Abstract
Post-operative radiation therapy (RT) reduces loco-regional recurrence rates and mortality in most patients with non-metastatic breast cancer. The aim of this critical review is to provide an overview of the applicability of moderately hypofractionated RT for breast cancer patients, focusing on factors influencing clinical decision-making. An international group of radiation oncologists agreed to assess, integrate, and interpret the existing evidence into a practical report to guide clinicians in their daily management of breast cancer patients. We conclude that moderately hypofractionated RT to the breast, chest wall (with/without breast reconstruction), and regional lymph nodes is at least as safe and effective as conventionally fractionated regimens and could be considered as the treatment option for the vast majority of the patients.For those who are still concerned about its generalised application, we recommend participating in ongoing trials comparing moderately hypofractionated RT to conventionally fractionated RT for breast cancer patients in some clinical circumstances.
Keywords: Breast cancer, Treatment, Radiation therapy, Radiation dose hypofractionation
1. Introduction
Post-operative radiation therapy (RT) reduces both loco-regional recurrence rates and breast cancer mortality in most patients who receive either breast-conserving surgery or mastectomy (Early Breast Cancer Trialists’ Collaborative G et al., 2011; Ebctcg et al., 2014).
For decades, conventional radiation doses ranged from 50 to 50.4 Gy, given in 25–28 fractions over a course of 5–6 weeks. This empirical schedule was based on the assumption that a total dose around 50 Gy, prescribed in 1.8–2.0 Gy fractions, might maximize tumour control while minimizing normal-tissue injury. This was reinforced by data analyses from early studies of hypofractionation in breast cancer using outdated and erroneous radiobiological models and archaic methods of treatment calculation and delivery, leading to high rates of late normal-tissue damage (Overgaard et al., 1987; Johansson et al., 2002).
In the early 90 s, a re-evaluation of the correlation between fraction size and normal-tissue injury in breast tumours suggested that an extended treatment duration was neither favourable for tumour control nor necessary to spare normal tissue. This led to the development of the moderately hypofractionated whole breast irradiation (HF-WBI) approach, which comprised fraction sizes up to 3 Gy combined with a reduced total dose, aimed at obtaining radiobiological equivalence to conventionally fractionated regimens (Whelan et al., 2002; Yarnold et al., 2005). Trials were developed using an (i) explanatory approach based on radiobiological assumptions, e.g. START A investigating hypofractionated regimens that were hypothesized to be isoeffective with 50 Gy in 25 fractions and (ii) pragmatic trials e.g. START B – based on historical patterns of practice predominantly in the north of the UK using traditionally 40 Gy in 15 fractions over 3 weeks whereas centres in the south tending to use 25 fractions (Group et al., 2008a; Group et al., 2008b). Of note, 40 Gy in 15 fractions has never being assumed to be isoeffective with 50 Gy in 25 fractions as radiobiologically it is estimated to be equal to 46−47 Gy in 2 Gy fractions, depending on the α/β ratio of the tissues in question.
Hypofractionated schedules decrease the overall treatment period by reducing the total number of fractions and thereby offer a therapeutic schedule that is more convenient for patients and health care providers. Hypofractionation may also improve patients` access to medical care (particularly in circumstances in which there is insufficient capacity), reduce indirect costs related to work interruptions and travel to the radiation oncology department, next to decreased direct treatment costs (Lievens et al., 2003).
The aim of this International Expert Opinion Report is to provide an overview of the current evidence and, derived therefrom, a guideline for the clinical application of moderate hypofractionation in patients with invasive breast cancer.
2. Data collection and endpoints
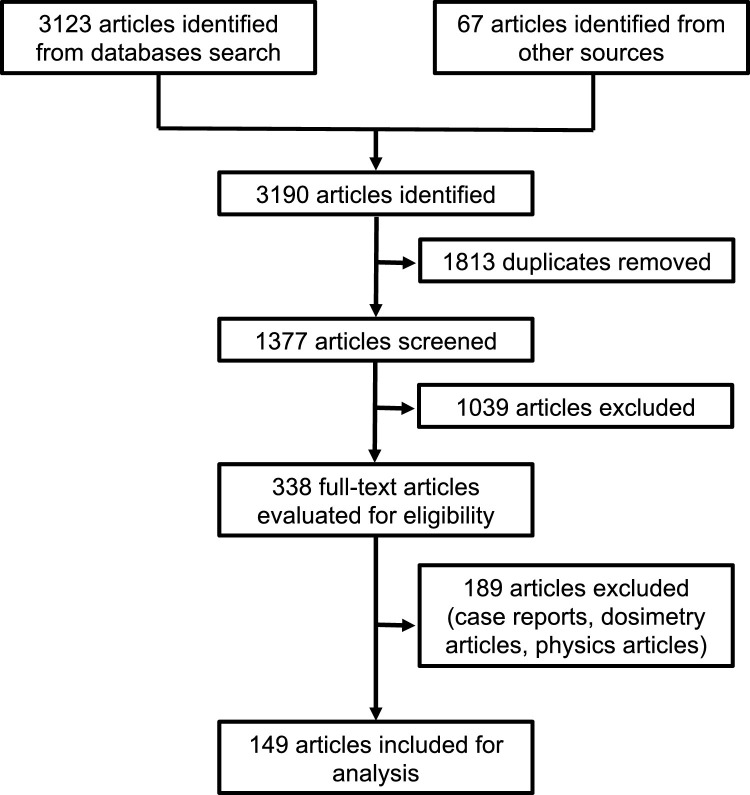
A systematic review in accordance with The Cochrane Collaboration Handbook of Interventions Systematic Reviews was performed (GSe, 2011). We conducted the resulting electronic literature search without any restrictions regarding language or publication year. We searched the electronic databases Cochrane Central Register of Controlled Trials (CENTRAL, 2019 issue 7, via Wiley - 25 August 2019), MEDLINE (1966 to 25 August 2019, via Pubmed), and EMBASE (1988 to 24 August 2019, via Elsevier). The terms and search strategy used were: (Breast Neoplasms OR Breast Neoplasm OR Neoplasm, Breast OR Neoplasms, Breast OR Tumors, Breast OR Breast Tumors OR Breast Tumor OR Tumor, Breast OR Mammary Neoplasms, Human OR Human Mammary Neoplasm OR Human Mammary Neoplasms OR Neoplasm, Human Mammary OR Neoplasms, Human Mammary OR Mammary Neoplasm, Human OR Mammary Carcinoma, Human OR Carcinoma, Human Mammary OR Carcinomas, Human Mammary OR Human Mammary Carcinomas OR Mammary Carcinomas, Human OR Human Mammary Carcinoma OR Breast Cancer OR Cancer, Breast OR Cancer of Breast OR Mammary Cancer OR Malignant Neoplasm of Breast OR Malignant Tumor of Breast OR Breast Carcinoma 0R Cancer of the Breast) and (Dose Hypofractionation OR Radiation Hypofractionation, Radiation Dose OR Radiotherapy Minibeams OR Minibeam, Radiotherapy OR Radiotherapy Minibeam OR Radiotherapy Dose Hypofractionation OR Dose Hypofractionation, Radiotherapy OR Hypofractionation, Radiotherapy Dose OR Hypofractionations, Radiotherapy Dose OR Hypofractionated Dose, Radiation OR Radiation Hypofractionated Dose). We also manually searched the reference lists of the included studies and review articles. We considered prospective trials, retrospective studies, systematic reviews, guidelines, and consensus papers for our analysis and discussion. Other types of studies were excluded. After an independent review of the references (by two authors – GNM and PP) and the removal of duplicates, 1558 potentially relevant abstracts remained. After further analysis, 149 articles were withheld for discussion within our international radiation oncologists breast cancer experts group (Fig. 1 ). Eligible citations were retrieved for full-text review. A committee performed an independent check and the definitive approval of the review.
