Abstract
Context
Controversy exists regarding if and how body mass index (BMI) impacts antimüllerian hormone (AMH) in women with and without polycystic ovary syndrome (PCOS). Understanding the BMI-AMH relationship has critical implications for clinical interpretation of laboratory values and could illuminate underlying ovarian physiology.
Objective
To test the hypotheses that (1) BMI is associated with reduced AMH in PCOS and ovulatory controls (OVAs) and (2) the reduction in AMH is not accounted for by dilutional effects.
Design/Setting
Multicenter cohort.
Participants
Women aged 25 to 40 years from 2 clinical populations: 640 with PCOS, 921 women as OVAs.
Main Outcome Measures
Ovarian reserve indices: AMH, antral follicle count (AFC), and AMH to AFC ratio (AMH/AFC) as a marker of per-follicle AMH production.
Results
In both cohorts, increasing BMI and waist circumference were associated with reductions in AMH and AMH/AFC, after adjusting for age, race, smoking, and site in multivariate regression models. Increasing BMI was associated with reduced AFC in PCOS but not OVAs. Body surface area (BSA), which unlike BMI is strongly proportional to plasma volume, was added to investigate a potential dilutive effect of body size on AMH concentrations. After controlling for BSA, BMI retained independent associations with AMH in both cohorts; BSA no longer associated with AMH.
Conclusions
In an adjusted analysis, BMI, but not BSA, was associated with reduced AMH; these data do not support a role for hemodilution in mediating the relationship between increased body size and reduced AMH. Decreased AMH production by the follicle unit may be responsible for reduced AMH with increasing BMI.
Keywords: polycystic ovary syndrome, obesity, antimüllerian hormone, ovarian reserve, antral follicle count
Ovarian reserve” is a ubiquitous clinical concept in reproductive endocrinology, designed to capture “reproductive potential as a function of the number and quality of remaining oocytes.” (1) Although ovarian reserve invariably declines with aging (2), indices of this reserve vary markedly between women of any particular age (3), with less than 30% of overall variability attributable to age in 1 study (4). A better understanding of the factors that determine absolute ovarian reserve would enable improved counseling of individual women and would likely elucidate pathways that contribute to ovarian aging.
Two primary tools are used in the quantitative assessment of ovarian reserve today: serum antimüllerian hormone (AMH) levels and antral follicle count (AFC) as measured by transvaginal ultrasonography. AMH is a dimeric glycoprotein hormone of the transforming growth factor beta superfamily. It is produced by granulosa cells of preantral and small antral ovarian follicles and plays a paracrine role in follicular recruitment (5). AFC is measured by transvaginal ultrasound as the number of gonadotropin-sensitive antral follicles typically 2 to 10 mm in diameter (1). Both AMH and AFC correspond to the underlying pool of primordial follicles and, thus, remaining oocyte inventory (6-9). These indices are used frequently as principle quantitative markers of ovarian reserve and have been shown to be effective in predicting an individual’s response to gonadotropin stimulation in the in vitro fertilization (IVF) clinic (1).
Whether and how adiposity and body size might influence ovarian reserve is unclear. Current literature has not identified a relationship between body mass index (BMI) and AFC (10). In contrast, a relationship between AMH and BMI has been suggested but is the source of ongoing controversy. Several authors report an inverse association between AMH and BMI (10-13), while others have observed a positive association (14) and still others, no association at all (15). It has been suggested that hemodilution might underlie the reduced serum AMH values observed in women with higher BMI (15), however no data exist to support or challenge this notion. Alternatively, there may be a direct negative effect of excess adiposity on AMH production at the level of the granulosa cell (16). Distinguishing between these potential mechanisms might illuminate our understanding of ovarian physiology and assist in the interpretation of these increasingly utilized clinical tests.
Women with polycystic ovary syndrome (PCOS), have characteristically increased AMH levels (17) and AFC, the latter constituting 1 of 3 diagnostic criteria toward a Rotterdam diagnosis (18). Women with PCOS are also at higher risk of being overweight or obese (19). It is unknown whether the relationship between ovarian reserve and BMI is different in women with PCOS compared with ovulatory peers. Inclusion of a PCOS cohort offers a model for exploration of an expanded range of both ovarian reserve and body size dimensions.
The objective of this paper was to test the hypotheses that (1) BMI is associated with reduced AMH in PCOS and normal controls and (2) the reduction in AMH observed with increased BMI is not accounted for by reduced follicle number or by dilutional effects related to body size. To assess for a potential hemodilution effect on AMH, we incorporated measurements of body surface area (BSA). BSA, rather than BMI, is directly proportional to plasma volume (20). Thus, by challenging BSA and BMI both separately and together in a regression model predicting AMH, we aimed to determine whether blood volume (represented by BSA) versus adiposity (represented by BMI) had a greater independent effect on AMH levels.
