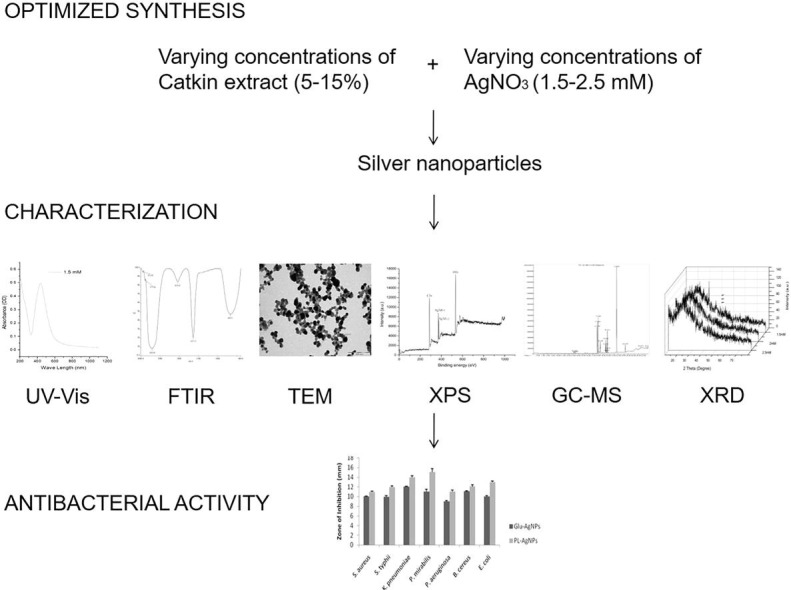
Graphical abstract
Keywords: Piper longum, Catkins, AgNPs, XPS, XRD, Antibacterial
Highlights
-
•
Catkin extract of Piper longum was used to optimize and biosynthesize AgNPs.
-
•
Biosynthesized AgNPs were characterized using UV, FTIR, TEM, XPS, GC-MS and XRD.
-
•
The antibacterial activity of the AgNPs was determined against 7 different food-borne pathogens.
Abstract
Inspired with an increasing environmental awareness, we performed an eco-friendly amenable process for the synthesis of silver nanoparticles (AgNPs) using the catkins of Piper longum as an alternative approach with the existing methods of using plant extracts. The fabrication of nanoparticles occurred within 10 min. This was initially observed by colour change of the solution. UV–visible spectroscopic studies (UV–Vis) were performed for further confirmation. The analysis elucidated that the surface plasmon resonance (SPR) was specifically corresponding to AgNPs. Fourier transform infrared spectrophotometry (FTIR) studies indicated that polyphenols could possibly be the encapsulating agents. The size and shape of the nanoparticles was analysed using Transmission electron microscopy (TEM). The nanoparticles were predominant spheres ranging between 10 and 42 nm at two different scales. The formation of elemental silver was confirmed further by X-ray photoelectron spectroscopy (XPS) and X-ray powder diffraction (XRD). GC-MS analysis was used to identify the possible encapsulates on the nanoparticles. The antibacterial effect of the biosynthesized AgNPs was tested against two gram-positive (Bacillus cereus and Staphylococcus aureus), and five gram-negative (Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa and Salmonella typhi) bacteria. Outcomes of the study suggest that these pathogens were susceptible to the AgNPs. This is the first ever international report on correlating the antibacterial effect of silver nanoparticles using mathematical modelling with a conventional antimicrobial assay. The results indicate that nanoparticles of silver synthesized using catkin extract of P. longum can be exploited towards the development of potential antibacterial agents.
1. Introduction:
Due to the gaining interest in studies of nano-range of 1–100 nm for biomedical applications, the biosynthesis of AgNPs using valuable medicinal plant extracts has considerably increased [1], [2], [3]. However, the synthesis of nanoparticles with the desired quality is one of the most important aspects of modern nanotechnological approaches [4].
There have been a number of studies describing the different methods for metal nanoparticle synthesis involving reductions performed by chemical, electrochemical, photochemical and other means [5], [6], [7]. But it has been reported that biological methods are superior to chemical ones in terms of economic feasibility and environmental safety. Biosynthesis of AgNPs by microorganisms is a recently well thought exploitation [8]. Though it has levelled a considerable success, the use of plant-based materials has gained much attention rather than microorganisms for green synthesis of metal nanoparticles because of limited toxicity, less time and the added advantage of available natural capping agents. Moreover, it reduces the cost of isolation of microorganisms and enhancement of culture media for microbe assisted biosynthesis and uses several sources of reductants such as leaves, flowers and catkins [2], [9], [10], [11], [12], [13].