Fig. 1.
PRISMA flow diagram.
The primary endpoint was effectiveness (loco-regional recurrence rates). The second endpoint were safety (side effects) and calculated radiobiological equivalence.
3. Current evidence for moderate hypofractionation
3.1. Clinical trials
Six randomized phase III trials were published with formal comparisons between moderate hypofractionation and conventionally fractionated irradiation for breast cancer patients (Table 1 and Table 2 ).
Table 1.
Characteristics of the prospective randomised studies comparing conventional with hypofractionation schedules in breast-cancer patients.
| RMH/GOC611 | START A712 | START B812 | OCOG514 | Beijing Trial17 | Total N (%) | |
|---|---|---|---|---|---|---|
| Number of patients | 1410 | 2236 | 2215 | 1234 | 820 | 7915 (100) |
| Years of inclusion | 1986 - 1998 | 1998 - 2002 | 1999 - 2001 | 1993 - 1996 | 2008−2016 | – |
| Inclusion criteria | T1−3;N01;M0 | T1−3;N0−1;M0 | T1−3;N0−1;M0 | T1−2;N0;M0 | T3-T4;N2−3;M0 | – |
| Median follow-up - years (range) | 9.7 (7.8−11.8) | 9.3 (8.0−10.0) | 9.9 (7.5−10.1 | 12.0 (a) | 4.9 (3.7−6.8) | – |
| Type of surgery N (%) | ||||||
| Breast-conserving surgery | 1214 (86) | 1900 (85) | 2038 (92) | 1098 (89) | 0 | 6250 (79) |
| Mastectomy | 0 | 336 (15) | 177 (8) | 0 | 820 (100) | 1665 (21) |
| Chemotherapy N (%) | 196 (14) | 793 (35) | 491 (22) | 136 (11) | 820 (100) | 2436 (31) |
| Boost N (%) | 1051 (75) | 1152 (61) | 875 (43) | 0 | 0 | 3078 (39) |
| Regional nodal irradiation N (%) | 290 (21) | 318 (14) | 161 (7) | 0 | 840 (100) | 1609 (20) |
RMH/GOC = Royal Marsden Hospital/Gloucestershire Oncology Centre.
OCOG = Ontario Clinical Oncology Group.
START = Standardization of Breast Radiotherapy Trial.
CF-WBI = conventionally-fractionated whole breast irradiation.
HF-WBI = hypofractionated whole breast irradiation.
The information was not available in the original publication.
Table 2.
Outcomes and equivalent doses of the prospective randomised studies comparing conventional with hypofractionation schedules in breast-cancer patients.
| RMH/GOC611 | START A712 | START B812 | OCOG514 | Beijing Trial17 | |
|---|---|---|---|---|---|
| Hypofractionated schedule | 42.9 Gy / 13 fractions | 39 Gy / 13 fractions | 40 Gy / 15 fractions | 42.6 Gy / 16 fractions | 43.5 / 15 fractions |
| 39 Gy / 13 fractions | 41.6 Gy/ 13 fractions | ||||
|
EQD2 α/β = 3(Dose level 100%) |
54.05 Gy (for 42.9 Gy) 46.80 Gy (for 39 Gy) |
51.58 Gy (for 41.6 Gy) 46.80 Gy (for 39 Gy) |
45.42 Gy | 48.18 Gy | 51.33 Gy |
| 10-year local recurrence (%) | |||||
| CF-WBI arm (50 Gy /25 fractions) | 12.1 | 6.7 | 5.2 | 7.5 | 8.1a |
| HF-WBI (42.9 Gy / 13 fractions) | 9.6 | – | – | – | – |
| HF-WBI (39 Gy / 13 fractions) | 14.8 | 8.1 | – | – | – |
| HF-WBI (41.6 Gy / 13 fractions) | – | 5.6 | – | – | – |
| HF-WBI (42.6 Gy / 16 fractions) | – | – | – | 7.4 | – |
| HF-WBI (40 Gy / 15 fractions) | – | – | 3.8 | – | – |
| HF-WBI (43.5 / 15 fractions) | – | – | – | – | 8.3a |
| Late toxic effects | |||||
| Skin (grade 3) - % | 2.7 (CF-WBI) versus 2.5 (HF-WBI) | 0.0 (CF-WBI) versus < 1.0 (HF-WBI) | |||
| Subcutaneous tissue (grade 3) - % | 3.6 (CF-WBI) versus 2.5 (HF-WBI) | – | |||
| Lymphoedema (grade 3) - % | 16.3 (CF-WBI) versus 22.5(HF – 41.6 Gy) versus 8.2 (HF – 39 Gy) | 13.5 (CF-WBI) versus 4.7 (HF-WBI | – | 1.0 (CF-WBI) versus 1.0 (HF-WBI) | |
| Breast shrinkage | 36.2 (CF-WBI) versus 34.2 (HF – 42.9 Gy) versus 44.4 (HF – 39 Gy) | 34.2 (CF-WBI) versus 31.4 (HF – 41.6 Gy) versus 30.0 (HF – 39 Gy) | 31.2 (CF-WBI) versus 26.2 (HF-WBI | ||
| Cosmetic outcome (EORTC Scale) | |||||
| Excellent or good - % | 71.3 (CF-WBI) versus 69.8 (HF-WBI) | ||||
| Fair /poor - % | 28.8 (CF-WBI) versus 25.6 (HF – 42.9 Gy) versus 42.0 (HF – 39 Gy) | ||||
Equivalent doses are calculated to be compared with a 2 Gy per fraction schedule (EQD2).
RMH/GOC = Royal Marsden Hospital/Gloucestershire Oncology Centre.
OCOG = Ontario Clinical Oncology Group.
START = Standardization of Breast Radiotherapy Trial.
CF-WBI = conventionally-fractionated whole breast irradiation.
HF-WBI = hypofractionated whole breast irradiation.
5-year local recurrence.
The Royal Marsden Hospital and Gloucestershire Oncology Centre study included 1410 breast-cancer patients who were at least 50 years of age and who had T1−3 N0−1 M0 cancer with a maximum of one involved node (Yarnold et al., 2005; Owen et al., 2006). Patients were randomly assigned to receive 50 Gy in 25 fractions (i.e., conventionally fractionated whole breast irradiation [CF-WBI]) or one of two dose schedules (39 or 42.9 Gy in 13 fractions) (EDQ2, equivalent dose in 2 Gy fractions based on an α/β of 4, of 45.5 and 52.19, respectively). The 10-year risks of ipsilateral breast cancer recurrence were 12.1 %, 14.8 %, and 9.6 %, respectively. Although local recurrence significantly differed between the HF-WBI groups (P = .027), neither HF-WBI group significantly differed from the CF-WBI group. Overall, side effects were limited, regardless of the treatment group (e.g., telangiectasia, shrinkage, induration, distortion, shoulder stiffness, and oedema), with the lowest rates in patients treated with a total dose of 39 Gy. This “pre-START” trial had an explanatory radiobiological design, the overall time (5 weeks) being kept constant, with two test arms representing a dose/fractionation that could be isoeffective based on the lower and higher estimates of the α/β ratio. This allowed estimation of the “true” isoeffective dose/fractionation regimen for both tumour and normal tissue. The 42.9 Gy in 13 reactions schedule was shown to have a higher effect compared to 50 in 25 fractions for normal tissues, leading to a modest dose reduction for the UK START A to 41.6 Gy total dose.
