Abstract
Purpose
We describe the presenting characteristics and hospital course of 11 novel coronavirus (COVID-19) patients who developed spontaneous subcutaneous emphysema (SE) with or without pneumomediastinum (SPM) in the absence of prior mechanical ventilation.
Materials and methods
A total of 11 non-intubated COVID-19 patients (8 male and 3 female, median age 61 years) developed SE and SPM between March 15 and April 30, 2020 at a multi-center urban health system in New York City. Demographics (age, gender, smoking status, comorbid conditions, and body-mass index), clinical variables (temperature, oxygen saturation, and symptoms), and laboratory values (white blood cell count, C-reactive protein, D-dimer, and peak interleukin-6) were collected. Chest radiography (CXR) and computed tomography (CT) were analyzed for SE, SPM, and pneumothorax by a board-certified cardiothoracic-fellowship trained radiologist.
Results
Eleven non-intubated patients developed SE, 36% (4/11) of whom had SE on their initial CXR. Concomitant SPM was apparent in 91% (10/11) of patients, and 45% (5/11) also developed pneumothorax. Patients developed SE on average 13.3 days (SD: 6.3) following symptom onset. No patients reported a history of smoking. The most common comorbidities included hypertension (6/11), diabetes mellitus (5/11), asthma (3/11), dyslipidemia (3/11), and renal disease (2/11). Four (36%) patients expired during hospitalization.
Conclusion
SE and SPM were observed in a cohort of 11 non-intubated COVID-19 patients without any known cause or history of invasive ventilation. Further investigation is required to elucidate the underlying mechanism in this patient population.
Keywords: Subcutaneous emphysema, Pneumomediastinum, COVID-19
Highlights
-
•
COVID-19 patients were observed to form subcutaneous air and pneumomediastinum in the absence of mechanical ventilation.
-
•
Patients in this cohort presented mainly with respiratory symptoms including mild hypoxia, but otherwise stable vital signs.
-
•
On initial presentation, all patients demonstrated abnormalities in inflammatory markers.
-
•
On initial presentation, all patients demonstrated abnormalities in inflammatory markers.
1. Introduction
Subcutaneous emphysema (SE) and pneumomediastinum refer to the presence of air in the subcutaneous tissue and mediastinum, respectively. Spontaneous pneumomediastinum (SPM) results from a sudden rise in intra-alveolar pressure (such as in the setting of reactive airways disease, Valsalva maneuver, cough, emesis, and barotrauma), resulting in the rupture of alveoli and subsequent dissection of air along the bronchovascular sheath into the mediastinum (Macklin effect) [1]. Air may then enter the pleural, pericardial, and peritoneal spaces or the soft tissues of the chest wall, neck, or face causing subcutaneous cervicothoracic emphysema. On their own, these conditions are not typically life-threatening and often resolve with conservative treatment. However, they may indicate the presence of severe underlying pathology. While SE and SPM have been observed in patients with a variety of viral pneumonias as a complication of mechanical ventilation, the development of these conditions in non-intubated patients suggests an alternative etiology [2].
We have identified 11 patients at our institution with COVID-19 who developed SE and SPM in the absence of any known underlying cause or mechanical ventilation (Fig. 1, Fig. 2 ). Similar observations have been described in three COVID-19 case reports [[3], [4], [5]].
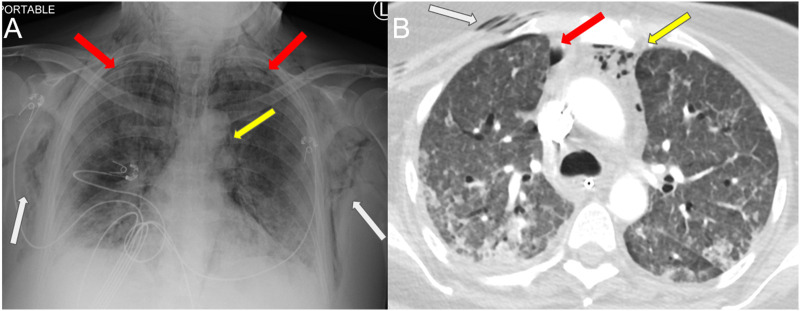
Fig. 1.
