Graphical abstract
Keywords: Measles, Adults, Hospitalized, Pneumonitis, Lymphopenia, C-reactive protein
Highlights
-
•
Measles is an unresolved issue for unvaccinated or incompletely vaccinated adults.
-
•
Data for outcomes, complications and risk factors in adults are limited.
-
•
Pneumonitis and hepatic involvement are the most frequent complications in adults.
-
•
Older age, low lymphocyte count and male sex are associated with pneumonitis.
-
•
Case fatality ratio in adults is low despite the high frequency of complications.
Abstract
Objectives
Measles outbreaks are increasingly reported among countries that were close-to-eliminate measles infection. There are few reports of clinical characteristics of measles in adults in the contemporary literature. In this study we aim to describe the clinical characteristics and complications of measles infection in hospitalized adults during the recent epidemic in Greece.
Methods
A multicentre observational retrospective study was conducted in three tertiary hospitals in Greece. All adult hospitalized patients (≥18 years old) with serologically confirmed and/or clinical features compatible with measles were included. Pediatric patients and patients with missing data were excluded.
Results
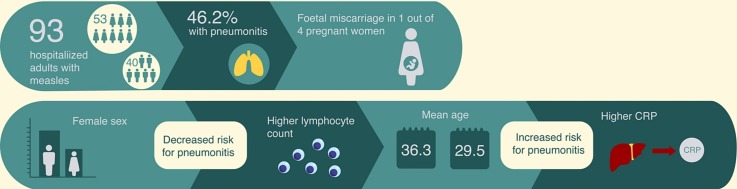
In total, 93 patients, 40 males (43 %) and 53 females (57 %), mostly young patients were included. Most of them (87 %) had no past medical history. Among women, 4 were pregnant. 56 (60.2 %) and 25 (26.9 %) patients reported either unknown or incomplete vaccination for measles. Ribavirin was administered in 8 (8.6 %) patients. Pneumonitis and hepatic involvement were the most common complications, occurring in 43 (46.2 %) and 75 (80.6 %) patients respectively. Pneumonitis was significantly associated with male sex, older age, lower lymphocyte counts and higher C-reactive protein (CRP) on admission. One pregnant woman suffered spontaneous fetal miscarriage and one patient died due to acute respiratory distress syndrome (ARDS) and high-risk pulmonary embolism.
Conclusion
Considerable proportions of incompletely vaccinated or unvaccinated adults have led to the re-emergence of measles in countries with reported close-to-elimination rates. Pneumonitis is a major complication among adults with measles. More studies are imperative in order to explore the role of immune paresis in measles.
1. Introduction
Measles infection is a highly contagious, air-borne, acute febrile illness caused by a paramyxovirus of the Morbillivirus genus [1] and still remains an unresolved global issue with considerable mortality and morbidity rates [2]. The World Health Organization (WHO) has recently reported an upsurge of measles cases in the Eastern Mediterranean, the European and the Americas Regions, in areas with reported close-to-elimination or elimination of measles [[2], [3], [4]]. The re-emergence of measles infection possibly signifies the gap in previous vaccination strategies. According to the latest WHO-UNICEF report only five European countries reported >95 % vaccination coverage with both doses of measles-containing vaccine; the respective rates in Greece for the first and second dose were 97–99 % and 77–83 % between 2008–2018 [5]. Although the epidemic was contained in Greece during 2019, there was a substantial upsurge in other European countries during the same year with a total of 13,460 cases [6]. Similarly, an outbreak with 1282 cases (of whom 29 % adults) was reported in the United States of America (USA) during 2019, a country that declared measles elimination in 2000 [7].
The natural course of the infection has only been scantly defined in adults, making management challenging even for experienced clinicians. In this study, we describe our experience from the recent outbreak of measles in adult hospitalized patients in Greece [8].
2. Patients and methods
2.1. Data collection
We conducted a multi-centre retrospective observational study of adult patients with measles in three tertiary teaching hospitals in Greece (Attikon University Hospital of Athens – AUH, Hippokration General Hospital of Athens – HGH and Thriasio General Hospital – TGH) between October 2017 and September 2019. All adult patients (≥18 years old) with compatible clinical features and/or serologically confirmed measles requiring hospitalization were included. Measles antibodies were measured with Enzyme-linked Immunosorbent Assay (MEASLES ELISA IgG/IgM kit, Vircell, Grenada, Spain). Patients with multiple missing data were excluded. All cases were then adjudicated with regards to clinical course, complications and/or outcome by three senior clinicians.
2.2. Ethics statement
This study was approved by the Research Ethics Committee of each participating institution (protocol numbers: AUH: 1821A/22−9-16, TGH: 433/18−12-19, HGH: 2613/18−2-2020) and was conducted according to the Outbreak Reports and Intervention studies Of Nosocomial infection (ORION) reporting guidelines [9]. Patients’ data were collected and analyzed under strict anonymity in agreement with the Declaration of Helsinki. Since this was a retrospective study, no informed consent was obtained.