The UK START A included 2236 breast cancer patients (pT1−3a pN0−1 M0) undergoing either breast-conserving surgery (n = 1990; 85 %) or mastectomy (n = 336; 15 %) (Group et al., 2008a; Haviland et al., 2013). Randomisation was between CF-WBI 50 Gy in 25 daily fractions, HF-WBI 41.6 Gy in 13 fractions (EDQ2 = 49.92 Gy), or HF-WBI 39 Gy in 13 fractions (EDQ2 = 45.5 Gy), all over 25 days (HF fractions given every other day). An optional boost dose of 10 Gy (in 5 fractions) was given per institutional policy. At 10-year follow-up, loco-regional recurrence was 6.7 % in the conventional fractionation group, 5.6 % in the 41.6 Gy hypofractionation group, and 8.1 % in the 39 Gy hypofractionation group, respectively (p = NS). At 10-year follow-up, normal-tissue effects (telangiectasia, breast oedema, and moderate or marked breast induration) were significantly reduced in the 39 Gy hypofractionation group, as compared to the conventional fractionation group. There were no significant differences between the conventional fractionation group and the 41.6 Gy hypofractionation group. Likewise, other side effects (such as shoulder stiffness, arm oedema, and breast shrinkage) were similar between the hypofractionation and conventional fractionation groups.
The UK START B randomized 2 215 women (pT1−3a pN0−1 M0) who underwent breast-conserving surgery (n = 2038; 92 %) or mastectomy (n = 177; 7%) to receive either CF-WBI 50 Gy in 25 fractions over 5 weeks or HF-WBI 40.05 Gy in 15 daily fractions over 3 weeks (Group et al., 2008b; Haviland et al., 2013). An optional boost dose of 10 Gy in 5 fractions was allowed per institutional protocol. At 10-year follow-up, the loco-regional recurrence rates did not significantly differ between the conventional and hypofractionation groups (5.5 % and 4.3 %, respectively; P = 0.21). Normal-tissue effects, such as breast oedema, telangiectasia, and breast shrinkage, were significantly less common in the hypofractionation than in the conventional fractionation group; no significant differences existed in arm oedema, shoulder stiffness, or breast induration. This pragmatic trial design, employing a biologically estimated lower dose led to, not surprisingly, a lower rate of acute side effects as the total dose was reduced, but it also produced lower late normal tissue side effects, which are more dependent on dose per fraction. Importantly, local control was at least as good. Overall survival was unexpectedly better, raising the exciting hypothesis that a shorter overall treatment time could be beneficial for survival, which could not be concluded in view of the non-inferiority design of this trial for local control. The explanatory START trials showed that α/β ratio for both breast tumours and late reacting normal tissues appears similar, demonstrating no advantage in using conventional 2 Gy per fraction regimens (Haviland et al., 2013; Yarnold et al., 2011).
The Ontario Clinical Oncology Group trial included 1234 women with T1−2 N0 M0 breast cancer after breast-conserving surgery who were randomized to receive CF-WBI 50 Gy in 25 fractions or HF-WBI 42.56 Gy in 16 fractions (EDQ2 47.24 Gy) (Whelan et al., 2002; Whelan et al., 2010). The 10-year local relapse rate was 6.7 % for the CF-WBI group as compared to 6.2 % for the HF-WBI group, for a non-significant absolute difference of 0.5 % (95 % CI, −2.5 to 3.5). Similarly, at 10 years the overall survival rates were 84.4 % in the CF-WBI and 84.6 % in the HF-WBI group, for a non-significant absolute difference of 0.2 % (95 % CI, −4.3–4.0). Moreover, the cosmetic outcomes were good or excellent for most patients: 69.8 % for HF-WBI and 71.3 % for CF-WBI, for a non-significant absolute difference of 1.5 % (95 % CI, –6.9–9.8). No differences in late adverse events in the skin or subcutaneous tissue were noted.
The M. D. Anderson Cancer Center trial included 287 patients stage 0 to II after breast-conserving surgery who underwent CF-WBI 50 Gy in 25 fractions or HF-WBI 42.56 Gy in 16 fractions (EDQ2 = 46.77 Gy) (Shaitelman et al., 2015). The patients in the CF-WBI group received a boost of 10–14 Gy (in 5 or 7 fractions, respectively), and those in the HF-WBI group received 10–12.5 Gy (in 4 or 5 fractions, respectively). The reported toxicities during treatment were statistically significantly lower in the HF-WBI group than in the CF-WBI group for the following outcomes: hyperpigmentation (9% vs. 20 %; P = .002), breast pain (55 % vs. 74 %; P = .001), acute dermatitis (36 % vs. 69 %; P < .001), fatigue (9% vs. 17 %; P = .02), and pruritus (54 % vs. 81 %; P < .001). Similarly, grade 2 or higher acute side effects were much less frequent in the HF-WBI group than in the CF-WBI group (47 % vs. 78 %; P < .001). At 6-month follow-up, the HF-WBI group showed lower rates than the CF-WBI group in lack of energy (23 % vs. 39 %; P < .001), and fatigue (0% vs. 6%; P = .01). The 3-year poor cosmetic outcomes were less frequent in the HF-WBI group than in the CF-WBI group (8.2 % vs. 13.6 %; P = 0 .002). The 3-year local relapse-free survival rate was 99 % for both groups (P = 0.37), but it is recognised that this trial is under-powered for this endpoint (Shaitelman et al., 2018).
The Beijing trial included 820 pT3−4 pN2−3 post-mastectomy breast-cancer patients, who were randomized to receive post-mastectomy RT of the chest wall and select nodal irradiation (supraclavicular and level 3) of 50 Gy in 25 fractions weeks or 3-week hypofractionation 43.5 Gy in 15 fractions (EDQ2 = 50 Gy) (Wang et al., 2019). The primary endpoint of this study was local control. The 5-year loco-regional relapse rates were 8.1 % and 8.3 % for the conventional dose and hypofractionation groups, respectively, resulting in a non-significant absolute difference of 0.2 % (90 % CI, –3.0–2.6) and a hazard ratio of 1.10 (90 % CI, 0.72–1.69; P < .001 for non-inferiority). The hypofractionation group had a lower rate of grade 3 acute skin toxicity than the conventional dose group did (3% vs. 8%; P < .001). There were no significant differences between the groups in terms of other acute or late toxicities.
4. Radiobiological considerations
The linear-quadratic model, based on the α/β value, is assumed to reliably predict the different fractionation sensitivity of early and late normal tissues for prescribed fraction sizes between 1.8 and 3 Gy (Qi et al., 2011). It can be used to predict the biological effective dose (BED) and thereby to calculate equivalent dose/fractionation schedules. For example, based on an α/β ratio of 3 Gy for late normal tissue response, a 15-fraction schedule leading to an equivalent rate of late effects of 50 Gy in 25 fractions requires a decrease in total dose to 42.8 Gy in fractions of 2.85 Gy to maintain the same risks of late side effects, not taking into account the shorter overall treatment time (Withers et al., 1983; Fowler, 1989; Jones et al., 2001).