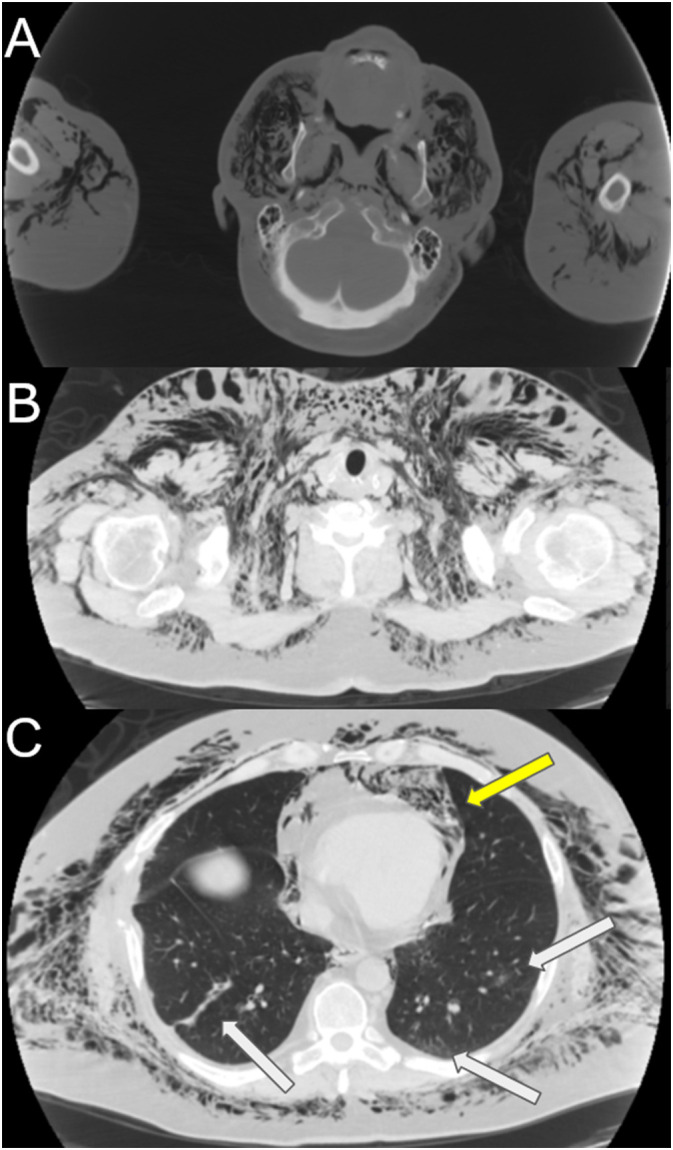
Axial CT in a 60 year old male with COVID-19 who presented with increasing facial swelling, demonstrating extensive subcutaneous emphysema through the skull base on bone windows. Pneumomediastinum (yellow arrow) and mild lower lobe opacities (white arrows) are labeled. Figures show (A) lower neck (B) upper thorax and (C) lung windows. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Chest radiography in a 72-year-old male presenting with dry cough and shortness of breath, who subsequently was found to be in acute hypoxic respiratory failure. The radiograph demonstrates diffuse ground glass opacities, spontaneous subcutaneous emphysema (white arrows), and pneumomediastinum (yellows arrows).
This case series characterizes 11 patients who presented with COVID-19 and developed SE or SPM with or without pneumothorax, in the absence of (or prior to) endotracheal intubation at five different hospitals in a multi-center urban health system in New York City.
2. Materials and methods
This retrospective case series was approved by the institutional review board (IRB), and informed consent was waived. The authors used the electronic medical record (EMR) to attain clinical variables and admission details. A picture archiving and communication system (PACS) was used to access all relevant imaging studies. All images were reviewed by a board-certified cardiothoracic-fellowship trained radiologist with 10 years of experience and a cardiothoracic radiology fellow with complete agreement on presence or absence of SE, SPM, and/or pneumothorax.
2.1. Subject population
Patients who were confirmed COVID-19 positive via real-time reverse transcription polymerase chain reaction (RT-PCR) and were noted to have SE on chest imaging without evidence of mechanical ventilation from March 10 to April 30, 2020 were reviewed. Any patient that first developed a radiographically apparent pneumothorax before identification of SE was not included, as this was felt to be a confounding variable possibly related to a separate mechanism.
2.2. Clinical data collection
In order to describe the admission characteristics of the cohort, demographic, clinical, and laboratory variables were collected for each patient. Demographic parameters included age, gender, smoking status, comorbid conditions, and body-mass index (BMI). Clinical variables included initial temperature on presentation, initial peripheral capillary oxygen saturation (SpO2%), and initial mean arterial pressure (in mmHg). Symptoms including chills, cough, dyspnea, and shortness of breath upon presentation were also noted. Date of symptom onset was also collected. Hospitalization parameters including details of noninvasive and invasive ventilation and mortality were collected. Finally, laboratory values collected included the admission white blood cell (WBC) count and absolute lymphocyte number as well as levels of lactate dehydrogenase (LDH), C-reactive protein (CRP), D-dimer, ferritin, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, procalcitonin and peak interleukin-6 (IL-6).
2.3. Imaging characteristics
Chest radiography (CXR) in all patients consisted of standard portable anteroposterior views. Chest computed tomography (CT) images were also examined in patients where available. Each patient's imaging was analyzed to identify the presence of SE, SPM, and pneumothorax (Fig. 3 ). Additionally, the severity of opacities on initial CXR and at the time of SE or SPM development was calculated as an objective measure of severity of pulmonary viral disease.
Fig. 3.
Chest radiography of a hospitalized 53-year-old COVID-19 patient (A) prior to intubation with extensive subcutaneous emphysema (white arrows), mild small pneumomediastinum (yellow arrows), trace apical and right lateral pneumothoraces (red arrows), and bilateral lower lobe predominant opacities. (B) Axial chest CT angiography on follow-up 22 days later demonstrates persistence of reticular and patchy ground-glass pulmonary opacities related to the viral pneumonia in addition to subcutaneous emphysema, pneumomediastinum, and trace right pneumothorax. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The severity score was determined using a previously validated lung zone scoring system whereby the lungs were each divided into upper, middle, and lower zones, and each zone was given one point if an opacity was present, for a maximum score of 6 (Fig. 4 ) [6]. Additional similar scoring methods have been proposed for assessing the severity of lung disease on chest radiography, but the 6-point scoring system was utilized as it has been previously validated for prognostication [[7], [8], [9]].
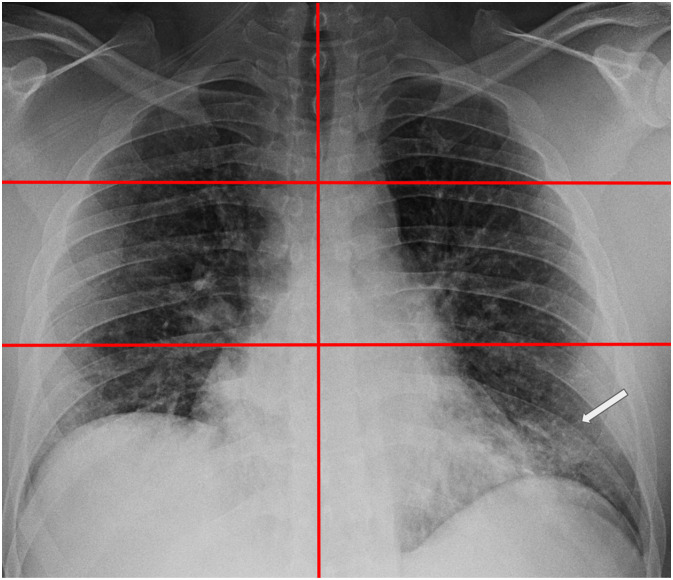
Fig. 4.
