ABSTRACT
Background
Efficient prevention of posttraumatic stress disorder (PTSD) needs to target individuals with an increased risk for adverse outcome after trauma. Prognostic or prescriptive biological markers assessed early posttrauma may inform personalized treatment recommendations.
Objective
To test prognostic and prescriptive effects of early (posttraumatic) autonomic and endocrine markers on PTSD symptom development.
Method
Autonomic and endocrine markers were assessed within 12 days posttrauma and before treatment initiation within a randomized placebo-controlled trial investigating repeated oxytocin administration as preventive intervention for PTSD. Linear mixed effects models were used to test the effects of heart rate (variability), resting cortisol, morning cortisol and cortisol awakening response (CAR), cortisol suppression by dexamethasone and resting oxytocin on PTSD symptoms 1.5, 3 and 6 months posttrauma in men (n = 54), women using hormonal contraception (n = 27) and cycling women (n = 19).
Results
We found significant prognostic effects of resting oxytocin and cortisol suppression. In women using hormonal contraception, higher oxytocin was associated with higher PTSD symptoms across follow-up. Stronger cortisol suppression by dexamethasone, reflecting increased glucocorticoid receptor feedback sensitivity, was associated with lower PTSD symptoms across follow-up in men, but with higher symptoms at 1.5 months in women using hormonal contraception. These effects were independent of treatment condition. No further significant prognostic or prescriptive effects were detected.
Conclusion
Our exploratory study indicates that resting oxytocin and glucocorticoid receptor feedback sensitivity early posttrauma are associated with subsequent PTSD symptom severity. Notably, prognostic effects depended on sex and hormonal contraception use, emphasizing the necessity to consider these factors in biomedical PTSD research.
KEYWORDS: Biomarker, Prevention, prognosis, glucocorticoids, oxytocin, heart rate
Antecedentes: La prevención eficiente del trastorno de estrés postraumático (TEPT) necesita dirigirse a personas con un mayor riesgo de consecuencias adversas después de un trauma. Los marcadores biológicos pronósticos o preceptivos evaluados tempranamente luego del trauma pueden informar recomendaciones de tratamiento personalizadas.
Objetivo: Evaluar los efectos pronósticos y preceptivos de los marcadores tempranos (postraumáticos) autonómicos y endocrinos sobre el desarrollo de síntomas de TEPT.
Método: Fueron evaluados marcadores autonómicos y endocrinos dentro de los 12 días postrauma y antes de la iniciación del tratamiento dentro de un estudio aleatorio placebo-control, investigando la administración repetida de oxitocina como intervención preventiva para TEPT. Se utilizaron modelos lineales de efectos mixtos para evaluar los efectos de la frecuencia cardiaca (variabilidad), cortisol en reposo, cortisol matutino y respuesta al despertar de cortisol (CAR por sus siglas en inglés), supresión del cortisol por dexametasona y oxitocina en reposo sobre los síntomas de TEPT a los 1.5, 3 y 6 meses postrauma en hombres (N=54), mujeres que usaban contracepción hormonal (N=27) y mujeres ciclantes (N=19).
Resultados: Encontramos efectos pronósticos significativos de la oxitocina en reposo y de la supresión de cortisol. En las mujeres que usaban contracepción hormonal, los niveles de oxitocina más altos se asociaron con más síntomas de TEPT a lo largo del seguimiento. La supresión mayor del cortisol por dexametasona, que refleja una mayor sensibilidad a la retroalimentación del receptor de glucocorticoides, se asoció con menos síntomas de TEPT a lo largo del seguimiento en los hombres, pero con mayores síntomas a los 1.5 meses en las mujeres que usaban contracepción hormonal. Estos efectos fueron independientes de la condición de tratamiento. No se detectaron más efectos pronósticos o preceptivos significativos.
Conclusión: Nuestro estudio exploratorio indica que la oxitocina en reposo y la sensibilidad a la retroalimentación del receptor de glucocorticoides tempranamente luego del trauma se asocian con la subsecuente severidad de los síntomas de TEPT. Notablemente, los efectos pronósticos dependen del sexo y del uso de contracepción hormonal, lo que enfatiza la necesidad de considerar estos factores en la investigación biomédica en TEPT.
PALABRAS CLAVE: Biomarcador, Prevención, Pronóstico, Glucocorticoides, Oxitocina, Frecuencia cardíaca
HIGHLIGHTS: • Biological markers can inform clinicians about an individual’s PTSD risk after recent trauma. • Biological markers predicting PTSD risk differed for men, cycling women and women on hormonal contraception. • Biological markers did not predict the effects of nasal spray with oxytocin as preventive intervention for PTSD.
背景: 有效预防创伤后应激障碍 (PTSD) 需要针对创伤后不良后果风险增加的个体。创伤后早期评估的预后 (prognostic) 或处方性 (prescriptive) 生物学标志物可能会为个性化治疗建议提供依据。
目的: 考查早期 (创伤后) 自主神经和内分泌标志物对PTSD症状发展的预后和处方性效应。
方法: 在一项考查重复服用催产素作为PTSD预防性干预的随机安慰剂对照试验中, 于创伤后12天内和开始治疗之前评估了自主神经和内分泌标志物。使用线性混合效应模型考查心率 (变异性), 静息皮质醇, 早晨皮质醇和皮质醇唤醒反应 (CAR), 地塞米松皮质醇抑制和静息催产素对创伤后1.5, 3和6个月的男性 (n = 54), 使用激素避孕的女性 (n = 27) 和正常生理周期女性 (n = 19) 的PTSD症状的影响。
结果: 我们发现静息催产素和皮质醇抑制具有显著的预后效应。在使用激素避孕的女性中, 较高的催产素与整个随访期间较高的PTSD症状相关。地塞米松对皮质醇的更强抑制作用, 反映糖皮质激素受体反馈敏感性的提高, 与男性整个随访期间PTSD症状的降低相关, 而与使用激素避孕的女性在1.5个月时较高的症状相关。这些效应与治疗条件无关。未发现进一步的显著预后或处方效应。
结论: 我们的探索性研究表明, 创伤后早期静息催产素和糖皮质激素受体的反馈敏感性与随后的PTSD症状严重程度有关。值得注意的是, 预后效果取决于性别和激素避孕的使用, 强调在生物医学PTSD研究中必须考虑这些因素。
关键词: 生物标志物, 预防, 预后, 糖皮质激素, 催产素, 心率
1. Introduction
Traumatic events, i.e. exposure to actual or threatened death, serious injury or sexual violence (American Psychiatric Association, 2013) are experienced by over 70% of the general population (Benjet et al., 2016; Kessler et al., 2017). A considerable proportion of traumatized individuals subsequently develop posttraumatic distress disorder (PTSD). A meta-analysis showed that 27.0% of individuals develop initial PTSD symptoms but then recover, 10.3% develop chronic and 6.4% delayed PTSD (Galatzer-Levy, Huang, & Bonanno, 2018). In addition to treating PTSD, over the past years, efforts have increased to prevent its development in the first place (see Dunlop, Mansson, & Gerardi, 2012; Horn, Charney, & Feder, 2016; Sijbrandij, Kleiboer, Bisson, Barbui, & Cuijpers, 2015). Recently, we reported the results of a randomized controlled trial (RCT) evaluating the potential preventive effects of intranasal oxytocin administration early posttrauma on PTSD symptom development (Frijling et al., 2014; van Zuiden et al., 2017). Beneficial effects were found in individuals with high acute PTSD symptoms. This supports the growing notion that instead of offering preventive interventions to all traumatized individuals, targeted interventions should be offered only to those who are at increased risk for adverse outcomes and most likely to benefit from the interventions. At the same time, interventions should not interfere with the adaptive recovery observed in the majority of traumatized individuals (Shalev & Barbano, 2019). Such targeted preventive interventions could be informed by prognostic markers, predicting PTSD symptom development irrespective of treatment, and prescriptive markers, moderating an intervention’s effectiveness (Garcia & Delahanty, 2017; National Institute of Clinical Excellence, 2018).
A variety of biological systems associated with perceiving, reacting to, and recovering from (traumatic) stress appear to be involved in PTSD symptom development, including the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis (Heim & Nemeroff, 2016; Olff & van Zuiden, 2017). During acute stress, the sympathetic branch of the ANS provides a rapid response, amongst others increasing heart rate (HR), peripheral vasoconstriction and energy mobilization. During recovery, the parasympathetic branch’s activity increases, exerting opposing effects on the ANS’s target organs (Andrews, Ali, & Pruessner, 2013; Chrousos & Gold, 1992). The interplay of sympathetic and parasympathetic activity determines heart rate variability (HRV), i.e. the beat-to-beat variation in heart rate over time. HRV represents an index for the ANS’s ability to respond appropriately to environmental challenges (Thayer, Ahs, Fredrikson, Sollers, & Wager, 2012). A meta-analysis summarizing prognostic studies reported that PTSD development was associated with high resting HR, assessed within 72 hours posttrauma (Morris, Hellman, Abelson, & Rao, 2016).
To date, three prospective studies investigated pretrauma HRV. These reported that pretrauma lower resting HRV was associated with higher posttrauma PTSD symptoms in male military personnel (Minassian et al., 2015) and a mixed-sex sample of children (Mikolajewski & Scheeringa, 2018). The third study, again in a predominantly male, military personnel sample, found this association was specific to the context of high pretrauma symptoms (Pyne et al., 2016). Further, lower 24 hours HRV 2 days posttrauma (Shaikh Al Arab et al., 2012) as well as during rapid eye movement sleep at 5 to 30 days posttrauma (Mellman, Knorr, Pigeon, Leiter, & Akay, 2004) were associated with subsequent PTSD development in mixed-sex samples. Thus, evidence indicates that autonomic dysregulation before, acutely and early after trauma is associated with increased PTSD risk.
