ABSTRACT
Studies on the prevalence of Cryptosporidium infection in People Living with HIV/AIDS (PLWHA) are inconsistent and heterogeneous. Therefore, this systematic review with meta-analysis was performed to assess the burden of the infection relative to the proportion of CD4 + T cell count among PLWHA in Ethiopia. Articles published before 15 May 2019, have been retrieved for this systematic review using five databases; PubMed, Scopus, Web of Science, Google Scholar and ProQuest, supplemented by the search for gray literature. The overall pooled prevalence and pooled Odds Ratio (OR) with their 95% Confidence Intervals (CI) were estimated using STATA 14 statistical Software. Of the 255 studies retrieved, 31 were considered for the final analysis. As many as 8,645 Ethiopians infected with HIV were included in the final quantitative synthesis. The overall pooled prevalence estimate of Cryptosporidium infection among PLWHA in Ethiopia was 11% (95%CI: 0.09–0.13). HIV-infected people with low CD4 + T cell count (CD4 < 200 cells/mm3) were 13.07 times more likely to become infected with Cryptosporidium than those with high CD4 + T cell count (CD4 > 500 cells/mm3) (OR: 13.07 (95%CI: 6.38–26.75). Cryptosporidium infection in PLWHA in Ethiopia showed decreasing patterns in 2001–2010, 2011–2014, and in 2015–2019, 14.6% (95%CI: 0.076–0.217), 12.71% (95%CI: 0.086–0.167) and 6.7% (95%CI: 0.044–0.090), respectively (p < 0.001). Though the pattern of Cryptosporidium infection in HIV-infected Ethiopians showed a declining trend; it remains a considerable problem that requires improvement in routine screening for Cryptosporidium in HIV-infected people, particularly with poor or declining CD4 + T cell count.
KEYWORDS: Cryptosporidium, human Immunodeficiency virus, CD4+ T cell count, Ethiopia, meta-analysis
Introduction
Acquired Immune Deficiency Syndrome (AIDS) is a significant consequence of infection with a human immune virus (HIV), characterized by the progressive depletion of CD4 + T cells and weakened immune system, leaving the individual at risk of various opportunistic infections [1,2]. Opportunistic infections among people infected with HIV/AIDS could be parasitic, viral, bacterial, and fungal origin [3,4]. As of 2014, two-thirds of PLWHA globally, specifically 25.7 million, were living in Sub-Saharan Africa, where parasitic infections are a major health concern [5]. Immunodeficiency, as a consequence, promotes HIV-parasitic co-infection in the region. In 2017, the number of PLWHA in Eastern and Southern Africa amounts to 19.6 million [6].
Cryptosporidium is a ubiquitous protozoan parasite of humans and a wide range of animals [7]. Globally, the majority of human Cryptosporidium infections are caused by Cryptosporidium hominis and Cryptosporidium parvum: however, Cryptosporidium meleagridis, Cryptosporidium felis, Cryptosporidium canis, Cryptosporidium muris, Cryptosporidium suis, and Cryptosporidium andersoni were also infrequently isolated from humans [8–10]. Cryptosporidium is transmitted mainly by the fecal-oral route. Human to human (anthroponotic) as well as animal to human (zoonotic) transmissions have also been documented. Though cryptosporidiosis has a worldwide distribution, its occurrence is higher in low-income countries with limited access to the necessary infrastructure and potable water. Consequently, people are left struggling with the necessary facilities to prevent food and water contaminated by infective oocysts [11]. Cryptosporidium usually causes asymptomatic or self-limiting diarrhea in healthy immunocompetent persons; however, it can progress to chronic diarrhea in immunocompromised individuals, including PLWHA [7]. This may results in significant morbidity and mortality if appropriate measures are not promptly instituted [12].
Anti-retroviral therapy (ART) has dramatically enhanced survival and quality of life and reduces the risk of acquiring opportunistic pathogens in PLWHA. However, opportunistic parasites such as Cryptosporidium species (spp), Toxoplasma gondii, Cyclospora cayetanensis, Isospora belli, and Microsporidia spp, continue to be reported in PLWHA across the globe [1,13] including Ethiopia [14–17]. It is estimated that 80% of deaths from AIDS are attributed to opportunistic infections rather than the virus itself, and of these, up to a third of death from AIDS is attributed to opportunistic intestinal parasitic infections [1,18].
In Ethiopia, multiple individual studies have been conducted on the magnitude of cryptosporidiosis in HIV/AIDS patients [16,17,19–22]. These studies reported an inconsistent and wide-ranging prevalence of cryptosporidiosis among HIV/AIDS patients at different times and geographical regions. Hitherto, there is concrete or representative information on the magnitude of cryptosporidiosis in HIV/AIDS patients in Ethiopia. We, therefore, hoped that this report would have implications for future planning of effective control strategies against opportunistic parasitic infections including cryptosporidiosis in PLWHA in Ethiopia and the entire region in general.
Materials and methods
This systematic review and meta-analysis were conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [23]. Study reports which were pertinent to the outcomes of interest were included in the quantitative synthesis using the guideline. The primary outcome of interest was the proportion of HIV/AIDS patients concurrently infected with Cryptosporidium. Cryptosporidium infection was confirmed either by examining stool for the presence of Cryptosporidium oocysts using modified Ziehl Neelson staining method or by examining for Cryptosporidium presence in stool sample using polymerase chain reaction (PCR), genotyping and subtyping. Secondary outcomes of interest were: (1) CD4 + T cell count: in HIV/AIDS patients CD4 + T cell count was carried out by BD FACS count or BD FACScalibur flow cytometry machines; (2) Diarrhea status: clinical symptom characterized by loose or watery stool as reported by patients’ interview. Data from relevant studies were synthesized to provide pooled country-level prevalence estimates of cryptosporidiosis in PLWHA and its magnitude of associations with CD4 + T cell count in Ethiopia.