Materials and Methods
This cohort study incorporates data from 2 study populations: (1) a multicenter randomized controlled trial (Pregnancy in Polycystic Ovary Syndrome II—PPCOSII) and (2) a prospective observational cohort study (OVAs—controls). Protocols were approved by the Institutional Review Board at each study center, and individual participating women provided written informed consent.
Participants
PCOS.
The PPCOS II cohort was derived from a Reproductive Medicine Network-sponsored multicenter, randomized controlled trial comparing letrozole with clomiphene citrate in the treatment of infertility in women with PCOS (NCT00719186) (21). Women were enrolled between 2009 and 2012 and were actively seeking pregnancy. PCOS was diagnosed by modified Rotterdam criteria, requiring ovulatory dysfunction in addition to hyperandrogenism and/or polycystic ovarian morphology, as this was an infertility treatment trial. Ovulatory dysfunction was defined as fewer than 9 menses per year, an intermenstrual interval of at least 45 days, or chronic anovulatory bleeding with serum progesterone less than 3 ng/mL. Hyperandrogenism was defined as elevated serum testosterone level, free androgen index, or modified Ferriman-Gallwey hirsutism score greater than 8. Polycystic ovarian morphology was defined as increased volume (at least 1 ovary greater than 10 cm3) or 12 or more antral follicles in at least 1 ovary. Mimicking disorders were excluded, and patients were otherwise healthy. A 2-month washout period for hormonally active medications was required prior to screening. Further details regarding the study protocol and inclusion and exclusion criteria of participants are publicly available (22).
Of the PPCOS II cohort, women aged 25 to 40 years (n = 640) were selected for inclusion in the present study, corresponding to the available age range of women participating in the ovulatory control cohort.
Controls.
The ovulatory controls (OVAs) were derived from the Ovarian Aging study, a prospective, longitudinal observational study exploring the clinical correlates and consequences of reproductive aging. Participants were recruited between 2006 and 2010 from eligible female members of the Kaiser Permanente of Northern California Health Plan, an integrated health care delivery system encompassing nearly one-third of the regional population and who were not actively seeking pregnancy. Participants were evaluated at the University of California San Francisco. Eligible women reported a regular menstrual cycle interval between 22 and 35 days. A multiethnic sample was targeted in the sampling strategy, including White, Asian, Hispanic, and African American heritage in roughly equal proportions. Women had no major medical illnesses and had their ovaries and uterus intact. A 3-month washout period for hormonally active medications was required prior to clinical assessment. No women in the ovulatory control cohort had hyperandrogenism and polycystic ovarian morphology. Additional details regarding the OVA study protocol and inclusion and exclusion criteria have been previously published (4, 23).
Women aged 25 to 40 years (n = 921) were selected for inclusion in the present study to correspond with the PPCOS II cohort.
Clinical assessment
AMH.
All AMH values were assayed using the Beckman Coulter enzyme-linked immunoassay 2-site sandwich immunoassay. This assay has a sensitivity of 0.25 ng/mL, intra-assay coefficient of variation of 3.0%, and inter-assay coefficient of variation of 7.0% (24). Among the ovulatory control cohort, AMH was assayed using the first-generation Beckman Coulter assay for the majority of women (84%); the second-generation (Gen II) assay was used for the remainder of samples. Regression analysis showed excellent correspondence between the 2 assays (R2 = 0.94) on a subset of women for whom both assays were performed.
AFC.
Transvaginal ultrasound examinations were performed at each study site at screening to count total number of antral follicles (2-10 mm). In the PCOS cohort, a different observer performed the ultrasounds at each of the 11 study sites. In the ovulatory control cohort, this examination was performed between cycle day 2 and 4 by 1 of 2 observers using a Shimadzu SDU-450XL machine with a variable transducer frequency of 4 to 8 mHz.
Anthropometrics and other clinical data.
Demographics were systematically assessed by self-report questionnaires, including race/ethnicity, educational attainment, income, and tobacco use.
Anthropometrics included measurement of height, weight, and waist circumference (WC) at baseline. BMI, a measure of body fat expressed in kg/m2, was calculated from height in meters squared and weight in kilograms:
BSA, measured in m2, was calculated using the DuBois formula (25):
BSA, not BMI, is directly proportional to estimated plasma volume (20). Notably, BSA is directly proportional to height (formula above), while BMI is inversely proportional to height squared. Thus, we challenged AMH against BMI, WC, as well as BSA to assess whether overall adiposity (as measured by BMI), central adiposity (as measured by WC), versus hemodilution (as measured by BSA), respectively, might play a greater role in the association between serum AMH and body size.