The HPA axis response is part of the comparatively slower, neuroendocrine stress response. Acute stress recovery is crucially modulated by HPA axis’s end product cortisol, via glucocorticoid receptor (GR)-induced inhibition of the HPA axis (Andrews et al., 2013; Chrousos & Gold, 1992). Most studies that tested prognostic effects of posttrauma cortisol used a single cortisol measurement and included mixed-sex samples (Bonne et al., 2003; McFarlane, Atchison, & Yehuda, 1997; Mouthaan et al., 2014; Price, Kearns, Houry, & Rothbaum, 2014; Resnick, Yehuda, Pitman, & Foy, 1995; Walsh et al., 2013). Assessment times varied from within the first hours to weeks posttrauma. Not surprisingly, given the methodological variability between studies, a meta-analysis showed that cortisol concentrations, assessed within 72 hours posttrauma, did not predict subsequent PTSD (Morris et al., 2016). Prospective studies assessing pretrauma hair cortisol concentrations in male military populations found negative associations with subsequent PTSD development when intermittent trauma exposure was adequately accounted for (Steudte-Schmiedgen et al., 2015).
High pretrauma GR function and GR-sensitivity to inhibitory effects of glucocorticoids in immune cells predicted subsequent PTSD symptoms in male, military populations (van Zuiden et al., 2012, 2011, 2012; van Zuiden, Kavelaars, Geuze, Olff, & Heijnen, 2013). One study reported a trend towards stronger cortisol suppression by the synthetic glucocorticoid dexamethasone, indicating higher GR sensitivity at the level of hypothalamus and pituitary, 2 days posttrauma in male and female emergency care patients with subsequent PTSD diagnosis (McFarlane, Barton, Yehuda, & Wittert, 2011), but the sample was rather small and findings have not been replicated yet. Thus, despite substantial methodological heterogeneity, preliminary evidence indicates that high GR feedback sensitivity and, potentially as a consequence, low long-term cumulative cortisol output might predict PTSD risk.
The neuropeptide oxytocin is a well-known modulator of the HPA axis, such that stress-induced oxytocin release dampens HPA axis reactivity (Alley, Diamond, Lipschitz, & Grewen, 2019; Engelmann, Landgraf, & Wotjak, 2004; Winter & Jurek, 2019). Moreover, oxytocin has anxiolytic effects (Jurek & Neumann, 2018), and promotes various aspects of prosocial behaviour (Heinrichs, von Dawans, & Domes, 2009) but these effects strongly depend on individual factors (e.g. secure vs. insecure attachment style; presence vs. absence of psychopathology) and on contextual factors (e.g. environment perceived as safe vs. as unsafe (Olff et al., 2013)). A recent meta-analysis showed increased expression of oxytocin pathway genes within functional brain networks associated with stress, fear, anxiety and cognitive processes relevant for social behaviours, such as reward and motivation (Quintana et al., 2019). As these processes presumably underlie vulnerability to PTSD after trauma, these findings provided the rationale to investigate intranasal oxytocin as candidate preventive intervention for PTSD (Frijling et al., 2014). They also suggest that blood oxytocin concentrations early posttrauma may impact PTSD risk or preventive treatment effectiveness. To date, two studies assessed blood oxytocin concentrations and PTSD symptoms prospectively. In a male, military sample, no impact of oxytocin concentrations, assessed before, 1 and 6 months post-deployment, on PTSD symptoms up to 5 years post-deployment was found (Reijnen, Geuze, & Vermetten, 2017). Likewise, in male and female emergency care patients, no significant association between oxytocin concentrations upon hospital admittance and PTSD symptoms 1 month later was found (Nishi, Hashimoto, Noguchi, Kim, & Matsuoka, 2015). Prescriptive effects of oxytocin concentrations have not been investigated yet.
One aspect that has largely been neglected is the impact of participants’ sex and, accordingly, gonadal steroid-related status on the prognostic value of biological markers for PTSD symptom development. There is accumulating evidence that cross-sectional associations between PTSD symptoms and HRV (Kamkwalala et al., 2012), as well as diurnal cortisol output (Nicolson & Ponnamperuma, 2019), are sex-dependent. Furthermore, HPA axis function and oxytocin concentrations differ between men and women, and, within women, are additionally influenced by hormonal contraception use and menstrual cycle phase (Engel, Klusmann, Ditzen, Knaevelsrud, & Schumacher, 2019; Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999; Stock, Karlsson, & von Schoultz, 1994). We also previously reported that hormonal contraception use promoted mid- to long-term recovery of early PTSD symptoms (Engel et al., 2019). However, to date, most published studies on biological prognostic markers for PTSD risk relied on single-sex, most commonly all-male samples. Even if studies included both men and women, sex has seldomly been taken into account adequately. Moreover, gonadal steroid-related statuses, such as menstrual cycle phase or hormonal contraception use, have rarely been considered as factors of influence in this line of research. In this context, it seems important to stress out that even statistically controlling for these factors impedes a precise estimation of the specific prognostic effects within the specific group participants (i.e. within men vs. within cycling women). This can only be achieved by stratifying analyses for sex and gonadal steroid-related statuses.
To sum up, a variety of autonomic and endocrine systems respond to traumatic events and might predict subsequent PTSD symptoms, although their predictive value may be dependent on sex and hormonal contraception use. It seems promising to concomitantly explore ANS functioning, HPA axis activity and oxytocin concentrations early posttrauma as dysregulations in these systems may be biological underpinnings of PTSD development. However, studies examining this broader range of stress response systems parameters early posttrauma are still sparse and to date no study comprehensively assessed all these systems within the same design, nor were previous analyses stratified for sex and hormonal contraception use. Moreover, biological prescriptive factors for preventive, pharmacological interventions for PTSD remain understudied to date, let alone whether these effects may be dependent on sex and hormonal contraception use. Therefore, the present study explored prognostic and prescriptive effects of ANS and HPA axis markers and oxytocin concentrations, all assessed early posttrauma, on PTSD symptom development, while stratifying for sex and hormonal contraception use. This was a secondary analysis of a trial studying the potential of oxytocin administration to prevent PTSD development (van Zuiden et al., 2017).
2. Methods and materials
This study encompasses follow-up analyses of a double-blind, placebo-controlled, multicenter RCT evaluating the effects of repeated intranasal oxytocin administration on PTSD symptom development (see (Frijling et al., 2014) for study protocol). Results of the main analyses of the RCT have been published elsewhere (van Zuiden et al., 2017).
2.1. Participants
Potential participants were eligible for inclusion in the RCT if they were recently (≤8 days) traumatized (fulfiling DSM-IV PTSD A1 criterion) adults (18–65 years) with moderate to severe peritraumatic or acute posttrauma distress (defined by cut-off scores ≥5 on the Trauma Screening Questionnaire (Mouthaan, Sijbrandij, Reitsma, Gersons, & Olff, 2014; Walters, Bisson, & Shepherd, 2007) or ≥17 on the Peritraumatic Distress Inventory (Nishi et al., 2010). Exclusion criteria were current PTSD or depression, psychotic, bipolar, substance-related (either reported by participants during screening or objectified during the pre-treatment clinical interview), and personality disorder (in case of a previous formal diagnosis reported by participants), severe or chronic systemic disease, mental retardation, neurological or endocrine disorder, ongoing traumatization, use of medications potentially interfering with oxytocin administration, oxytocin allergy, persistent impaired consciousness or amnesia, pregnancy and breastfeeding at the pre-treatment assessment. For an overview of the study design, participants flow and availability of autonomic and endocrine data, see Table 1.
Table 1.
Study design, participant flow and available data for psychological, endocrine and autonomic parameters of interest.
| |
 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Available data | n (CAPS) |
n (cortisol AUCg) n (CAR AUCi) n (DST AUCg) n (DST AUCi) |
n (oxytocin) n (cortisol) n (ANS) |
Condition n (oxytocin) n (placebo) |
n (CAPS) | n (CAPS) | n (CAPS) | ||
| All participants | 100 | 68 | 74 | 49 | 84 | 78 | 77 | ||
| 68 | 84 | 51 | |||||||
| 31 | 83 | ||||||||
| 31 | |||||||||
| Men | 54 | 36 | 45a | 26 | 43 | 42 | 40 | ||
| 35b | 43b | 28 | |||||||
| 15 | 46 | ||||||||
| 15 | |||||||||
| Women using hormonal contraception | 27 | 20 | 17 | 17 | 26 | 24 | 25 | ||
| 20 | 23c,d | 10 | |||||||
| 11 | 20 | ||||||||
| 11 | |||||||||
| Cycling women | 19 | 12b | 12 | 6 | 15 | 12 | 12 | ||
| 13 | 18 | 13 | |||||||
| 5e | 17 | ||||||||
| 5e | |||||||||
The number of available data is based on the intention-to-treat sample. With regard to autonomic and endocrine parameters, outliers deviating more than 3 SD from the mean were removed. There were no differences in availability of heart rate, high-frequency heart rate variability (HRV) and low-frequency HRV measures; thus, these parameters are summarized as autonomous nervous system (ANS) markers. Missing CAPS (Clinician-Administered PTSD Scale) data at T4, T5 and T6 were imputed for analyses. The pre-defined maximum number of 12 days between trauma and T2 limited the number of performed dexamethasone suppression tests (DSTs). CAR = cortisol awakening response; AUCg = area under the curve with respect to the ground; AUCi = area under the curve with respect to increase. aOne value was removed due to technical problems bOne value was removed as an outlier cTwo values were removed as outliers dOne value was removed as coefficient of variation was ≥10% eLacking sufficient data, cycling women were not considered in the DST linear mixed models.
2.2. Study design
Within 8 days after emergency department admission due to acute exposure to various kinds of traumatic events (most frequently: motor vehicle accidents), participants were screened for eligibility for the RCT via telephone or at the bedside if still hospital admitted (T0, screening). Within 10 days posttrauma, the remaining exclusion criteria were checked and psychometric data gathered, by means of clinical interviews and questionnaires (T1, pre-treatment assessment). Subsequently, participants were enrolled in the RCT. Within 12 days posttrauma, they administered their first, randomly assigned dose of intranasal oxytocin or placebo, under experimenter supervision (T2, treatment initiation). If there was sufficient time before initiation of the intervention (within 12 days posttrauma), post-awakening cortisol output, cortisol awakening response (CAR) and cortisol suppression by dexamethasone were assessed at home at two subsequent days between T1 and T2. Furthermore, at T2, resting biological markers were collected prior to the first administered dose. T2 assessments were scheduled in the afternoon or early evening. Sixty-eight per cent of assessments took place between 12:00pm and 18:00pm. On average, assessments began at 14:30pm. The earliest assessment started at 9:33am and the latest at 20:51pm. Participants watched a nature documentary depicting plants for 25 minutes while HR(V) was recorded. Immediately afterwards, saliva was collected in order to measure resting cortisol and thereafter blood was drawn in order to measure resting oxytocin.