Literature search strategies
We searched relevant studies published in English medium from five electronic bibliographic databases, including PubMed, Web of Science, Scopus, Google Scholar, and ProQuest Dissertations & Theses until 5 May 2019. Furthermore, the reference list of all selected studies were screened for studies related to Cryptosporidium infection in HIV-infected individuals and its relationships with CD4 + T cell count in the country. Grey literature has also been searched from the Ethiopian University online library research repository. A systematic literature search was performed using keywords and Medical Subject Headings (MeSH) terms, separately and in combination by Boolean operators such as ‘OR’ or ‘AND.’ Keywords and MeSH terms used for the search were ”Cryptosporidium”[MeSH], ”cryptosporidiosis”[MeSH], ”Cryptosporidium parvum”[MeSH], ”opportunistic infections”, ”HIV/AIDS”[MeSH], ”immunodeficiency”[MeSH], ”immunocompromised”[MeSH] and Ethiopia. An example of a search strategy for relevant articles using PubMed was (((cryptosporidium OR cryptosporidiosis OR cryptosporidium parvum OR opportunistic parasites OR intestinal parasites)) AND (HIV OR AIDS OR immunodeficiency OR immunocompromised)) AND Ethiopia) in all fields. To manage and remove duplicates, all searched literature were imported to EndNote X7 software (Thompson Reuter, CA, USA). In two rounds, eligible articles were screened for the final analysis. In the first round, we excluded studies not suitable for the outcome of interest and in the second round, eligible studies were screened for the final synthesis according to the inclusion and exclusion criteria.
Inclusion and exclusion criteria
For this work, only studies on the prevalence of cryptosporidiosis in PLWHA and its correlation with CD4 + T cell count conducted in Ethiopia were selected, regardless of the study design, study time, study area, and publication status (included both published and unpublished studies). We omitted studies that used secondary data, studies with sample size less than 35 and those that do not report at least one outcome of interest. Besides, studies published only in English were included for analysis.
Data extraction and quality assessment
Pre-designed excel data extraction sheet was prepared for data extraction for each study and the following data extracted; author/s, publication year, study area, study design, number of PLWHA, number of individuals infected with both Cryptosporidium and HIV/AIDS, CD4 + T cell count, diagnosis method, and clinical symptom. The Hoy 2012 tool was used to assess the quality of the included studies [24]. The tool included 10 items plus summary assessment: 4 external validity items such as (I) representation of the population, (II) sampling frame, (III) methods of participant selection, and, (IV) non-response bias and 6 internal validity assessment items were: (I) data collection directly from the subjects, (II) appropriateness of case definition, (III) reliability and validity of study instrument, (IV) method of data collection, (V) duration of prevalence period, and (VI) correctness of numerator and denominator. For each item, the risk of bias was assessed as low, moderate, or high. When there is no adequate information regarding a particular item, it was deemed as high risk. Summary risk of bias was evaluated and then rated based on the number of the high risk of bias per study; Low (≤2), moderate (3–4), and high (≥5).
Data analysis
Using a prepared Microsoft Excel spreadsheet, the required data was extracted from each original study and then imported into STATA 14 statistical software for further analysis. Inverted variance method was generally used for proportion meta-analysis and applicable when the prevalence is approximately 0.5. However, two problems arise, when the proportions get closer to zero and one range limits: I) CI does not preclude confidence limits outside 0–1 range II) when the proportion becomes too small or too large [25] a study gets a significant weighting. Hence, we transformed point prevalence estimates of studies by variance stabilizing double arcsine transformation using the following formula: t = arcsin (sqrt (r/(n + 1))) + arcsin(sqrt((r + 1)/(n + 1))), where t = transformed prevalence, r = positive numbers, and n = sample size; se(t) = sqrt(1/(n + 0.5)), where se = standard error and the back transformation to a proportion are done using: p = (sin(t/2))2. Index of heterogeneity (I2 statistics) was used to evaluate the degree of heterogeneity across the studies. When I2 is 25%, 50% and 75%, respectively, heterogeneity was regarded as low, medium, and high. Univariate meta-regression analysis was also performed to detect the potential source of heterogeneity by considering the year of publication and sample size. Funnel plot symmetry, Egger’s regression asymmetry test, and Begg rank correlation methods were used to evaluate publication bias. The pooled effect size was performed in the form of prevalence and the association measured by OR using the random model. Analysis of sensitivity was done by excluding a single study step-by-step.
Results
A total of 255 articles was retrieved after systematic searching of existing literature, using the international bibliographic databases and gray literature search. Of the total items retrieved, 134 duplicates were removed, 87 were excluded after the title and abstract screening, and finally, 31 studies were considered for the meta-analysis (Figure 1).
Figure 1.
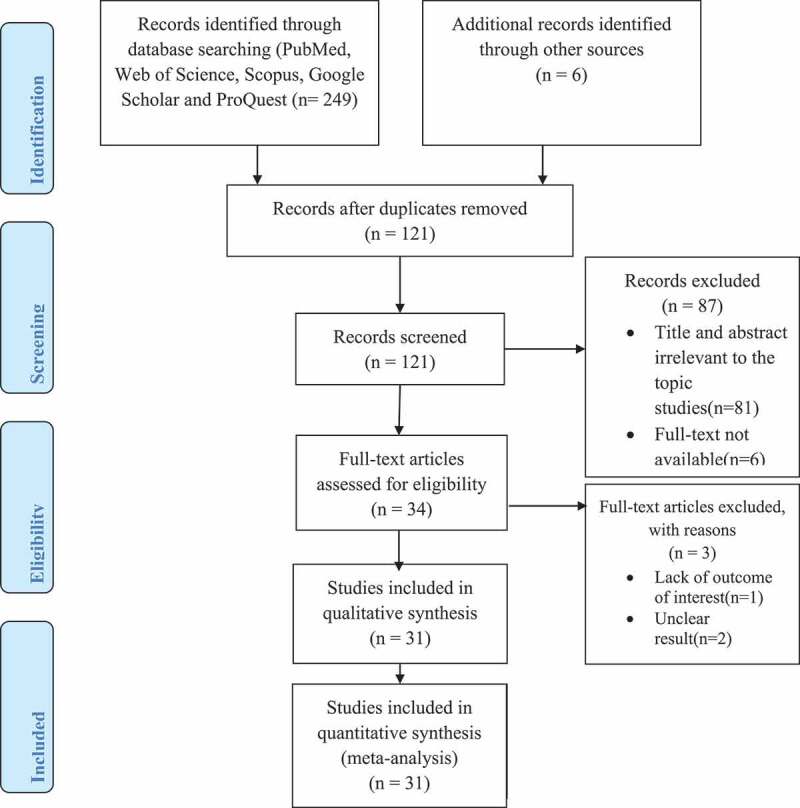
PRISMA flowchart for selecting eligible studies on the prevalence of cryptosporidiosis among PLWHA and its associations with CD4 + T cell count, in Ethiopia, 2001 to 2019.