Chest radiograph with each lung divided into three lung zones for scoring. 38-year-old male patient presented with fever, cough, and dyspnea shows an opacity in the left lower lung zone only (white arrow); severity score = 1 (opacities noted in ⅙ zones). Subsequent laboratory testing confirmed COVID-19 positivity.
2.4. Analysis
The clinical, laboratory, and demographic features of the patients on initial presentation were tabulated and are presented (Table 1 ). Imaging and ventilation parameters of the patients are described (Table 2 ). Two representative cases are presented in detail.
Table 1.
Initial demographic, clinical, and laboratory characteristics of COVID-19 patients developing spontaneous subcutaneous emphysema and/or pneumomediastinum
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 82 | 61 | 53 | 66 | 38 | 72 | 69 | 89 | 60 | 38 | 57 |
| Sex | Male | Male | Female | Female | Male | Male | Male | Male | Male | Male | Female |
| Smoking status | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker | Nonsmoker |
| Presence of comorbid conditions | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| BMI (kg/m2) | 27.5 | 31.4 | 21.3 | 22.3 | 20.2 | 26.6 | 27.9 | 26.6 | 25.1 | 30.3 | – |
| Initial temperature (°C) | 39.4 | 40.0 | 38.8 | 36.8 | 36.6 | 35.8 | 37.8 | 36.7 | 37.3 | 39.0 | 36.0 |
| Initial oxygen saturation (%) | 88 | 92 | 96 | 68 | 97 | 87 | 99 | 94 | 86 | 85 | 84 |
| Initial mean arterial pressure (mmHg) | 103 | 94 | 80 | 71 | 108 | 92 | 68 | 114 | 96 | 96 | 103 |
| Presence of chills | No | Yes | Yes | No | No | No | No | No | Yes | Yes | No |
| Presence of cough | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Presence of dyspnea | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Initial WBC count (4.5–11.0 x10E3 cells/uL) | 10.0 | 4.6 | 12.7 | 9,0 | 11.0 | 16.7 | 6.2 | 14.3 | 8.8 | 11.5 | 15.4 |
| Initial lymphocyte count (1.0–4.5 x10E3 cells/uL) | 0.8 | 0.6 | 1.0 | 0.3 | 0.7 | 0.6 | 0.9 | 5.2 | 0.7 | 1.3 | 0.7 |
| Initial LDH (100–220 u/L) | 442 | 632 | 593 | 775 | 704 | 623 | 518 | 624 | 491 | 747 | 957 |
| Initial CRP (0.0–5.0 mg/L) | 253 | 59 | 145 | 193 | 27 | 378 | 61 | 259 | 146 | 96 | 96 |
| Initial d-dimer (<0.50 μg/mL FEU) | 1.1 | 0.8 | 2.1 | 3.8 | 1.2 | 11.3 | 1.2 | 20.0 | 1.8 | 1.1 | 20.0 |
| Initial ferritin (30–400 ng/ML) | 156 | 1995 | 993 | 6531 | 264 | 379 | 1588 | 658 | 1385 | 1982 | 1351 |
| Initial AST/ALT (<35/<45 u/L) | 122/79 | 213/117 | 43/41 | 47/21 | 14/11 | 33/56 | 70/80 | 29/14 | 74/100 | 84/89 | 45/41 |
| initial procalcitonin (<0.49 ng/mL) | 0.50 | 0.53 | 0.12 | 1.76 | 1.14 | 1.28 | 0.12 | 0.23 | 0.96 | 0.13 | 3.90 |
| Peak IL-6 (0.0–5.0 pg/mL) | 175.0 | 49.1 | 132.0 | 166.0 | 10.1 | 710.0 | 81.4 | 231.8 | 5.5 | 1648.0 | 82.3 |
Legend: (−) indicates a missing value. BMI: body mass index; SOB: shortness of breath; WBC: white blood cell count; CRP: c-reactive protein; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Il-6: interleukin 6. Reference values and units for laboratory markers included parenthetically in first column.
Table 2.