Statistical analysis
Descriptive statistics were provided for baseline participant characteristics using parametric and categorical testing. Data were visualized using scatterplots to understand the overall relationship between the outcome measures of ovarian reserve (AMH and AFC) and the predictor variables of body size and composition (BMI, WC, and BSA). Univariate linear regression models were explored to assess unadjusted relationships between predictors and outcome variables. In light of the different BMI ranges observed between cohorts, we performed stratified analyses using a BMI threshold of 30 kg/m2.
Data were checked for normality. AMH, AFC, and AMH/AFC were transformed using natural logarithms to normalize the distribution of these right-skewed variables.
Next, multivariate linear regression models were used to control for potential confounders of the relationship between each predictor and log-transformed outcome; the variables adjusted for included age, smoking, race, and study center in each study cohort. Finally, BMI and BSA were incorporated together as predictors into the multivariate models to assess whether body fat versus plasma volume better accounted for the observed effect of body size on AMH levels and test the hypothesis that hemodilution might contribute to a reduction in serum AMH.
An interaction analysis using the pooled data investigated whether the relationship between BMI and AMH systematically differed based on cohort (PCOS vs controls). The impact of specific racial groups on AMH levels was also explored using this pooled dataset.
Results
Participant characteristics of the 2 cohorts are listed in Table 1. Women with PCOS had higher BMI and WC than the ovulatory control cohort. A greater proportion of women with PCOS were White, owing to differences in recruitment strategies.
Table 1.
Participant characteristics
| Characteristic | OVA N = 921 | PCOS N = 640 |
|---|---|---|
| Age, years (SD) | 33.0 (4.3) | 30.0 (3.6) |
| BMI, kg/m2 (SD) | 26.7 (7.0) | 35.1 (9.3) |
| Waist circumference, cm (SD) | 83.5 (15.4) | 105.7 (20.5) |
| Race, % | ||
| White | 27 | 63 |
| African American | 21 | 12 |
| Asian | 28 | 4 |
| Hispanic | 24 | 18 |
| Other | - | 3 |
| Education, % | ||
| ≤High school | 19 | 21 |
| Some college | 22 | 33 |
| College graduate | 38 | 33 |
| Graduate degree | 21 | 13 |
| Income, % | ||
| <$50k | 48 | 37 |
| $50-100k | 34 | 44 |
| $≥100k | 18 | 6 |
| Decline | -- | 13 |
| Cigarette smoking, % | 8 | 13 |
Mean (SD) or % as indicated
Abbreviations: BMI, body mass index; SD, standard deviation.
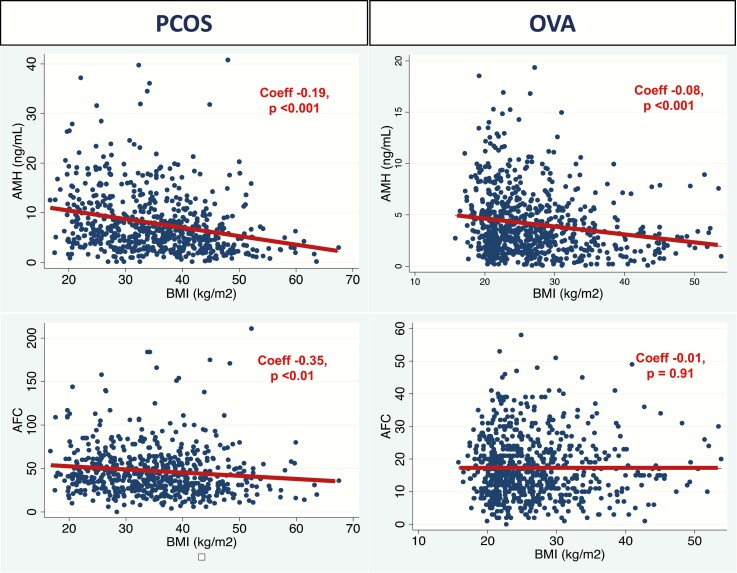
The relationship between BMI and markers of ovarian reserve is visualized in Figure 1. A linear best fit model is represented by the maroon line. The coefficient and corresponding P value from the unadjusted linear regression model is also found in the plots. Among women with PCOS, AMH declined with increasing BMI; specifically, there was a 0.19 ng/mL reduction in serum AMH with each increasing 1 kg/m2 of BMI. AFC also declined with increasing BMI (coefficient [coeff] –0.35; P < 0.01). Among the ovulatory control cohort, there was also a decline in AMH with increasing BMI (coeff –0.08; P < 0.001); however, no statistically significant relationship was observed between BMI and AFC in this cohort (Fig. 1). When we stratified the populations by BMI using a cutoff of 30 kg/m2, the relationship between BMI and AMH was sustained among women without obesity with PCOS (n = 210, coeff –0.38; P = 0.03) as well as women with obesity with PCOS (n = 430, coeff –0.1; P < 0.001). The association between AMH and BMI was also sustained in the control cohort without obesity (n = 664, coeff –0.11; P = 0.01), but not among the control cohort with obesity (n = 206, coeff –0.05; P = 0.13).