Characteristics of the included studies and risk of bias assessment
Thirty-one studies included in this meta-analysis, except for one case–control study, were cross-sectional in design. Five of the studies were not published. A total of 8,648 individuals was included in the analysis. All studies included in the analysis were conducted between 2001 and 2019. The highest prevalence of the cryptosporidiosis was 44% (95%CI: 0.37–0.51) and the lowest 1% (95%CI: −0.00–0.03). Regarding the risk of bias assessment, 29 studies (93.4%) had the low risk, while 0.3% (1 study) each was of medium and high risk, respectively (Table 1).
Table 1.
General characteristics of included studies.
| S.N | Author/s and year of Publication |
Number of HIV infected people |
Number of individuals with Cryptosporidium coinfection |
Region | Prevalence (%) |
Diagnostic Method |
Diarrhea status | Risk of bias assessment |
|---|---|---|---|---|---|---|---|---|
| 1 | Abate et al., 2001 [19] | 100 | 17 | Oromia | 17 | Modified Ziehl- Neelsen Staining |
Unclear | Low risk |
| 2 | Adamu and Petros, 2009 [14] | 200 | 16 | Oromia | 8 | Modified Ziehl- Neelsen Staining |
Diarrheic and non-diarrheic | Low risk |
| 3 | Adamu et al., 2013 [33] | 378 | 32 | Oromia | 8 | Modified Ziehl- Neelsen Staining |
Diarrheic and non-diarrheic | Low risk |
| 4 | Adamu et al., 2014 [20] | 520 | 140 | Addis Ababa | 27 | Molecular Method | Diarrheic and non-diarrheic | Low risk |
| 5 | Alemayehu et al., 2017 [21] | 377 | 9 | South | 2 | Modified Ziehl-Neelsen Staining | Unclear | Medium risk |
| 6 | Alemseged et al., 2015 [34] | 139 | 13 | South | 9 | Modified Ziehl-Neelsen Staining | Unclear | Low risk |
| 7 | Alemu et al., 2011 [35] | 188 | 82 | Amhara | 44 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 8 | Alemu et al., 2018 [15] | 220 | 19 | South | 9 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 9 | Assefa et al., 2009 [36] | 214 | 43 | South | 20 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 10 | Awole et al., 2003 [37] | 192 | 6 | Oromia | 3 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 11 | Endeshaw, 2005 [38] | 245 | 70 | Ethiopia | 29 | Modified Ziehl Neelsen Staining and Molecular | Diarrheic | Low risk |
| 12 | Eshetu et al., 2017 [39] | 223 | 7 | Amhara | 3 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 13 | Fekadu et al., 2013 [40] | 343 | 10 | South | 3 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | High risk |
| 14 | Gebretsadik et al., 2018 [41] | 223 | 3 | Amhara | 1 | Modified Ziehl-Neelsen Staining | Unclear | Low risk |
| 15 | Gebrewahid et al., 2019 [42] | 242 | 15 | Tigray | 6 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 16 | Gedle et al., 2017 [43] | 323 | 19 | South | 6 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 17 | Getaneh et al., 2010 [16] | 192 | 48 | South | 25 | Modified Ziehl-Neelsen Staining | Unclear | Low risk |
| 18 | Girma et al., 2014 [17] | 268 | 92 | South | 34 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 19 | Kiros et al., 2015 [44] | 399 | 23 | Amhara | 6 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 20 | Mariam et al., 2008 [45] | 223 | 7 | Oromia | 3 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 21 | Menbereleul and Sissay,2014 [46] | 422 | 60 | South | 14 | Modified Ziehl-Neelsen Staining | Unclear | Low risk |
| 22 | Menkir and Mengestie, 2014 [47] | 192 | 12 | Amhara | 6 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 23 | Missaye et al., 2013 [48] | 272 | 2 | Amhara | 1 | Modified Ziehl-Neelsen Staining | Unclear | Low risk |
| 24 | Raga and Menkir, 2013 [49] | 384 | 68 | Oromia | 18 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 25 | Seid et al., 2018 [50] | 100 | 11 | Amhara | 11 | Molecular Method | Diarrheic | Low risk |
| 26 | Tadesse et al., 2013 [51] | 397 | 26 | Oromia | 7 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 27 | Taye et al., 2014 [52] | 316 | 3 | Addis Ababa | 1 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 28 | Shimelis et al., 2016 [53] | 491 | 65 | South | 13 | Modified Ziehl-Neelsen Staining | Diarrheic | Low risk |
| 29 | Teklemariam et al., 2013 [54] | 371 | 8 | Amhara | 2 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 30 | Yitagesu et al., 2018 [55] | 403 | 42 | Dire Dawa | 10 | Modified Ziehl-Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
| 31 | Zeynudin et al., 2013 [56] | 91 | 8 | Oromia | 9 | Modified Ziehl Neelsen Staining | Diarrheic and non-diarrheic | Low risk |
Heterogeneity and publication bias
The included studies were evaluated for the presence of potential publication bias by direct observation of the funnel plot. The funnel plot symmetry showed the absence of publication bias. Furthermore, Begg’s rank correlation test (p = 0.610) and Egger’s weighted regression test (bias coefficient (B) = 0.455 (95%CI = -8.950–9.860; p = 0.922) confirmed the absence of statistically significant publication bias. The extent of heterogeneity among studies was statistically assessed using the I2 test and Cochran’s Q test. The I2 value suggested a statistically significant variation among the studies (I2 = 96.3; p < 0.001), hence the random-effects model applied to estimate the Der Simonian and Laird’s pooled effect.
Pooled prevalence estimates of cryptosporidiosis in PLWHA in Ethiopia
As many as 8,648 Ethiopians infected with HIV were considered in the study, 976 of whom were infected with Cryptosporidium from 2001 to 2019. This results in an overall prevalence estimate of 11% (95%CI: 0.09–0.13; I2 = 96.3%, p < 0.001) (Figure 2). To identify potential sources of variations across the studies, univariate meta-regression between prevalence and both sample size and year of publication was performed. Sample size did not show a statistically significant reduction in the prevalence of infection following an increase in the sample size (B =.0001271, p = 0.808). Meta-regression using the year of publication has shown a decreasing trend in the prevalence of Cryptosporidium from 2001 to 2019. This trend of decline in cryptosporidiosis prevalence over time was not shown to be statistically significant in meta-regression analysis (B = −0.0191292, p = 0.152). We also performed a sensitivity analysis of a step-by-step one study omitted from analysis to assess the impact of each study on pooled prevalence estimate of the co-infection. The outcome of sensitivity evaluation confirmed that our findings were robust and not one-study dependent. The pooled prevalence estimates of cryptosporidiosis in HIV-infected Ethiopians ranged from 9.86% (95%CI: 0.077–0.12) to 11.24% (95%CI: 0.086–0.133) after a single study was deleted.