Imaging and ventilation characteristics of COVID-19 patients developing spontaneous subcutaneous emphysema and/or pneumomediastinum
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SE on initial CXR | No | No | No | Yes | Yes | Yes | No | Yes | No | No | No |
| Development of SPX | No | Yes | Yes | Yes | No | No | Yes | Yes | No | No | No |
| Development of SPM | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Maximal oxygen support [settings] | HFNC [40 L/min at Fi0260%] |
HFNC+ NRB [40 L/min at Fi02100%] and [10 L/min] |
HFNC + NRB [50 L/min at Fi0260%] and [10 L/min] |
BiPAP [12/7 cm H20 at Fi0270%] |
None | HFNC [40 L/min at Fi0260%] |
CPAP [12 cm H20 at Fi02100%] |
HFNC [50 L/min at Fi0260%] |
CPAP [16 cm H20 at Fi0280%] |
NRB [15 L/min] | BiPAP [12/7 cm H20 at Fi0260%] |
| Intubation (days from positive CXR) | No | Yes (1) | Yes (9) | Yes (1) | No | Yes (7) | Yes (3) | No | Yes (7) | Yes (1) | No |
| Expired (days from positive CXR) | No | Yes (9) | No | No | No | Yes (18) | Yes (4) | Yes (10) | No | No | No |
| Severity score of opacity on initial CXR in ED | 5 | 4 | 2 | 6 | 3 | 6 | 2 | 4 | 3 | 1 | 6 |
| Severity score of opacity when SE developed | 5 | 4 | 4 | 6 | 3 | 6 | 2 | 4 | 3 | 6 | 6 |
| Days from symptom onset to discovery of SE | 18 | 18 | 17 | 7 | 3 | 14 | 22 | 3 | 16 | 15 | 14 |
Legend: SE: Subcutaneous emphysema; SPX: spontaneous pneumothorax; SPM: spontaneous pneumomediastinum; HFNC: high flow nasal cannula; NRB: non-rebreather mask; BiPAP: bilevel positive airway pressure; CPAP: continuous positive airway pressure; FiO2: fraction of inspired oxygen; CXR: chest radiography; ED: emergency department. Maximal oxygenation settings denoted in brackets. HFNC settings given as flow rate followed by FiO2. NRB settings given as flow rate. BiPAP settings given as positive end-expiratory pressure/positive inspiratory pressure followed by FiO2. CPAP settings given as pressure followed by FiO2. Details of CXR severity scoring discussed in Materials and Methods section and Fig. 4. Development of PTX and SP refer to the discovery of these findings at any point during hospital course.
3. Results
Eleven patients met inclusion criteria including confirmed COVID-19 RT-PCR, absence of prior mechanical ventilation, and SE and/or SPM seen on imaging (Table 1). The median age of the cohort was 61 years (range: 38–89 years). There were 8 males and 3 females. No patients reported a history of smoking. The mean BMI was 25.9 (standard deviation [SD]: 3.7) [10].
In this cohort, all patients had recorded pre-existing comorbidities. The most common comorbidities included hypertension (6/11), diabetes mellitus (5/11), asthma (3/11), dyslipidemia (3/11), and renal disease (2/11).
On initial presentation to the emergency department (ED), the mean temperature was 37.6 °C (SD: 1.4 °C), mean oxygen saturation was 89% (SD: 8.6%), and mean arterial pressure was 93 mmHg (SD: 14.7 mmHg). Upon review of patients' most common presenting symptoms, four patients (36%) presented with fever (defined as initial temperature greater than 38 °C), three (27%) displayed chills, nine (82%) had a cough, and ten (91%) displayed dyspnea or shortness of breath.
Average laboratory marker values on presentation included a WBC count of 10.9 × 103 cells/uL (SD: 3.7 × 103), an absolute lymphocyte count of 1.2 × 103/uL (SD: 1.4 × 103 cells/uL), an LDH level of 646 u/L (SD: 145 u/L), a CRP level of 155 mg/L (SD: 107 mg/L), a D-dimer level of 5.85 μg/mL (SD: 7.60 UG/ML), a ferritin level of 1571 ng/mL (SD: 1770 NG/ML), a procalcitonin level of 0.97 ng/mL (SD: 1.1 ng/mL) and an AST/ALT ratio of 70/59 (SD: 56/36). Average peak IL-6 values during hospitalization were 290.2 pg/mL (SD: 487.9 pg/mL). References values are included in Table 1.
With respect to imaging findings, all patients had SE and 36% (4/11) of patients presented to the hospital with subcutaneous air on initial CXR. Of the four patients who initially presented with SE, one had SE alone, two had concomitant SPM, and one patient had SE and SPM in addition to a small pneumothorax on initial CXR. Of the patients who developed SE on subsequent radiographic follow-up, 57% (4/7) developed SE and SPM concurrently, and 43% (3/7) developed SE, SPM and small unilateral pneumothoraces concurrently.
In total, 91% (10/11) patients presented with or developed SE and SPM on the same radiograph, suggesting a common mechanism.
The average severity score of opacities on initial CXR was 3.8 (SD: 1.8), classified as opacities within at least three or four lung zones, most frequently in the middle to lower lung zones. The average severity score of opacities upon development of SE/SPM was 4.4 (SD: 1.4).
Patients developed SE/SPM on average 13.3 days (SD: 6.3) following symptomatology onset. Ninety-one percent (10/11) had received a form of supplemental oxygen prior to the discovery of SE/SPM. Thirty-six percent (4/11) of patients received noninvasive ventilation (NIV) in the form of either bilevel positive airway pressure (BiPAP) (2/11) with peak end expiratory pressure up to 7 cm or continuous positive airway pressure (CPAP) (2/11) with peak continuous pressure up to 16 cm. Forty five percent (5/11) of patients had received high flow nasal cannula with a peak flow up to 50 L/min while one patient received supplemental oxygen through a non-rebreather (NRB) mask with a peak flow up to 15 L/min. One patient (9%) did not receive any form of supplemental oxygen prior to discovery of SE/SPM on CXR.
Only one patient received a jugular central venous catheter; however, this was placed two weeks after discovery of SE. Seven (64%) patients were eventually intubated on average 4.5 (SD: 3.4) days. No patients underwent placement of a chest tube. Four (36%) patients expired during their hospitalization, on average 10.2 (SD: 5.7) days following detection of SE/SPM.
Two illustrative cases from the patient cohort are highlighted to present a more comprehensive view of the hospital course of individuals with these findings.