Figure 1.
Changes in ovarian reserve measures (AMH, AFC) with increasing BMI. The relationship between BMI and ovarian reserve measures (AMH, AFC) is depicted, with data from the women with PCOS in the left hand column and data from the ovulatory controls (OVA) in the right hand column. AFC, antral follicle count; AMH, antimüllerian hormone; BMI, body mass index; coeff, coefficient; OVA, ovarian aging control cohort; PCOS, polycystic ovary syndrome.
Multivariate linear regression models examining the relationship between body size predictors (BMI, WC, BSA) and log-transformed ovarian reserve outcomes (AMH, AFC, AMH/AFC) adjusted for age, race, smoking, and study center are represented in Table 2. In the PCOS cohort, all measures of body size, including BMI, WC, and BSA, remained inversely correlated with AMH after controlling for these potential confounders (P < 0.001). In addition, AFC, as well as AMH/AFC, was also inversely associated with measures of body size (Table 2).
Table 2.
Multivariate linear regression analysis of the association between anthropometrics (predictor) and ovarian reserve indices (outcome)
| AMHa | AFCa | AMH/AFCa | ||||
|---|---|---|---|---|---|---|
| Coeff (95% CI) | P | Coeff (95% CI) | P | Coeff (95% CI) | P | |
| PCOS | ||||||
| BMI (kg/m2) | –0.023 (–0.030, -0.016) | <0.001 | –0.008 (–0.012, 0.003) | 0.001 | –0.015 (–0.021, –0.009) | <0.001 |
| Waist circumference (cm) | –0.010 (–0.013, –0.006) | <0.001 | –0.003 (–0.005, 0.001) | 0.005 | –0.006 (–0.009, –0.004) | <0.001 |
| BSA (m2) | –0.729 (–0.978, –0.481) | <0.001 | –0.231 (–0.394, –0.068) | 0.006 | –0.479 (–0.679, –0.279) | <0.001 |
| OVA | ||||||
| BMI (kg/m2) | –0.019 (–0.027, –0.010) | <0.001 | 0.002 (–0.005, 0.008) | 0.63 | –0.019 (-0.026, –0.013) | <0.001 |
| Waist circumference (cm) | –0.008 (–0.012, –0.004) | <0.001 | 0.000 (–0.003, 0.003) | 0.82 | –0.008 (–0.011, –0.005) | <0.001 |
| BSA (m2) | –0.492 (–0.756, –0.224) | <0.001 | 0.141 (–0.052, 0.333) | 0.15 | –0.603, (–0.795, –0.411) | <0.001 |
Controlled for age, race, smoking, and study center.
Predictors across rows represent separate models for each of the 3 outcomes listed in columns.
Abbreviations: AFC, antral follicle count; AMH, antimüllerian hormone; BMI, body mass index; BSA, body surface area; CI, confidence interval; coeff, coefficient OVA, ovarian aging control cohort; PCOS, polycystic ovary syndrome.
a AMH, AFC, and AMH/AFC outcomes transformed by natural logarithm.
In the ovulatory control cohort, all measures of body size were again inversely correlated with AMH as well as AMH/AFC. However, AFC was not correlated with body size predictors in this cohort (Table 2).
Finally, we challenged BMI against BSA by including both predictors in the fully adjusted models for AMH to determine whether 1 index of body size remained more strongly associated with the AMH outcome while holding the other constant (Table 3). In the PCOS cohort, the inclusion of both BMI and BSA in the model rendered the association between BSA and AMH null (coeff –0.21; P = 0.41), while the association between BMI and AMH was sustained (coeff –0.02; P = 0.02). This was interpreted to indicate that adiposity, rather than plasma volume, was the more significant driver of the correlation between body size and serum AMH. In the ovulatory cohort, after controlling for BSA, BMI remained associated with AMH (coeff –0.02; P = 0.04), while BSA no longer was associated with AMH, echoing the findings in the PCOS cohort (Table 3).
Table 3.
