatments. The order of treatment (i.e. control or habitat) for habitat trials and the position of structures (i.e. complex and simple structure) were randomized amongst trials. All fish were fasted for 24 h prior to trials to remove the influence of digestive processes on the measurements (Niimi and Beamish, 1974). For each trial, the fish and habitat were placed into the system at 1430 h, and given 1.5 h to familiarize with the system prior to the heating and cooling pumps being activated. Fish were given an additional 17-h learning period prior to data collection. Data collection began at 0900 h the next morning and continued for 5 h (i.e. until 1400 h). Following each trial, the shuttlebox was drained, cleaned and refilled with fresh seawater in preparation for the next trial.
For each treatment, preferred temperature (Tpref) was defined as the temperature where the fish spent the most time (i.e. modal temperature) within each trial. The lower and upper threshold temperatures were defined as the lowest and highest absolute temperatures, respectively; each individual fish experienced when associated with either a complex or rubble habitat. For the control, the lower and upper threshold temperatures were defined as the lowest and highest temperatures experienced by each fish during a trial. For the habitat trials, the proportion of time spent associated with each habitat type (i.e. complex or simple) was calculated for each individual.
Data analyses
All analyses were performed in R (Version 3.5.12018, R Core Development Team) using ‘lme4’. Generalized linear mixed-effects models (GLMM) using the gamma distribution and ‘log’ link function were used to compare Tpref and upper and lower threshold temperatures between control and complex or rubble habitats. The most appropriate statistical family and error distribution for each analysis was determined by examining the distribution of the response variable and visually inspecting the residuals for the saturated models. Treatment was used as a fixed effect, and holding tank was included as a random effect. All assumptions were checked by visual inspection of residuals, Shapiro–Wilk normality tests, variance inflation factors and Q-Q plots. Tukey post hoc tests were used for all a priori analyses. All values are reported as mean ± SEM.
Results
Lower preferred temperatures (blue) were established when complex habitat structure was placed into the ‘cooler’ chamber, while upper preferred temperatures (red) were established from trials where complex habitat structure was placed in the ‘warmer’ chamber (Fig. 1). The lower (blue) and upper (red) threshold temperatures are the minimum and maximum temperatures experienced by a focal C. atripectoralis for each of the treatments (Fig. 2).
Figure 1.

Boxplots representing mean Tpref (dashed lines), median Tpref (solid lines), interquartiles, and upper (open triangles) and lower (solid circles) thermal preferences for each individual, for fish occupying the control vs. complex habitat environment
Figure 2.

Boxplots representing mean threshold temperature (dashed lines), median threshold temperature (solid lines), interquartiles, and upper (open triangles) and lower (solid circles) threshold temperatures for each individual, for fish occupying the control vs. complex habitat environment
In the absence of any habitat (i.e. control), C. atripectoralis avoided temperatures below 22.9 ± 0.8°C and temperatures above 31.9 ± 0.6°C, while preferred a temperature of 28.1 ± 0.9°C (Figs 1 and 2). When the alternative habitat types (rubble versus complex habitat of A. nasuta) were added to the shuttlebox, fish preferentially associated with the complex habitat spending 62.7 and 78.8% of each trial associating with the complex habitat, as opposed to rubble (Fig. 3).
Figure 3.
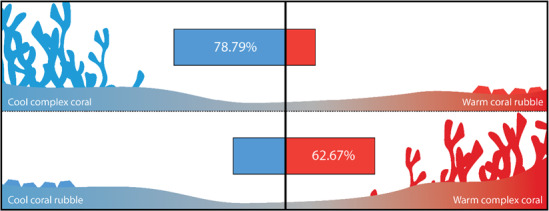
The proportion of time Chromis atripectoralis spent associated with structurally complex (i.e. A. nasuta skeleton) versus structurally simple (i.e. rubble) habitat within the shuttlebox. The upper panel displays the proportion of time when the complex habitat was positioned in the ‘cooler’ chamber, and the lower panel displays the proportion of time when the complex habitat was positioned in the ‘warmer’ chamber of the shuttlebox
When associated with the complex habitat, C. atripectoralis would tolerate lower (18.6 ± 0.7°C, z = 4.37; P < 0.001), but not higher (31.7 ± 0.4°C, z = 0.27; P = 0.79; Fig. 2) threshold temperatures than control fish. Further, C. atripectoralis preferred temperatures of 18.9 ± 1.0°C (z = 8.27; P < 0.001) or 28.3 ± 0.7°C (z = −0.752; P = 0.73) depending on the placement of the complex habitat in the ‘cooler’ or ‘warmer’ chamber, respectively (Fig. 1).