In all biological assessments, participants were asked to record their behaviour with regard to potentially confounding variables, that is, time of day, food, caffeine and alcohol intake, smoking and sports. Participants were asked to refrain from eating, drinking (except water), smoking and sporting 2 hours before T2 initiation. Compliance was inquired upon arrival at T2. The potential covariates were considered in the statistical analyses.
For the following 7 days, participants administered nasal spray twice daily at their homes. Participants were instructed to maintain approximately 12 hours between administrations. Adherence to the intervention regimen and potential adverse events were registered in a diary on a daily basis (see van Zuiden et al., (2017) for more details and related findings). At T1, as well as upon treatment completion at 1.5 (T4), 3 (T5) and 6 (T6) months posttrauma, participants’ PTSD symptoms were assessed with the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995). All interviewers had at least a Bachelor’s degree in psychology or medicine and received standardized training in administering and scoring the interview. There were no significant differences in CAPS total scores from T1 to T6 between interviewers (F(19, 300) = 1.17, p = .29). For a graphical representation of the study design see Table 1. Further information concerning participants, study design, psychometric instruments and pharmacological details, including CONSORT flow diagram and checklist is reported in our previous publication (van Zuiden et al., 2017).
2.3. Assessment of biological parameters
2.3.1. Autonomic nervous system (ANS) markers
HR(V) was recorded using the Polar RS800CX (wristwatch and chest strap), during the 25 minutes resting time participants spent at T2, watching a nature documentary depicting plants. Participants were placed in a sitting position, while spontaneously breathing. Five minute HR(V) samples were obtained from the 25 minutes measurements. All samples were obtained after at least 10 minutes of recording, to ensure a minimum consistent resting period (Shaffer & Ginsberg, 2017). The 5 minutes HR(V) samples were collected approximately 40–45 minutes after start of the T2 assessment. The sampling rate of the device was 1000 Hz, which is sufficiently rapid for accurate HRV measurements (Kuusela, 2013; Shaffer, McCraty, & Zerr, 2014). Raw unfiltered interbeat intervals (RR-intervals) were processed and analysed using the process software Kubios HRV (Standard Software version 3.0.2; University of Eastern Finland). HRV indices were computed with the power spectrum analysis using an autoregressive model without factorization (Wahbeh & Oken, 2013). For statistical analyses, three different parameters were calculated. HR, defined as mean number of heartbeats per minute, was used as indicator of autonomic, predominantly sympathetic activity. Additionally, we investigated HRV, i.e. the beat-to-beat variation in heart rate over time, as index of autonomic regulation. We investigated high-frequency HRV (HF-HRV) power (0.15 to <0.40 Hz frequency band) and low-frequency HRV (LF-HRV) power (0.04 to <0.15 Hz frequency band (Malik et al., 1996; Quintana, Alvares, & Heathers, 2016)). To facilitate interpretation, we opted to report the normalized instead of absolute power. To calculate the percentage spent in each frequency band, we divided the absolute power (in ms2/Hz) of the respective frequency band by the summed absolute power of HF, LF and very-LF bands and multiplied it by 100 (Burr, 2007). Trend components were removed using a time-varying high pass filter (smoothness priors) with a cut-off frequency of 0.018 Hz (lambda 500; Tarvainen, Ranta-Aho, & Karjalainen, 2002)
2.3.2. Hypothalamic-pituitary-adrenal (HPA) axis markers
At T2, after HR(V) recording, participants provided a single saliva sample for measuring resting cortisol concentrations. To account for cortisol’s diurnal rhythm, time of day was considered as a potential covariate. Saliva was collected by Salivettes (Sarstedt, Rommelsdorf, Germany). Samples were immediately stored at −20ºC until analysis. Free cortisol concentrations were determined by an enzyme immunoassay (IBL, Hamburg, Germany). Samples were analysed in duplicate, and all samples of the same participants were run in a single assay. The mean intra-assay coefficient of variation was 2%.
For measuring total morning cortisol output, CAR and its suppression by the dexamethasone suppression test (DST), participants collected saliva samples at home on two subsequent days between T1 and T2. Participants received precise instructions with regard to the sampling protocol and reported sampling protocol adherence in a diary. Collection times were immediately, 15, 30 and 60 min after awakening. At 11pm on the first day, participants took a low dose (0.25 mg) of dexamethasone, aimed to suppress cortisol production on the second day. We decided to use this low dose of dexamethasone instead of the more commonly used 0.5 mg because of previous findings of high prevalence of super-suppression early posttrauma (McFarlane et al., 2011). Participants stored saliva samples in their refrigerators and brought them back to the laboratory for the T2 assessment, where they remained stored at −20°C until further analysis by the same assay as the resting cortisol samples. Although our data collection started prior to publication of expert consensus guidelines for CAR assessments (Stalder et al., 2016), our procedures follow the guidelines’ recommendations, except that waking times were not objectively monitored. For statistical analyses, four different indicators of HPA axis activity were calculated. Area under the curve coefficients were calculated for natural (first day) and the dexamethasone suppressed (second day) morning cortisol, using the formulas provided by Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (2003). As recommended, we distinguished between the area under the curve with respect to the ground (AUCg), indicating total cortisol production during the first hour after awakening, and the AUC with respect to increase (AUCi), indicating the CAR, i.e. amount of cortisol production relative to the first measurement after awakening (Stalder et al., 2016).
2.3.3. Oxytocin
Resting oxytocin concentrations were measured in blood plasma at T2 after HR(V) recording and saliva collection. Plasma was collected into 7 ml EDTA tubes, placed on ice immediately after sampling, centrifuged at 4°C and stored at −80°C until analysis. Oxytocin was quantified by a highly specific and sensitive radioimmunoassay (RIA) using 0.8 ml of plasma (RIAgnosis, Munich, Germany) as previously described (Kagerbauer et al., 2013). All samples were extracted prior to analysis and analysed simultaneously in the same assay. The sensitivity was 0.1 pg per sample and the intra- and inter-assay variability was <10%. All samples were within detection limit.
2.4. Statistical analyses
Prognostic and prescriptive effects were tested by means of linear mixed models. The baseline model included the variables treatment condition and time as well as their interactions, as predictors, and – in accordance with the main RCT publication (van Zuiden et al., 2017) – square root-transformed CAPS scores at T4, T5 and T6 as outcomes. Concerning the predictor treatment condition, we applied unweighted contrast coding (−0.5 for placebo condition and 0.5 for oxytocin condition, as done by Fournier et al. (2009)) this means, the effects of oxytocin administration were compared with the effects of placebo administration as a reference. The predictor time was metric and indicated time in months between T4, T5 and T6 (0 = T4, 1.5 = T5, 4.5 = T6). Accordingly, in addition to modelling random intercept effects and fixed slope effects, the predictors’ effects on CAPS at T4 were indicated by the intercept and their effects on the change from T4 to T6 were indicated by the slope. The outcome CAPS was metric and positively skewed and therefore square root-transformed. In line with our aim to specifically consider sex and hormonal contraception use, analyses were performed separately in men, women using hormonal contraception and cycling women. In order to subsequently analyse prognostic and prescriptive effects of biological parameters early posttrauma, we followed the stepwise approach recommended by Fournier et al. (2009), as we also did in our previous paper (van Zuiden et al., 2017). Following this approach, we subsequently added the biological parameters of interest as predictors to the baseline model. A linear mixed effects model was tested per biological parameter (i.e. HR, HF-HRV, LF-HRV, resting cortisol, morning cortisol AUCg, CAR AUCi, DST AUCg, DST AUCi and resting oxytocin) and per group (men, women using hormonal contraception and cycling women). Distributions of HPA axis parameters and resting oxytocin concentrations were positively skewed. Therefore, values were log-transformed. For all biological parameters, outliers deviating more than 3 SDs from the mean were removed (see Table 1 for an overview on outlier removal). All biological parameters were z-transformed in order to yield standardized regression coefficients in the analyses, enabling comparisons of the magnitude of effects between parameters.
In a first step, main and interaction effects between the respective biological parameter, treatment condition and time were included in the model. Main effects on the intercept indicated a prognostic effect on PTSD symptoms at T4. Interactive effects of the respective biological variable with time (i.e. on the slope) indicated a prognostic effect on the change from T4 to T6. Interaction effects of the respective biological variable with treatment condition indicated a prescriptive effect at T4.
In a second, third, and fourth step, effects with ps <.20, <.10 and <.05, respectively, were maintained and effects with p-values above these thresholds were excluded. If interaction effects were maintained, the corresponding main effects were maintained too. If p was <.05 in the final model, the effect was considered statistically significant (Fournier et al., 2009).
The robustness of the respective final models was tested by adding relevant covariates. As such, we considered general potential confounders (age, body mass index (BMI), habitual smoking, current employment, current sick leave, recent drug use, having children, number of children, (non-)Dutch ethnicity and time since trauma at T2). For ANS parameters, resting cortisol and oxytocin, we additionally considered time of day at assessment, food, caffeine and alcohol intake, smoking and sports prior to assessment. For morning cortisol, CAR and DST parameters, we considered deviations >5 minutes from the defined sampling timepoints, smoking and caffeine intake during the sampling period, alcohol intake on the sampling day or day before, breakfast intake at the sampling day and whether the sampling day was normal or busy, as potential confounders. In order to select confounders for the mixed effects models, we conducted one-way ANOVAS and χ2 tests to investigate whether men, women using hormonal contraception and cycling women differed with regard to general potential confounders. In case of significant group differences, Bonferroni and Scheffé posthoc tests were performed. Hereby, we ensured that any detected prognostic of prescriptive effect within a specific group was not due to group differences in potential confounders.