Applying this concept to the dose distribution for locoregional radiation therapy for breast cancer obtained with 3D-CRT, it is possible to calculate various scenarios regarding the relative radiobiological effect to both tumour and normal tissues to compare the former standard of 50 Gy in 25 fractions with the 40 Gy in 15 fractions as tested in the START-B trial (Jones et al., 2001; Haviland et al., 2016). Table 3 illustrates the mathematical estimations, clearly showing that for a broad range of α/β values for normal tissue and breast cancer, the reduction of the total dose with the 40 Gy in 15 fractions schedule leads to a lower expected effect both for the volumes inside and outside the therapeutic radiation doses. This relative sparing proportionally even increases for dose levels below the prescribed dose level, as typically extending into neighbouring normal tissue including heart and lungs.
Table 3.
RBE dose calculations considering different α/β values for normal tissue and breast cancer for the fractionation schedules compared in the START-B trial10. The RBE calculations are shown at dose levels of 107 %, 105 %, 100 %, 70 % and 50 % for 3 different scenarios.
| Realistic scenario α/β 2 for Normal Tissue and 3.5 for Tumour | |||||
|---|---|---|---|---|---|
| Schedule and dose level | Numerical dose | EQD2 α/β = 2 |
BED α/β = 2 |
EQD2 α/β = 3.5 |
BED α/β = 3.5 |
| 50/25 Dose level 107 % |
25*2.14 = 53.5 Gy | 55.37Gy | 110.75Gy | 54.86Gy | 86.21Gy |
| 40/15 Dose level 107 % |
15*2.85 = 42.8 Gy | 51.90 Gy | 103.70 Gy | 49.41Gy | 77.65Gy |
| 50/25 Dose level 105 % |
25*2.1 = 52.5 Gy | 53.81Gy | 107.62 Gy | 53.45Gy | 84.00 Gy |
| 40/15 Dose level 105 % |
15*2.8 = 42 Gy | 50.40 Gy | 100.8Gy | 48.11Gy | 75.60 Gy |
| 50/25 Dose level 100 % |
25*2 = 50 Gy | 50.00 Gy | 100.00 Gy | 50.00 Gy | 78.57Gy |
| 40/15 Dose level 100 % |
15*2.67 = 40.05 Gy | 46.76Gy | 93.52 Gy | 44.93Gy | 70.6Gy |
| 50/25 Dose level 70 % |
25*1.4 = 35 Gy | 29.75Gy | 59.5Gy | 31.18Gy | 49Gy |
| 40/15 Dose level 70 % |
15*1.87 = 28.04 Gy | 27.12 Gy | 54.23Gy | 27.37Gy | 43.01Gy |
| 50/25 Dose level 50 % |
25*1 = 25 Gy | 18.75Gy | 37.5Gy | 20.45Gy | 32.14Gy |
| 40/15 Dose level 50 % |
15*1.34 = 20.03 Gy | 16.7Gy | 33.39Gy | 17.6Gy | 27.66Gy |
| Optimistic scenario α/β 3 for both Normal Tissue and Tumour | |||
| Schedule and dose level | Numerical dose | EQD2 α/β = 3 |
BED α/β = 3 |
| 50/25 Dose level 107 % |
25*2.14 = 53.5 Gy | 55Gy | 91.66Gy |
| 40/15 Dose level 107 % |
15*2.85 = 42.8 Gy | 50.08Gy | 83.46Gy |
| 50/25 Dose level 105 % |
25*2.1 = 52.5 Gy | 53.55Gy | 89.25Gy |
| 40/15 Dose level 105 % |
15*2.8 = 42 Gy | 48.72 Gy | 81.2 Gy |
| 50/25 Dose level 100 % |
25*2 = 50 Gy | 50 Gy | 83.33Gy |
| 40/15 Dose level 100 % |
15*2.67 = 40.05 Gy | 45.42 Gy | 75.69Gy |
| 50/25 Dose level 70 % |
25*1.4 = 35 Gy | 30.8Gy | 51.33Gy |
| 40/15 Dose level 70 % |
15*1.87 = 28.04 Gy | 27.3Gy | 45.5Gy |
| 50/25 Dose level 50 % |
25*1 = 25 Gy | 20 Gy | 33.33Gy |
| 40/15 Dose level 50 % |
15*1.34 = 20.03 Gy | 17.36Gy | 28.94Gy |
| Worst case scenario α/β 1 for Normal Tissue and 5 for Tumour | |||||
| Schedule and dose level | Numerical dose | EQD2 α/β = 1 |
BED α/β = 1 |
EQD2 α/β = 5 |
BED α/β = 5 |
| 50/25 Dose level 107 % |
25*2.14 = 53.5 Gy | 56.00 Gy | 167.99Gy | 54.57Gy | 76.40 Gy |
| 40/15 Dose level 107 % |
15*2.85 = 42.8 Gy | 54.93Gy | 164.78Gy | 48Gy | 67.2 Gy |
| 50/25 Dose level 105 % |
25*2.1 = 52.5 Gy | 54.25Gy | 162.75Gy | 53.25Gy | 74.55Gy |
| 40/15 Dose level 105 % |
15*2.8 = 42 Gy | 53.20 Gy | 159.60 Gy | 46.80 Gy | 65.52 Gy |
| 50/25 Dose level 100 % |
25*2 = 50 Gy | 50.00 Gy | 150.00 Gy | 50.00 Gy | 70.00 Gy |
| 40/15 Dose level 100 % |
15*2.67 = 40.05 Gy | 48.99Gy | 146.9Gy | 43.88Gy | 61.44Gy |
| 50/25 Dose level 70 % |
25*1.4 = 35 Gy | 28.00 Gy | 84.00 Gy | 32.00 Gy | 44.80 Gy |
| 40/15 Dose level 70 % |
15*1.87 = 28.04 Gy | 26.81Gy | 80.43Gy | 27.51Gy | 43.01Gy |
| 50/25 Dose level 50 % |
25*1 = 25 Gy | 16.67Gy | 50.00 Gy | 21.43Gy | 30.00 Gy |
| 40/15 Dose level 50 % |
15*1.34 = 20.03 Gy | 15.59Gy | 46.76Gy | 18.12 Gy | 25.37Gy |
RBE = Relative Biological Effective; EQD2 = Equivalent Dose relative to 2 Gy per fraction; BED = Biologically Effective Dose.
Both the explanatory- and the pragmatically- designed trials demonstrate indeed that moderate hypofractionation leads to a lower rate for side effects, especially for the 40 Gy in 15 fractions schedule, thereby confirming the appropriateness of the radiobiological estimations (Owen et al., 2006; Haviland et al., 2016). The at least as good local control might be explained by the influence of the shortened overall treatment time as a contributor to anti-tumoural efficacy due to tumour cell proliferation, as hypothesised by the START triallists, estimating that 0.6 Gy per calendar day could be lost in the period between 3 and 5 weeks of treatment (Haviland et al., 2016).