P. longum (Piperaceae, long pepper) is cultivated almost all over India and is known to possess antimicrobial activities [14], [15]. It is an active ingredient of Kabasura Kudineer, a traditional siddha formulation with non-toxic, drug-like properties and enhanced bioavailability. It is used to treat viral fevers and respiratory infections. A recent in silico study indicates that the binding efficacy of this formulation is significant with the spike protein of COVID-19 [16].
AgNPs have been reported for their antibacterial, anti-biofilm and antiviral properties at limited concentrations [17], [18], [19], [20], [21]. Management of infections in medicine to several other industries is critical and an alternative for chemical agents has become a necessity. Disinfection has become a critical parameter for pandemics such as COVID-19 and colloidal silver can be used in intensive care units of hospitals as novel standard for prophylactic treatment of ventilator acquired pneumonia. Silver nanoparticles alone or in combination with existing antibiotics are suitable and efficient antimicrobial agents [22], [23], [24]. Pharmaceutically valuable molecules could be retained after bioreduction by the biosynthesized AgNPs that might have potential application in therapeutics [25]. Silver nanoparticles are used widely in packaging industries to increase the shelf-life of food products pertaining to their antimicrobial effects [26], [27], [28], [29].
Therefore, considering the importance of medicinal value of P. longum, its catkin extract was used to synthesize AgNPs by the reduction of silver ions and to exploit its antibacterial efficacy. The results indicate that AgNPs could be efficient antibacterial agents.
2. Experimental section:
Catkins of P. longum were collected and shade dried at room temperature for a period of 7 days. Silver nitrate (AgNO3) was purchased from Qualigens Fine Chemicals, Mumbai, India (99.9% pure).
2.1. Preparation of catkin extract
Desired amounts of the finely ground catkin powder (5, 10 and 15 gm) were added to desired volume of menstruum used (100 mL of sterile deionised water) and stirred well. The resulting mixture was then heated at 60 °C for 10 min to produce a galenical. This solution was then allowed to cool and further filtered using Whatman paper No. 1. The hydrosol obtained was used for further experimentation following previous reports with slight modifications [30].
2.2. Synthesis of AgNPs
For the bioreduction process to occur, catkin broth was mixed with AgNO3 and incubated at varying temperatures and varying concentrations of catkin and AgNO3. Initially, 5 mL of 5%, 10% and 15% catkin extracts were added to 95 mL of 10-3 M AgNO3 solution individually and allowed for thermal reduction to occur at temperatures ranging from 30 ◦ to 95 °C. Later, the concentrations of AgNO3 (1.5–2.5 mM) were varied, setting 5% catkin extract as the standard. After the reduction of 1.5 mM AgNO3 by the 5% P. longum catkin extract, AgNPs were separated from the solution by centrifuging at 10000 rpm for 20 min. The pellet was re-dispersed in 25 mL of fresh deionised water and centrifuged at 10000 rpm for 20 min as before for three times after being replaced with fresh deionised water every time. The final pellet after centrifugation and resuspension in fresh water was used for further analysis [31]. The control nanoparticles were synthesized by treating glucose as a reductant with silver oxide (1 mM) as the precursor following the same procedure [32].
2.3. Characterization of the synthesized nanoparticles
UV–visible spectroscopic analysis was done by using Hitachi double beam equipment (Model Lambda 35) spectrophotometer. For FTIR measurements, Spectrum RX 1 instrument was used. TEM analysis was performed using Tecnai 10 instrument for characterizing the size and shape of the synthesized AgNPs at 100 and 200 nm scales. XPS analysis was performed with Omicron ESCA Probe spectrometer. GC-MS analysis was performed using Agilent GC 7890A / MS5975C instrument using Agilent DB5MS capillary column. The crystalline nature of AgNPs was studied using Phillips PW 1830 model XRD instrument.