Furthermore, we correlated general and specific potential confounders with each biological parameter in each group (results presented in supplementary material 1). Potential confounders that yielded significant results in these analyses were statistically controlled for in the respective mixed effects model. Included metric potential confounders were also z-transformed and for dichotomous potential confounders, weighted contrast coding was applied. If case numbers of dichotomous potential confounders were very small, sensitivity analyses were performed, in order to test whether excluding the respective participants changed the results.
The statistical approach we chose was specifically designed to balance type I and type II error risks in exploratory analyses (Fournier et al., 2009). Therefore, we refrained from correcting for multiple testing and instead would like to emphasize that our approach serves for the purpose of generating, but not testing hypotheses. All mixed effects models were performed in 40 datasets with missing CAPS scores imputed, using auxiliary variables treatment, demographic, trauma, and baseline clinical characteristics as reported before (van Zuiden et al., 2017). Pooled results are reported. All analyses were performed with SPSS software (version 22; IBM Corp., Armonk, NY).
3. Results
3.1. Participants
The original intention-to-treat sample, consisting of n = 107 participants, was split up according to sex and hormonal contraception use. Due to their small number (n = 6), menopausal women were not included in the present analyses and one woman from the original sample had to be excluded because of unknown fertility status. The present analyses are thus based on n = 100 participants: n = 54 men (M = 37.94, SD = 13.68 years), n = 27 women using hormonal contraception (M = 28.74, SD = 9.30 years) and n = 19 naturally cycling women (M = 31.58, SD = 10.71 years). Concerning hormonal contraception, all used methods delivered female gonadal steroids. The following methods were applied: oral contraceptives (n = 19), hormonal intrauterine device (n = 6), hormonal injection (n = 1) and vaginal ring (n = 1). Participants information is presented in Table 2.
Table 2.
Sample description.
| Group differences |
|||||
|---|---|---|---|---|---|
| Men | Women using hormonal contraception | Cycling women | F/χ2 | p | |
| General information | |||||
| Age | 37.94 (13.68) | 28.74 (9.30) | 31.58 (10.72) | 5.76 | <.01 |
| BMI | 25.10 (3.09) | 23.99 (5.24) | 24.86 (5.17) | .63 | .53 |
| Time between traumatic event and T2 | 9.09 (1.64) | 7.85 (1.96) | 9.58 (1.39) | 7.04 | <.01 |
| Habitual smoking (yes/no, %(yes)) | 19/35 (35.19) | 3/24 (11.11) | 4/15 (21.05) | 5.72 | .06 |
| Current employment (yes/no, %(yes)) | 44/10 (81.48) | 25/2 (92.59) | 14/5 (73.68) | 3.02 | .22 |
| Current sick leave (yes/no, %(yes)) | 28/26 (51.85) | 12/15 (44.44) | 11/8 (57.89) | .84 | .66 |
| Recent drug use (yes/no, %(yes)) | 13/41 (24.07) | 8/19 (29.63) | 2/17 (10.53) | 2.37 | .30 |
| Dutch ethnicity (yes/no, %(yes)) | 42/12 (77.78) | 23/4 (85.19) | 11/8 (57.89) | 4.76 | .09 |
| Children (yes/no, %(yes)) | 29/25 (53.70) | 8/19 (29.63) | 10/9 (52.63) | 4.49 | .11 |
| Number of children | 2.22 (1.29) | 1.62 (.77) | 1.88 (.78) | 1.64 | .20 |
| Autonomic and endocrine parameters | |||||
| HR | 68.88 (8.43) | 74.10 (12.73) | 71.62 (9.23) | 2.08 | .13 |
| HF-HRV | 20.52 (13.72) | 31.39 (13.31) | 30.11 (12.59) | 6.08 | <.01 |
| LF-HRV | 50.81 (13.20) | 45.73 (11.06) | 48.56 (12.74) | 1.15 | .32 |
| Resting cortisol (ug/cl) | .38 (.23) | .38 (.16) | .33 (.22) | .62 | .54 |
| Cortisol AUCg | 59.04 (29.09) | 69.20 (31.72) | 68.57 (33.45) | 1.27 | .29 |
| CAR AUCi | .76 (1.18) | .60 (1.21) | 1.18 (1.57) | .74 | .48 |
| DST AUCg | 34.46 (25.47) | 26.37 (22.93) | () | .86 | .36 |
| DST AUCi | .49 (.71) | .30 (.59) | () | .49 | .49 |
| Resting oxytocin (pg/ml) | 1.77 (1.93) | 7.15 (8.57) | 2.70 (4.16) | 6.19 | <.01 |
| PTSD symptoms over time | |||||
| CAPS T1 | 37.20 (18.00) | 44.78 (17,18) | 51.53 (21.28) | 4.67 | .01 |
| CAPT T4 | 24.67 (20.57) | 24.42 (21.60) | 30.07 (27.01) | .11 | .90 |
| CAPS T5 | 17.81 (17.95) | 15.92 (17.51) | 24.08 (25.67) | .29 | .75 |
| CAPS T6 | 14.25 (15.11) | 8.56 (7.91) | 16.58 (18.76) | .86 | .42 |
If not indicated differently, M (SD) is reported. Group differences were determined by means of one-way ANOVAs (for metric variables) or χ2 tests (for dichotomous variables). To facilitate interpretation, non-transformed cortisol and oxytocin concentrations, area under the curve coefficients and post-treatment (T4, T5 and T6) Clinician-Administered PTSD Scale (CAPS) values are reported, although these parameters were log- (biological parameters) or square root-transformed (CAPS scores) for the statistical analyses. Bold values indicate significant p values. BMI = body mass index, HR = heart rate; HF-HRV = heart rate variability, percentage of time spent in the high frequency band (.15 to <.40 Hz); LF-HRV = heart rate variability, percentage of time spent in the low frequency band (.04 to <.15 Hz); CAR = cortisol awakening response; AUCg = area under the curve with respect to the ground; AUCi = area under the curve with respect to increase; DST = dexamethasone suppression test; PTSD = posttraumatic stress disorder; () = parameter was not analysed, lacking sufficient number of participants.
Men, women using hormonal contraception, and cycling women significantly differed in age (F(2, 97) = 5.76, p < .01). Posthoc tests indicated men were significantly older than women using hormonal contraception. Significant group differences were also present with regard to time since trauma at T2 (F(2, 97) = 7.04, p < .01). Posthoc tests indicated that women using hormonal contraception had a shorter time since trauma than both other groups. Moreover, the three groups significantly differed with regard to initial PTSD symptoms (F(2,97) = 4.67, p = .01), with posthoc tests showing that cycling women had higher initial PTSD symptoms than men. No further significant group differences were found.
3.2. Group differences and correlations
Group differences in biological variables are also presented in Table 2. A comprehensive overview of correlations of biological variables with pre-treatment PTSD symptoms is given in supplementary material 2.
HR and LF-HRV did not significantly differ between groups and these parameters were not significantly correlated with pre-treatment PTSD symptoms. Significant group differences emerged for HF-HRV (F(2, 80) = 6.08, p < .01). Posthoc tests showed that men had lower HF-HRV than both female groups. HF-HRV was not significantly correlated with pre-treatment PTSD symptoms.
There were no significant group differences in resting cortisol. In cycling women, higher concentrations were significantly correlated with higher pre-treatment symptoms (r = .48, p < .05). No significant group differences or correlations were detected with regard to morning cortisol AUCg, CAR AUCi, DST AUCg or DST AUCi, but significant differences were found in resting oxytocin (F(2, 71) = 6.19, p < .01). Posthoc tests showed that men had significantly lower concentrations than women using hormonal contraception. Oxytocin was not significantly correlated with pre-treatment PTSD symptoms.
3.3. Prognostic and prescriptive effects
3.3.1. ANS markers
An overview of all linear mixed effects models’ results is provided in supplementary material 3.
No significant prognostic or prescriptive effects of ANS parameters were observed in men, women using hormonal contraception or cycling women. In women using hormonal contraception, we initially detected a significant main effect of HR on the slope. However, when we performed sensitivity analyses, excluding women who reported acute exercise, caffeine or alcohol intake prior to measurement (n = 2), the effect was no longer significant.
3.3.2. HPA axis markers
No significant prognostic or prescriptive effect of resting cortisol, morning cortisol AUCg or CAR AUCi was found.
In men, the final model for DST AUCg yielded a significant main effect on the intercept. It remained significant when controlling for age and time between trauma and T2 but was no longer significant when taking pre-treatment PTSD symptoms into account. In women using hormonal contraception, we detected significant main effects of DST AUCg on the intercept and slope, indicating that stronger morning cortisol suppression was associated with higher PTSD symptoms at 1.5 months, and subsequently, a stronger symptom decrease from 1.5 to 6 months (see Table 3 and Figure 1). These effects remained significant when controlling for age and time between trauma and T2, and when additionally controlling for pre-treatment PTSD symptoms.
Table 3.