3.1. Selected cases
3.1.1. Patient #1
An 82-year-old male with a past medical history of intracranial hemorrhage, cerebrovascular accident (CVA), dementia, and hypertension was admitted following presentation to the ED with a fever of 39.4 °C, altered mental status, and shortness of breath. Notably, 10 days prior to presentation he had tested positive for COVID-19 (via RT-PCR) in the ED after a syncopal episode. He was discharged at that time following a course of antibiotic treatment. On re-presentation, his condition had deteriorated, with a blood pressure of 150/80 mmHg and an SpO2 of 88%. Abnormal laboratory markers included an elevated CRP, LDH, D-dimer, and AST:ALT ratio. Initial CXR demonstrated moderate to severe opacities, consistent with atypical viral pneumonia. Abnormal laboratory and CXR severity score values are included in Table 1, Table 2, respectively.
In the ED, the patient was started on a NRB mask at 15 L/min as well as empiric ceftriaxone and azithromycin, which upon admission was transitioned to intravenous cefepime and vancomycin. The patient was also started on methylprednisolone (40 mg/day). On hospital day 10, the patient was placed on HFNC starting at 50 L/min. Multiple attempts to wean oxygen below 30 L/min of HFNC were unsuccessful. On hospital day 13, repeat CXR was performed, revealing new extensive SE with diffuse reticular and bilateral hazy opacities (Fig. 5 ). On hospital day 14, the patient received convalescent plasma therapy and remains hospitalized at the time of writing with an oxygen requirement of HFNC at 40 L/min.
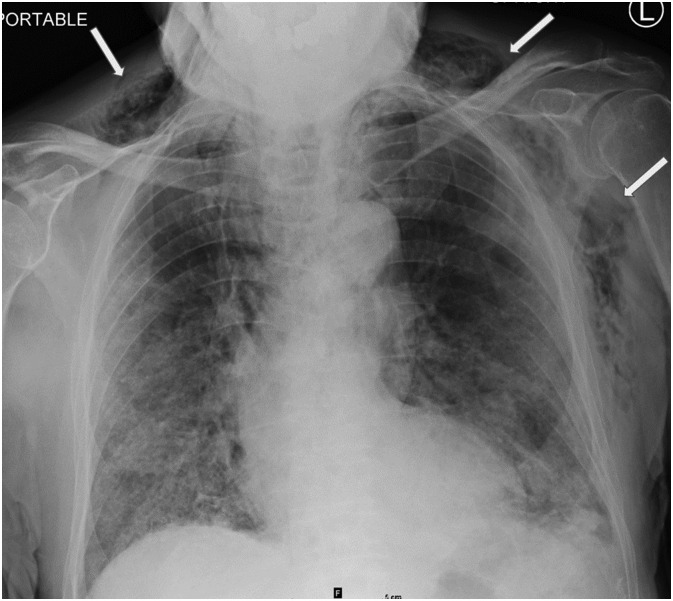
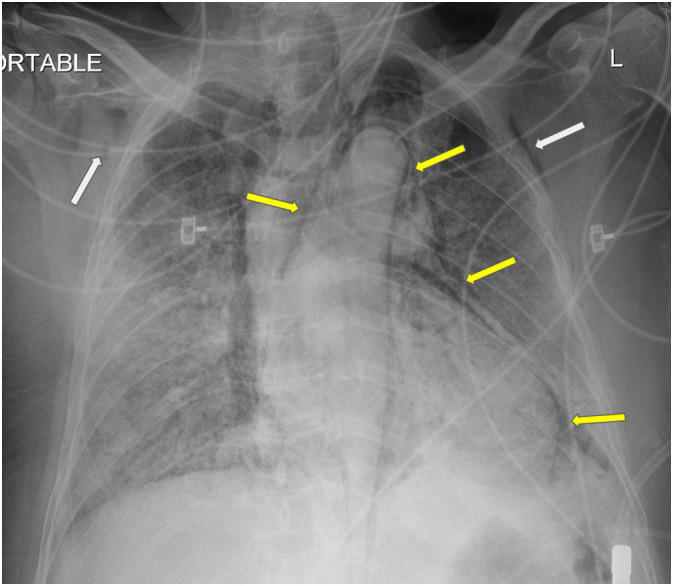
Fig. 5.
Chest radiograph from 82-year-old male (patient #1) on hospital day 13 demonstrating moderate new subcutaneous emphysema (white arrows) who had been placed on a combination of non-rebreather mask (NRB) at 15 L/min and high flow nasal cannula (HFNC) at 50 L/min.
3.1.2. Patient #2
A 61-year-old male with a past medical history of hypertension, diabetes mellitus and dyslipidemia presented to the ED with fever, chills, shortness of breath, productive cough, malaise, and vomiting starting the previous week. The patient's temperature was 40 °C, and his SpO2 was 92%. Complete blood count (CBC) was notable for lymphopenia. Abnormal laboratory markers included an elevated CRP, LDH, D-dimer, and AST:ALT ratio (Table 1). COVID-19 RT-PCR was performed and was positive. CXR demonstrated bibasilar and peripheral patchy opacities, findings suspicious for atypical viral pneumonia (Fig. 6 ).
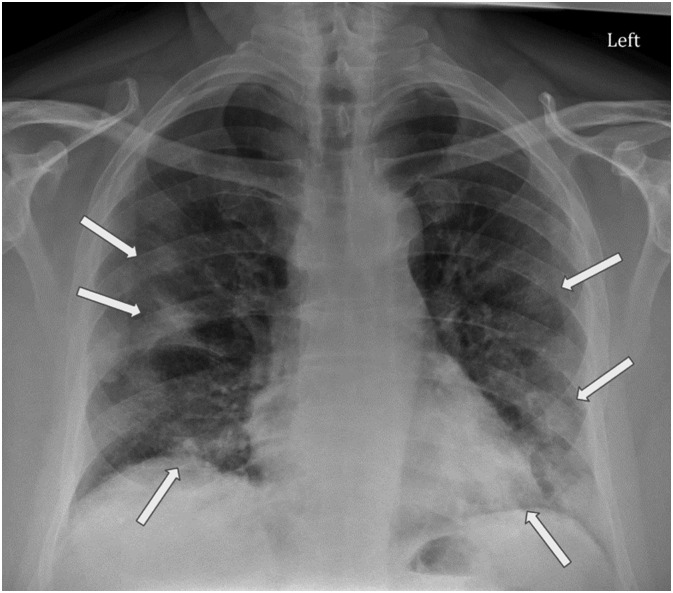
Fig. 6.