2.4. Antimicrobial studies
Antibacterial activity of nanoparticles were tested against Bacillus cereus (MTCC 1272), Escherichia coli (MTCC 1687), Klebsiella pneumoniae (MTCC 530), Proteus mirabilis (MTCC 425), Pseudomonas aeruginosa (MTCC 1688), Salmonella typhi (MTCC 531) and Staphylococcus aureus (MTCC 96) cultures procured from the Microbial Type Culture Collection Centre (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India. Since disk diffusion is the common standard for bacterial sensitivity test, standard disks of 6 mm were purchased from HIMEDIA (Mumbai, India), impregnated with nanoparticles (50 μl), placed on LB agar medium, seeded with the test pathogens and incubated at 37 °C. The sensitivity of bacterial cultures was measured in triplicate after 24 h [33], [34].
3. Results and discussion
3.1. Colour change
Colour change in solutions incubated with precursors and reductants could be considered an initial confirmation for the synthesis of nanomaterials [35]. A pale brown colour was observed after catkin extract and AgNO3 were mixed and incubated. This is due to the excitation of SPR vibrations after incubation as indicated in Fig. 1A [36], [37], [38]. Although the colour change was observed at 30 °C and 60 °C, prominent changes were observed in 90 °C and 95 °C. Therefore, incubations at 90 °C and 95 °C were considered for further study for rapid synthesis.
Fig. 1A.
Colour change on incubating 1.5 mM AgNO3 and 5% catkin broth together as a solution at varying temperatures (30 °C, 60 °C, 90 °C, 95 °C).
3.2. UV–Vis analysis
3.2.1. Effect of temperature, reductant and precursor concentrations
The characteristic SPR of silver nanoparticles at 5% (434 nm), 10% (427 nm) and 15% (439 nm) catkin broth concentrations were observed keeping 1.5 mM AgNO3 concentration as a standard [39]. To involve minimal reactants, 5% catkin extract was used for further analysis. The SPR peaks for solutions tried using 5% catkin broths as standard with varying concentrations of the precursor AgNO3 in the range of 1.5–2.5 mM were 435 nm (1.5 mM), 427 nm (2 mM) and 430 nm (2.5 mM). The study also analysed the incubation of the precursor and reductant at temperatures ranging between 30 °C, 60 °C, 90 °C and 95 °C. The peaks were consistent in the range between 420 nm and 440 nm (Fig. 1B ). According to previously published reports, the nanoparticles in the range of 410–450 nm have been known to be spherical [40], [41]. The particles with the SPR range of around 450 nm have been known to be in the size range of 2 to 100 nm [42], [43].
Fig. 1B.
UV– visible spectra of the synthesized AgNPs at varying AgNO3 and catkin broth concentrations incubated at 90 °C and pH 7.
3.2.2. Optimization of synthesis and characterization by UV–Vis analysis
Though the synthesis was observed at other catkin extract and AgNO3 concentrations as predicted by all UV–Vis observations in this study, they were not characterized further because, the above-mentioned optimized concentration and pH provides an eco-friendly approach to the maximum, with minimal reactants (Fig. 1B). Furthermore, it is of interest to note that the reduction of silver ions completed within 10 min of incubation, indicating the rapid biosynthesis of AgNPs. Therefore, to use limited reactant conditions such as temperature and also involve rapid synthesis, the hydrosol prepared by incubating 1.5 mM AgNO3 and 5% catkin extract at 90 °C was considered to be optimized and used for further studies rather than 95 °C.
Since the particle sizes were miniature and the usage of harmful reactants were avoided, 5% catkin extract and 1.5 mM AgNO3 concentration were preferred at pH 7 and 90 °C for further characterization and antimicrobial studies. Regarding stability, the synthesized nanoparticles were kept for three months at 4 °C in a refrigerator for stability analysis. The AgNPs were stable for more than three months. This was proven as the UV–vis peak remained the same reflecting the SPR for AgNPs when tested even after the period of three months. The antibacterial effect also remained the same after the described period.