Figure 2.
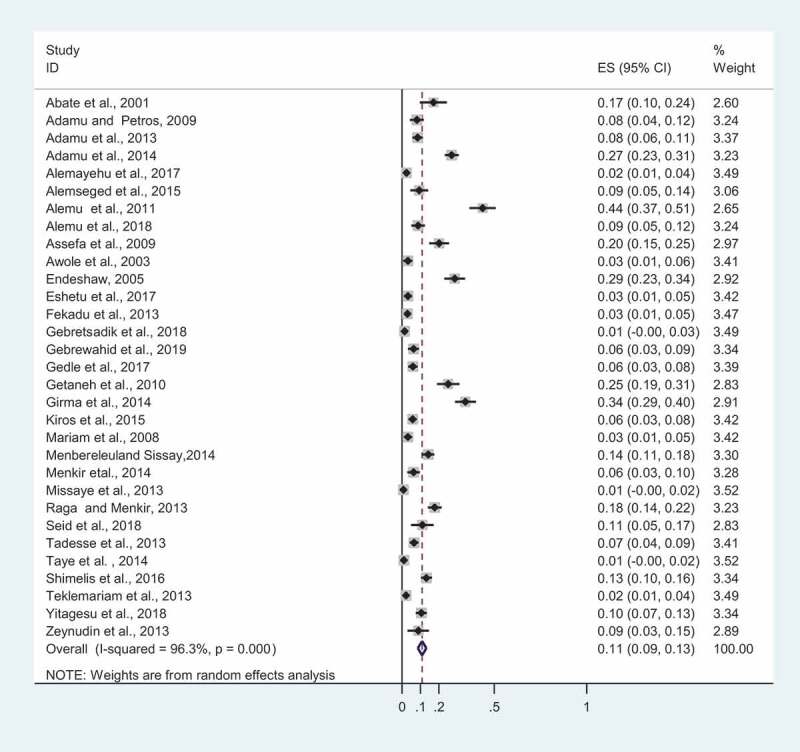
An overall pooled prevalence estimates of cryptosporidiosis in PLWHA in Ethiopia, 2001 to 2019. The X-axis of ES represents a study’s effect size estimate with a point and 95% confidence interval with a horizontal line. The weight (in %) denotes the influence an individual study has had on the pooled outcome. The vertical line is the line of zero prevalence. The diamond block indicates pooled prevalence estimate. P-value (p < 0.001) signifies a statistically significant heterogeneity among studies.
Sub-group analysis
We performed a subgroup pooled prevalence estimate of Cryptosporidium based on clinical symptoms, particularly diarrhea status. Eight studies reported the prevalence of Cryptosporidium based on the symptom (diarrheic vs non-diarrheic). The result of subgroup analysis demonstrated that the overall prevalence of Cryptosporidium in diarrheic and non-diarrheic patients was found to be 13% (95%CI; 0.07–0.20%) and 2% (95%CI: 0.01–0.04%), respectively (Figure 3). In addition, we also carried out subgroup overall estimate according to diagnostic methods. The vast majority of studies (28 out of 31) included in this study used acid fast staining (i.e. modified Zeihl-Neelsen staining method), whereas small proportion of studies (3 out of 31) used molecular method. The pooled prevalence of Cryptosporidium was found to be 10% (95%CI: 0.07–0.12%) and 22% (95%CI: 0.12–0.32%) by modified Zeihl-Neelsen staining method and molecular method, respectively, according to subgroup analysis by diagnosis methods (Figure 4). The prevalence of cryptosporidiosis in PLWHA in Ethiopia was further analyzed by stratification of the study into the study regions and periods. Sub-group analysis by differences in the study areas showed a wide variation in the prevalence, 6.2% (95%CI: 0.032–0.092) in the Tigray Region and 13.9% (95%CI: −0.116–0.393) in the Addis Ababa. The prevalence in other regions such as Southern, Oromia, Dire Dawa, and Amhara was found to be 13.2% (95%CI: 0.083–0.180), 10.8% (95%CI: 0.066–0.151), 10.4% (95%CI: 0.074–0.134), and 7.6% (95%CI: 0.041–0.111), respectively. In addition, subgroup analysis was carried out based on the study periods, which show a prevalence of 14.6% (95%CI: 0.076–0.217) in 2001–2010, 12.7% (95%CI: 0.086–0.167) in 2011–2014 and 6.7% (95%CI: 0,047–0,090) in between 2015 and 2019. This finding indicated a declining trend in the prevalence of the cryptosporidiosis.
Figure 3.