Chest radiograph (CXR) of 61-year-old male (patient #2) presenting to the ED with one week of fever, chills, shortness of breath, productive cough, malaise, and vomiting. CXR demonstrates irregular mild to moderate peripheral predominant hazy opacities in the bilateral middle to lower lungs (white arrows), suggestive of atypical viral pneumonia.
After two days in the ED, the patient was admitted and started on nasal cannula and treated with intravenous methylprednisolone (40 mg/day) and hydroxychloroquine (400 mg/day for three days). Over the following ten days, the patient began requiring escalating oxygen support, requiring HFNC at 40 L/min. On hospital day 14, the patient was noted to be tachypneic and mildly hypoxic (SpO2 of 91%) despite being on HFNC at 40 L/min and NRB at 10 L/min; daily laboratory results revealed increasing leukocytosis from 12 to 17 × 103 cells/uL. Repeat CXR was performed revealing new mild subcutaneous air and pneumomediastinum (Fig. 7A).
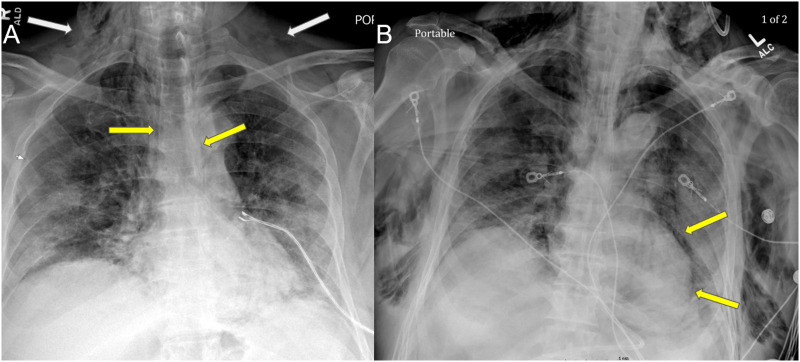
Fig. 7.
After requiring increasing oxygen support with high flow nasal cannula (HFNC) bilevel positive airway pressure (BiPAP) for 14 days, Patient #2's chest radiograph (A) demonstrates new mild subcutaneous emphysema in the soft tissues of the neck and upper chest wall (white arrows) with mild pneumomediastinum (yellow arrows). (B) Chest radiography three days later demonstrates more extensive subcutaneous emphysema and pneumomediastinum (yellow arrows) with lack of pneumothorax. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The following day, on hospital day 15, the patient became acutely hypoxic, and settings were increased to HFNC at 60 L/min and NRB at 15 L/min, while SpO2 remained below 90%. The rapid response team was called, and he was intubated, paralytically proned, and transferred to the intensive care unit (ICU). In the ICU, the patient's course was complicated by acute kidney injury (AKI) and acute respiratory distress syndrome (ARDS) requiring pressor support. The patient eventually progressed to multiorgan failure due to increasing circulatory and ventilatory demand. Serial CXRs demonstrated worsening SPM, severe SE, and stable diffuse lung disease (Fig. 7B). Despite maximal medical support, the patient died on hospital day 22.
4. Discussion
COVID-19 has caused a dramatic rise in the number of patients requiring critical care. In the ICU setting, the incidence of pulmonary air leak disorders in patients on mechanical ventilation range from 15 to 40% [1]. However, our institution has noted an increasing frequency of COVID-19 patients developing these pathologies in the absence of mechanical ventilation. Given that the incidence of SE and SPM are exceedingly rare in the general population (1.2 and 3.0 per 100,000 respectively) this trend warrants further inquiry [11,12]. We aim to bolster the literature by presenting experiences from our multi-system institution.
Most patients in this group presented with slight hypoxia, but otherwise initially stable vital signs. Symptoms were non-specific and predominantly respiratory, with the primary symptom being dyspnea in all eleven patients. This cohort also displayed anomalies in inflammatory markers with elevated average LDH, CRP, D-dimer, ferritin, and procalcitonin on presentation, features well-described in many reports on COVID-19 patients [13].
Our patients were mostly male, older aged, and predominantly overweight. The majority of patients had comorbid hypertension. This is consistent with studies identifying these characteristics as risk factors for progression of COVID-19 [[14], [15], [16]]. However, all patients in our cohort were self-reported nonsmokers. Albeit in a small sample, this was unexpected given reports demonstrating an association between smoking, SPM and pneumothorax [17,18].
Four of the patients who developed SPM and SE had received noninvasive ventilation (NIV), including BiPAP and CPAP. Though the risk of barotrauma and subsequent complications are low with NIV, such cases have been reported in the literature [19,20]. The proposed mechanism is that the end-expiratory pressure provided by BiPAP or CPAP increases the pressure gradient between alveoli and the interstitial space, causing alveolar rupture with extension of air into the mediastinum, pleura, and subcutaneous tissues. While these observations may partially confound the role that COVID-19 may play in SE and SPM, it is noteworthy that the vast majority of patients on NIV do not experience these complications. This may suggest that extensive airspace disease caused by COVID-19 makes normally low-risk patients susceptible to SE/SPM, perhaps related to the constellation of lung damage, supplemental oxygen, and decreased lung compliance.