Assessment of dilution
| AMHa | ||
|---|---|---|
| PPCOS | Coeff | P |
| BMI (kg/m2) | –0.02 (–0.03, –0.00) | 0.02 |
| BSA (m2) | –0.21 (–0.71, 0.29) | 0.41 |
| Age (years) | –0.03 (–0.05, –0.02) | <0.001 |
| White | 0.09 (–0.06, 0.23) | 0.26 |
| AMHa | ||
| OVA | Coeff | P |
| BMI (kg/m2) | –0.02 (–0.04, 0.00) | 0.04 |
| BSA (m2) | –0.02 (–0.55, 0.51) | 0.94 |
| Age (yrs) | –0.06 (–0.07, –0.04) | <0.001 |
| White | 0.33 (0.18, 0.47) | <0.001 |
Single multivariate model incorporating all predictors shown in rows and further adjusted for smoking and study center.
Abbreviations: AMH, antimüllerian hormone; BMI, body mass index; BSA, body surface area; coeff, coefficient; OVA, ovarian aging control cohort; PPCOS, pregnancy in polycystic ovary syndrome.
a AMH transformed by natural logarithm.
As anticipated, age was associated with reduced AMH levels in both cohorts (Table 3). We further observed that White race was associated with higher AMH levels as compared with non-Caucasian women in both cohorts. In a more granular exploratory analysis using the pooled dataset of both cohorts, we identified Asian women and Hispanic women to have lower AMH compared with Caucasian women in the fully adjusted model (Asian coeff –0.27; 95% confidence interval [CI], –0.42 to –0.12; P < 0.001; Hispanic coeff –0.34; 95% CI, –0.48 to –0.12; P < 0.001).
An interaction analysis investigated whether the impact of BMI on AMH differed as a function of whether a woman had PCOS or not, after adjusting for all aforementioned covariates. We did not identify an interaction between cohort and BMI in this model (P = 0.80), suggesting the impact of each additional unit of BMI on percentage reduction in AMH serum levels is similar across women with and without PCOS. In an exploratory analysis, we fit an interaction model using the raw AMH data rather than the log-transformed outcome in order to examine the impact of BMI on absolute change in serum AMH levels (rather than percentage change as indicated by the model with a log-transformed outcome). In this analysis, a significant interaction was detected (coeff –0.11; 95% CI, –0.18 to –0.04; P = 0.001), suggesting each 1-kg/m2 unit increase in BMI was associated with a small but significantly larger negative impact on AMH (by 0.11 ng/mL) in women with PCOS when compared with ovulatory control participants. This relationship was further sustained after restricting the analysis to the participants without obesity (coeff –0.24; 95% CI, –0.49 to 0.00; P = 0.05).
Discussion
In this cohort study examining the relationship between body size and markers of ovarian reserve, we demonstrate a consistent inverse association between body size indices and serum AMH levels in women with PCOS as well as community-based ovulatory control women. In fully adjusted models, the negative impact of body size on ovarian reserve indices appears primarily to impact AMH levels, with relative sparing of AFC in the control cohort. Notably, we provide the first evidence against the hemodilution hypothesis (ie, the idea that AMH declines with increasing BMI due to a dilutional effect of increased blood volume). When controlling for both BMI (a measure of adiposity) and BSA (a direct correlate of plasma volume) in regression models, it is only BMI that appears to maintain an independent negative effect on serum AMH levels.
Our results also have implications for current clinical practice. Serum AMH is ubiquitously marketed and used as a convenient and patient-friendly indicator of the ovarian follicular pool that is concordant with the gold standard ultrasound-based assessment of antral follicles (1). However, our findings reveal that the presumed linear relationship between AMH and AFC is disrupted by adiposity in a clinically meaningful way in ovulatory women: a BMI difference of + 5 kg/m2 in ovulatory women would associate with an 8.9% reduction in AMH and 9.2% reduction in AMH/AFC but no change in actual follicle count. Thus, if AMH is to be used as a correlate for AFC, our results suggest that improved accuracy will be achieved through the use of algorithms that adjust for BMI.
In the PCOS cohort, alternatively, we observed a reduction in AFC in addition to AMH with increasing BMI. However, there was a proportionally greater negative impact on AMH levels compared with AFC, as indicated by the reduction in AMH/AFC with increasing BMI. In this cohort, a BMI difference of + 5 kg/m2 would associate with a 10.7% reduction in AMH, 7.2% reduction in AMH/AFC, and 3.8% decline in AFC. This might reflect a quantitative decline in overall ovarian reserve or, alternatively, AFC estimations might be affected by reduced ultrasonic resolution with increasing BMI leading to systematic underestimates; notably 31% of women in the PCOS cohort had BMI in the morbidly obese range (≥40kg/m2).