Discussion
Increasing ocean temperatures will have both direct and indirect effects on coral reef fishes (Stuart-Smith et al., 2015), whereby changes in the distribution of fishes relative to thermal environments may be moderated by temperature-induced changes in habitat structure. Here, we demonstrate that at lower temperatures, a common coral reef fish, C. atripectoralis, appears to trade-off between the ecological benefits of associating with a complex habitat and physiological costs of occupying a suboptimal thermal environment. In the absence of any habitat, C. atripectoralis avoided temperatures <23 and > 32°C, with a preferred temperature of 28.1°C. When associated with the complex habitat, individual C. atripectoralis experienced temperatures 4.5°C lower than control fish (i.e. in the absence of any habitat), resulting in a 9.2°C decrease in their preferred temperature. In contrast, we found no evidence that C. atripectoralis would experience temperatures above 31.9°C when associated with complex habitat, likely due to the close proximity to their upper thermal limits (i.e. critical thermal maximum CTMax). Our results support previous studies that have shown several tropical damselfishes (including C. atripectoralis), and cardinal fishes occupy thermal environments that are close to their upper thermal limits (Gardiner et al., 2010, Rummer et al., 2013). While numerous studies have investigated the effects of habitat degradation and loss of structural complexity (e.g. Richardson et al., 2018, Roberts and Ormond, 1987) or changing temperatures (e.g. Donelson et al., 2010, Habary et al., 2017) on reef fishes, few, if any, have considered how habitat availability may affect temperature choice and vice versa (see Matis et al., 2018 for exception). Understanding the nature and magnitude of the costs and benefits of associating with different habitat/s and thermal environments is crucial to predict how populations and distributions of coral reef fishes will respond to future conditions under ongoing ocean warming.
Reductions in live coral and the consequent loss of structural complexity are known to reduce the abundance and diversity of coral reef fish assemblages, with those species that rely directly on corals for food and/or shelter being the most vulnerable (Caley and John, 1996, Coker et al., 2009, Pratchett et al., 2008). While C. atripectoralis is considered a facultative coral dweller, a meta-analysis has shown their abundances are relatively insensitive to the loss of live coral (Pratchett et al., 2016). The results of the present study suggest that the preference of C. atripectoralis for the complex habitat, although important, may be lesser than the effects of increasing temperature on physiological function and survival. Chromis atripectoralis would not tolerate temperatures greater than 31.9°C, even when the preferred complex habitat was available, which is likely due to close proximity of preferred temperatures to the upper thermal limits. However, this response may have been stronger if the complex habitat provided was a live coral colony, given the benefits of live coral versus dead coral skeleton in providing food, moderating competition and predation (Coker et al., 2013). If an obligate coral dweller was examined, there was a threat of predation, or if microthermal refugia were present within the structure. This is supported by a previous study that demonstrated a reduction in maximum oxygen uptake rate and aerobic scope of Chromis viridis, the sister species to C. atripectoralis with similar preferred temperature (28.9°C), at temperatures above 31°C (Habary et al., 2017). The lack of change in upper threshold temperatures when associating with complex coral structure suggests that behavioural thermal thresholds for this species may be close to upper (acute) critical thermal limits (CTMax), as seen in other tropical taxa (e.g. CTMax of ~37°C for C. viridis; Habary et al., 2017).
Coral reef fish associations with complex coral structure are well established (Caley and John, 1996, Coker et al., 2009, Pratchett et al., 2008); however, such strong habitat associations could result in an ‘ecological trap’ (i.e. a situation where a given trait is no longer beneficial given the changing environment; Wong and Candolin, 2015). For instance, a focal fish remaining with complex coral structure would benefit from the structural habitat, but risk exposure to suboptimal thermal conditions. Such fish would benefit from a more plastic behavioural response to the changing environment. Ecological traps such as these could cause further exposure to suboptimal thermal conditions, ultimately leading to changes such as lower reproductive output or slower growth, and changes in population structure and dynamics. Indeed, exposure to 31°C for up to 3 months resulted in slower growth for a common coral reef fish, Acanthochromis polyacanthus (Munday et al., 2008a), indicating that the growth of some coral reef fish populations may be limited with exposure to suboptimal conditions. Although these effects were not tested here, remaining with complex coral structure and potentially enduring suboptimal conditions could have longer-term effects for coral reef fish populations.