2.3. Definitions
All patients were classified either as probable or as confirmed cases according to the case classification by the European Centers for Disease Control and prevention (ECDC) (Table 1 ). Definitions of pneumonitis and hepatic involvement are provided in Table 1.
Table 1.
Definitions of measles cases, pneumonitis and hepatic involvement.
| Condition | Definition |
|---|---|
| Probable case of measles infection [10] | Any person meeting the clinical criteria with an epidemiological link. |
| Clinical Criteria: Fever AND Maculo-papular rash AND at least one of: Cough, Coryza, Conjunctivitis. | |
| Confirmed case of measles infection [10] | Any person not recently vaccinated and meeting the clinical and the laboratory criteria |
| Clinical Criteria: Fever ANDMaculo-papular rash AND at least one of: Cough, Coryza, Conjunctivitis. | |
| AND | |
| Laboratory Criteria: At least one of: Isolation of measles virus from a clinical specimen AND/OR Detection of measles virus nucleic acid in a clinical specimen AND/OR Measles virus specific antibody response characteristic for acute infection in serum or saliva AND/OR Detection of measles virus antigen by DFA in a clinical specimen using measles specific monoclonal antibodies | |
| Pneumonitis | The presence of cough and/or dyspnea |
| AND | |
| Clinical signs and/or imaging compatible with lower respiratory tract infection. | |
| Hepatic involvement | Elevation of ALT above the ULNon admission (according to each centre’s reference range) |
| The severity was divided into 2 Grades; | |
| Grade I: ALT≤5xULN | |
| Grade II: ALT>5xULN. |
ALT: Alanine aminotransferase, DFA: Direct fluorescent antibody, ULN: Upper limit of normal.
2.4. Statistical analysis
Demographic and descriptive continuous variables with normal distribution are reported as mean (standard deviation, SD), whereas non-normally distributed data are presented as median values (interquartile range, IQR). Categorical variables are expressed as percentages. Chi square or Fisher’s exact test was used for comparison of dichotomous and Mann-Whitney or t-test for continuous variables. Univariate and multivariable logistic regression analysis was used in order to identify baseline factors associated with pneumonitis. Variables that were statistically significant in univariate analyses (p < 0.1) were included in the multivariable model. Variables of biological significance (sex and age), were retained until the final stage of multivariable analysis (backward selection). Outcomes of logistic regression analysis are displayed as odds ratios (OR) and their respective 95 % confidence intervals (95 %CI). All statistical analyses were two-tailed and performed with SPSS (IBM SPSS Statistics for Windows, v. 20.0. Armonk, NY: IBM Corp) and Stata 12 (StataCorp).
3. Results
3.1. Demographics
In total 93 patients, 40 males (43 %) and 53 females (57 %) aged between 18 and 62 years old, were included (Table 2 ).
Table 2.
Patient demographics and clinical characteristics.
| Demographics | |
|---|---|
| Female (n, %) | 53 (57 %) |
| Age [mean (SD)] | 32.6 (10.2) |
| Pregnancy (n, % of female) | 4 (7.5 %) |
| Days of symptoms before admission [median (IQR)] | 4 (3−5) |
| Antibiotic use during disease course (n, %) | 55 (59.8 %) |
| Ribavirin (n, %) | 8 (8.6 %) |
| Vaccination status (n, %) | |
| None | 12 (12.9 %) |
| Incomplete | 25 (26.9 %) |
| Unknown | 56 (60.2 %) |
| Ethnicity (n, %) | |
| Non-Greek | 19 (20.4 %) |
| Greek | 74 (79.6 %) |
| Roma ethnic subgroup (% of Greek patients) | 11 (14.9 %) |
| Comorbidities (n, %) | |
| None | 81 (87 %) |
| Diabetes | 4 (4.3 %) |
| Chronic pulmonary disease | 6 (6.5 %) |
| Coronary artery disease | 5 (5.4 %) |
| Heart failure | 2 (2.2 %) |
| Current cancer | 0 (0%) |
| Cirrhosis | 1 (1.1 %) |
| Symptoms and signs, n (%) | |
| Rash | 89 (95.7 %) |
| Cough | 52 (55.9 %) |
| Koplik spots | 30 (34.1 %) |
| GI symptoms | 29 (31.9 %) |
| Pharyngitis | 28 (30.1 %) |
| Conjunctivitis | 27 (29 %) |
| Nasal discharge | 16 (17.2 %) |
| Lymphadenopathy | 14 (16.1 %) |
| Dyspnea | 10 (10.8 %) |
| Complications, n (%) | |
| Pneumonitis | 43 (46.2 %) |
| Hepatic involvement | 75 (80.5 %) |
| Grade I | 47 (50.5 %) |
| Grade II | 28 (30.2 %) |
| Outcomes | |
| Discharged (n, %) | 92 (98.9 %) |
| Died (n, %) | 1 (1.1 %) |
| Hospitalization in days [median (IQR)] |
6 (4−7) |
GI: gastrointestinal, IQR: interquartile range, n: number of patients, SD: standard deviation.