3.3. FTIR analysis
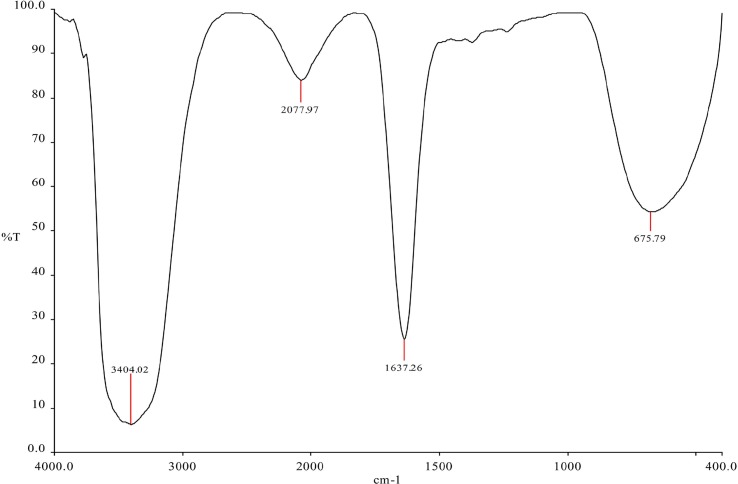
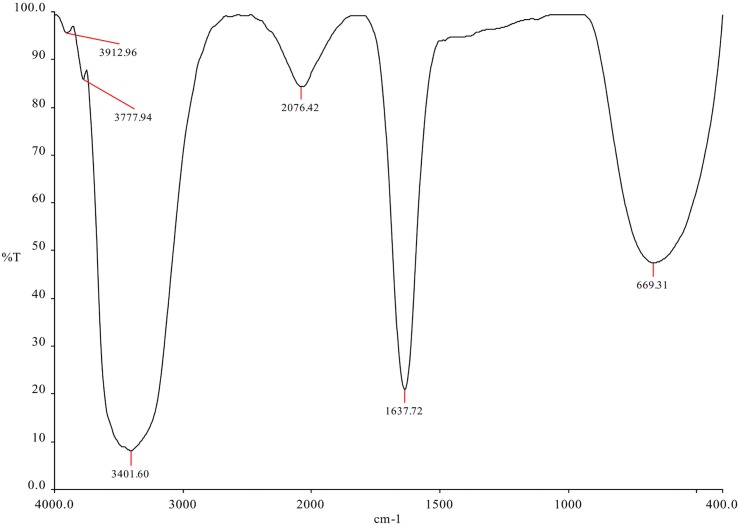
FTIR analysis performed to identify the functional groups for determination of capping agents indicates that sharp absorption peaks were located at 3404 cm−1, 2077 cm−1, 1637 cm−1 and 675 cm−1 in the catkin extract (Fig. 2A ). Similar peaks were retained at 3401 cm−1, 2076 cm−1, 1637 cm−1 and 669 cm−1 were observed in the hydrosol containing nanoparticles obtained at 90 °C (Fig. 2B ). Similar peaks were observed for hydrosols at 60 °C and 95 °C (Supplementary Information 1). The new peaks observed for samples at 90 °C around 3912 cm−1 and 3777 cm−1 could be due to overtones and combination bands in the mid IR region and the moisture content in the sample [44]. The peaks at 3401 and 3404 cm−1 in both solutions indicates free O—H stretches related to alcohols and phenols [45]. The absorption peaks at 1637 cm−1, 667 and 669 cm−1 are assigned close to that of native proteins [46]. The peaks around 2076 and 2077 cm−1 could be attributed to alkynes [47]. These results indicate that the polyphenols and proteins in the catkin extract have acted as reducing, capping and stabilizing agents, for the nanoparticles, as predicted by the FTIR peaks. They might also have acted as agents for elevated antimicrobial activity as compared to the nanoparticles synthesized by chemical means [48], [49], [50], [51], [52], [53].
Fig. 2A.
FTIR measurements of the catkin extract.
Fig. 2B.
FTIR measurements of the AgNPs synthesized using 1.5 mM AgNO3 and 5% catkin broth incubated at 90 °C and pH 7.
3.4. TEM analysis
TEM is a widely used microscopic technique for the characterization of nanomaterials based on their size and morphology [54]. It was inferred from TEM analysis that the catkin extract derived nanoparticles were predominantly spherical in shape with varying sizes ranging from 26 to 42 nm at the 200 nm scale (Fig. 3 ). Previous reports using electron microscopy indicate that the sizes of biosynthesized silver nanoparticles range between 20 and 50 nm [55], [56], [57], [58].
Fig. 3.

TEM image of the biosynthesized AgNPs at 200 nm scale.