Exploration of possible prognostic or prescriptive effects of autonomic and endocrine parameters on posttraumatic stress disorder (PTSD) symptoms 1.5 (intercept), 3 and 6 months posttrauma (linear slope).
| Effect on the intercept |
Effect on the linear slope |
|||||||
|---|---|---|---|---|---|---|---|---|
| b (SEM) | t | p | 95% CI | b (SEM) | t | p | 95% CI | |
| Effect of DST AUCg in women using hormonal contraception (n = 11) | ||||||||
| Reference | 4.34 (.65) | 6.70 | <.01 | 3.07; 5.61 | −.44 (.09) | −4.75 | <.01 | −.62; −.26 |
| Age | −.11 (.36) | −.31 | .76 | −.82; .60 | ||||
| Days between trauma and T2 | .63 (.81) | .78 | .44 | −.96; 2.23 | ||||
| Pre-treatment PTSD symptoms | .78 (.33) | 2.36 | .02 | .13; 1.43 | ||||
| Treatment | 1.92 (.90) | 2.13 | .03 | .16; 3.68 | −.35 (.18) | −1.90 | .06 | −.71; .01 |
| DST AUCg | −1.17 (.28) | −4.11 | <.01 | −1.73; −.61 | .19 (.09) | 2.16 | .03 | .02; .37 |
| Effect of DST AUCi in men (n = 15) | ||||||||
| Reference | 4.15 (.33) | 11.68 | <.01 | 3.45; 4.84 | −.16 (.08) | −2.03 | .04 | −.32; −.00 |
| Age | −.04 (.33) | −.12 | .90 | −.68; .60 | ||||
| Days between trauma and T2 | .13 (.40) | .32 | .75 | −.66; .92 | ||||
| Pre-treatment PTSD symptoms | .49 (.33) | 1.46 | .14 | −.17; 1.14 | ||||
| Treatment | 1.04 (.70) | 1.49 | .14 | −.33; 2.41 | ||||
| DST AUCi | .75 (.34) | 2.17 | .03 | .07; 1.42 | ||||
| Effect of resting oxytocin in women using hormonal contraception (n = 17) | ||||||||
| Reference | 4.63 (.35) | 13.28 | <.01 | 3.95; 5.32 | −.43 (.10) | −4.16 | <.01 | −.63; −.23 |
| Age | .37 (.30) | 1.24 | .22 | −.21; .95 | ||||
| Days between trauma and T2 | −.10 (.30) | −.32 | .75 | −.68; .49 | ||||
| Pre-treatment PTSD symptoms | .34 (.35) | .96 | .34 | .36; 1.03 | ||||
| Treatment | −.26 (.79) | −.34 | .74 | −1.80; 1.28 | −.17 (.21) | −.83 | .40 | −.58; .23 |
| Resting oxytocin | .87 (.33) | 2.67 | .01 | .23; 1.51 | ||||
The table summarizes the final models with significant prognostic or prescriptive effects of autonomic or endocrine parameters. Effects of morning cortisol suppression by dexamethasone (DST, area under the curve with respect to the ground, AUCg), cortisol awakening response suppression by DST (area under the curve with respect to increase AUCi) and resting endogenous oxytocin concentrations on post-traumatic stress disorder (PTSD) symptoms over follow-up time points in men and women using hormonal contraception are presented. Age, days between trauma and T2, pre-treatment PTSD symptoms and the endocrine parameters are metric and were z-transformed. Treatment condition, a dichotomous variable, was unweighted contrast coded (−.5 = placebo condition and .5 = oxytocin condition). Reference indicates the effect of all parameters set at their mean. Bold values indicate significant p values.
Figure 1.
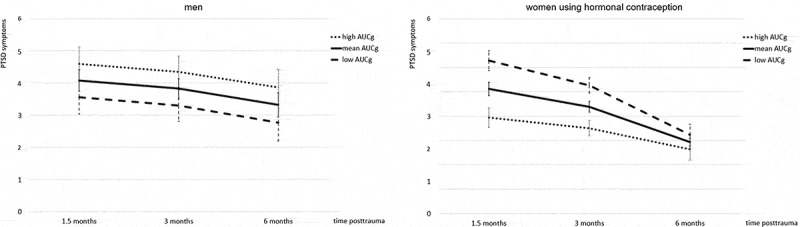
Effect of morning cortisol suppression by dexamethasone (area under the curve with respect to the ground, AUCg) on posttraumatic stress disorder (PTSD) symptoms over follow-up time points in men and women using hormonal contraception. Predicted M and SEM of square root-transformed total Clinician-Administered PTSD Scale (CAPS) scores at the respective measurement point are presented for participants with high (M + 1 SD), mean (M) and low (M – 1SD) AUCg. Lower AUCg values indicate stronger suppression by dexamethasone. i.e. stronger glucocorticoid feedback sensitivity. Predictions are based on the final model for women using hormonal contraception (n = 11), as presented in Table 3. The same model was applied to men. In this group, it did not result in significant effects. Predictions are controlled for age, time between traumatic event and T2 as well as pre-treatment PTSD symptoms.
In men, the final model for DST AUCi included a significant prognostic effect on the intercept, indicating that stronger CAR suppression by dexamethasone was associated with lower PTSD symptoms 1.5 months posttrauma (see Table 3 and Figure 2). The effect remained significant when controlling for age and time between trauma and T2 and also when additionally controlling for pre-treatment PTSD symptoms. In women using hormonal contraception, no significant prognostic or prescriptive effect of DST AUCi was found.
Figure 2.

Effect of cortisol awakening response suppression by dexamethasone (area under the curve with respect to the ground, AUCg) on post-traumatic stress disorder (PTSD) symptoms over follow-up time points in men and women using hormonal contraception. Predicted M and SEM of square root-transformed total Clinician-Administered PTSD Scale (CAPS) scores at the respective measurement point are presented for participants with high (M + 1 SD), mean (M) and low (M – 1SD) AUCg. Lower AUCg values indicate stronger suppression by dexamethasone. i.e. stronger glucocorticoid feedback sensitivity. Predictions are based on the final model for men (n = 15), as presented in Table 3. The same model was applied to women using hormonal contraception. In this group, it did not result in significant effects. Predictions are controlled for age, time between traumatic event and T2 as well as pre-treatment PTSD symptoms.
3.3.3. Oxytocin
In men and cycling women, we detected no significant prognostic or prescriptive effect of resting oxytocin. In women using hormonal contraception, a significant main effect on the intercept was found, indicating that higher oxytocin concentrations were associated with higher PTSD symptoms at 1.5 months (see Table 3 and Figure 3). The effect remained significant when controlling for age and time between trauma and T2 and when we additionally took pre-treatment PTSD symptoms into account.
Figure 3.

Effect of resting endogenous oxytocin concentrations on post-traumatic stress disorder (PTSD) symptoms over follow-up time points in women using hormonal contraception. Predicted M and SEM of square root-transformed total Clinician-Administered PTSD Scale (CAPS) scores at the respective measurement point are presented for women with high (M + 1 SD), mean (M) and low (M – 1SD) oxytocin concentrations. Predictions are based on the final model presented in Table 3, based on n = 17 women and controlled for age, time between traumatic event and T2 as well as pre-treatment PTSD symptoms.
4. Discussion
We investigated possible prognostic and prescriptive effects of biological parameters, measured within 12 days posttrauma, in a secondary analysis of an RCT evaluating repeated intranasal oxytocin administration as a preventive intervention for PTSD. Specifically, we investigated effects of HR(V), resting cortisol, morning cortisol and CAR, cortisol suppression by the DST, and resting oxytocin in men, women using hormonal contraception and cycling women. The analyses revealed no prescriptive effects (i.e. no significant interaction effect between the respective biological variables and treatment condition), indicating that the main effect of intranasal oxytocin administration was independent of pre-treatment biological states. Also, no prognostic effects of HR, HF-HRV, LF-HRV, resting cortisol, morning cortisol and CAR were found. Yet, the stratified analyses revealed prognostic effects of cortisol suppression and resting oxytocin on PTSD symptom severity that were sex- and hormonal contraception use-dependent. Stronger CAR suppression upon dexamethasone ingestion was associated with lower PTSD symptoms at 1.5 months in men, an effect that remained stable across follow-up. In contrast, in women using hormonal contraception, stronger morning cortisol suppression was associated with higher symptoms at 1.5 months, but also with a stronger subsequent decrease in symptoms from 1.5 to 6 months posttrauma; resulting in no difference in long-term outcome between those with high and low suppression. In women using hormonal contraception, higher oxytocin concentrations were also associated with higher PTSD symptoms at 1.5 months and also across follow-up timepoints.
Previous research has shown that autonomic dysregulation during the acute posttrauma period predicted subsequent PTSD risk (Mellman et al., 2004; Mikolajewski & Scheeringa, 2018; Minassian et al., 2015; Morris et al., 2016; Pyne et al., 2016; Shaikh Al Arab et al., 2012) We assessed HR(V) parameters during rest within the first 12 days posttrauma and did not observe any prognostic effects. The comparison with previous findings suggests that resting HR(V) measured before or acutely after trauma has prognostic value, whereas this value diminishes over time, supporting the recommendation to apply a stage-dependent approach to investigate biological mechanisms associated with PTSD (McFarlane, Lawrence-Wood, van Hooff, Malhi, & Yehuda, 2017). It also indicates that in the early period posttrauma, prospective information provided by biological assessments may vary on a day-to-day or week-to-week timescale.
We assessed HPA axis activity by means of various complementary parameters. To the best of our knowledge, this was the first study conducted in the early posttrauma period that assessed the dynamics of cortisol output in response to awakening. Concurring with our results, several prospective studies that assessed pretrauma morning cortisol and CAR did not find prognostic effects (Heinrichs et al., 2005; van Zuiden et al., 2011). Our negative finding on resting cortisol seems to be in line with the absence of a significant meta-analytic prognostic effect of cortisol, assessed within 72 hours posttrauma (Morris et al., 2016), and with several additional studies assessing the prognostic effect of cortisol with higher time intervals since trauma (Bonne et al., 2003; Ehring, Ehlers, Cleare, & Glucksman, 2008; McFarlane et al., 1997; Price et al., 2014; Resnick et al., 1995). However, it was previously reported that while lower cortisol measured within hours posttrauma predicted higher PTSD symptoms 2 weeks and 6 months posttrauma, cortisol concentrations measured just 1 day later did not (Ehring et al., 2008). This observation is interesting as this time window within the first hours posttrauma coincides with the initial phase of acute stress recovery, during which GR-induced negative feedback on the HPA axis occurs. This fits with previously observed prognostic effects of GR sensitivity and function, as assessed pretrauma and 2 days posttrauma (McFarlane et al., 1997; van Zuiden et al., 2012, 2011, 2012, 2013). However, these studies observed that higher pretrauma GR sensitivity and function predicted subsequent PTSD symptoms in male and mixed-gender samples, while in our study, lower GR sensitivity, reflected in decreased cortisol suppression by dexamethasone, within 12 days posttrauma was predictive for higher PTSD symptoms in men. Thus, again, the comparison with previous evidence suggests that time since trauma at the assessment is crucial for prognostic effects.