Most patients were immunocompetent and young, with only 4 (4.3 %) being > 50 years old. Among the 53 women, 4 (7.5 %) were pregnant. Seventy-two patients (77 %) had serologically confirmed measles with positive IgM antibody on admission. From the remaining 21 patients, 12 (13 %) had probable infection with negative serology on admission despite of the compatible clinical presentation, whereas serological testing was not performed in 9 (10 %) patients. Fifty-six (60.2 %) and 25 (26.9 %) subjects had either unknown or incomplete vaccination status (1 dose) for measles, with the combined measles/mumps/rubella (MMR) vaccine, respectively. Twelve (12.9 %) patients reported no MMR vaccination. Treating physicians considered all included measles cases as primary infections and none of the patients reported a prior measles infection.
3.2. Signs, symptoms and laboratory results
All 93 patients presented with high grade fever (>38.5 °C), while 89 (95.7 %) patients developed generalized rash. Other common symptoms and signs were cough (55.9 %), Koplik spots (34.1 %), gastrointestinal symptoms (31.9 %) and pharyngitis (30.1 %) (Table 2). Laboratory results are summarized in Table S1.
3.3. Pneumonitis
In total, 43 (46.2 %) patients (24 men) fulfilled the definition of pneumonitis. No bacterial superinfections were diagnosed. Pneumonitis was more prevalent among male compared to female patients (60 % vs. 36 %, p = 0.02). Furthermore, the patients in pneumonitis group were significantly older than those without pneumonitis (36.3 ± 11.1 vs. 29.5 ± 8.2 years old, p = 0.001) (Table 3 ).
Table 3.
Comparison of patients with and without pneumonitis.
| Variable | Pneumonitis group (n = 43) |
No pneumonitis group (n = 50) | p value |
|---|---|---|---|
| Age [mean (SD)] | 36.3 (11.1) | 29.5 (8.2) | 0.001 |
| Female (n, %) | 19 (44) | 34 (68) | 0.02 |
| Vaccination status (n, %) | <0.001 | ||
| None | 6 (14) | 6 (12) | |
| Incomplete | 20 (46.5) | 5 (10) | |
| Unknown | 17 (39.5) | 39 (78) | |
| Antibiotic use (n, %) | 31 (72) | 24 (49) | 0.02 |
| Symptoms duration before admission in days [median (IQR)] | 4 (3−5) | 4 (3−5) | 0.54 |
| Hospitalization duration in days [median (IQR)] | 6 (3) | 5 (3) | 0.37 |
| Conjunctivitis (n, %) | 16 (37) | 11 (22) | 0.10 |
| Lymphadenopathy (n, %) | 4 (10) | 10 (21) | 0.15 |
| Nasal discharge (n, %) | 10 (23) | 6 (12) | 0.15 |
| Rash (n, %) | 42 (98) | 47 (94) | 0.38 |
| Koplik spots (n, %) | 13 (32) | 17 (36) | 0.66 |
| Pharyngitis (n, %) | 12 (28) | 16 (32) | 0.67 |
| GI symptoms | 13 (30) | 16 (33) | 0.75 |
| WBC count (x109/L [mean (SD)] | 4.7 (1.9) | 4.9 (2.5) | 0.72 |
| Lymphocyte count nadir (x106/L) [mean (SD)] | 420 (220) | 610 (400) | 0.005 |
| Lymphocyte count on admission (x106/L) [mean (SD)] | 490 (290) | 740 (510) | 0.006 |
| Neutrophil count on admission (x109/L) [mean (SD)] | 4.1 (1.7) | 3.9 (2.4) | 0.68 |
| Monocyte count on admission (x106/L) [mean (SD)] | 441 (280) | 400 (254) | 0.46 |
| CRP (mg/L) [mean (SD)] | 40.5 (25.0) | 54.6 (38.7) | 0.04 |
| ALT, U/L [mean (SD)] | 149 (146) | 148 (124) | 0.97 |
ALT: alanine aminotransferase, CRP: C-reactive protein, GI: gastrointestinal, IQR: interquartile range, L: liter, n: number of patients, SD: standard deviation, U: units, WBC: white blood cell count.