3.5. XPS spectra
Scan survey denoted strong peaks of C1s, O1s and Ag3d core levels. The major C1s peak occurred at 286.2 eV. This corresponds to α-carbon. The O1s value was centred at 529.8 eV [59]. Correspondingly, the two spin–orbit components Ag3d5/2 and Ag3d3/2 were observed at the binding energies of 368.22 and 374.28 eV, separated by 6 eV [60] (Fig. 4 ). The core values for metallic Ag lies in this range as established by previous studies [61], [62], [63].
Fig. 4.
XPS survey spectra of the synthesized AgNPs.
3.6. XRD
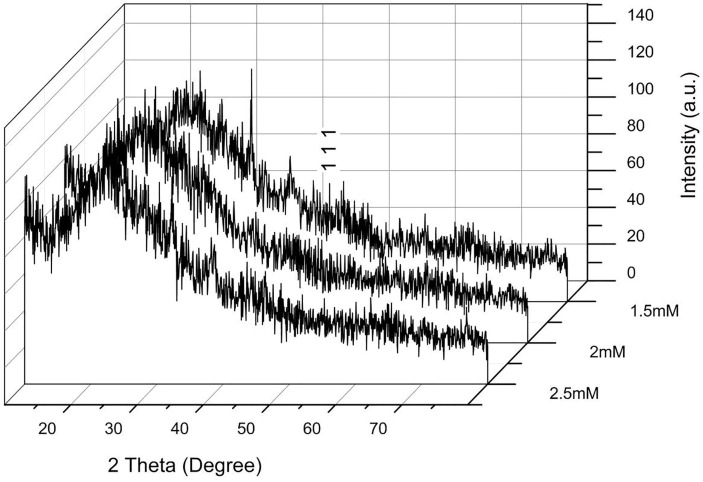
Comparing the hydrosols containing nanoparticles prepared at different concentrations, 1.5 mM clearly represents the main peak at 38.34 which corresponds to plane 111 (JCPDS file no: 89-3722). From the determination of width and using Debye–Scherrer’s equation, the average particle size measured was 7 nm [64]. Additionally, a variety of peaks were produced due to the hindrance of various bioorganic agents or bioactive compounds possibly from the catkin extract present on the surface of AgNPs [56], [65]. Therefore, the XRD pattern of AgNPs synthesized using P. niruri reveals that the particles are crystalline in nature (Fig. 5 ).
Fig. 5.
XRD pattern of the synthesized nanoparticles at varying concentrations of silver nitrate (1.5, 2, 2.5 mM) and 5% catkin broth concentration.
3.7. GC-MS analysis
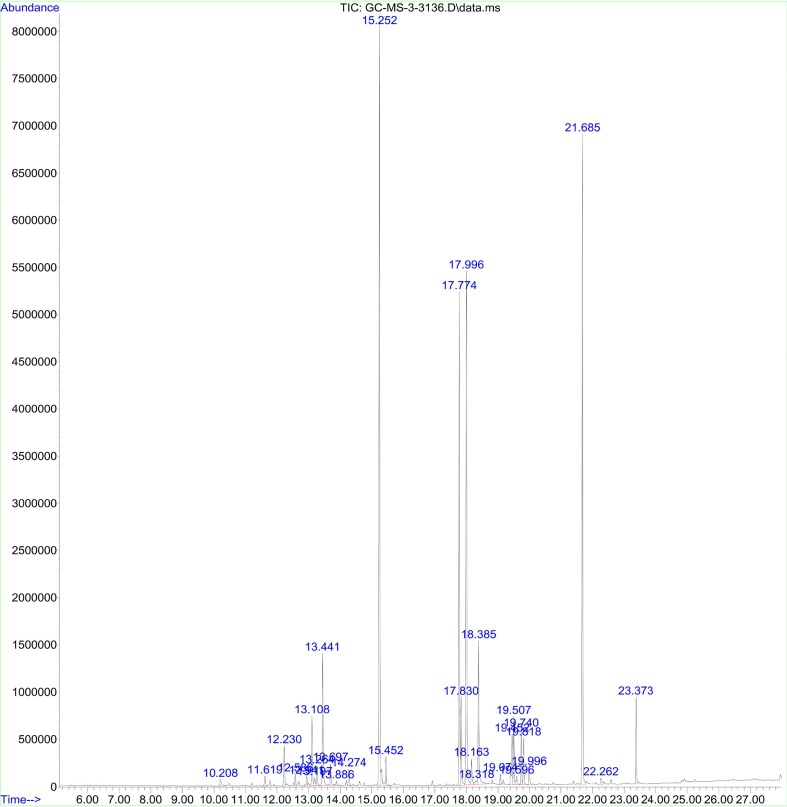
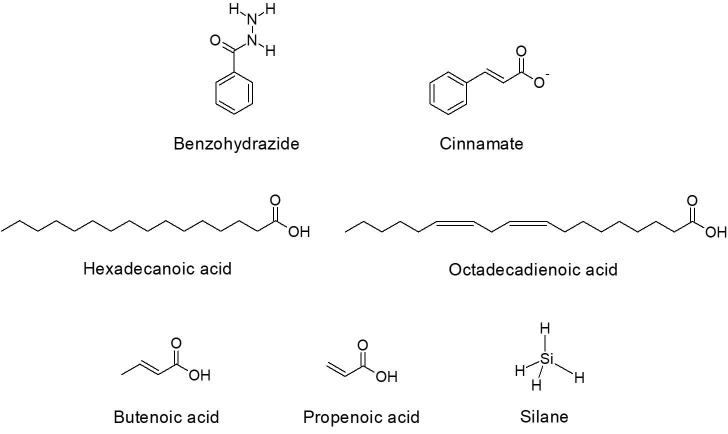
The comparative analysis of peak numbers indicates that both the catkin extract and hydrosol with nanoparticles constituted several similar active components (Fig. 6A, Fig. 6B ). The structures of these compounds are presented in Fig. 6C . Although, several peaks were lost in this comparison, no novel peaks with established biological activity were observed in nanoparticle containing hydrosol. Among these constituents, benzohydrazide is a lead compound with significant therapeutic effects on cancer, killing of microbial pathogens and treatment of neurological disorders such as epilepsy [66]. Cinnamate derivatives are organic aromatic melatonin scavenger with potent antibacterial agents [67], [68], [69], [70]. Fatty acids such as hexadecanoic acid are effective against inflammatory disorders and microbial pathogens [71], [72], [73]. Extracts that constitute octadecadienoic acid, butenoic acid, propenoic acid and silane derivatives are known to possess antibacterial activities [74], [75], [76], [77], [78]. This analysis indicates that these constituents observed in the catkin extract has most probably reduced the precursor and likewise encapsulated the nanoparticles leading to enhanced antimicrobial activity compared to the chemically synthesized nanoparticles.