With regard to the prognostic effects of GR feedback sensitivity detected in our study, one additional aspect seems particularly worth discussing, namely that the detected effects were sex- and hormonal contraception use-specific. As discussed, in men, low GR feedback sensitivity, reflected in higher AUCi after DST, was associated with PTSD symptom development. In contrast, in women using hormonal contraception, high GR feedback sensitivity, reflected in lower AUCg after DST, was a prognostic factor. In men, the prognostic effect of the AUCg was no longer significant after accounting for acute PTSD symptoms before intervention onset, which can be explained by the medium-sized correlation between the two parameters (see supplementary material 2). In women using hormonal contraception, the effect remained significant after controlling for acute symptom severity. The observed differential effects of biological prognostic markers might be explained by participants’ gonadal steroid-related statuses, as these regulate the biological stress response systems (Kirschbaum et al., 1999). Specifically, interactions between testosterone, oestrogens and cortisol can be assumed (Ferree & Cahill, 2009; Glover et al., 2012; Milad et al., 2010). Hormonal contraception use is associated with low but stable levels of endogenous oestradiol and elevated levels of exogenous oestrogens, and generally decreases testosterone output (Glover et al., 2012). Based on experimental evidence (Ferree & Cahill, 2009; Ferree, Kamat, & Cahill, 2011; Glover et al., 2012; Milad et al., 2010; Soni, Curran, & Kamboj, 2013), we previously hypothesized that elevated oestrogens concentrations might promote extinction of traumatic memories and thereby prevent PTSD development in women using hormonal contraception (Engel et al., 2019). At the same time, these experimental studies investigated naturally cycling women with different concentrations of endogenous oestradiol. Another study which specifically investigated women using hormonal contraception found that their responses during extinction learning and recall were more comparable to those of naturally cycling women with low concentrations of endogenous oestradiol (Hwang et al., 2015). This implies that the effects of endogenous and exogenous oestrogens differ and it remains an interesting question whether and if so, which precise oestrogens- or testosterone-related biological mechanisms underlie the observed prognostic value of GR sensitivity for PTSD risk in men and women using hormonal contraception. Even though the impact of sex and gonadal steroids has largely been ignored in previous studies, there are indications for its relevance for understanding the biological underpinnings of PTSD development (Kornfield, Hantsoo, & Epperson, 2018). The necessity to consider these factors has also been emphasized within the current sample, as we previously observed that women using hormonal contraception had lower PTSD symptoms at 3 and 6 months posttrauma than men and cycling women, irrespective of treatment (Engel et al., 2019).
As suggested by previous evidence (Stock et al., 1994) and confirmed in our data, women using hormonal contraceptives showed higher oxytocin concentration than men and cycling women. Within this group, higher oxytocin concentrations pre-treatment predicted higher symptoms across timepoints, suggesting that relatively high oxytocin concentrations in the early posttrauma period may increase subsequent PTSD risk. In line with this, one of our analyses detected adverse effects of intranasal oxytocin administration in women using hormonal contraception. The treatment effect on symptoms 1.5 months posttrauma was not significant in the main analysis within women using hormonal contraception (Engel et al., 2019). However, it became significant when considering GR feedback sensitivity. Women who used hormonal contraception and were treated with oxytocin, as compared to placebo, reported higher PTSD symptoms at 1.5, but no longer at 3 and 6 months (see Table 3). It needs to be noted that this effect only emerged under the very specific condition of controlling for GR feedback sensitivity, which incidentally was a prognostic factor for increased PTSD symptom severity at 1.5 months post-trauma but not at later time points. Thus, our findings provide a preliminary indication that intranasal oxytocin administration should be applied cautiously to women using hormonal contraception. Moreover, it further supports the notion that this randomized controlled trial warrants replication with an even more detailed evaluation of sex-specific biological mechanisms underlying recovery from acute trauma.
4.1. Strengths and limitations
Concomitantly assessing a range of parameters reflecting three distinct biological systems involved in the acute stress response allowed us to describe and compare their respective prognostic and prescriptive effects. The time point of assessment within 12 days posttrauma provided insights into their potential role during the phase of early recovery from traumatic stress, specifically. This is important as several dysregulations in biological pathways previously associated with increased PTSD vulnerability are specifically involved in this phase (Bryant, 2003; Zuj, Palmer, Lommen, & Felmingham, 2016). Moreover, the timepoint has practical relevance for the implementation of preventive interventions since patients are often in contact with the health system acutely or early after trauma.
To date, sex and gonadal steroid use have seldomly been adequately accounted for in this field of biomedical research. Neglecting sex and hormonal contraception use can lead to imprecise results, that are valid only for a population subgroup, if at all. Considering these factors can open up options for personalized patient care (Ferretti, Santuccione-Chadha, & Hampel, 2019). Therefore, our statistical analyses were stratified for sex and hormonal contraception use. This allowed us to consider gonadal steroids as possible source of heterogeneity in previous research and responds to the call for more sex- and gender-sensitive biomedical research (Bale & Epperson, 2017; Olff, 2016).
Despite these strengths of this study, some limitations need to be mentioned, as well. The concomitant investigation of different prognostic or prescriptive markers in three different groups could only be implemented with multiple statistical tests. We chose the specific statistical approach as it was developed to balance type I and type II error risks (Fournier et al., 2009). However, due to the large number of statistical tests reported here, the risk of type I error is still increased. Therefore, all analyses we reported are exploratory. Further hypotheses-testing studies could specifically target oxytocin and GR feedback sensitivity, as well as their interactions with gonadal steroids.
In order to specifically target a high-risk group for PTSD, we only included patients that indicated high peritraumatic and immediate distress. Furthermore, in order to specifically investigate the efficacy of oxytocin in preventing PTSD development instead of a potential treatment effect on pre-existing psychiatric symptoms, we excluded participants with current PTSD, depression, psychotic, bipolar, substance-related and personality disorder at the time of traumatic event exposure. Therefore, the interpretation of prognostic effects is limited to this specific population. We cannot exclude the possibility that the biological markers we investigated would be differentially associated with PTSD development within individuals less psychologically distressed in the acute aftermath of a traumatic event or in individuals with previous psychiatric disorders. Therefore, our findings should not only be replicated within a larger, independent, but also within a more diverse sample of traumatized individuals.
All predictions we made in this study were based on methods that enable the identification of risk and protective factors on a group level. However, other methodological approaches requiring larger samples enable more precise estimation of individual risk based on unique characteristics of an individual (Schultebraucks & Galatzer-Levy, 2019). Such machine learning approaches could additionally improve personalized patient care in the future.
We did not detect any effects in cycling women, which might be attributable to their small sample size – the effects of GR feedback sensitivity could not even be tested in this group – or to the remaining heterogeneity within this group due to different cycle phases. It was a specific concern of our study to differentiate effects of sex and hormonal contraception use, in order to consider interactions with gonadal steroids that have previously been suggested (Engel et al., 2019; Kamkwalala et al., 2012; Kirschbaum et al., 1999; Nicolson & Ponnamperuma, 2019; Stock et al., 1994). However, future studies should specifically investigate cycling women and the impact of menstrual cycle phases. In addition, as their small number disabled us to conduct our analyses in menopausal women, more attention should be given to this group, too.
Additionally, total PTSD symptom severity was assessed as outcome. However, biologically mechanisms underlying development and maintenance of specific PTSD symptoms should be investigated in more detail. In this regard, as GR feedback sensitivity and oxytocin impact memory consolidation and retrieval (de Quervain, Schwabe, & Roozendaal, 2017; McEwen, 2004), it seems interesting to investigate their effects with regard to the development of intrusions specifically. However, to refrain from additional statistical testing, we decided not to pursue these analyses here.
Although we preferentially scheduled T2 assessments in the afternoon or early evening, this was not always possible. As a result, variability in the timing of the resting cortisol and oxytocin assessments occurred. Therefore, we considered timing of the T2 assessment as potential covariate. Additionally, it remains unknown whether variability in timing of the repeated oxytocin administration between and within participants may have influenced the clinical effects of the intervention to some extent. Participants were asked to maintain approximately 12 hours between administrations, but the exact administration times were not standardized. Meta-analytic evidence supports the existence of a diurnal rhythm in endogenous oxytocin (Engel et al., 2019). However, to the best of our knowledge, it was not previously investigated whether timing of day during exogenous oxytocin administration, let alone repeated administration, influences its effects.
4.2. Conclusion
Our study is part of a promising, growing field of study in search of biological predictors of PTSD development and (preventive) treatment response. With the aim of personalizing patient care and precision medicine, one might envision that biological markers could ultimately be used to inform patients about their likelihood to develop PTSD symptoms, to provide them with the preventive treatment they are most likely to benefit from and to prevent them from receiving unnecessary or ineffective treatment. Our exploratory analyses investigated a broad range of biological parameters, enabling a comparative assessment of their use as prognostic or prescriptive markers when assessed within few days posttrauma. We did not detect prescriptive effects but found prognostic effects of GR feedback sensitivity and resting oxytocin that depended on sex and hormonal contraception use and can be followed up by future research.
Supplementary Material
Acknowledgments
We express our sincere gratitude to J.C. Goslings, PhD, MD; J.S.K. Luitse, MD; T. Biesheuvel, MD; and A. Honig, PhD, MD and all other involved emergency department and trauma surgery department personnel for their invaluable facilitation of and contribution to our study. We also thank Kim van Dijk, MSc, and Saleha Tariq, MSc, for their valuable assistance in data collection, as well as Inga Neumann, PhD for her support regarding analyses and interpretation of the oxytocin assays.