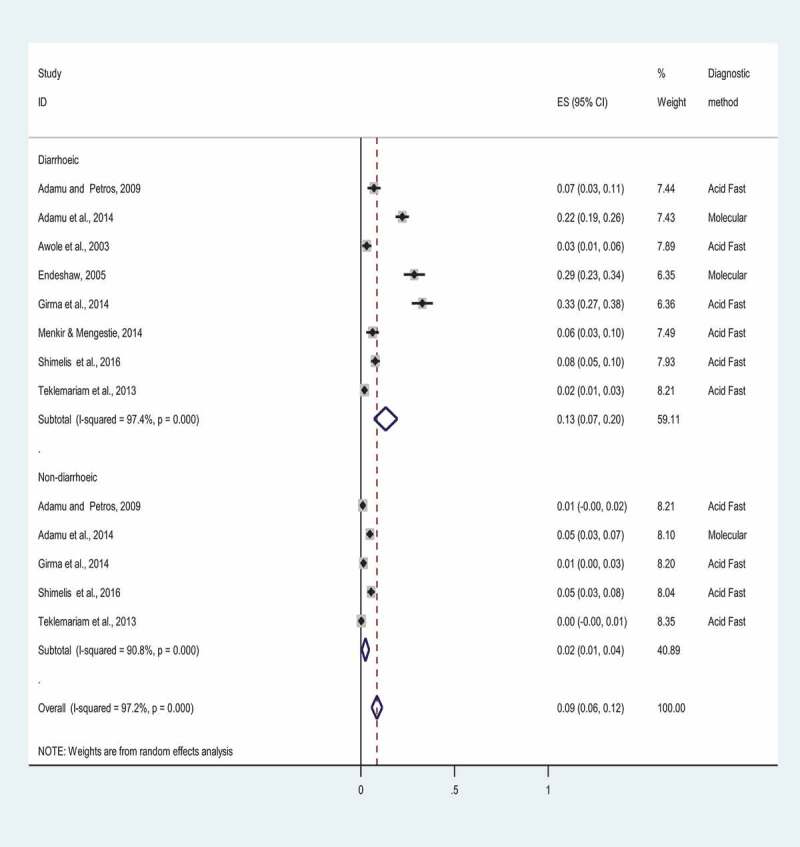
Forest plot showing the prevalence of cryptosporidiosis among PLWHA in Ethiopia based on diarrhea status, 2001 to 2019. ES indicates a study’s effect size estimate with a point and 95% confidence interval with a horizontal line. The weight (in %) designates the impact an individual study has had on the overall prevalence estimate. The vertical line is the line of null prevalence. The diamond block shows the overall prevalence estimate in Diarrheic and Non-diarrheic patients. P-value (p < 0.001) suggests a statistically significant heterogeneity among studies.
Figure 4.
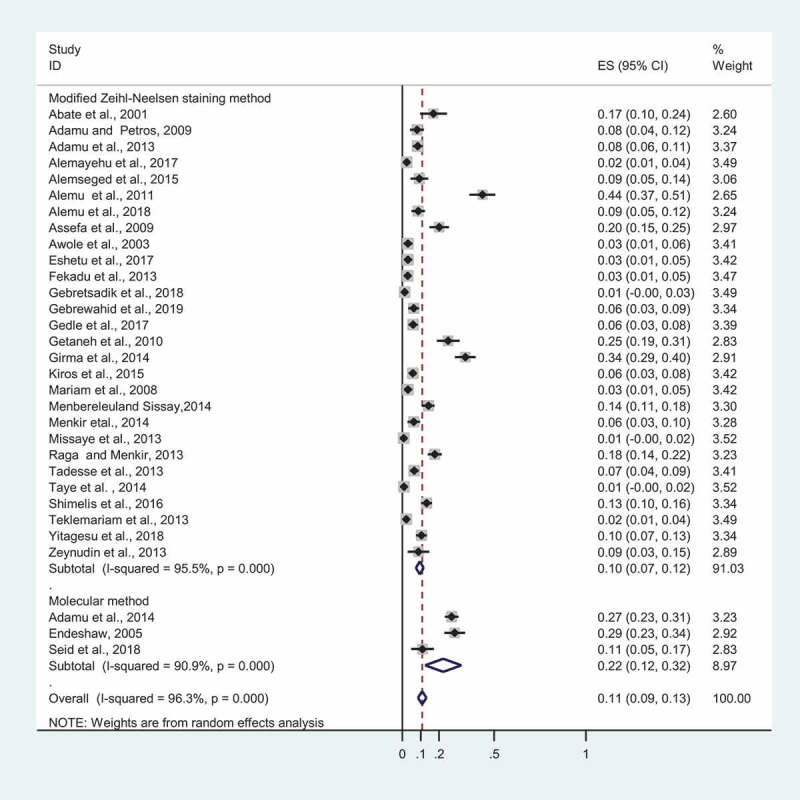
Forest plot depicting prevalence estimates of cryptosporidiosis in PLWHA in Ethiopia based on diagnosis methods, 2001 to 2019.
Correlation between cryptosporidiosis and CD4 + T cell count in PLWHA in Ethiopia
In total, six studies reported Cryptosporidium infection among HIV-infected Ethiopians and its association with CD4 + T cell count. We equally performed sensitivity analysis here by omitting a single study step-by-step to evaluate the effect of a single study on the pooled estimate of OR. Results of sensitivity analysis indicated that none of the studies produced a considerable impact on the pooled OR estimate of the relationship of Cryptosporidium infection with CD4 + T cell count among people who were infected with HIV. Accordingly, an overall estimate of OR of the association between Cryptosporidium infection and CD4 + T cell count ranged from 10.84 (95%CI: 5.04–23.29) to 16.43 (95%CI: 7.38–36.57). HIV-infected people with low CD4 + T cell count (CD4 < 200 cells/mm3) were 13.07 times more likely to have Cryptosporidium compared to high CD4 + T cell count (CD4 > 500 cells/mm3) (OR: 13.07, 95% CI: 6.38–26.75). Ninety-five percent CI of the OR does not include 1, it could be used as a proxy for the presence of statistical significance assuming there is no bias or confounding (Figure 5).
Figure 5.
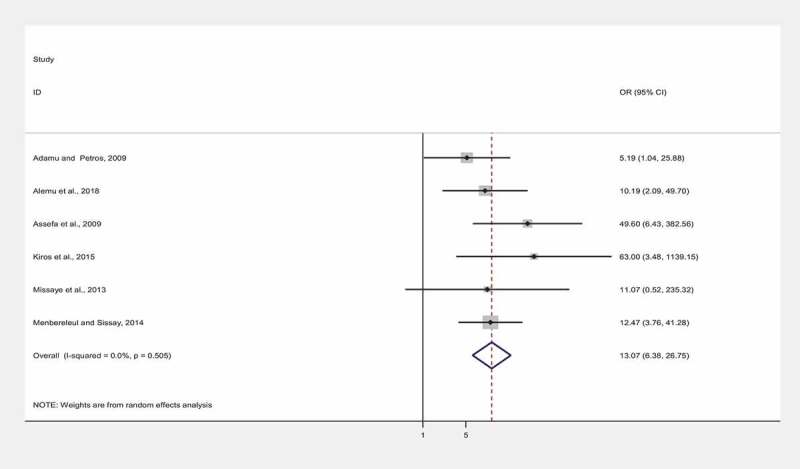
A pooled odds ratio of the correlation between cryptosporidiosis with CD4 + T cell count among Ethiopians infected with HIV, 2001 to 2019. The points on the plot represent the odds ratio (OR) of individual studies and the horizontal line with the points show the confidence interval of individual studies. The vertical axis (OR = 1) denotes the line of no association between cryptosporidiosis with CD4 + T cells count. The diamond block shows the overall pooled odds ratio of correlation between cryptosporidiosis with CD4 + T cells count in PLWHA in Ethiopia. P-value (p > 0.05) signifies a statistically insignificant homogeneity among studies.