None of the remaining seven patients who had developed SPM and SE had received any sort of assisted ventilation. Five patients received high-flow nasal cannula (HFNC), an intermediate level of support between conventional oxygen delivery and NIV. While a few cases of possible barotrauma during HFNC have been reported in the pediatric population, no clear association has been established [21]. Further, the incidence of pneumothoraces related to HFNC in the pediatric population is reportedly as low as 1% [22]. In adults, a single case report of barotrauma while on HFNC has been seen in a patient with hemophagocytic lymphohistiocytosis, however, this association was confounded by recent prior mechanical ventilation [23]. Of the seven patients not placed on NIV, one received solely supplemental oxygen through a non-rebreather mask up to 15 L/min and the other received no oxygen support. Barotrauma induced SPM or SE would be unlikely in these patients.
SPM unrelated to intubation or positive pressure ventilation has been previously reported in respiratory infections such as pneumocystis pneumonia (PCP), Staphylococcus aureus pneumonia, cytomegalovirus (CMV), influenza bronchiolitis, and even severe acute respiratory syndrome (SARS) [12,24,25]. Additional predisposing factors have been observed such as asthma, corticosteroids, respiratory irritants, and other anatomical abnormalities such as tracheomalacia [[26], [27], [28], [29]].
The pathophysiological basis of air-leak disease in COVID-19 patients is not well-known, though postulations can be made by applying current understandings of the disease to existing concepts. COVID-19 pneumonia, like SARS, has been shown to cause severe diffuse alveolar damage (DAD) [30]. The rupturing of alveoli secondary to DAD may cause pulmonary interstitial emphysema (PIE), a rare disease process that most commonly occurs in neonates on ventilatory support [31]. Interstitial air can then dissect into the mediastinum, pleural cavity and subcutaneous tissues. Similar pathological progressions have been previously observed in a variety of viral pneumonias [32,33]. Impaired oxygenation as a result of alveolar rupture and PIE, whether on a macroscopic or microscopic level, may also play a role in the profound hypoxia common with COVID-19 lung disease through impairment of ventilation and pulmonary blood flow. Identification of SE and pneumomediastinum on CXR may be one of the few objective signs of significant alveolar rupture and PIE development. Of note, our results show that radiographic manifestations of subcutaneous air and pneumomediastinum almost always occur at the same time, most often with little to no associated pneumothorax. One patient demonstrated SE without an apparent SPM on initial imaging, but we posit that SPM exists in all cases but might be difficult to visualize on radiography. Also potentially difficult to visualize on radiography are small apical pneumothoraces, which were absent in six of our eleven patients. While the possibility that small apical radiographically occult pneumothoraces may have been present, an alternative explanation may lie in the current or prior pulmonary inflammatory process, which can form pleural adhesions between parietal and visceral layers of the pleura. Such adhesions can protect against development of pneumothoraces, as illustrated by the concept of pleurodesis in which the pleural space is artificially obliterated and subsequently adhesed to treat recurrent spontaneous pneumothorax [34].
Recent studies have demonstrated COVID-19 viral entry via angiotensin converting enzyme-2 (ACE-2) receptor into target cells including surfactant-producing type II pneumocytes [35]. Such cellular injury could theoretically lead to dysregulation of surfactant production contributing to the development of SE and pneumomediastinum from impaired lung compliance, analogous to the pathophysiology of PIE development and barotrauma-related air leak in premature neonates. Similarly, the upregulation of ACE-2 expression found in chronic hypertension and diabetes may potentially explain these being the most common comorbidities in our patient population (and all infected COVID-19 patients) [36].
The clinical importance of COVID-19 induced SE and SPM has yet to be elucidated. In their experiences with the SARS epidemic, Chu et al. demonstrated that the development of SPM in SARS patients not on mechanical ventilation was independently associated with increased intubation and an increased chance of death [12]. It is possible that the development of SE and SPM, while itself benign, may be an indicator of poor prognosis. Future studies are needed to further characterize such prognostic implications.
Outcomes were generally poor in this set of patients with over half requiring intubation, and over a third having expired at the time of writing. Those who did expire did so within approximately ten days of SE and SPM development. It is interesting that, on average, a moderate disease burden was present on initial CXR (average score of 3.8) with only minimal progression of disease at the time of subsequent SE or pneumomediastinum development (average score of 4.4), as determined by slight interval change in severity score.
Limitations of this study that limit its generalizability include the small sample size and retrospective design, which can introduce observer bias. Additionally, the time of symptom onset was largely self-reported, leaving the possibility of recall bias. There is a possibility that a patient might have received invasive ventilation during a prior hospitalization. However, among the presenting narratives of the eleven patients in the cohort during chart review, there was no mention of previous hospitalizations making this possibility unlikely. Similar to the prior limitation, however, these narratives are subject to the reliability of historian and history taker. An additional limitation of the study is that the lungs were retrospectively assessed on CXR and not CT. While it is true that trace pneumothoraces may be radiographically occult and only evident on CT, the degree of air in the mediastinum and soft tissues would be unlikely to result from a trace pneumothorax.
4.1. Conclusion
In conclusion, non-intubated patients with COVID-19 infection were observed to have SE and SPM, which may be a component of disease pathogenesis and may be a poor prognostic indicator.
Declaration of competing interest
The authors have no conflicts of interests to disclose. No financial or material support was received for this work.
Acknowledgments
The authors would like to express their gratitude to all front-line providers and all essential staff for their selfless efforts during this time.