5. Clinical applicability of moderate hypofractionation
Most of the patients who participated in the trials had early-stage breast cancer and underwent breast conserving therapy including WBI without regional nodal irradiation. Therefore, the use of hypofractionated RT in post-mastectomy patients and/or those requiring regional nodal irradiation still remains a matter of debate. However, there is no radiobiological reason why these patients should have different outcomes, even on the contrary taking the radiobiological calculations from Table 3 into account. Notwithstanding this, several current guidelines support the use of moderate hypofractionated RT for breast cancer patients on the one hand rather broadly independent of disease stage, patient’s age, and the use of systemic agents, but remain reluctant towards chest wall and regional lymph node irradiation (Smith et al., 2018; BSoR et al., 1992). In the 2019 St Gallen consensus conference, the panellists were divided as to whether hypofractionated treatment was appropriate for these women (Balica et al., 2019). In contrast, the recently updated ESMO guidelines recommend moderate hypofractionation for routine postoperative RT of breast cancer, advising to carefully monitor, evaluate, and compare outcomes of patients treated with hypofractionation outside of the inclusion criteria of the published studies (Cardoso et al., 2019). The reluctance to do so is illustrated in the United States National Cancer Database study, the results of which demonstrate that between 2004–2014 the use of hypofractionated post-mastectomy RT of the chest wall (with or without the regional lymph nodes) is as low as 1.1 % of all patients (Venigalla et al., 2018).
Nevertheless, the data from the START studies (the Royal Marsden Hospital study, as well as both START A and START B) show that 14.7 % (n = 864) of the patients received lymphatic radiation, with another 8.5 % (n = 513) undergoing mastectomy (Haviland et al., 2018). After 10-year follow-up, the cumulative incidence rates of both patient- and physician-assessed side effects (marked or moderate severity) were similar for these patients as well, without statistically significant differences in local recurrence rates after mastectomy or breast conserving surgery (Haviland et al., 2013). Additionally, similar results were found in the Beijing trial, even for locally advanced breast-cancer patients and using older techniques (Wang et al., 2019; Marta and Poortmans, 2019).
Some clinicians argue that, due to the long interval that side effects might occur, especially with regards to heart, lung function and nerve tissue, moderate hypofractionation for regional nodal irradiation should be assessed with caution until the results of other prospective randomized clinical trials are available (Vinh-Hung et al., 2019). Also, some clinicians have expressed concern because chemotherapy was used in only 11 %, 35 %, and 22 % of patients in the Ontario Clinical Oncology Group, START A, and START B trials, respectively, with most patients receiving a currently non-standard regimen (Whelan et al., 2002; Group et al., 2008a; Haviland et al., 2013; Whelan et al., 2010). However, standard chemotherapy, including anthracycline and taxane- or anthracycline-based regimens, was used in both the Beijing trial and the MD Anderson trial, with satisfactory toxicity results, albeit after relatively short follow-up periods (Shaitelman et al., 2015; Wang et al., 2019). Reassuringly, the START trials’ data showed very low rates of ischemic heart disease and lung fibrosis (<2%) (Haviland et al., 2018). Even though reported rate may be higher when using modern diagnostic instruments due to increased detection of subclinical heart or lung disease, patients currently rarely develop cardiac or pulmonary toxicity symptoms that require medical intervention (Verbanck et al., 2016; Liss et al., 2017; Chan et al., 2014).
A point to consider is the very low rate of brachial plexopathy in patients who received regional nodal radiation (Haviland et al., 2018). The 40 Gy in 15 fractions (START B trial) hypofractionated regimen is estimated at an equivalent EQD2Gy biological dose of 45.42 Gy and 47.72 Gy to the brachial plexus, for α/β ratios of 3 Gy and 1.5 Gy, respectively (Yarnold et al., 2011). Only one patient who received RT to breast and supraclavicular fossa area in the START trials developed mild brachial plexopathy. This patient had a family record of polydactyly on the affected site, suggesting a non-confirmed genetic susceptibility (Haviland et al., 2018).
The EORTC 22,881/10,882 “boost” trial demonstrated that a boost dose to the primary tumour bed after breast conserving surgery and whole breast irradiation decreases local recurrences rates to a similar relative extent across all risk groups (Vrieling et al., 2017). However, patients with risk-factors for local recurrences, including young age, high grade, and involved margins, derive a larger absolute benefit from a boost dose, and should thereby be advised to receive this independent of the fractionation schedule. While in most trials a conventionally fractionated boost of 5–8 fractions of 2 Gy was used, the UK and The Netherlands as well as some Italian institutions gathered many years of experience using hypofractionated boost schedules, demonstrating its safety and tolerance (Bloomfield, 2017; Palumbo et al., 2019). Some countries, like The Netherlands, mostly integrate the boost simultaneously (SIB) combined with adding a number (in general 5) of fractions and without increasing the maximum fraction size. The IMPORT High trial, integrating the boost while maintaining a constant number of fractions, assessed more than 2600 patients and showed that a hypofractionated SIB is safe in term of 3 year toxicity and showed an expected a dose response for adverse effects with increasing dose, but not with fraction size (Coles et al., 2019). Moreover, an analysis of tumour factors that might be predictive of response to hypofractionated RT based on a central review of tumour samples showed that molecular subtype clearly predicted local recurrence, while tumour grade, molecular subtype and hypoxia did not predict the response to hypofractionation RT, suggesting that patients of all grades and molecular subtypes may be safely treated with hypofractionated RT regimens (Bane et al., 2014).
Treatment-related toxicities are probably more related to the type of RT technique than to the dose schedule. This concept is accepted in some countries, including the Netherlands and the UK, where moderate hypofractionation has been the standard for practically all indications of RT in breast-cancer patients for many years (Bloomfield, 2017; Lansu et al., 2015; NICE, 2019). The results of prospectively collected databases and other real-life retrospective studies confirm that hypofractionated RT, with or without regional nodal irradiation, is safe, well-tolerated, and associated with satisfactory local control rates (Khan et al., 2017; Bellefqih et al., 2017; Shin et al., 2016; Ko et al., 2015; Miranda et al., 1992; Rastogi et al., 2018; Leong et al., 2017; Chatterjee et al., 2016; Guenzi et al., 2015; Eldeeb et al., 2012).
Perhaps there is excessive concern about toxicity related to moderate hypofractionated RT to the regional nodal areas, an issue that is not considered as a subject of discussion for most other types of cancer. For example, the fractionation schedules for head and neck cancer are independent from the anatomical sub-site, even though high biological-equivalent doses of radiation (60–70 Gy) are usually delivered, even concomitant with chemotherapy in the treatment of patients with locally advanced disease (Marta et al., 2014; Mendez et al., 2016).