Discussion
Though studies on the prevalence of cryptosporidiosis among PLWHA in Ethiopia are numerous and dispersed throughout the country, a country-level pooled prevalence estimate was not reported yet. Estimating country-level and regional pooled prevalence, trends of infection and relationship of Cryptosporidium with CD4 + T cell count could play a central role toward developing suitable strategies of cryptosporidiosis diagnosis, prevention, treatment, and control in Ethiopia, especially in PLWHA. This study is the first, to estimate the overall pooled prevalence of cryptosporidiosis and its association with CD4 + T cell count in PLWHA in Ethiopia. The overall pooled prevalence estimate of Cryptosporidium in HIV-infected Ethiopians was 11% (95%CI: 0.09–0.13).
The overall pooled prevalence of cryptosporidiosis in HIV-infected Ethiopian was relatively higher than pooled prevalence estimates of Iran (4.54%) [26] and Western and Central Europe and North America (7.3%) [27]. This relatively higher pooled prevalence of cryptosporidiosis in HIV-infected Ethiopians may be explained by a higher prevalence of PLWHA in Ethiopia than Iran and Europe, poor sanitation practice, inadequate access to safe water, close contact with animals, and a high burden of HIV/AIDS [28]. On the other hand, the finding of this systematic review is lower than the pooled prevalence estimate of the whole SAA, which was estimated to be 26.1% [27]. Lower prevalence might be due to the difference in sensitivity and specificity of diagnostic techniques used and the difference in time trend of analysis and number of studies included for meta-analysis. A large proportion of studies included in this review used modified acid-fast staining techniques for the detection of Cryptosporidium.
The result of this study shows a declining trend in the prevalence of Cryptosporidium over the last two decades. Cryptosporidium infection in PLWHA in Ethiopia showed decreasing patterns in 2001–2010, 2011–2014, and in 2015–2019, 14.6% (95%CI: 0.076–0.217), 12.71% (95%CI: 0.086–0.167) and 6.7% (95%CI: 0.044–0.090), respectively (p < 0.001). Using meta-regression analysis, trend of decline in cryptosporidiosis prevalence over time was not shown to be statistically significant in meta-regression analysis (B = −0.0191292, p = 0.152). This decrease could be linked to reduced prevalence of new HIV cases, improved use of antiretroviral treatment, improved sanitary practice, and access to safe potable water. From 2005 to 2017, number of new HIV infections, AIDS-related death, and PLWHA in Ethiopia were reduced from 31,000 to 16,000, 80,000 to 15,000 and 880,000 to 610,000, respectively [6]. Similarly, decrease in Cryptosporidium detection in rising sample size was statistically insignificant (B = 0. 0001271, p = 0.808). This could be due to studies that have a large sample size are few. Sub-group analysis showed that the pooled prevalence of Cryptosporidium among HIV-infected Ethiopians was heterogenic across the regions. The highest prevalence was recorded in Addis Ababa, 13.9% (95%CI: −0.116–0.393) followed by Southern region 13.2% (95% CI: 0.083–0.180) while the lowest prevalence was observed in Tigray region 6.2% (95% CI: 0.032–0.092). The difference could be explained by variations in geographical locations, study periods, number of studies included for analysis, seasonal variation, antiretroviral treatment status, sociocultural factors, and level of general hygiene.
In relation to the clinical symptom, Cryptosporidium infection showed nearly seven folds’ increase in prevalence in diarrheic patients (13% (95%CI; 0.07–0.20%)) compared to non-diarrheic patients (2% (95%CI: 0.01–0.04%)). Similarly, cross-sectional studies conducted in Ethiopia [29] and Venezuela [30] showed that Cryptosporidium infection is associated with diarrhea in HIV/AIDS patients. In immune-competent individuals, Cryptosporidium infection is self-limiting: However, in immune-compromised individuals like HIV/AIDS patients, Cryptosporidium infection is accounting for about one-third of diarrhea [18].
In this meta-analysis, HIV-infected patients with low CD4 + T cell count (<200 cells/mm3) were 13.07 times more likely to have Cryptosporidium compared to high CD4 + T cells count (CD4 > 500 cells/mm3) (OR: 13.07, 95%CI: 6.38–26.75). This finding is similar to cross-sectional studies conducted in Ethiopia that indicated that the prevalence of opportunistic parasites tends to increase with reduced CD4 + T cell count [14]. Similar findings were also reported in India [1], and Thailand [31] indicating that opportunistic infections were more likely encountered as the CD4 + T cell count decline below 200 cells/mm3. CD4 + T lymphocytes are the crucial immune cell that can mount active cell-mediated immunity against pathogens. The decline in CD4 + T cell count results in an impaired immune system, making an individual susceptible to multiple infections. The higher prevalence of opportunistic parasites with lower CD4 + T cell count might be due to deprivation of the immune cells that make the patients more vulnerable to get infected with particular parasites and unable to remove once the infection is established. Nevertheless, the underlying mechanism that helps the establishment of specific parasites in immunosuppressed individuals remains obscure [12,32].
Cryptosporidium infection is notable in HIV-infected people in Ethiopia, especially those with suboptimal CD4 + T cell count. Thus, routine screening for Cryptosporidium parasite in HIV patients should be incorporated as part of routine care, especially in patients with lower CD4 + T cell count. Early diagnosis and prompt treatment coupled with improved hygienic practices and access to clean potable water could help in controlling and reducing the burden of the infection. Furthermore, country-wide studies should be conducted on the epidemiology of circulating species and subtypes among HIV-infected individuals and the general public.
However, this research has some limitations: First, we could not find studies conducted from some regions of Ethiopia, which reduce the representativeness of the pooled estimate. Secondly, a large proportion of studies considered in the analysis was cross-sectional, as such, our result should be interpreted with caution. Third, multiple socio-cultural factors, ART status, and viral loads were not considered in the analysis since they were not reported in many included studies.