The lymphocyte count on admission as well as the lymphocyte nadir count, were both significantly lower in the pneumonitis group (490 × 106/L vs. 740 × 106/L, p = 0.006 and 420 × 106/L vs. 610 × 106/L, p = 0.005 respectively) (Table 3). Patients with pneumonitis received antibiotics more frequently (p = 0.02). Neither duration of symptoms before admission nor any specific symptoms or signs were significantly associated with the presence of pneumonitis. In multivariable analysis, both female sex and higher lymphocyte counts on admission had a protective effect against pneumonitis (OR = 0.26, 95 %CI: 0.09−0.752 and OR = 0.997, 95 %CI: 0.995−0.999 respectively) (Table 4 ). On the contrary, advanced age and C-reactive protein (CRP) were independently associated with the development of pneumonitis (OR = 1.14, 95 %CI: 1.038–1.249 and OR = 1.07, 95 %CI: 1.013–1.14) (Table 4).
Table 4.
Multivariable analysis for pneumonitis risk factors.
| Variable | OR | Lower 95 %CI | Upper 95 %CI | p value |
|---|---|---|---|---|
| CRP (mg/L) | 1.07 | 1.013 | 1.14 | 0.017 |
| Female sex | 0.26 | 0.09 | 0.752 | 0.013 |
| Age | 1.14 | 1.038 | 1.249 | 0.006 |
| Total lymphocyte count (x106/L) | 0.997 | 0.995 | 0.999 | 0.002 |
CI: confidence intervals, CRP: C-reactive protein, L: liter, mg: milligram, OR: odds ratio.
Age-CRP interaction was also included in the final model, however it was non-significant.
Intravenous ribavirin administered in 8 (18.6 %) patients with pneumonitis and, compared to those who were managed conservatively, tended to be prescribed in numerically lower arterial partial oxygen pressure to inspired oxygen fraction (PaO2/FiO2) ratios, without prolongation of hospitalization correlation (Table 5 ). Of note, most of the ribavirin-treated patients (7/8) were from one of the participating centers.
Table 5.
. Characteristics and outcomes of ribarivin group in patients with pneumonitis and hepatic involvement.
|
Pneumonitis |
Hepatic involvement |
|||||
|---|---|---|---|---|---|---|
| Variable | Ribavirin (+) (n = 8) | Ribavirin (-) (n = 35) | p value | Ribavirin (+) (n = 6) | Ribavirin (-) (n = 69) | p value |
| Lymphocyte count nadir (x106/L) [mean (SD)] | 541 (186) | 389 (221) | 0.08 | 546 (197) | 505 (327) | 0.76 |
| Lymphocyte count on admission (x106/L) [mean (SD)] | 718 (389) | 447 (241) | 0.15 | 781 (429) | 583 (381) | 0.23 |
| PaO2/FiO2 ratio* [mean (SD)] | 254 (63) | 297 (65) | 0.17 | 262 (65) | 379 (70) | 0.001 |
| Hospitalization duration in days [median (IQR)] | 7.5 (6−9) | 6 (4−7) | 0.28 | 7.5 (5.75−9) | 6 (5−7) | 0.24 |
| Age [mean (SD)] | 38 (16.2) | 35.9 (9.8) | 0.65 | 36 (18.4) | 32 (9.4) | 0.48 |
| Female, n (%) | 4 (50) | 15 (43) | 0.71 | 3 (50) | 39 (56.5) | 0.76 |
| ALT (U/L) [mean (SD)] | 141 (188) | 151 (138) | 0.86 | 179 (206) | 179 (126) | 0.99 |
*Data from 21 patients (pneumonitis comparison) and 46 patients (hepatic involvement comparison).
ALT: alanine aminotransferase, FiO2: fraction of inspired oxygen, IQR: interquartile range, L: liter, n: number of patients, PaO2: arterial partial pressure of oxygen, SD: standard deviation, U: units.
3.4. Other complications
Hepatic involvement was found in 77 (82.8 %) patients. Among them, 47 (50.5 %) and 28 (30.2 %) patients had Grade I and Grade II hepatic involvement respectively. When patients divided into those without or with Grade I liver injury and those with Grade II hepatic involvement, no statistically significant differences regarding baseline characteristics and presenting symptoms or signs were noted apart from the gastrointestinal (GI) symptoms that were more frequent in those without or with Grade I liver involvement (Table S2). A similar comparison between those with normal alanine aminotransferase (ALT) and those with any degree of hepatic involvement did not reveal any statistically significant difference.
A patient with confirmed measles was presented with a generalized vasculitic eruption instead of the typical rash, while another two males developed haematuria with dysmorphic red blood cells in urine sediment, suggestive of acute glomerulonephritis. In one of them, further work-up with C3, C4, rheumatoid factor and immunoglobulin levels disclosed normal values. Furthermore, 1 out of the 4 pregnant women suffered spontaneous fetal miscarriage during her hospital stay at the 8th week of gestation. No neurological complications were reported in this cohort.