Funding Statement
This study was supported by grants from the Netherlands organization for Health research and Development (ZonMw, grant no. 91210041) and from the Academic Medical Center Research Council (grant no. 110614). The ‘Stiftung der Deutschen Wirtschaft’ provided doctoral funding for Sinha Engel. Additionally, Mirjam van Zuiden was supported by a Veni grant from the Netherlands organization for Health research and Development (ZonMw, grant no. 91617037). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
All authors have contributed to the manuscript and have approved the present version. Miranda Olff designed the initial concept of the RCT and obtained funding. Mirjam van Zuiden, Jessie Frijling, Saskia Koch, Laura Nawijn and Dick Veltman contributed to the design of the study. Mirjam van Zuiden coordinated data acquisition by Jessie Frijling, Saskia Koch and Laura Nawijn. Sinha Engel and Mirjam van Zuiden conceptualized the current study concerning secondary analyses of the RCT. Sinha Engel performed all data analyses. Jos Bosch oversaw the cortisol assays and aided in critical interpretation of resulting statistical findings. HR(V) data preparation was performed by Rinde Yildiz and Jessie Frijling. Sinha Engel and Mirjam van Zuiden drafted the manuscript. All authors were involved in critical interpretation of results and have approved and added to the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Alley, J., Diamond, L. M., Lipschitz, D. L., & Grewen, K. (2019). Associations between oxytocin and cortisol reactivity and recovery in response to psychological stress and sexual arousal. Psychoneuroendocrinology, 106, 47–17. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) , (Ed.). (2013). Diagnostic and statistical manual of mental disorders. DSM-5. Arlington, VA: Author. [Google Scholar]
- Andrews, J., Ali, N., & Pruessner, J. C. (2013). Reflections on the interaction of psychogenic stress systems in humans: The stress coherence/compensation model. Psychoneuroendocrinology, 38(7), 947–961. [DOI] [PubMed] [Google Scholar]
- Bale, T. L., & Epperson, C. N. (2017). Sex as a biological variable: Who, what, when, why, and how. Neuropsychopharmacology, 42, 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet, C., Bromet, E., Karam, E. G., Kessler, R. C., McLaughlin, K. A., Ruscio, A. M., & Koenen, K. C. (2016). The epidemiology of traumatic event exposure worldwide: Results from the World Mental Health Survey Consortium. Psychological Medicine, 46(2), 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Bonne, O., Brandes, D., Segman, R., Pitman, R. K., Yehuda, R., & Shalev, A. Y. (2003). Prospective evaluation of plasma cortisol in recent trauma survivors with posttraumatic stress disorder. Psychiatry Research, 119(1–2), 171–175. [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. (2003). Early predictors of posttraumatic stress disorder. Biological Psychiatry, 53(9), 789–795. [DOI] [PubMed] [Google Scholar]
- Burr, R. L. (2007). Interpretation of normalized spectral heart rate variability indices in sleep research: A critical review. Sleep, 30, 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos, G. P., & Gold, P. W. (1992). The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA, 267(9), 1244–1252. [PubMed] [Google Scholar]
- de Quervain, D., Schwabe, L., & Roozendaal, B. (2017). Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nature Reviews Neuroscience, 18, 7–19. [DOI] [PubMed] [Google Scholar]
- Dunlop, B. W., Mansson, E., & Gerardi, M. (2012). Pharmacological innovations for posttraumatic stress disorder and medication-enhanced psychotherapy. CPD, 18, 5645–5658. [DOI] [PubMed] [Google Scholar]
- Ehring, T., Ehlers, A., Cleare, A. J., & Glucksman, E. (2008). Do acute psychological and psychobiological responses to trauma predict subsequent symptom severities of PTSD and depression? Psychiatry Research, 161, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, S., Klusmann, H., Ditzen, B., Knaevelsrud, C., & Schumacher, S. (2019). Menstrual cycle-related fluctuations in oxytocin concentrations: A systematic review and meta-analysis. Frontiers in Neuroendocrinology, 52, 144–155. [DOI] [PubMed] [Google Scholar]
- Engel, S., Laufer, S., Miller, R., Niemeyer, H., Knaevelsrud, C., & Schumacher, S. (2019). Demographic, sampling- and assay-related confounders of endogenous oxytocin concentrations: A systematic review and meta-analysis. Frontiers in Neuroendocrinology, 54, 100775. [DOI] [PubMed] [Google Scholar]
- Engel, S., van Zuiden, M., Frijling, J. L., Koch, S. B. J., Nawijn, L., Schumacher, S., … Olff, M. (2019). Patterns of recovery from early posttraumatic stress symptoms after a preventive intervention with oxytocin: Hormonal contraception use is a prognostic factor. Biological Psychiatry, 85(12), e71–e73. [DOI] [PubMed] [Google Scholar]
- Engelmann, M., Landgraf, R., & Wotjak, C. T. (2004). The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: An old concept revisited. Frontiers in Neuroendocrinology, 25, 132–149. [DOI] [PubMed] [Google Scholar]
- Ferree, N. K., & Cahill, L. (2009). Post-event spontaneous intrusive recollections and strength of memory for emotional events in men and women. Consciousness and Cognition, 18(1), 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree, N. K., Kamat, R., & Cahill, L. (2011). Influences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Consciousness and Cognition, 20(4), 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti, M. T., Santuccione-Chadha, A., & Hampel, H. (2019). Account for sex in brain research for precision medicine. Nature, 569, 40. [DOI] [PubMed] [Google Scholar]
- Fournier, J. C., DeRubeis, R. J., Shelton, R. C., Hollon, S. D., Amsterdam, J. D., & Gallop, R. (2009). Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. Journal of Consulting and Clinical Psychology, 77(4), 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling, J. L., van Zuiden, M., Koch, S. B. J., Nawijn, L., Goslings, J. C., Luitse, J. S., … Olff, M. (2014). Efficacy of oxytocin administration early after psychotrauma in preventing the development of PTSD: Study protocol of a randomized controlled trial. BMC Psychiatry, 14(1), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy, I. R., Huang, S. H., & Bonanno, G. A. (2018). Trajectories of resilience and dysfunction following potential trauma: A review and statistical evaluation. Clinical Psychology Review, 63, 41–55. [DOI] [PubMed] [Google Scholar]
- Garcia, M. A., & Delahanty, D. L. (2017). Oxytocin and other pharmacologic preventive interventions for posttraumatic stress disorder: Not a one-size-fits-all approach. Biological Psychiatry, 81(12), 977–978. [DOI] [PubMed] [Google Scholar]
- Glover, E. M., Jovanovic, T., Mercer, K. B., Kerley, K., Bradley, B., Ressler, K. J., & Norrholm, S. D. (2012). Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry, 72(1), 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim, C., & Nemeroff, C. B. (2016). Neurobiological pathways involved in fear, stress, and PTSD. In Liberzon I. & Ressler K. J. (Eds.), Neurobiology of PTSD: From brain to mind (pp. 220–238). Oxford, UK: Oxford University Press. [Google Scholar]
- Heinrichs, M., von Dawans, B., & Domes, G. (2009). Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology, 30(4), 548–557. [DOI] [PubMed] [Google Scholar]
- Heinrichs, M., Wagner, D., Schoch, W., Soravia, L. M., Hellhammer, D. H., & Ehlert, U. (2005). Predicting posttraumatic stress symptoms from pretraumatic risk factors: A 2-year prospective follow-up study in firefighters. American Journal of Psychiatry, 162(12), 2276–2286. [DOI] [PubMed] [Google Scholar]
- Horn, S. R., Charney, D. S., & Feder, A. (2016). Understanding resilience: New approaches for preventing and treating PTSD. Experimental Neurology, 284, 119–132. [DOI] [PubMed] [Google Scholar]
- Hwang, M. J., Zsido, R. G., Song, H., Pace-Schott, E. F., Miller, K. K., Lebron-Milad, K., & Milad, M. R. (2015). Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry, 15(1), 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek, B., & Neumann, I. D. (2018). The oxytocin receptor: From intracellular signaling to behavior. Physiological Reviews, 98(3), 1805–1908. [DOI] [PubMed] [Google Scholar]
- Kagerbauer, S. M., Martin, J., Schuster, T., Blobner, M., Kochs, E. F., & Landgraf, R. (2013). Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. Journal of Neuroendocrinology, 25(7), 668–673. [DOI] [PubMed] [Google Scholar]
- Kamkwalala, A., Norrholm, S. D., Poole, J. M., Brown, A., Donley, S., Duncan, E., … Jovanovic, T. (2012). Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: Putative sex-specific correlates of posttraumatic stress disorder. Psychosomatic Medicine, 74(2), 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C., Aguilar-Gaxiola, S., Alonso, J., Benjet, C., Bromet, E. J., Cardoso, G., & Koenen, K. C. (2017). Trauma and PTSD in the WHO World Mental Health Surveys. European Journal of Psychotraumatology, 8(sup5), 1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162. [DOI] [PubMed] [Google Scholar]
- Kornfield, S. L., Hantsoo, L., & Epperson, C. N. (2018). What does sex have to do with it? The role of sex as a biological variable in the development of posttraumatic stress disorder. Current Psychiatry Reports, 20(6), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela, T. (2013). Methodological aspects of heart rate variability analysis. In Kamath M. V., Watanabe M., & Upton A. (Eds.), Heart rate variability (HRV) signal analysis (pp. 9–41). Boca Raton: CRC Press. [Google Scholar]
- Malik, M., Camm, A. J., Bigger, J. T., Breithardt, G., Cerutti, S., Cohen, R. J., ... Singer, D. H. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043–1065. [PubMed] [Google Scholar]
- McEwen, B. B. (2004). Brain–fluid barriers: Relevance for theoretical controversies regarding vasopressin and oxytocin memory research. In McEwen B. B. (Ed.), The roles of vasopressin and oxytocin in memory processing (pp. 531–592). Amsterdam: Elsevier Academic Press. [DOI] [PubMed] [Google Scholar]
- McFarlane, A. C., Atchison, M., & Yehuda, R. (1997). The acute stress response following motor vehicle accidents and its relation to PTSD. Annals of the New York Academy of Sciences, 821, 437–441. [DOI] [PubMed] [Google Scholar]
- McFarlane, A. C., Barton, C. A., Yehuda, R., & Wittert, G. (2011). Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology, 36, 720–727. [DOI] [PubMed] [Google Scholar]