Acknowledgments
We would like to acknowledge Tehran University of Medical Sciences, International Campus, Tehran, Iran, for providing a conducive atmosphere to conduct this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Gupta S, Narang S, Nunavath V, et al. Chronic diarrhoea in HIV patients: prevalence of coccidian parasites. Indian J Med Microbiol. 2008;26(2):172. [DOI] [PubMed] [Google Scholar]
- [2].Dash M, Padhi S, Panda P, et al. Intestinal protozoans in adults with diarrhea. N Am J Med Sci. 2013;5(12):707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim YJ, Woo JH, Kim MJ, et al. Opportunistic diseases among HIV-infected patients: a multicenter-nationwide Korean HIV/AIDS cohort study, 2006 to 2013. Korean J Intern Med. 2016;31(5)953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rubaihayo J, Tumwesigye NM, Konde-Lule J, et al. Frequency and distribution patterns of opportunistic infections associated with HIV/AIDS in Uganda. BMC Res Notes. 2016;9(1)501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Organization WH: Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. 2002:https://apps.who.int/iris/handle/10665/42588. [PubMed]
- [6].[https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf]. Accessed 15 July 2019.
- [7].Bamaiyi PH, Redhuan NEM.. Prevalence and risk factors for cryptosporidiosis: a global, emerging, neglected zoonosis. Asian Biomed. 2017;10(4):309–325. [Google Scholar]
- [8].Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124(1):80–89. [DOI] [PubMed] [Google Scholar]
- [9].Xiao L, Bern C, Limor J, et al. Identification of 5 types of cryptosporidium parasites in children in Lima, Peru. J Infect Dis. 2001;183(3):492–497. [DOI] [PubMed] [Google Scholar]
- [10].Checkley W, Jr AC W, Jaganath D, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis. 2015;15(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burnet J-B, Penny C, Ogorzaly L, et al. Spatial and temporal distribution of cryptosporidium and giardia in a drinking water resource: implications for monitoring and risk assessment. SciTotal Environ. 2014;472:1023–1035. [DOI] [PubMed] [Google Scholar]
- [12].Hunter PR, Nichols G.. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15(1):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Akinbo F, Okaka C, Machado R, et al. Cryptosporidiosis among HIV-infected patients with diarrhea in Edo State, Midwestern Nigeria. Malaysian J Microbiol. 2010;6(1):99-101. [Google Scholar]
- [14].Adamu H, Petros B. Intestinal protozoan infections among HIV positive persons with and without antiretroviral treatment (ART) in selected ART centers in Adama, Afar and Dire-Dawa, Ethiopia. Ethiop J Health Dev. 2009;23(2):133–140. [Google Scholar]
- [15].Alemu G, Alelign D, Abossie A. Prevalence of opportunistic intestinal parasites and associated factors among HIV patients while receiving ART at Arba Minch Hospital in Southern Ethiopia: a cross-sectional study. Ethiop J Health Sci. 2018;28(2):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Getaneh A, Medhin G, Shimelis T. Cryptosporidium and strongyloides stercoralis infections among people with and without HIV infection and efficiency of diagnostic methods for strongyloides in Yirgalem Hospital, southern Ethiopia. BMC Res Notes. 2010;3(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Girma M, Teshome W, Petros B, et al. Cryptosporidiosis and Isosporiasis among HIV-positive individuals in south Ethiopia: a cross sectional study. BMC Infect Dis. 2014;14(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marcos LA, Gotuzzo E. Intestinal protozoan infections in the immunocompromised host. Curr Opin Infect Dis. 2013;26(4):295–301. [DOI] [PubMed] [Google Scholar]
- [19].Abate E, Mekonnen E, Awol M, et al. Cryptosporidiosis and isosporiasis among HIV/AIDS patients in Jimma, Southwest Ethiopia. J Ethiopian Medical Practice. 2001;3(2):64–69. [Google Scholar]
- [20].Adamu H, Petros B, Zhang G, et al. Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS Negl Trop Dis. 2014;8(4):e2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alemayehu M, Yisehak Y, Alaro W, et al. Opportunistic infections among HIV/AIDS patients taking ante-retroviral therapy at tertiary care hospital in wolaita zone, southern Ethiopia. J AIDS Clin Res. 2017;8(2). DOI: 10.4172/2155-6113.1000665 [DOI] [Google Scholar]
- [22].Raga DK, Menkir S: Prevalence of Isospora belli and Cryptosporidium parvuminfections among HIV sero-positive patients in Asella Hospital, Arsi Zone, Central Ethiopia. Thesis: Haramaya University. Unpublished results. [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- [24].Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. [DOI] [PubMed] [Google Scholar]
- [25].Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. [DOI] [PubMed] [Google Scholar]
- [26].Berahmat R, Spotin A, Ahmadpour E, et al. Human cryptosporidiosis in Iran: a systematic review and meta-analysis. Parasitol Res. 2017;116(4):1111–1128. [DOI] [PubMed] [Google Scholar]
- [27].Wang Z-D, Liu Q, Liu -H-H, et al. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasit Vectors. 2018;11(1). DOI: 10.1186/s13071-017-2558-x28-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nsagha DS, Njunda AL, Assob NJC, et al. Intestinal parasitic infections in relation to CD4+ T cell counts and diarrhea in HIV/AIDS patients with or without antiretroviral therapy in Cameroon. BMC Infect Dis. 2015;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Girma M, Teshome W, Petros B, et al. Cryptosporidiosis and Isosporiasis among HIV-positive individuals in south Ethiopia: a cross sectional study. BMC Infect Dis. 2014;14(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Certad G, Arenas-Pinto A, Pocaterra L, et al. Cryptosporidiosis in HIV-infected Venezuelan adults is strongly associated with acute or chronic diarrhea. Am J Trop Med Hyg. 2005;73(1):54–57. [PubMed] [Google Scholar]