Others have observed a relationship between AMH and BMI. Alebic et al investigated discrepancies between AMH and AFC and reported that BMI, menstrual cycle length, testosterone, and follicle-stimulating hormone were associated with the diminution in AMH among women in whom AMH was less than expected based on AFC (26). Our results further corroborate that incorporation of BMI would allow a more nuanced approach to future investigations of the relationship of AMH to AFC. Moreover, AMH has been investigated as a health indicator more broadly. Recent publications have reported an inverse relationship between AMH and markers of cardiometabolic health in women with PCOS as well as ovulatory control participants, after adjusting for age (12, 27-31). Notably, upon further adjustment for BMI in 1 study, the associations between ovarian reserve and cardiometabolic health were virtually nullified (12).
We found that White women had higher serum AMH levels than non-White women, particularly Hispanic and Asian women, after accounting for confounders. The impact of race on AMH has been previously investigated in the OVA cohort (32). This finding highlights the potential contribution of race/ethnicity to ovarian reserve indices and warrants additional targeted exploration in additional clinical cohorts.
We observed reduced AMH per antral follicle with increasing adiposity in both cohorts, implicating a potential reduction in AMH production. Although the pathways by which increased adiposity might impact AMH production are not known, in vitro studies have highlighted a potential role for several adipokines. In particular, leptin, an adipokine functioning in energy homeostasis, is increased in the serum of individuals with obesity (33) and is also found in follicular fluid of women undergoing IVF, where its levels positively correlated with BMI (16). In vitro administration of leptin suppresses AMH and AMHR-II messenger ribonucleic acid expression in granulosa cells, suggesting a direct effect on follicular AMH production and signaling (16). Similarly, adiponectin, an adipokine with reduced concentration in women with obesity, could also impact AMH production (34). Adiponectin receptors are expressed by granulosa cells, and signaling is thought to inhibit aromatase activity. A release from adiponectin-mediated inhibition due to reduced adiponectin could result in an increased estrogen to androgen ratio, which might in turn suppress AMH production (35). Finally, increased catabolism or clearance of AMH have been speculated to contribute to the reduction of levels observed in women with increased BMI (35), however data to support this idea are lacking. Taken together, there are clearly biologically plausible pathways by which adiposity could impair AMH production.
While it is known that women with obesity experience diminished fecundity, ovulatory dysfunction, and diminished outcomes in IVF (36, 37), the etiology of this reduced reproductive performance has been a matter of debate. Decreased IVF success in women with obesity can be, at least in part, overcome using donor oocytes (38, 39), providing evidence for a reduction in oocyte quality with elevated BMI as a contributing factor. Whether our observations of a reduction in AMH per follicle have critical downstream implications for the decreased fertility seen with obesity is not known.
Finally, in the primary interaction analysis, we did not observe a differential effect of BMI on percent reduction in AMH levels in women with PCOS compared with control participants. However, in an exploratory analysis using raw AMH data as the outcome (rather than the log-transformed data), we observed a larger effect of BMI on reduction in AMH levels in women with PCOS compared with control participants. This observation sustained even after restricting the analysis to the women without obesity (BMI < 30 kg/m2). This likely, at least in part, reflects the greater range of AMH serum levels observed in women with PCOS, but also leaves open the possibility that PCOS might amplify the effect of BMI on AMH. Women with PCOS have decreased insulin sensitivity and a more inflammatory form of adiposity, independent of BMI (40); thus, it is possible that inflammatory mediators, insulin signaling, and/or elevated androgens contribute to a differential effect of BMI on AMH production in women with PCOS compared with those without. Further clarification of potential mechanisms linking BMI and serum AMH might lead to important insights related to ovarian physiology.
Limitations
This study was a secondary analysis of data from 2 deeply phenotyped, systematically examined cohorts of women. Yet, notable limitations remain. (1) Characterization of AFC is inherently subject to within and between-observer error. In the multicenter PCOS cohort, examinations might be subject to some variance in inter-rater reliability, which was not quantified. However, study center was controlled for in the models to account for potential differences in technique. In the control cohort, transvaginal ultrasound examinations were performed by 1 of 2 observers with excellent correlation demonstrated between the 2 (r2 = 0.92) (4). We cannot exclude the possibility that the inverse association between BMI and AFC observed in the PCOS cohort could be due to inadequate visualization of the ovaries in the setting of high BMI. However, the striking absence of an association between BMI and AFC in the OVA cohort, albeit with a more restricted BMI range, as well as the consistent reduction in AMH/AFC with increased BMI in both cohorts, corroborates our overall interpretation of these data as relative sparing of AFC in comparison with AMH with increasing BMI. (2) A strength of the study is that all participants had AMH measured by the Beckman-Coulter enzyme-linked immunoassay for AMH. Of note, however, in subsequent years newer assays with enhanced sensitivity have become available. Future studies should seek to replicate our findings in newer assay platforms. (3) The control cohort was recruited from a deliberately multiethnic Northern California sample, while the PCOS cohort had a broader geographical base yet more homogeneous racial distribution, affecting a direct comparison between the groups. Yet, the consistency of our findings between groups, and the further adjustment for race and study site in the models, should mitigate the impact of this limitation. (4) As noted above, serum AMH samples may not accurately reflect the local paracrine impact at the level of the follicle, affecting our ability to gauge the magnitude of the effect at the site of action. (5) Finally, while our data challenge the notion of a hemodilution effect, we do not purport to identify the underlying mechanisms linking BMI and AMH. Future investigations should aim to better understand these mechanisms.