Little data are available about the use of hypofractionation before or after breast reconstruction, which is increasingly done using implants or autologous tissue (Santosa et al., 2018). RT might increase the frequency of complications including capsular contracture rates and reconstruction failures (Bachour et al., 2018; Tallet et al., 2003; Anderson et al., 2009; Cowen et al., 2010). A phase II prospective trial by Khan et al. included 69 stage II-III breast cancer patients who received hypofractionated post-mastectomy RT (36.63 Gy over 11 days) to the chest wall and the regional lymph-nodes, followed by an optional boost to the mastectomy scar of 13.32 Gy in 4 fractions (Khan et al., 2017). Breast reconstruction using temporary expanders or implants was performed in 41 patients (59 %). The 3-year local relapse-free survival rate was 89.2 % for all patients. Three of the patients who had breast reconstruction had the expanders removed due to infection before RT. The rate of implant failure was 24 %, and 8% of the patients needed additional surgical correction. These complication rates are similar to those observed after conventional RT in breast-reconstruction patients (Eriksson et al., 2013; Bostwick and Jurkiewicz, 1980; Bostwick, 1980). We expect that moderate hypofractionation for patients after breast reconstruction will compare favourably to conventional fractionation, provided a homogenous dose distribution is given, as most breast-related side effects that are associated with radiation-related toxicities (e.g., skin retraction, fibrosis, and breast shrinkage) show a trend to be less frequent and less severe in patients who underwent hypofractionation (Group et al., 2008a; Group et al., 2008b; Owen et al., 2006; Haviland et al., 2013; Whelan et al., 2010; Wang et al., 2019). The recently published ESTRO-ACROP guidelines for target volume delineation for the chest wall irradiation after implant-based breast reconstruction allow, by limiting the target volumes to anatomically defined zones at risk only, for a reduction of the irradiated volume, which is expected to lower the risk for side effects (Kaidar-Person et al., 2019). Delivery of hypofractionated RT to the reconstructed chest wall with methods that deliver homogenous dose distributions such as IMRT/VMAT is currently being investigated whether they may further reduce toxicities.
Several randomized ongoing clinical trials are investigating the effectiveness and safety of moderate hypofractionation, as compared to conventional fractionation, in various other clinical settings (e.g., NCT02690636, NCT02700386, NCT02958774, NCT02384733, and NCT03127995). The results of these studies will create supplementary evidence from over another 4000 breast cancer patients receiving hypofractionated RT, adding to the existing evidence the ultimate confirmation supporting further extension of hypofractionation to all breast cancer patients in countries where this is not yet the case.
Moreover, there is now an even more urgent need than before to share the available evidence, offering emergency guidance for breast radiation therapy during the COVID-19 pandemic. As per the World Health Organisation (WHO) statement, our aim and obligation should be “to stop, contain, control, delay and reduce the impact of this virus at every opportunity”. In our roles as healthcare professionals and breast cancer experts this translates into minimising the exposure of our patients to COVID-19 without compromising the oncological outcome. In this context, the use of moderate hypofractionation is an appropriate approach even more at this specific worldwide moment in time.
6. Technical considerations
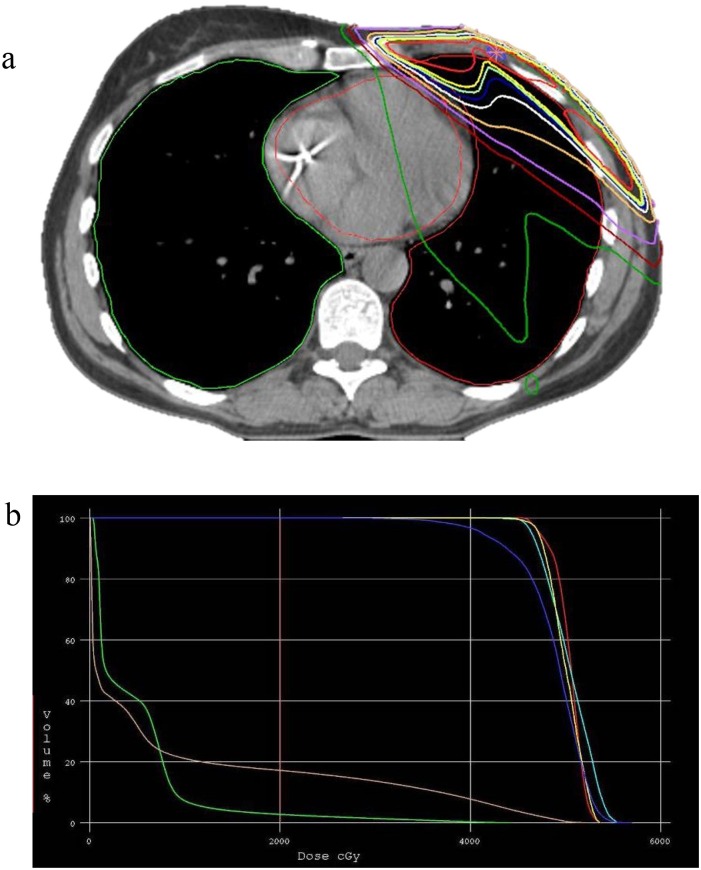
Over the last decades, RT techniques have improved considerably for the treatment of breast cancer patients (Boyages and Baker, 2018). Two-dimensional field-based treatment set-up was routinely used in the prior decades, with limited options to estimate the predicted radiation effect on the organs at risk (e.g. heart, lung, contralateral breast, oesophagus, brachial plexus etc). Thanks to the progress made in both hard- and software, RT transitioned progressively to individualised treatment planning taking into account patient’s anatomy (Yeboa and Evans, 2016) – Fig. 2 . Essential in this is that treatment planning should be based on anatomically defined target volumes, as defined by cooperative groups or societies such as ASTRO or ESTRO (Offersen et al., 2015). Finally, accurate image-guided position verification is required to limit treatment set-up variation for increased precision of dose delivery and thereby smaller safety margins around the target volumes (Dawson and Jaffray, 2007).
Fig. 2.
a 3D-CRT dose distribution for 50 Gy in 25 fractions locoregional radiation therapy. b Dose-volume histogram for a 50 Gy in 25 fractions planning, displaying the dose to the heart in green and to both lungs combined in brown (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Using modern RT techniques, the absolute risks for severe side effects including secondary malignancies and heart disease are very low compared to the past (Taylor et al., 2017). Contemporary, homogeneously delivered, volume-based RT permits even more, irrespective of the target volumes, the adoption of moderately hypofractionated RT for breast cancer patients. Dose inhomogeneity or regions of “hot spots” increase both the total dose and the dose per fraction. The renowned radiation biologist, Rodney Withers recognised this situation with conventional 2 Gy/fraction radiation therapy plans and called it “double trouble” (Yarnold et al., 2011; Bartelink and Arriagada, 2008). If moderate hypofractionation is also added as a third factor, then this could be called “triple trouble” as the equivalent dose in 2 Gy fractions can become higher relative to conventional fractionation with large doses per fraction in case of marked dose homogeneity (Fowler, 1989; Jones et al., 2000). As illustrated in Table 2, this effect should remain however absent for hotspots up to 107 %, thanks to the moderately increase fraction size accompanied with a decrease in total dose. Moreover, modern radiation therapy techniques reduce dose inhomogeneity to the minimum and the clinical effect of this becomes negligible. This is illustrated by the FAST breast radiotherapy that tested 50 Gy in 25 fractions over 5 weeks with 5 fractions of either 5.7 or 6.0 Gy over 5 weeks: dose inhomogeneity showed no difference in cosmesis at 2 years with hypofractionation compared with conventional fractionation (Tsang et al., 2012).
7. Conclusions
Existing data confirms that moderately hypofractionated RT for breast cancer is efficient, convenient and safe for all indications, target volumes and techniques, with most evidence available for 2D- and 3D techniques for treatment of the breast and the chest wall, with and without a boost. Based on a more limited set of data, combined with radiobiological considerations, we recommend extrapolating these results to other indications, target volumes and techniques including regional nodal irradiation and treatment after mastectomy with or without breast reconstruction.
We recognise that some colleagues are still concerned about a generalised application of hypofractionation. Therefore, we recommend that centres participate in the ongoing trials addressing the use of moderately hypofractionated RT for breast cancer in some pertinent clinical circumstances. Centres that already apply hypofractionation as a standard should record, review and analyse their outcomes as this can contribute to provide further evidence and thereby confidence in that treatment.