Fig. 6A.
GC–MS chromatogram of the catkin extract.
Fig. 6B.
GC–MS chromatogram of the silver nanoparticles containing hydrosol.
Fig. 6C.
Structures of compounds identified in common between catkin extract and silver nanoparticles containing hydrosol using GC–MS.
The mechanism of green synthesis of nanoparticles are attributed to the secondary metabolites, enzymes such as aspartic proteases, phytochelatins and metallothioneins which are considered to be metal-binding ligands presented in the plant extracts. Derivatives of phenols such as tannins are identified to be more efficient in reduction of silver salts into silver nanoparticles. Concentration of reducing agents and the silver salt used, pH, temperature and duration of incubation are critical parameters for the biosynthesis to occur [79], [80], [81], [82], [83], [84]. Therefore, the phytochemicals identified by GC-MS, in this study, could be possible reductants and/or encapsulates on the nanoparticles from the catkin extract.
3.8. Conventional disk diffusion assay
Bacillus cereus, Staphylococcus aureus, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa and Salmonella typhi are reported to be food borne pathogens [85], [86], [87]. Biosynthesized nanoparticles with its large surface area were tested for their antibacterial activity against both gram-negative and gram-positive microorganisms. The diameter of the inhibition zone (mm) around the disks impregnated with AgNPs against test strains was measured after the incubation period.
Comparative analysis of the overall zone of inhibition indicates a percentage increase of 16.4% between the test nanoparticles and control nanoparticles. P. mirabilis was the most and S. aureus the least susceptible to the test nanoparticles. The control nanoparticles showed increased activity against K. pneumoniae and minimal activity against P. aeruginosa (Fig. 7 ). Existing data for sensitivity to vancomycin, the most effective antibiotic against multiple drug resistant microbes, was used to compare with test nanoparticles [33]. The sensitivity of these microorganisms to vancomycin (30 μg) does range between 7 and 25 mm, according to previous studies. In our study, the nanoparticle solution loaded at 50 μl per disk were one half equivalent to activity of vancomycin according to existing reports [88], [89], [90], [91]. The zone of inhibition is significantly similar to the previously published reports [92], [93].