3.5. Outcomes
All but one patient, were discharged alive from hospital after a median hospitalization of 6 days (IQR = 4−7). Pneumonitis was not correlated with the length of hospital stay (Table 3). One obese female patient with a Grade II hepatic involvement and pneumonitis that progressed rapidly into acute respiratory distress syndrome (ARDS) requiring mechanical ventilation, died within 6 days of her admission due to high-risk pulmonary embolism (PE) despite being treated with ribavirin.
4. Discussion
In this study we describe the clinical features and outcomes of mostly healthy and young adult hospitalized patients with measles. Our data show that the most prevalent symptoms and signs are high-grade fever, cough and generalized maculopapular rash in concordance with previous reports in adults [11,12]. Hepatic involvement and pneumonitis frequencies were as high as 80.6 % and 46.2 % respectively. When compared to other recent studies including adults, our cohort was quite similar in terms of mean age, sex distribution, presenting symptoms and baseline laboratory findings [13]. Despite the high prevalence of pneumonitis in this study, case fatality rate remained low (1.1 %), possibly explained by the cohort’s characteristics (young previously healthy patients). Low case fatality rates were also reported in Europe (10/13,460, 0.007 %) and in the USA, where no deaths were reported to the CDC during the 2019 outbreak [7].
MMR vaccine was introduced in the Greek National Immunization Program in 1989 for all who were born after 1970. During the latest outbreak, the schedule was revised and it currently includes two mandatory doses with the first administered in children aged between 12–15 months and the second between the age of 24–47 months. Genotype B3 was the most commonly circulating strain during the 2017–2019 outbreak [8]. On the contrary, genotypes D4 and D6 co-circulated during a major outbreak occurred between 2005–2006 [14].
Pneumonitis is probably the most serious complication of measles. The prevalence of pneumonitis in our study (46.2 %) was slightly higher than the one recently reported in Israel (30.5 %), in Serbia (26.8 %) and China (33 %), although the latter two studies included pediatric and adolescent cases that could have affected the pooled results [13,15,16]. Here, we show that male sex, older age, higher CRP and low lymphocyte counts are significantly associated with the presence of pneumonitis. To our knowledge, only one recent study has focused on identifying risk factors for pneumonitis. Tu et al. reported that measles-associated pneumonia was associated with younger age, longer fever duration, higher white blood cell (WBC) counts and normal ALT [16]. Again, we believe that the inclusion of all age groups in this analysis may have a significant impact on the outcomes, making the extrapolation of the results in adults less sounding. Indeed, children <8 months old had significantly higher rates of pneumonitis; moreover, normal lymphocytosis of childhood may have attenuated the impact of lymphopenia as an independent associated factor for pneumonitis.
Another interesting finding is the low proportion of patients with Koplik spots seen in our cohort (30.4 %). This could be possibly attributed to the long median time from symptom onset to hospital admission (4 days), given that Koplik spots appear 1–2 days prior to skin rash and usually disappear within the second day of rash eruption.
Measles-induced lymphocytopenia as well as its grade, possibly has a role either as a disease severity biomarker or as a predisposing factor for short- and long-term infectious complications. There is growing evidence that measles infection induces immunosuppression; a recent study in measles-infected children found significantly higher rates of hospitalization (during the first month), of non-measles infections and of antimicrobial prescriptions up to 5 years post-measles, compared to children without measles [17]. Mina et al. showed that measles infection, but not MMR vaccination, reduces the humoral immune memory against numerous viruses in children [18]. Another study demonstrated that measles compromises the protection acquired by vaccinations or previous infections by altering the memory B-lymphocyte diversity and leads to incomplete naïve B-cell reconstitution, characterized by a narrower post-infection B-cell receptor repertoire [19].
Although it is generally believed that CRP is not increased in patients with viral infections in the absence of pneumonitis, several studies have shown modest increases of CRP in patients with influenza and other viruses even in the context of non-severe disease sparing the lower respiratory tract [[20], [21], [22]]. As for measles, Griffin et al. reported in an older study including children, 4- to 5-fold increases in CRP even in uncomplicated cases. In concordance with the above, we do not consider the increased CRP in our patients with and without pneumonitis unusual [23].
Diagnosis of pneumonitis was followed by antibiotic prescription in 72 % of patients, though we had no proven superinfections in this cohort. In contrast, an older study by Loukides et al. reported a bacterial pneumonia rate of 26 % in measles-infected young males during an epidemic in the Greek army, with Streptococcus pneumoniae and Klebsiella pneumonia being the most commonly isolated pathogens [24].
In our cohort, only 8 patients received ribavirin. Due to the small number of treated patients and the heterogeneity in its use among the participating centers, our study could not reach to any specific conclusions regarding its exact role in the management of patients with measles. Ribavirin use in this setting is mainly supported by case reports or small case series [25,26].