- McFarlane, A. C., Lawrence-Wood, E., van Hooff, M., Malhi, G. S., & Yehuda, R. (2017). The need to take a staging approach to the biological mechanisms of PTSD and its treatment. Current Psychiatry Reports, 19(2), 10. [DOI] [PubMed] [Google Scholar]
- Mellman, T. A., Knorr, B. R., Pigeon, W. R., Leiter, J. C., & Akay, M. (2004). Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biological Psychiatry, 55(9), 953–956. [DOI] [PubMed] [Google Scholar]
- Mikolajewski, A. J., & Scheeringa, M. S. (2018). Examining the prospective relationship between pre-disaster respiratory sinus arrhythmia and post-disaster posttraumatic stress disorder symptoms in children. Journal of Abnormal Child Psychology, 46(7), 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, M. R., Zeidan, M. A., Contero, A., Pitman, R. K., Klibanski, A., Rauch, S. L., & Goldstein, J. M. (2010). The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience, 168(3), 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian, A., Maihofer, A. X., Baker, D. G., Nievergelt, C. M., Geyer, M. A., & Risbrough, V. B. (2015). Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry, 72(10), 979–986. [DOI] [PubMed] [Google Scholar]
- Morris, M. C., Hellman, N., Abelson, J. L., & Rao, U. (2016). Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review, 49, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthaan, J., Sijbrandij, M., Luitse, J. S. K., Goslings, J. C., Gersons, B. P. R., & Olff, M. (2014). The role of acute cortisol and DHEAS in predicting acute and chronic PTSD symptoms. Psychoneuroendocrinology, 45, 179–186. [DOI] [PubMed] [Google Scholar]
- Mouthaan, J., Sijbrandij, M., Reitsma, J. B., Gersons, B. P. R., & Olff, M. (2014). Comparing screening instruments to predict posttraumatic stress disorder. PloS One, 9(5), e97183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Clinical Excellence . (2018). Clinical guideline 116: Post-traumatic stress disorder: Evidence reviews for psychological, psychosocial and other non-pharmacological interventions for the treatment of PTSD in adults. Retrieved from https://www.nice.org.uk/guidance/ng116 [PubMed]
- Nicolson, N. A., & Ponnamperuma, T. (2019). Gender moderates diurnal cortisol in relation to trauma and PTSD symptoms: A study in Sri Lankan adolescents. Psychoneuroendocrinology, 104, 122–131. [DOI] [PubMed] [Google Scholar]
- Nishi, D., Hashimoto, K., Noguchi, H., Kim, Y., & Matsuoka, Y. (2015). Serum oxytocin, posttraumatic coping and C-reactive protein in motor vehicle accident survivors by gender. Neuropsychobiology, 71, 196–201. [DOI] [PubMed] [Google Scholar]
- Nishi, D., Matsuoka, Y., Yonemoto, N., Noguchi, H., Kim, Y., & Kanba, S. (2010). Peritraumatic Distress Inventory as a predictor of post-traumatic stress disorder after a severe motor vehicle accident. Psychiatry and Clinical Neurosciences, 64(2), 149–156. [DOI] [PubMed] [Google Scholar]
- Olff, M. (2016). Five years of European Journal of Psychotraumatology. European Journal of Psychotraumatology, 7, 31350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff, M., Frijling, J. L., Kubzansky, L. D., Bradley, B., Ellenbogen, M. A., Cardoso, C., & van Zuiden, M. (2013). The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38(9), 1883–1894. [DOI] [PubMed] [Google Scholar]
- Olff, M., & van Zuiden, M. (2017). Neuroendocrine and neuroimmune markers in PTSD: Pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Current Opinion in Psychology, 14, 132–137. [DOI] [PubMed] [Google Scholar]
- Price, M., Kearns, M., Houry, D., & Rothbaum, B. O. (2014). Emergency department predictors of posttraumatic stress reduction for trauma-exposed individuals with and without an early intervention. Journal of Consulting and Clinical Psychology, 82(2), 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Pyne, J. M., Constans, J. I., Wiederhold, M. D., Gibson, D. P., Kimbrell, T., Kramer, T. L., & McCune, T. R. (2016). Heart rate variability: Pre-deployment predictor of post-deployment PTSD symptoms. Biological Psychology, 121, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana, D. S., Alvares, G. A., & Heathers, J. A. J. (2016). Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Translational Psychiatry, 6(5), e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana, D. S., Rokicki, J., van der Meer, D., Alnæs, D., Kaufmann, T., Córdova-Palomera, A., & Westlye, L. T. (2019). Oxytocin pathway gene networks in the human brain. Nature Communications, 10(1), 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnen, A., Geuze, E., & Vermetten, E. (2017). Individual variation in plasma oxytocin and vasopressin levels in relation to the development of combat-related PTSD in a large military cohort. Journal of Psychiatric Research, 94, 88–95. [DOI] [PubMed] [Google Scholar]
- Resnick, H. S., Yehuda, R., Pitman, R. K., & Foy, D. W. (1995). Effect of previous trauma on acute plasma cortisol level following rape. The American Journal of Psychiatry, 152, 1675–1677. [DOI] [PubMed] [Google Scholar]
- Schultebraucks, K., & Galatzer-Levy, I. R. (2019). Machine learning for prediction of posttraumatic stress and resilience following trauma: An overview of basic concepts and recent advances. Journal of Traumatic Stress, 32(2), 215–225. [DOI] [PubMed] [Google Scholar]
- Shaffer, F., & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology, 5, 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh Al Arab, A., Guédon-Moreau, L., Ducrocq, F., Molenda, S., Duhem, S., Salleron, J., … Vaiva, G. (2012). Temporal analysis of heart rate variability as a predictor of post traumatic stress disorder in road traffic accidents survivors. Journal of Psychiatric Research, 46(6), 790–796. [DOI] [PubMed] [Google Scholar]
- Shalev, A. Y., & Barbano, A. C. (2019). PTSD: Risk assessment and early management. Psychiatric Annals, 49(7), 299–306. [Google Scholar]
- Sijbrandij, M., Kleiboer, A., Bisson, J. I., Barbui, C., & Cuijpers, P. (2015). Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: A systematic review and meta-analysis. Lancet Psychiatry, 2, 413–421. [DOI] [PubMed] [Google Scholar]
- Soni, M., Curran, V. H., & Kamboj, S. K. (2013). Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiology of Learning and Memory, 104, 32–38. [DOI] [PubMed] [Google Scholar]
- Stalder, T., Kirschbaum, C., Kudielka, B. M., Adam, E. K., Pruessner, J. C., Wüst, S., … Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen, S., Stalder, T., Schönfeld, S., Wittchen, H.-U., Trautmann, S., Alexander, N., … Kirschbaum, C. (2015). Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology, 59, 123–133. [DOI] [PubMed] [Google Scholar]
- Stock, S., Karlsson, R., & von Schoultz, B. (1994). Serum profiles of oxytocin during oral contraceptive treatment. Gynecological Endocrinology, 8, 121–126. [DOI] [PubMed] [Google Scholar]
- Tarvainen, M. P., Ranta-Aho, P. O., & Karjalainen, P. A. (2002). An advanced detrending method with application to HRV analysis. IEEE Transactions on Bio-medical Engineering, 49, 172–175. [DOI] [PubMed] [Google Scholar]
- Thayer, J. F., Ahs, F., Fredrikson, M., Sollers, J. J., & Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Frijling, J. L., Nawijn, L., Koch, S. B. J., Goslings, J. C., Luitse, J. S., … Olff, M. (2017). Intranasal oxytocin to prevent posttraumatic stress disorder symptoms: A randomized controlled trial in emergency department patients. Biological Psychiatry, 81(12), 1030–1040. [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Geuze, E., Willemen, H. L. D. M., Vermetten, E., Maas, M., Amarouchi, K., & Heijnen, C. J. (2012). Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: A prospective study. Biological Psychiatry, 71(4), 309–316. [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Geuze, E., Willemen, H. L. D. M., Vermetten, E., Maas, M., Heijnen, C. J., & Kavelaars, A. (2011). Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. American Journal of Psychiatry, 168(1), 89–96. [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Heijnen, C. J., Maas, M., Amarouchi, K., Vermetten, E., Geuze, E., & Kavelaars, A. (2012). Glucocorticoid sensitivity of leukocytes predicts PTSD, depressive and fatigue symptoms after military deployment: A prospective study. Psychoneuroendocrinology, 37(11), 1822–1836. [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Kavelaars, A., Geuze, E., Olff, M., & Heijnen, C. J. (2013). Predicting PTSD: Pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventive interventions. Brain, Behavior, and Immunity, 30, 12–21. [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Kavelaars, A., Rademaker, A. R., Vermetten, E., Heijnen, C. J., & Geuze, E. (2011). A prospective study on personality and the cortisol awakening response to predict posttraumatic stress symptoms in response to military deployment. Journal of Psychiatric Research, 45, 713–719. [DOI] [PubMed] [Google Scholar]
- Wahbeh, H., & Oken, B. S. (2013). Peak high-frequency HRV and peak alpha frequency higher in PTSD. Applied Psychophysiology and Biofeedback, 38(1), 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, K., Nugent, N. R., Kotte, A., Amstadter, A. B., Wang, S., Guille, C., & Resnick, H. S. (2013). Cortisol at the emergency room rape visit as a predictor of PTSD and depression symptoms over time. Psychoneuroendocrinology, 38(11), 2520–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, J. T. R., Bisson, J. I., & Shepherd, J. P. (2007). Predicting post-traumatic stress disorder: Validation of the Trauma Screening Questionnaire in victims of assault. Psychological Medicine, 37(1), 143–150. [DOI] [PubMed] [Google Scholar]
- Winter, J., & Jurek, B. (2019). The interplay between oxytocin and the CRF system: Regulation of the stress response. Cell and Tissue Research, 375(1), 85–91. [DOI] [PubMed] [Google Scholar]
- Zuj, D. V., Palmer, M. A., Lommen, M. J. J., & Felmingham, K. L. (2016). The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neuroscience & Biobehavioral Reviews, 69, 15–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- National Institute of Clinical Excellence . (2018). Clinical guideline 116: Post-traumatic stress disorder: Evidence reviews for psychological, psychosocial and other non-pharmacological interventions for the treatment of PTSD in adults. Retrieved from https://www.nice.org.uk/guidance/ng116 [PubMed]