Conclusions
BMI, rather than hemodilution, appears to influence the reduction in serum AMH levels observed in women with increasing body size. This effect is more pronounced than the impact on AFC among women with PCOS; in ovulatory women, AFC is spared altogether from the negative effects of increased BMI. Taken together, these findings suggest a reduction in follicular production of AMH in the presence of elevated BMI. This opens the possibility of a direct toxic effect of excess adipose tissue on ovarian granulosa cells.
Acknowledgments
Financial Support: OVA: Supported by a National Institute of Child Health and Human Development/National Institute of Aging grant R01HD044876 and the National Institutes of Health/Neurobehavioral Core for Rehabilitation Research, University of California at San Francisco–Clinical and Translational Science Institute grant UL1 RR024131). PPCOS: Supported by National Institutes of Health/National Institute of Child Health and Human Development grant U10 HD38992. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or NICHD Reproductive Medicine Network.
Individual Support: We acknowledge Esther Eisenberg for her leadership role with the NICHD Reproductive Medicine Network, which assisted in the design, implementation, and supervision of the PPCOS Trial.
Glossary
Abbreviations
- AFC
antral follicle count
- AMH
antimüllerian hormone
- BMI
body mass index
- BSA
body surface area
- CI
confidence interval
- coeff
coefficient
- IVF
in vitro fertilization
- OVA
ovulatory control
- PCOS
polycystic ovary syndrome
- PPCOSII
Pregnancy in Polycystic Ovary Syndrome II
- WC
waist circumference
Additional Information
Disclosure Summary: E.G.J., J.S.R., M.G.P., M.I.C., and H.G.H. have nothing to disclose. R.S.L. is consultant for Ferring, Fractyl, AbbVie, and Bayer and receives research funds from Guerbet. N.F.S. serves on the Scientific Advisory Board for Astellas/Ogeda and Menogenix, Inc, and has equity interests in Menogenix, Inc.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2012;98(6):1407-1415. [DOI] [PubMed] [Google Scholar]
- 2. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5): 465-493. [DOI] [PubMed] [Google Scholar]
- 3. Greenwood EA, Cedars MI, Santoro N, et al. ; National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Cooperative Reproductive Medicine Network . Antimüllerian hormone levels and antral follicle counts are not reduced compared with community controls in patients with rigorously defined unexplained infertility. Fertil Steril. 2017;108(6):1070-1077. [DOI] [PubMed] [Google Scholar]
- 4. Rosen MP, Johnstone E, McCulloch CE, et al. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril. 2012;97(1):238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385. [DOI] [PubMed] [Google Scholar]
- 6. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER. Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril. 1999;72(5):845-851. [DOI] [PubMed] [Google Scholar]
- 7. Gougeon A. Caracteres qualitatifs et quantitatifs de la population folliculaire dans l’ovaire humaine adulte. Contracept Fertil Sex. 1984;12:527-535. [Google Scholar]
- 8. van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065-3071. [DOI] [PubMed] [Google Scholar]
- 9. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468-471. [DOI] [PubMed] [Google Scholar]
- 10. Moslehi N, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P, Azizi F. Is ovarian reserve associated with body mass index and obesity in reproductive aged women? A meta-analysis. Menopause. 2018;25(9):1046-1055. [DOI] [PubMed] [Google Scholar]
- 11. Lefebvre T, Dumont A, Pigny P, Dewailly D. Effect of obesity and its related metabolic factors on serum anti-müllerian hormone concentrations in women with and without polycystic ovaries. Reprod Biomed Online. 2017;35(3):325-330. [DOI] [PubMed] [Google Scholar]
- 12. Rios JS, Greenwood EA, Pavone MEG, et al. Associations between anti-mullerian hormone and cardiometabolic health in reproductive age women are explained by body mass index. J Clin Endocrinol Metab. 2020;105(1):e555-e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kriseman M, Mills C, Kovanci E, Sangi-Haghpeykar H, Gibbons W. Antimullerian hormone levels are inversely associated with body mass index (BMI) in women with polycystic ovary syndrome. J Assist Reprod Genet. 2015;32(9):1313-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albu D, Albu A. The relationship between anti-müllerian hormone serum level and body mass index in a large cohort of infertile patients. Endocrine. 2019;63(1):157-163. [DOI] [PubMed] [Google Scholar]