Seawater temperature and habitat structure are widely recognized as two of the major drivers of reef fish communities (Pratchett et al., 2008, Robinson et al., 2019, Stuart-Smith et al., 2009, Waldock et al., 2019), yet are often viewed at different spatial scales. Increasing ocean temperatures have typically been related to shifts in the geographic distribution of reef fishes (e.g. Feary et al., 2014, Sunday et al., 2012), while changes in habitat structure have been related to changes in fish communities within or amongst proximal locations (e.g. Darling et al., 2017, Messmer et al., 2011). The results of this study highlight the need to consider both thermal environments and habitat structure when considering how fishes may be affected by changing environmental conditions. Indeed, the lack of suitable habitat has been suggested to constrain the poleward expansion of some reef fish species (Feary et al., 2014, Munday et al., 2008b). The only other study we are aware of that investigated the effects of temperature on habitat choice of coral reef fishes suggested that exposure to 22, 28 or 31°C influenced habitat selectivity of three species of juvenile damselfishes, and although some differences were reported, the effect sizes were small (Matis et al., 2018).
Global declines in coral cover, and the subsequent reductions in the goods and services that they provide has led to an increased emphasis on coral reef restoration projects to aid in coral reef recovery (Fox et al., 2019, Hein et al., 2017, Rinkevich, 2015). While there are a growing number of approaches to coral restoration (e.g. enhanced larval supply: Cruz and Harrison, 2017, assisted evolution of thermally tolerated corals: van Oppen et al., 2017, growth and outplanting of coral nubbins: Suggett et al., 2019, structural complexity enhancement: Yanovski and Abelson, 2019), all are aimed at increasing the cover of live coral and/or the physical structure of reef habitats. It is often assumed, either implicitly or explicitly, that the provisioning of physical structure will facilitate the recovery of reef fish assemblages (Ladd et al., 2019). However, the physiological tolerances of reef fishes to increasing temperatures are rarely considered. The results of the present study suggest that provisioning habitat structure alone may not be sufficient to restore or maintain fish populations, especially at their lower latitude boundaries, under ongoing climate change scenarios.
Changes in the abundance, diversity and composition of reef fish assemblages have typically been related to changes in coral cover and/or the physical structure of the habitat (Pratchett et al., 2008, Wilson et al., 2006). However, the results of this study suggest that, as oceans continue to warm, the physiological effects of local environmental temperatures are likely to overwhelm any benefit of associating with their preferred habitats and may lead to shifts in the distributions of species to cooler (i.e. deeper and/or higher latitude) habitats. This is particularly important as both theoretical predictions and empirical evidence suggest that many coral reef fish species have limited thermal safety margins as their preferred, and often realized temperatures are close to the thermal maximum (Gardiner et al., 2010, Pinsky et al., 2019, Rummer et al., 2013, Tewksbury et al., 2008). This is critical given the increasing focus on coral restoration efforts to conserve reefs under ongoing climate change (e.g. Boström-Einarsson et al., 2020). There are many obstacles to successful reef restoration (i.e. cost, small spatial scale, high coral mortality; Bellwood et al. 2019; Ware et al., 2020), and even if strategies are successful, they may be unable to support associated fish assemblages if the local temperatures exceed the fish species’ preferred thermal temperatures. Further, the trade-off at lower temperatures may influence the poleward range extensions of some fishes. While they may be able to tolerate cooler temperatures, they may not do so in the absence of their preferred (i.e. complex) habitat. An urgent rethinking of conservation actions for coral reefs is required and reinforces the need for action on limiting future increases in global temperatures. Integrating habitat quality with along with thermal conditions will be critical in predicting how fishes will respond to future ocean warming and the potential of restorative techniques for maintaining future fish populations.
Funding
This work was supported by the ARC Centre of Excellence for Coral Reef Studies at James Cook University (A.S.H.).
Acknowledgements
The authors would like to thank the Orpheus Island Research Station, Teish Prescott and Kelly Hannan for field assistance; Sybille Hess for assistance in animal husbandry; Connor Gervais for his insightful editorial guidance and the Marine Aquaculture Research Facilities Unit at JCU for their laboratory assistance.