Only one death was seen among 93 patients. This case was a 36-year old previously healthy female who developed measles–induced ARDS and PE with hemodynamic instability. The interplay between viruses and pro-coagulant state has been previously reported; influenza has been associated with acute coronary syndromes [27] and possibly de novo PE in the absence of deep venous thrombosis [28], while similar cases have also been reported with other viral infections [29]. Apart from obesity, our patient did not have any other risk factors for PE, in agreement with a recently reported pediatric case [30]. This potentially fatal complication could be attributed to direct endothelial damage caused by the measles virus, given that endothelial dysfunction has already been reported in children with fatal measles encephalitis [31]. This particular complication in acutely ill patients with respiratory viral infections has become even more relevant in light of the COVID-19 pandemic, where venous thromboembolism (VTE) is increasingly reported, even in the presence of prophylaxis [32]. Moreover, some evidence supports a survival benefit with VTE treatment in critically ill patients with COVID-19 (but not in those with non−COVID-19 illness) and increased d-dimers [33]. Current guidelines show significant divergence ranging between more conservative [34] and more liberal approaches [35]. Regarding measles infection, we suggest that hospitalized patients with measles should be treated according to the existing VTE prophylaxis guidelines in patients with acute medical illness.
Another finding of great interest is the disease pattern in pregnant women with measles. In our cohort, 4 women (7.5 % of female patients) were pregnant and we recorded one miscarriage among them (25 %). Pregnant women are more likely to be hospitalized, develop pneumonia and die when compared to non-pregnant women and, although not regularly associated with a higher miscarriage rate, measles has been correlated with preterm labor, low birth weight and higher rates of admission in neonatal ICU [36]. The rate of obstetric complication in our cohort was similar to the one reported by Rafat et al. (25 % vs. 20 %) in a series of adult patients admitted in the ICU [37]. These findings emphasize the need for a robust vaccination program in women of childbearing age before conception.
Strengths of our study are its multicenter design, with the three participating tertiary hospitals covering a substantial area of metropolitan Athens and Western Attica and the patient care by senior infectious diseases physicians in all three hospitals. Furthermore, we present data from a homogenous cohort of immunocompetent adults diagnosed during a short time period. In contrast to other contemporary studies, we focused on clinical and not exclusively on epidemiological aspects. Finally, we identified patient characteristics (age and sex) along with simple and widely available biomarkers (lymphocyte count and CRP) that could facilitate physicians to timely identify adults at high risk for pneumonitis. However, these findings need to be prospectively validated in future studies.
Our study also has some limitations. The retrospective design could attribute to several biases and missing data, although the medical files were thoroughly reviewed and the percentage of missing data was quite low for almost all variables. Moreover, treatment patterns across participating centers were not aligned, as it was depicted in the heterogeneity of ribavirin administration. This is not surprising, given the absence-to our knowledge-of guidelines establishing the role of ribavirin or other antivirals in immunocompetent adult patients with measles. Finally, the inclusion of hospitalized patients may not precisely reflect the physical history of measles-associated pneumonitis in general population, since a proportion of patients with a more indolent lung involvement might not require hospitalization.
In summary, in this study we presented the clinical characteristics of measles infection during the recent epidemic in hospitalized adults in Greece. We further identified independent baseline risk factors for pneumonitis and described several other serious complications. Measles remains an unresolved global issue and clusters of incompletely vaccinated or unvaccinated populations remain vulnerable during epidemics. Healthcare professionals should compel a high index of suspicion and familiarize themselves with the most common signs, symptoms and complications of measles in adults.
Author contributions
Conception and design of the study: PCF, DK, ST, TM. Acquisition of data: PCF, KT, TM, EK, MP, GL, ES, SS, HS. Analysis and interpretation of data: KT, MP, HS, SD, ES, SS, ST, DK. Drafting the article: PCF, KT, TM, EK, ES, HS, SD, GL. Revising critically the manuscript for important intellectual content: PCF, KT, MP, ES, SS, SD, ST, DK. All authors approved the final version of the manuscript submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data statement
The data that support the findings of this study are available from the corresponding author [PCF: evita.fragou@gmail.com] upon request.
Declaration of Competing Interest
None of the authors has any conflicts to declare.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104608.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Premaratna R., Luke N., Perera H., Gunathilake M., Amarasena P., Chandrasena T.G.A.N. Sporadic cases of adult measles: a research article. BMC Res. Notes. 2017;10:38. doi: 10.1186/s13104-017-2374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strebel P.M., Orenstein W.A. Measles. N. Engl. J. Med. 2019;381:349–357. doi: 10.1056/NEJMcp1905181. [DOI] [PubMed] [Google Scholar]
- 3.WHO | Measles – Madagascar, (n.d.). https://www.who.int/csr/don/17-january-2019-measles-madagascar/en/ (accessed February 26, 2020).