- [31].Wiwanitkit V. Intestinal parasitic infections in Thai HIV-infected patients with different immunity status. BMC Gastroenterol. 2001;1(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Evering T, Weiss LM. The immunology of parasite infections in immunocompromised hosts. Parasite Immunol. 2006;28(11):549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Adamu H, Wegayehu T, Petros B. High prevalence of diarrhoegenic intestinal parasite infections among non-ART HIV patients in Fitche Hospital, Ethiopia. PloS One. 2013;8(8). DOI: 10.1371/journal.pone.0072634e72634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alemseged M, Abebe R, Girma M. Prevalence and associated risk factors of opportunistic intestinal parasites among HIV positive and negative individuals in South Ethiopia: a case control study. BAOJ Hiv. 2015;1:001. [Google Scholar]
- [35].Alemu A, Shiferaw Y, Getnet G, et al. Opportunistic and other intestinal parasites among HIV/AIDS patients attending Gambi higher clinic in Bahir Dar city, North West Ethiopia. Asian Pac J Trop Med. 2011;4(8):661–665. [DOI] [PubMed] [Google Scholar]
- [36].Assefa S, Erko B, Medhin G, et al. Intestinal parasitic infections in relation to HIV/AIDS status, diarrhea and CD4 T-cell count. BMC Infect Dis. 2009;9(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Awole M, Gebre-Selassie S, Kassa T, et al. Prevalence of intestinal parasites in HIV-infected adult patients in southwestern Ethiopia. Ethiop J Health Dev. 2003;17(1):71–78. [Google Scholar]
- [38].Endeshaw T: Opportunistic and other intestinal parasites among HIV/AIDS patients in Ethiopia. Thesis, Addis Ababa, Ethiopia: Addis Ababa University; 2005. [Google Scholar]
- [39].Eshetu T, Sibhatu G, Megiso M, et al. Intestinal parasitosis and their associated factors among people living with HIV at University of Gondar Hospital, Northwest-Ethiopia. Ethiop J Health Sci. 2017;27(4):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fekadu S, Taye K, Teshome W, et al. Prevalence of parasitic infections in HIV-positive patients in southern Ethiopia: a cross-sectional study. J Infect Developing Countries. 2013;7(11). DOI: 10.3855/jidc.2906868-872 [DOI] [PubMed] [Google Scholar]
- [41].Gebretsadik D, Haileslasie H, Feleke DG. Intestinal parasitosis among HIV/AIDS patients who are on anti-retroviral therapy in Kombolcha, North Central, Ethiopia: a cross-sectional study. BMC Res Notes. 2018;11(1):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gebrewahid T, Gebrekirstos G, Teweldemedhin M, et al. Intestinal parasitosis in relation to CD4 count and anemia among ART initiated patients in St. Mary Aksum general hospital, Tigray, Ethiopia. BMC Infect Dis. 2019;19(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gedle D, Kumera G, Eshete T, et al. Intestinal parasitic infections and its association with undernutrition and CD4 T cell levels among HIV/AIDS patients on HAART in Butajira, Ethiopia. J Health Popul Nutr. 2017;36(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kiros H, Nibret E, Munshea A, et al. Prevalence of intestinal protozoan infections among individuals living with HIV/AIDS at Felegehiwot Referral Hospital, Bahir Dar, Ethiopia. Inter J Infect Dis. 2015;35:-80–86. [DOI] [PubMed] [Google Scholar]
- [45].Mariam ZT, Abebe G, Mulu A. Opportunistic and other intestinal parasitic infections in AIDS patients, HIV seropositive healthy carriers and HIV seronegative individuals in southwest Ethiopia. East Afr J Public Health. 2008;5(3):169–173. [PubMed] [Google Scholar]
- [46].Menbereleul M, Sissay M: Prevalence of opportunistic intestinal parasitic infection among HIV/AIDS patients attending Othona Hospital, Wolayita Sodo, Southern Ethiopia. Thesis Harmaya, Ethiopia: Harmaya University; 2014. [Google Scholar]
- [47].Menkir S, Mengestie H: Prevalence of intestinal parasitic infections among people with and without Hiv infection and their association with diarrhea in Debre Markos Town, East Gojjam Zone, Ethiopia. Thesis Haramaya, Ethiopia: Haramaya University;2014. [Google Scholar]
- [48].Missaye A, Dagnew M, Alemu A, et al. Prevalence of intestinal parasites and associated risk factors among HIV/AIDS patients with pre-ART and on-ART attending dessie hospital ART clinic, Northeast Ethiopia. AIDS Res Ther. 2013;10(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Raga DK, Menkir S: Prevalence of Isospora belliand Cryptosporidium parvum infections among HIV sero-positive patients in Asella Hospital, Arsi Zone, Central Ethiopia. Thesis. Haramaya, Ethiopia: Haramaya University; 2013. [Google Scholar]
- [50].Seid L, Stokes W, Bayih AG, et al. Molecular detection of enteropathogens from diarrheic stool of HIV positive patients in Gondar, Ethiopia. BMC Infect Dis. 2018;18(1). DOI: 10.1186/s12879-018-3265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tadesse G, Zeynudin A, Mekonnen Z, et al. Intestinal parasitosis among HIV sero positive in Jimma, Ethiopia. J Trop Dis. 2013;01(04). DOI: 10.4172/2329-891X.1000122 [DOI] [Google Scholar]
- [52].Taye B, Desta K, Ejigu S, et al. The magnitude and risk factors of intestinal parasitic infection in relation to human immunodeficiency virus infection and immune status, at ALERT Hospital, Addis Ababa, Ethiopia. Parasitol Int. 2014;63(3):550–556. [DOI] [PubMed] [Google Scholar]
- [53].Shimelis T, Tassachew Y, Lambiyo T. Cryptosporidium and other intestinal parasitic infections among HIV patients in southern Ethiopia: significance of improved HIV-related care. Parasit Vectors. 2016;9(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Teklemariam Z, Abate D, Mitiku H, et al. Prevalence of intestinal parasitic infection among HIV positive persons who are naive and on antiretroviral treatment in Hiwot Fana Specialized University Hospital, Eastern Ethiopia. Isrn Aids. 2013;2013:324329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yitagesu A, Kebede A, Menkir S: Prevalence of opportunistic intestinal protozoan parasitic infections and associated risk factors among HIV sero-positive individuals at dilchora referral hospital, Dire Dawa Town, Eastern Ethiopia. Thesis. Haramaya, Ethiopia: Haramaya University; 2018. [Google Scholar]
- [56].Zeynudin A, Hemalatha K, Kannan S. Prevalence of opportunistic intestinal parasitic infection among HIV infected patients who are taking antiretroviral treatment at Jimma Health Center, Jimma, Ethiopia. Eur Rev Med Pharmacol Sci. 2013;17(4):513–516. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- [https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf]. Accessed 15 July 2019.