References
- Alvarez-Filip L, Dulvy NK, Gill JA, Coˆté IM, Watkinson AR (2009, 1669) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc Royal Soc B: Biol Sci 276: 3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Institute of Marine Science (2020) Daily average ocean water temperatures against long term average water temperature. Viewed 25 May 2020 http://data.aims.gov.au/aimsrtds/yearlytrends.xhtml. [Google Scholar]
- Baird A, Marshall P (2002) Mortality, growth and reproduction in scleractinian coral following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237: 133–141. [Google Scholar]
- Beever EA, Hall LE, Varner J, Loosen AE, Dunham JB, Gahl MK, Smith FA, Lawler JJ (2017) Behavioral flexibility as a mechanism for coping with climate change. Front Ecol Envir 15: 299–308. [Google Scholar]
- Bellwood DR, et al. (2019) Coral reef conservation in the Anthropocene: confronting spatial mismatches and prioritizing functions. Biol Conser 236: 604–615. [Google Scholar]
- Bento R, Hoey AS, Bauman AG, Feary DA, Burt JA (2016) The implications of recurrent disturbances within the world's hottest coral reef. Mar Pollut Bull 105: 466–472. [DOI] [PubMed] [Google Scholar]
- Berumen ML, Pratchett MS (2006) Recovery without resilience: persistent disturbance and long-term shifts in the structure of fish and coral communities at Tiahura reef, Moorea. Coral Reefs 25: 647–653. [Google Scholar]
- Boström-Einarsson L, et al. (2020) Coral restoration–a systematic review of current methods, successes, failures and future directions. PloS One 15: e0226631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caley MJ, John JS (1996) Refuge availability structures assemblages of tropical reef fishes. J Anim Ecol 65: 414–428. [Google Scholar]
- Coker D, Pratchett ML, Munday P (2009) Coral bleaching and habitat degradation increase susceptibility to predation for coral-dwelling fishes. Behav Ecol 20: 1204–1210. [Google Scholar]
- Coker D, Wilson S, Pratchett M (2013) Importance of live coral habitat for reef fishes. Rev Fish Biol Fish 24: 89–126. doi: 10.1007/s11160-013-9319-5. [DOI] [Google Scholar]
- Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski W, Johns T, Krinner G (2013) Long-term climate change: projections, commitments and irreversibility. Cambridge University Press, New York. [Google Scholar]
- Comte L, Olden JD (2017) Climatic vulnerability of the world's freshwater and marine fishes. Nat Clim Change 7: 718. [Google Scholar]
- Cruz DWD, Harrison PL (2017) Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Sci Rep 7: 13985–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling ES, Graham NAJ, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36: 561–575. [Google Scholar]
- Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM (2010) Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar Ecol Prog Ser 401: 233–243. [Google Scholar]
- Feary DA, Pratchett MSJ, Emslie M, Fowler AM, Figueira WF, Luiz OJ, Nakamura Y, Booth DJ (2014) Latitudinal shifts in coral reef fishes: why some species do and others do not shift. Fish Fish 15: 593–615. [Google Scholar]
- Fox HE, Harris JL, Darling ES, Ahmadia GN, Estradivari GN, Razak TB (2019) Rebuilding coral reefs: success 16 years after low-cost, low-tech restoration. Restor Ecol 27: 862. [Google Scholar]
- Froese R, Pauly D.. Editors (2019) Fishbase. World Wide Web electronic publication. www.fishbase.org, version (12/2019).