Author contributions
GNM and PP conceived the project; GNM performed the literature search; all authors contributed to the literature analysis and synthesis of expert opinions; GNM and PP created the figures and tables; GNM wrote the review; and all authors were involved in further editing and finalising the manuscript.
Funding/Support
None.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Anderson P.R., Freedman G., Nicolaou N., et al. Postmastectomy chest wall radiation to a temporary tissue expander or permanent breast implant--is there a difference in complication rates? Int. J. Radiat. Oncol. Biol. Phys. 2009;74:81–85. doi: 10.1016/j.ijrobp.2008.06.1940. [DOI] [PubMed] [Google Scholar]
- Bachour Y., Oei L.J., Van der Veen A.J., et al. The influence of radiotherapy on the mechanical properties of silicone breast implants. Plast. Reconstr. Surg. Glob. Open. 2018;6:e1772. doi: 10.1097/GOX.0000000000001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balica M., Thomssenb C., Würstlein R., Gnantd M., Harbeck N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care (Basel) 2019;14(2):103–110. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bane A.L., Whelan T.J., Pond G.R., et al. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann. Oncol. 2014;25:992–998. doi: 10.1093/annonc/mdu090. [DOI] [PubMed] [Google Scholar]
- Bartelink H., Arriagada R. Hypofractionation in radiotherapy for breast cancer. Lancet. 2008;371:1050–1052. doi: 10.1016/S0140-6736(08)60349-9. [DOI] [PubMed] [Google Scholar]
- Bellefqih S., Elmajjaoui S., Aarab J., et al. Hypofractionated regional nodal irradiation for women with node-positive breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017;97:563–570. doi: 10.1016/j.ijrobp.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Bloomfield D.J. Core Group facilitated by The Royal College of R. Development of Postoperative Radiotherapy for Breast Cancer: UK Consensus Statements - a Model of Patient, Clinical and Commissioner Engagement? Clin Oncol (R Coll Radiol) 2017;29:639–641. doi: 10.1016/j.clon.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Bostwick J. Reconstruction of the breast. Acta Chir. Belg. 1980;79:125–129. [PubMed] [Google Scholar]
- Bostwick J., 3rd, Jurkiewicz M.J. Recent advances in breast reconstruction: transposition of the latissimus dorsi muscle singly or with the overlying skin. Am. Surg. 1980;46:537–547. [PubMed] [Google Scholar]
- Boyages J., Baker L. Evolution of radiotherapy techniques in breast conservation treatment. Gland Surg. 2018;7:576–595. doi: 10.21037/gs.2018.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BSoR Sbrt, Freitas N.M.A., Rosa A.A., et al. Recommendations for hypofractionated whole-breast irradiation. Rev. Assoc. Med. Bras. 1992;2018(64):770–777. doi: 10.1590/1806-9282.64.09.770. [DOI] [PubMed] [Google Scholar]
- Cardoso F., Kyriakides S., Ohno S., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:1674. doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- Chan E.K., Woods R., McBride M.L., et al. Adjuvant hypofractionated versus conventional whole breast radiation therapy for early-stage breast cancer: long-term hospital-related morbidity from cardiac causes. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:786–792. doi: 10.1016/j.ijrobp.2013.11.243. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Arunsingh M., Agrawal S., et al. Outcomes following a moderately hypofractionated adjuvant radiation (START B type) schedule for breast Cancer in an unscreened non-caucasian population. Clin Oncol (R Coll Radiol) 2016;28:e165–172. doi: 10.1016/j.clon.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Coles C.E., Griffin C.L., Kirby A.M., et al. Dose escalated simultaneous integrated boost radiotherapy for women treated by breast conservation surgery for early breast cancer: 3-year adverse effects in the IMPORT HIGH trial (CRUK/06/003) [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4-8; San Antonio, TX. Philadelphia (PA): AACR. Cancer Res. 2019;79(4 Suppl) Abstract nr GS4-05. [Google Scholar]
- Cowen D., Gross E., Rouannet P., et al. Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast Cancer Res. Treat. 2010;121:627–634. doi: 10.1007/s10549-010-0791-5. [DOI] [PubMed] [Google Scholar]
- Dawson L.A., Jaffray D.A. Advances in image-guided radiation therapy. J. Clin. Oncol. 2007;25:938–946. doi: 10.1200/JCO.2006.09.9515. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative G, Darby S., McGale P., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebctcg, McGale P., Taylor C., et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldeeb H., Awad I., Elhanafy O. Hypofractionation in post-mastectomy breast cancer patients: seven-year follow-up. Med. Oncol. 2012;29:2570–2576. doi: 10.1007/s12032-012-0192-1. [DOI] [PubMed] [Google Scholar]
- Eriksson M., Anveden L., Celebioglu F., et al. Radiotherapy in implant-based immediate breast reconstruction: risk factors, surgical outcomes, and patient-reported outcome measures in a large Swedish multicenter cohort. Breast Cancer Res. Treat. 2013;142:591–601. doi: 10.1007/s10549-013-2770-0. [DOI] [PubMed] [Google Scholar]
- Fowler J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- Group S.T., Bentzen S.M., Agrawal R.K., et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group S.T., Bentzen S.M., Agrawal R.K., et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GSe Higgins J.P.T. 2011. Cochrane Handbook for Systematic Reviews of Interventions: Version 5.0.2 [updated September 2011]. The Cochrane Collaboration 2008. [Google Scholar]
- Guenzi M., Blandino G., Vidili M.G., et al. Hypofractionated irradiation of infra-supraclavicular lymph nodes after axillary dissection in patients with breast cancer post-conservative surgery: impact on late toxicity. Radiat. Oncol. 2015;10:177. doi: 10.1186/s13014-015-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviland J.S., Owen J.R., Dewar J.A., et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- Haviland J.S., Bentzen S.M., Bliss J.M., et al. Prolongation of overall treatment time as a cause of treatment failure in early breast cancer: an analysis of the UK START (Standardisation of Breast Radiotherapy) trials of radiotherapy fractionation. Radiother. Oncol. 2016;121:420–423. doi: 10.1016/j.radonc.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Haviland J.S., Mannino M., Griffin C., et al. Late normal tissue effects in the arm and shoulder following lymphatic radiotherapy: results from the UK START (Standardisation of Breast Radiotherapy) trials. Radiother. Oncol. 2018;126:155–162. doi: 10.1016/j.radonc.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S., Svensson H., Denekamp J. Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2002;52:1207–1219. doi: 10.1016/s0360-3016(01)02743-2. [DOI] [PubMed] [Google Scholar]
- Jones B., Dale R.G., Deehan C., et al. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol) 2001;13:71–81. doi: 10.1053/clon.2001.9221. [DOI] [PubMed] [Google Scholar]
- Jones B., Dale R.G., Finst P., Khaksar S.J. Biological equivalent dose assessment of the consequences of hypofractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:1379–1384. doi: 10.1016/s0360-3016(00)00571-x. [DOI] [PubMed] [Google Scholar]