References
- 1.Maunder R.J., Pierson D.J., Hudson L.D. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch. Intern. Med. 1984;144:1447–1453. [PubMed] [Google Scholar]
- 2.Manna S. COVID-19: a multimodality review of radiologic techniques, clinical utility, and imaging features. Radiology: Cardiothoracic Imaging. 2020;2 doi: 10.1148/ryct.2020200210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Su X., Zhang T., Zheng C. Spontaneous Pneumomediastinum: a probable unusual complication of coronavirus disease 2019 (COVID-19) pneumonia. Korean journal of radiology: official journal of the Korean Radiological Society. 2020;21:627–628. doi: 10.3348/kjr.2020.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W., Gao R., Zheng Y., Jiang L. COVID-19 with spontaneous pneumothorax,pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020 doi: 10.1093/jtm/taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C., Gao C., Xie Y., Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020;20:510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toussie D., Voutsinas N., Finkelstein M. Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID-19 [published online ahead of print, 2020 May 14] Radiology. 2020 doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cellina M., Panzeri M., Oliva G. Chest radiograph features predict a favorable outcome in patients with COVID-19. Radiology. 2020 doi: 10.1148/radiol.2020202326. Online, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong H.Y.F. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296:E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghesi A., Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125:509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Organization, W. H. & Others . 2016. BMI classification; p. 2006.http://apps.who.int/bmi/index.jsp Avaiable at. [Google Scholar]
- 11.Onuki T. Primary and secondary spontaneous pneumothorax: prevalence, clinical features, and in-hospital mortality. Can Respir J. 2017;6014967:2017. doi: 10.1155/2017/6014967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu C.M. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23:802–804. doi: 10.1183/09031936.04.00096404. [DOI] [PubMed] [Google Scholar]
- 13.Richardson S. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W.-J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newcomb A.E., Clarke C.P. Spontaneous pneumomediastinum: a benign curiosity or a significant problem? Chest. 2005;128:3298–3302. doi: 10.1378/chest.128.5.3298. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y.-L. The impact of smoking in primary spontaneous pneumothorax. J Thorac Cardiovasc Surg. 2009;138:192–195. doi: 10.1016/j.jtcvs.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Carron M. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110:896–914. doi: 10.1093/bja/aet070. [DOI] [PubMed] [Google Scholar]
- 20.Piastra M., Morena T.C., Antonelli M., Conti G. Uncommon barotrauma while on high-flow nasal cannula. Intensive Care Med. 2018;44:2288–2289. doi: 10.1007/s00134-018-5279-5. [DOI] [PubMed] [Google Scholar]
- 21.Hegde S., Prodhan P. Serious air leak syndrome complicating high-flow nasal cannula therapy: a report of 3 cases. Pediatrics. 2013;131:e939–e944. doi: 10.1542/peds.2011-3767. [DOI] [PubMed] [Google Scholar]
- 22.Baudin F. Modalities and complications associated with the use of high-flow nasal cannula: experience in a pediatric ICU. Respir Care. 2016;61:1305–1310. doi: 10.4187/respcare.04452. [DOI] [PubMed] [Google Scholar]
- 23.Galanis E., Litzow M.R., Tefferi A., Scott J.P. Spontaneous pneumomediastinum in a patient with bronchiolitis obliterans after bone marrow transplantation. Bone Marrow Transplant. 1997;20:695–696. doi: 10.1038/sj.bmt.1700939. [DOI] [PubMed] [Google Scholar]
- 24.Olliff J.F., Williams M.P. Radiological appearances of cytomegalovirus infections. Clin Radiol. 1989;40:463–467. doi: 10.1016/s0009-9260(89)80245-4. [DOI] [PubMed] [Google Scholar]
- 25.Silva C., Almeida A.F., Ferraz C., Nunes T., Guedes Vaz L. Spontaneous pneumothorax with subcutaneous emphysema: a rare complication of respiratory syncytial virus infection. J Clin Med Res. 2016;8:260–262. doi: 10.14740/jocmr2353w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murayama S., Gibo S. Spontaneous pneumomediastinum and Macklin effect: overview and appearance on computed tomography. World J Radiol. 2014;6:850–854. doi: 10.4329/wjr.v6.i11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zylak C.M., Standen J.R., Barnes G.R., Zylak C.J. Pneumomediastinum revisited. Radiographics. 2000;20:1043–1057. doi: 10.1148/radiographics.20.4.g00jl131043. [DOI] [PubMed] [Google Scholar]
- 28.Lal A., Mishra A.K., Sahu K.K., Noreldin M. Spontaneous Pneumomediastinum: rare complication of Tracheomalacia. Arch Bronconeumol. 2020;56:185–186. doi: 10.1016/j.arbres.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Meireles J., Neves S., Castro A., França M. Spontaneous pneumomediastinum revisited. Respir Med CME. 2011;4:181–183. [Google Scholar]
- 30.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morisot C. Risk factors for fatal pulmonary interstitial emphysema in neonates. Eur J Pediatr. 1990;149:493–495. doi: 10.1007/BF01959402. [DOI] [PubMed] [Google Scholar]
- 32.Emiralioğlu N. Pneumomediastinum, pneumorrhachis and subcutaneous emphysema associated with viral infections: report of three cases. Pediatr Int. 2015;57:1038–1040. doi: 10.1111/ped.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo H.H., Sweeney R.T., Regula D., Leung A.N. Best cases from the AFIP: fatal 2009 influenza A (H1N1) infection, complicated by acute respiratory distress syndrome and pulmonary interstitial emphysema. Radiographics. 2010;30:327–333. doi: 10.1148/rg.302095213. [DOI] [PubMed] [Google Scholar]
- 34.Mierzejewski M., Korczynski P., Krenke R., Janssen J.P. Chemical pleurodesis - a review of mechanisms involved in pleural space obliteration. Respir Res. 2019;20(247) doi: 10.1186/s12931-019-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Úri K. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0087845. [DOI] [PMC free article] [PubMed] [Google Scholar]