- Fry FEJ. (1947) Effects of the environment on animal activity. Pub Ontario Fish Res Lab 68: 1–62. [Google Scholar]
- Gardiner NM, Munday PL, Nilsson GE (2010) Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS One 5: e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NAJ, Wilson SK, Jennings S, Nicholas VCP, Bijoux JP, Robinson J (2006) Dynamic fragility of oceanic coral reef ecosystems. Proc Natl Acad Sci U S A 103: 8425–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habary A, Johansen JL, Nay TJ, Steffensen JF, Rummer JL (2017) Adapt, move or die – how will tropical coral reef fishes cope with ocean warming? Glob Change Biol 23: 566–577. [DOI] [PubMed] [Google Scholar]
- Hein M, Willis B, Beeden R, Birtles A (2017) The need for broader ecological and socioeconomic tools to evaluate the effectiveness of coral restoration programs: socioecological effectiveness of coral restoration revisited. Restor Ecol 25: 873–883. [Google Scholar]
- Hoegh-Guldberg 0, Bruno JF (2010) The impact of climate change on the world's marine ecosystems. Science 328: 1523–1528.. [DOI] [PubMed] [Google Scholar]
- Hughes TD, et al. (2018a) Spatial and temporal patterns of mass bleaching of corals in the anthropocene. Science 359: 80–83. [DOI] [PubMed] [Google Scholar]
- Hughes T, et al. (2018b) Global warming transforms coral reef assemblages. Nature 556: 492–496. [DOI] [PubMed] [Google Scholar]
- Hughes TP, et al. (2017) Global warming and recurrent mass bleaching of corals. Nature 543: 373–377. [DOI] [PubMed] [Google Scholar]
- Kerr RA. (2011) Humans are driving extreme weather; time to prepare. Science 334: 1040–1040. [DOI] [PubMed] [Google Scholar]
- Ladd MC, Burkepile DE, Shantz AA (2019) Near-term impacts of coral restoration on target species, coral reef community structure, and ecological processes. Restor Ecol 27: 1166–1176. [Google Scholar]
- Ling SD, Johnson CR, Ridgway K, Hobday AJ, Haddon M (2009) Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Glob Change Biol 15: 719–731. [Google Scholar]
- Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4: 122–131. [Google Scholar]
- Madeira D, Narciso L, Cabral HN, Vinagre C (2012) Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. J Sea Res 70: 32–41. [Google Scholar]
- Madin JS, Baird AH, Bridge TCL, Connolly SR, Zawada KJA, Dornelas M (2018) Cumulative effects of cyclones and bleaching on coral cover and species richness at lizard island. Mar Ecol Prog Ser 604: 263–268. [Google Scholar]
- Matis P, Donelson J, Bush S, Fox R, Booth D (2018) Temperature influences habitat preference of coral reef fishes: will generalists become more specialised in a warming ocean? Glob Change Biol 24: 3158–3169. [DOI] [PubMed] [Google Scholar]
- McClanahan TR, Ateweberhan M, Graham NAJ, Wilson SK, Sebastian CR, Guillaume MMM, Bruggemann JH (2007) Western Indian Ocean coral communities: bleaching responses and susceptibility to extinction. Mar Ecol Prog Ser 337: 1–13. [Google Scholar]
- Messmer V, Jones GP, Munday PL, Holbrook SJ, Schmitt RJ, Brooks AJ (2011) Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 92: 2285–2298. [DOI] [PubMed] [Google Scholar]
- Munday P, Kingsford M, O’Callaghan M, Donelson J (2008a) Elevated temperature restricts growth potential of the coral reef fish Acanthochromis polyacanthus. Coral Reefs 27: 927–931. [Google Scholar]
- Munday PL, Jones GP, Pratchett MS, Williams AJ (2008b) Climate change and the future for coral reef fishes. Fish Fish 9: 261–285. [Google Scholar]
- Nagelkerken I, Munday PL (2016) Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob Change Biol 22: 974–989. [DOI] [PubMed] [Google Scholar]
- Nguyen KDT, Morley SA, Lai C-H, Clark MS, Tan KS, Bates AE, Peck LS (2011) Upper temperature limits of tropical marine ectotherms: global warming implications. PLoS One 6: e29340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi AJ, Beamish FWH (1974) Bioenergetics and growth of largemouth bass (Micropterus salmoides) in relation to body weight and temperature. Can J Zool 52: 447–456. [DOI] [PubMed] [Google Scholar]
- Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC, Chen I-C, Clark TD, Colwell RK, Danielsen F, Evengård B et al. (2017) Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Ocean Mar Biol Science:eaai9214. [DOI] [PubMed] [Google Scholar]
- Pinsky ML, Eikeset AM, McCauley DJ, Payne JL, Sunday JM (2019) Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569: 108–111. [DOI] [PubMed] [Google Scholar]