- 4.2019. WHO | Measles – Global Situation.https://www.who.int/csr/don/26-november-2019-measles-global_situation/en/ (accessed February 26, 2020) [Google Scholar]
- 5.2019. WHO Vaccine-preventable Diseases: Monitoring System. global summary, (n.d.). http://apps.who.int/immunization_monitoring/globalsummary/estimates?c=GRC (accessed February 26, 2020) [Google Scholar]
- 6.2020. European Centre for Disease Prevention and Control, Measles.https://www.ecdc.europa.eu/en/measles (accessed February 26, 2020) [Google Scholar]
- 7.Patel M., Lee A.D., Clemmons N.S., Redd S.B., Poser S., Blog D., Zucker J.R., Leung J., Link-Gelles R., Pham H., Arciuolo R.J., Rausch-Phung E., Bankamp B., Rota P.A., Weinbaum C.M., Gastañaduy P.A. National update on measles cases and outbreaks - United States, January 1-October 1, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019;68:893–896. doi: 10.15585/mmwr.mm6840e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgakopoulou T., Horefti E., Vernardaki A., Pogka V., Gkolfinopoulou K., Triantafyllou E., Tsiodras S., Theodoridou M., Mentis A., Panagiotopoulos T. Ongoing measles outbreak in Greece related to the recent European-wide epidemic. Epidemiol. Infect. 2018;146:1692–1698. doi: 10.1017/S0950268818002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone S.P., Cooper B.S., Kibbler C.C., Cookson B.D., Roberts J.A., Medley G.F., Duckworth G., Lai R., Ebrahim S., Brown E.M., Wiffen P.J., Davey P.G. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect. Dis. 2007;7:282–288. doi: 10.1016/S1473-3099(07)70082-8. [DOI] [PubMed] [Google Scholar]
- 10.COMMISSION IMPLEMENTING DECISION (EU) 2018/ 945 - of 22 June 2018 - on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions, (n.d.). URL: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2018.170.01.0001.01.ENG (accessed June 20, 2020).
- 11.Stahl J.P., Salmon D., Bruneel F., Caumes E., Freymuth F., Bru J.P., Morand P., Roblot F., Schmit J.L., Strady C., Timsit J.F., Rabaud C. Investigators, Adult patients hospitalized for measles in France, in the 21st century. Med. Mal. Infect. 2013;43:410–416. doi: 10.1016/j.medmal.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Wong R.D., Goetz M.B. Clinical and laboratory features of measles in hospitalized adults. Am. J. Med. 1993;95:377–383. doi: 10.1016/0002-9343(93)90306-a. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Chetrit E., Oster Y., Jarjou’i A., Megged O., Lachish T., Cohen M.J., Stein-Zamir C., Ivgi H., Rivkin M., Milgrom Y., Averbuch D., Korem M., Wolf D.G., Wiener-Well Y. Measles-related hospitalizations and associated complications in Jerusalem, 2018-2019. Clin. Microbiol. Infect. 2019 doi: 10.1016/j.cmi.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Kokotas S.N., Bolanaki E., Sgouras D., Pogka V., Logotheti M., Kossivakis A., Horefti E., Papadakos K., Mentis A. Cocirculation of genotypes D4 and D6 in Greece during the 2005 to 2006 measles epidemic. Diagn. Microbiol. Infect. Dis. 2008;62:58–66. doi: 10.1016/j.diagmicrobio.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Medić S., Petrović V., Lončarević G., Kanazir M., Begović Lazarević I., Rakić Adrović S., Bančević M., Muller C.P., Hübschen J.M. Epidemiological, clinical and laboratory characteristics of the measles resurgence in the Republic of Serbia in 2014-2015. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu B., Zhao J.-J., Hu Y., Fu J.-L., Huang H.-H., Xie Y.-X., Zhang X., Shi L., Zhao P., Zhang X.-W., Wu D., Xu Z., Zhou Z.-P., Qin E.-Q., Wang F.-S. Clinical and immunological analysis of measles patients admitted to a Beijing hospital in 2014 during an outbreak in China. Epidemiol. Infect. 2016;144:2613–2620. doi: 10.1017/S0950268816001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadroen K., Dodd C.N., Masclee G.M.C., de Ridder M.A.J., Weibel D., Mina M.J., Grenfell B.T., Sturkenboom M.C.J.M., van de Vijver D.A.M.C., de Swart R.L. Impact and longevity of measles-associated immune suppression: a matched cohort study using data from the THIN general practice database in the UK. BMJ Open. 2018;8:e021465. doi: 10.1136/bmjopen-2017-021465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mina M.J., Kula T., Leng Y., Li M., De Vries R.D., Knip M., Siljander H., Rewers M., Choy D.F., Wilson M.S., Benjamin Larman H., Nelson A.N., Griffin D.E., De Swart R.L., Elledge S.J. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. 2019;366:599–606. doi: 10.1126/science.aay6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrova V.N., Sawatsky B., Han A.X., Laksono B.M., Walz L., Parker E., Pieper K., Anderson C.A., De Vries R.D., Lanzavecchia A., Kellam P., Von Messling V., De Swart R.L., Russell C.A. Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aay6125. [DOI] [PubMed] [Google Scholar]