- Kaidar-Person O., Vrou Offersen B., Hol S., et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother. Oncol. 2019;137:159–166. doi: 10.1016/j.radonc.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Khan A.J., Poppe M.M., Goyal S., et al. Hypofractionated postmastectomy radiation therapy is safe and effective: first results from a prospective phase II trial. J. Clin. Oncol. 2017;35:2037–2043. doi: 10.1200/JCO.2016.70.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko D.H., Norriss A., Harrington C.R., et al. Hypofractionated radiation treatment following mastectomy in early breast cancer: the Christchurch experience. J. Med. Imaging Radiat. Oncol. 2015;59:243–247. doi: 10.1111/1754-9485.12242. [DOI] [PubMed] [Google Scholar]
- Lansu J.T., Essers M., Voogd A.C., et al. The influence of simultaneous integrated boost, hypofractionation and oncoplastic surgery on cosmetic outcome and PROMs after breast conserving therapy. Eur. J. Surg. Oncol. 2015;41:1411–1416. doi: 10.1016/j.ejso.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Leong N., Truong P.T., Tankel K., et al. Hypofractionated nodal radiation therapy for breast Cancer Was not associated with increased patient-reported arm or brachial plexopathy symptoms. Int. J. Radiat. Oncol. Biol. Phys. 2017;99:1166–1172. doi: 10.1016/j.ijrobp.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Lievens Y., van den Bogaert W., Kesteloot K. Activity-based costing: a practical model for cost calculation in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:522–535. doi: 10.1016/s0360-3016(03)00579-0. [DOI] [PubMed] [Google Scholar]
- Liss A.L., Marsh R.B., Kapadia N.S., et al. Decreased lung perfusion after Breast/Chest wall irradiation: quantitative results from a prospective clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2017;97:296–302. doi: 10.1016/j.ijrobp.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta G.N., Poortmans P. Moderately hypofractionated breast radiation therapy: is more evidence needed? Lancet Oncol. 2019;20:e226. doi: 10.1016/S1470-2045(19)30078-6. [DOI] [PubMed] [Google Scholar]
- Marta G.N., Silva V., de Andrade Carvalho H., et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother. Oncol. 2014;110:9–15. doi: 10.1016/j.radonc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Mendez L.C., Moraes F.Y., Poon I., Marta G.N. The management of head and neck tumors with high technology radiation therapy. Expert Rev. Anticancer Ther. 2016;16:99–110. doi: 10.1586/14737140.2016.1121111. [DOI] [PubMed] [Google Scholar]
- Miranda F.A., Vieira M.T.L., Moraes F.Y., et al. Cosmesis in patients with breast neoplasia submitted to the hypofractionated radiotherapy with of intensity-modulated beam. Rev. Assoc. Med. Bras. 1992;2018(64):1023–1030. doi: 10.1590/1806-9282.64.11.1023. [DOI] [PubMed] [Google Scholar]
- Offersen B.V., Boersma L.J., Kirkove C., et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- Overgaard M., Bentzen S.M., Christensen J.J., Madsen E.H. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother. Oncol. 1987;9:1–11. doi: 10.1016/s0167-8140(87)80213-x. [DOI] [PubMed] [Google Scholar]
- Owen J.R., Ashton A., Bliss J.M., et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- Palumbo I., Mariucci C., Falcinelli L., et al. Hypofractionated whole breast radiotherapy with or without hypofractionated boost in early stage breast cancer patients: a mono-institutional analysis of skin and subcutaneous toxicity. Breast Cancer. 2019;26:290–304. doi: 10.1007/s12282-018-0923-z. [DOI] [PubMed] [Google Scholar]
- Qi X.S., White J., Li X.A. Is alpha/beta for breast cancer really low? Radiother. Oncol. 2011;100:282–288. doi: 10.1016/j.radonc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Rastogi K., Jain S., Bhatnagar A.R., et al. A comparative study of hypofractionated and conventional radiotherapy in postmastectomy breast Cancer patients. Asia. J. Oncol. Nurs. 2018;5:107–113. doi: 10.4103/apjon.apjon_46_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa K.B., Qi J., Kim H.M., et al. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2018;153:891–899. doi: 10.1001/jamasurg.2018.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaitelman S.F., Schlembach P.J., Arzu I., et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaitelman S.F., Lei X., Thompson A., et al. Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: results of a randomized, noninferiority clinical trial. J. Clin. Oncol. 2018 doi: 10.1200/JCO.18.00317. JCO1800317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.M., No H.S., Vega R.M., et al. Breast, chest wall, and nodal irradiation with prone set-up: results of a hypofractionated trial with a median follow-up of 35 months. Pract. Radiat. Oncol. 2016;6:e81–88. doi: 10.1016/j.prro.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Smith B.D., Bellon J.R., Blitzblau R., et al. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract. Radiat. Oncol. 2018;8:145–152. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Tallet A.V., Salem N., Moutardier V., et al. Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:136–142. doi: 10.1016/s0360-3016(03)00526-1. [DOI] [PubMed] [Google Scholar]
- Taylor C., Correa C., Duane F.K., et al. Estimating the risks of breast Cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J. Clin. Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute for Health and Care Excellence (NICE) Available at: Recommendations - radiotherapy. Accessed April 27, 2019.
- Tsang Y., Haviland J., Venables K., et al. The impact of dose heterogeneity on late normal tissue complication risk after hypofractionated whole breast radiotherapy. Radiother. Oncol. 2012;104:143–147. doi: 10.1016/j.radonc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Venigalla S., Guttmann D.M., Jain V., et al. Trends and patterns of utilization of hypofractionated postmastectomy radiotherapy: a national Cancer database analysis. Clin. Breast Cancer. 2018;18:e899–e908. doi: 10.1016/j.clbc.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanck S., Hanon S., Schuermans D., et al. Mild lung restriction in breast Cancer patients after hypofractionated and conventional radiation therapy: a 3-Year follow-up. Int. J. Radiat. Oncol. Biol. Phys. 2016;95:937–945. doi: 10.1016/j.ijrobp.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Vinh-Hung V., Nguyen N.P., Verschraegen C. Hypofractionated nodal irradiation for breast Cancer: a case for caution. JAMA Oncol. 2019;5:13–14. doi: 10.1001/jamaoncol.2018.5061. [DOI] [PubMed] [Google Scholar]
- Vrieling C., van Werkhoven E., Maingon P., et al. Prognostic factors for local control in breast Cancer After long-term follow-up in the EORTC boost vs No boost trial: a randomized clinical trial. JAMA Oncol. 2017;3:42–48. doi: 10.1001/jamaoncol.2016.3031. [DOI] [PubMed] [Google Scholar]
- Wang S.L., Fang H., Song Y.W., et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20:352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- Whelan T., MacKenzie R., Julian J., et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J. Natl. Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- Whelan T.J., Pignol J.P., Levine M.N., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- Withers H.R., Thames H.D., Jr., Peters L.J. A new isoeffect curve for change in dose per fraction. Radiother. Oncol. 1983;1:187–191. doi: 10.1016/s0167-8140(83)80021-8. [DOI] [PubMed] [Google Scholar]
- Yarnold J., Ashton A., Bliss J., et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother. Oncol. 2005;75:9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Yarnold J., Bentzen S.M., Coles C., Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:1–9. doi: 10.1016/j.ijrobp.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Yeboa D.N., Evans S.B. Contemporary breast radiotherapy and cardiac toxicity. Semin. Radiat. Oncol. 2016;26:71–78. doi: 10.1016/j.semradonc.2015.09.003. [DOI] [PubMed] [Google Scholar]