- Pratchett MS, Coker DJ, Jones GP, Munday PL (2012) Specialization in habitat use by coral reef damselfishes and their susceptibility to habitat loss. Ecol Evol 2: 2168–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchett M, Hoey A, Wilson S, Messmer V, Graham N, Au N (2011) Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 3: 424–452. [Google Scholar]
- Pratchett MS, Hoey A, Wilson SK (2016) Habitat-use and specialisation among coral reef damselfishes In Parmentier E, Frederich B, eds, Biology of Damselfishes, CRC Press, pp 102–139. [Google Scholar]
- Pratchett MS, Munday PL, Wilson SK, Graham NAJ, Cinner JE, Bellwood DR, Jones GP, Polunin NVC, McClanahan TR (2008) Effects of climate-induced coral bleaching on coral-reef fishes - ecological and economic consequences. Ocean Mar Biol 46: 251–296. [Google Scholar]
- Richardson L, Graham N, Pratchett M, Eurich J, Hoey A (2018) Mass coral bleaching causes biotic homogenization of reef fish assemblages. Glob Change Biol 1: 3117–3129. [DOI] [PubMed] [Google Scholar]
- Rinkevich B. (2015) Climate change and active reef restoration-ways of constructing the "reefs of tomorrow". J Mar Sci Eng 3: 111–127. [Google Scholar]
- Roberts C, Ormond RFG (1987) Habitat complexity and coral reef fish diversity and abundance on red sea fringing reefs. Mar Ecol Prog Ser 41: 1–8. [Google Scholar]
- Robinson JPW, Wilson SK, Jennings S, Graham NAJ (2019) Thermal stress induces persistently altered coral reef fish assemblages. Glob Change Biol 25: 2739–2750. [DOI] [PubMed] [Google Scholar]
- Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, Munday PL (2013) Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob Change Biol 20: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann H, Steffensen JF, Lomholt JP (1991) The influence of hypoxia on the preferred temperature of rainbow trout Oncorhynchus mykiss. J Exp Biol 157: 75–86. [Google Scholar]
- Stella J, Pratchett M, Hutchings P, Jones G (2011) Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol 49: 43–104. [Google Scholar]
- Stuart-Smith RD, Barrett NS, Stevenson DG, Edgar GJ (2009) Stability in temperate reef communities over a decadal time scale despite concurrent ocean warming. Glob Change Biol 16: 122–134. [Google Scholar]
- Stuart-Smith RD, Edgar GJ, Barrett NS, Kininmonth SJ, Bates AE (2015) Thermal biases and vulnerability to warming in the world’s marine fauna. Nature 528: 88. [DOI] [PubMed] [Google Scholar]
- Stuart-Smith R, Brown C, Ceccarelli D, Edgar G (2018) Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560: 92–96. [DOI] [PubMed] [Google Scholar]
- Suggett DJ, Camp EF, Edmondson J, Boström-Einarsson L, Ramler V, Lohr K, Patterson JT (2019) Optimizing return-on-effort for coral nursery and outplanting practices to aid restoration of the Great Barrier Reef. Restor Ecol 27: 683–693. [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Change 2: 686–690(2012). [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch CA (2008) Putting the heat on tropical animals. Science 320: 1296–1297. [DOI] [PubMed] [Google Scholar]
- Oppen MJH, et al. (2017) Shifting paradigms in restoration of the world's coral reefs. Glob Change Biol 23: 3437–3448. [DOI] [PubMed] [Google Scholar]
- Vergés A, et al. (2016) Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proc Natl Acad Sci U S A 113: 13791–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinagre C, Leal I, Mendonça V, Madeira D, Narciso L, Diniz MS, Flores AAV (2016) Vulnerability to climate warming and acclimation capacity of tropical and temperate coastal organisms. Ecol Indic 62: 317–327. [Google Scholar]
- Waldock C, Stuart-Smith RJ, Edgar G, Bird T, Bates A (2019) The shape of abundance distributions across temperature gradients in reef fishes. Ecol Lett 22: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416: 389–395. [DOI] [PubMed] [Google Scholar]
- Ware M, Garfield EN, Nedimyer K, Levy J, Kaufman L, Precht W, Winters RS, Miller SL (2020) Survivorship and growth in staghorn coral (Acropora cervicornis) outplanting projects in the Florida Keys National Marine Sanctuary. PloS One 15: e0231817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernberg T, Thomsen MS, Tuya F, Kendrick GA, Staehr PA, Toohey BD (2010) Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol Lett 13: 685–694. [DOI] [PubMed] [Google Scholar]
- Wilson SK, Graham NAJ, Pratchett MS, Jones GP, Polunin NVC (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob Change Biol 12: 2220–2234. [Google Scholar]
- Wong BBM, Candolin U (2015) Behavioral responses to changing environments. Behav Ecol 26: 665–673. [Google Scholar]
- Yanovski R, Abelson A (2019) Structural complexity enhancement as a potential coral-reef restoration tool. Ecol Eng 132: 87–93. [Google Scholar]