- 20.Vasileva D., Badawi A. C-reactive protein as a biomarker of severe H1N1 influenza. Inflamm. Res. 2019;68:39–46. doi: 10.1007/s00011-018-1188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon J.-S., Rheem I., Kim J.K. C-reactive protein and respiratory viral infection. Korean J. Clin. Lab. Sci. 2017;49:15–21. doi: 10.15324/kjcls.2017.49.1.15. [DOI] [Google Scholar]
- 22.Melbye H., Hvidsten D., Holm A., Nordbø A., Brox J. The course of C-reactive protein response in untreated upper respiratory tract infection. Br. J. Gen. Pract. 2004;54:653–658. /pmc/articles/PMC1326064/?report=abstract (accessed June 26, 2020) [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin D.E., Hirsch R.L., Johnson R.T., De Soriano I.L., Roedenbeck S., Vaisberg A. Changes in serum C-reactive protein during complicated and uncomplicated measles virus infections. Infect. Immun. 1983;41:861–864. doi: 10.1128/iai.41.2.861-864.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loukides S., Panagou P., Kolokouris D., Kalogeropoulos N. Bacterial pneumonia as a suprainfection in young adults with measles. Eur. Respir. J. 1999;13:356–360. doi: 10.1183/09031936.99.13235699. [DOI] [PubMed] [Google Scholar]
- 25.Ortac Ersoy E., Tanriover M.D., Ocal S., Ozisik L., Inkaya C., Topeli A. Severe measles pneumonia in adults with respiratory failure: role of ribavirin and high-dose vitamin A. Clin. Respir. J. 2016;10:673–675. doi: 10.1111/crj.12269. [DOI] [PubMed] [Google Scholar]
- 26.Forni A.L., Schluger N.W., Roberts R.B. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin. Infect. Dis. 1994;19:454–462. doi: 10.1093/clinids/19.3.454. [DOI] [PubMed] [Google Scholar]
- 27.Kwong J.C., Schwartz K.L., Campitelli M.A., Chung H., Crowcroft N.S., Karnauchow T., Katz K., Ko D.T., McGeer A.J., McNally D., Richardson D.C., Rosella L.C., Simor A., Smieja M., Zahariadis G., Gubbay J.B. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 28.Dimakakos E., Grapsa D., Vathiotis I., Papaspiliou A., Panagiotarakou M., Manolis E., Syrigos K. H1N1-induced venous thromboembolic events? Results of a single-institution case series. Open Forum Infect. Dis. 2016;3 doi: 10.1093/ofid/ofw214. ofw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramacciotti E., Agati L.B., Aguiar V.C.R., Wolosker N., Guerra J.C., de Almeida R.P., Alves J.C., Lopes R.D., Wakefield T.W., Comerota A.J., Walenga J., Fareed J. Zika and Chikungunya virus and risk for venous thromboembolism. Clin. Appl. Thromb. Hemost. 2019;25 doi: 10.1177/1076029618821184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cambrea S.C., Balasa A.L., Arghir O.C., Mihai C.M. Fatal rare case of measles complicated by bilateral pulmonary embolism: a case report and short literature review. J. Int. Med. Res. 2019 doi: 10.1177/0300060519894120. 300060519894120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esolen L.M., Takahashi K., Johnson R.T., Vaisberg A., Moench T.R., Wesselingh S.L., Griffin D.E. Brain endothelial cell infection in children with acute fatal measles. J. Clin. Invest. 1995;96:2478–2481. doi: 10.1172/JCI118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.M., Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antithrombotic Therapy | Coronavirus Disease COVID-19, (n.d.). https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ (accessed June 26, 2020).
- 35.COVID-19 and VTE-Anticoagulation - Hematology.org, (n.d.). https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation (accessed June 26, 2020).
- 36.Rasmussen S.A., Jamieson D.J. What obstetric health care providers need to know about measles and pregnancy. Obstet. Gynecol. 2015;126:163–170. doi: 10.1097/AOG.0000000000000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafat C., Klouche K., Ricard J.-D., Messika J., Roch A., Machado S., Sonneville R., Guisset O., Pujol W., Guérin C., Teboul J.-L., Mrozek N., Darmon M., Chemouni F., Schmidt M., Mercier E., Dreyfuss D., Gaudry S. Severe measles infection: the Spectrum of disease in 36 critically ill adult patients. Bull. Sch. Med. Md. 2013;92:257–272. doi: 10.1097/MD.0b013e3182a713c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.