- 15. Lambert-Messerlian G, Plante B, Eklund EE, Raker C, Moore RG. Levels of antimüllerian hormone in serum during the normal menstrual cycle. Fertil Steril. 2016;105(1):208-13.e1. [DOI] [PubMed] [Google Scholar]
- 16. Merhi Z, Buyuk E, Berger DS, et al. Leptin suppresses anti-mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28(6):1661-1669. [DOI] [PubMed] [Google Scholar]
- 17. Dewailly D, Gronier H, Poncelet E, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123-3129. [DOI] [PubMed] [Google Scholar]
- 18. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47. [DOI] [PubMed] [Google Scholar]
- 19. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28-38.e25. [DOI] [PubMed] [Google Scholar]
- 20. Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247(4 Pt 2):F632-F636. [DOI] [PubMed] [Google Scholar]
- 21. Legro RS, Brzyski RG, Diamond MP, et al. ; NICHD Reproductive Medicine Network . Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Legro RS. RMN Amigos Protocol V. 4.3. Pregnancy in polycystic ovary syndrome II (PPCOSII): a 20-week double-blind randomized trial of clomiphene citrate and letrozole for the treatment of infertility in women with polycystic ovary syndrome Web site. ProMED-mail website. https://www.nejm.org/doi/suppl/10.1056/NEJMoa1313517/suppl_file/nejmoa1313517_protocol.pdf. Accessed 2019.
- 23. Bleil ME, Adler NE, Pasch LA, et al. Depressive symptomatology, psychological stress, and ovarian reserve: a role for psychological factors in ovarian aging? Menopause. 2012;19(11):1176-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Legro RS, Brzyski RG, Diamond MP, et al. The Pregnancy in Polycystic Ovary Syndrome II study: baseline characteristics and effects of obesity from a multicenter randomized clinical trial. Fertil Steril. 2014;101(1):258-269.e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303-313. [PubMed] [Google Scholar]
- 26. Alebic MŠ, Stojanovic N, Dewailly D. Discordance between serum anti-müllerian hormone concentrations and antral follicle counts: not only technical issues. Hum Reprod. 2018;33(6):1141-1148. [DOI] [PubMed] [Google Scholar]
- 27. Feldman RA, O’Neill K, Butts SF, Dokras A. Antimüllerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Fertil Steril. 2017;107(1):276-281. [DOI] [PubMed] [Google Scholar]
- 28. Cengiz H, Ekin M, Dagdeviren H, Yildiz Ş, Kaya C, Kanawati A. Comparison of serum anti-müllerian hormone levels in normal weight and overweight-obese adolescent patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2014;180:46-50. [DOI] [PubMed] [Google Scholar]
- 29. La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Mullerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82(4):970-972. [DOI] [PubMed] [Google Scholar]
- 30. Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24(11):2917-2923. [DOI] [PubMed] [Google Scholar]
- 31. Tehrani FR, Erfani H, Cheraghi L, Tohidi M, Azizi F. Lipid profiles and ovarian reserve status: a longitudinal study. Hum Reprod. 2014;29(11):2522-2529. [DOI] [PubMed] [Google Scholar]
- 32. Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI. Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril. 2014;101(1):199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33(1):35-45. [DOI] [PubMed] [Google Scholar]
- 34. Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun. 2012;425(3):560-564. [DOI] [PubMed] [Google Scholar]
- 35. Nouri M, Aghadavod E, Khani S, et al. Association between BMI and gene expression of anti-müllerian hormone and androgen receptor in human granulosa cells in women with and without polycystic ovary syndrome. Clin Endocrinol (Oxf). 2016;85(4):590-595. [DOI] [PubMed] [Google Scholar]
- 36. Obesity and reproduction: a committee opinion. Fertil Steril. 2015;104(5):1116-1126. [DOI] [PubMed] [Google Scholar]
- 37. Fedorcsák P, Dale PO, Storeng R, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod. 2004;19(11):2523-2528. [DOI] [PubMed] [Google Scholar]
- 38. Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R; SART Writing Group . Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26(1):245-252. [DOI] [PubMed] [Google Scholar]
- 39. Jungheim ES, Schon SB, Schulte MB, DeUgarte DA, Fowler SA, Tuuli MG. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod. 2013;28(10):2720-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shorakae S, Teede H, de Courten B, Lambert G, Boyle J, Moran LJ. The Emerging role of chronic low-grade inflammation in the pathophysiology of polycystic ovary syndrome. Semin Reprod Med. 2015;33(4):257-269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.