Fig. 7.
Mean zone of inhibition (mm) of seven different pathogens generated by AgNPs synthesized using glucose and catkin extract of Piper longum (5%). The disc diameter was 6 mm. All experiments were performed in triplicate. The results were expressed as mean ± SEM.
The elevated antibacterial effect of nanoparticles against gram-negative bacteria in comparison to gram-positive bacteria could be attributed to the thickness of gram-positive bacterial cell wall (30 nm). The cell wall thickness of gram-negative bacteria lies in between 3 and 4 nm [94]. Another rationale behind enhancement of antibacterial effect of biologically synthesized AgNPs could be the particle size. AgNPs of size less than 50 nm are considered effective antibacterial agents as elucidated in this study [95], [96].
Although the chemically synthesized nanoparticles indicated considerable zones of inhibition, the biologically synthesized nanoparticles inhibited the microbial growth better at the same volume of the hydrosol. Alkaloids, phenols, flavonoids and tannins are major constituents of piper fruits and could be probably responsible for the antibacterial efficacy of the same [97]. The possible encapsulation of phytochemicals of the catkins on AgNPs might be responsible for the enhanced antimicrobial activity compared to glucose derived AgNPs [98].
This antibacterial effect elucidates that larger surface area of the nanoparticles acts better on the bacterial cells and this is by membrane and its charge related aspects [99], [100]. Finding its use in thousands of products for human appliances, silver nanoparticles penetrate bacterial cell wall, interact with their genetic material and impair cell division. This causes changes in biological processes such as respiration, thereby leading to cell death. According to the inhibition zones observed in this study, these nanoparticles could be considered effective bacteria-inhibitory agents. Therefore, the mechanism of antibacterial effect of AgNPs primarily relies on the release and electrostatic attraction of silver cations to the negative charge on the bacterial cell wall surface and permeate through it based on the thickness of peptidoglycan layer. This can result in interactions of these nanoparticles with sizes less than 100 nm with DNA and protein leading to denaturation which can eventually lead to cell death by the production of reactive oxygen species [101], [102], [103], [104], [105], [106], [107], [108], [109], [110].
3.9. Mathematical modelling:
The Karl Pearson’s Co-efficient of Correlation, which usually exists between +1 and −1, among the tested nanoparticles was 0.7064. The calculated Pearsonian R value was indicative of a positive correlation (0 to +1) between the activity of biological and chemically synthesized nanoparticles [111]. P. mirabilis was the most deviated for both chemical and biosynthesized nanoparticles as calculated by the percentage of increase in activity between the both test agents and therefore, identified to be the most susceptible organism. Co-efficient of mean deviation determines that catkin derived AgNPs was most effective against B. cereus, whereas, chemically derived nanoparticles were most effective against P. aeruginosa in comparison to the other microbes (Table 1 ). Therefore, the mathematical modelling supports the interpretation that the biosynthesized nanoparticles were more efficient in comparative antimicrobial effect with glucose derived nanoparticles.
Table 1.
Sensitivities of food-borne pathogens to silver nanoparticles. Mean zone of inhibition (mm) of AgNPs synthesized using catkin extract of P. longum (5%) against seven different pathogens. The disc diameter was 6 mm. The average of percentage increase in antibacterial activity among the 7 tested strains was 16.4%. The table also represents the percentage of increase in activity and the coefficient of mean deviation of the nanoparticles. The coefficient of mean deviation was calculated by dividing the mean deviation using the average.
| Organism | PL-AgNPs | Coefficient of Mean Deviation | Glu-AgNPs | Coefficient of Mean Deviation | % of increase in activity |
|---|---|---|---|---|---|
| S. aureus | 10.97 ± 0.17 | 0.0101 | 10.03 ± 0.08 | 0.01107 | 8.56 |
| S. typhii | 11.97 ± 0.26 | 0.00928 | 9.97 ± 0.18 | 0.01114 | 16.70 |
| K. pneumoniae | 13.97 ± 0.35 | 0.00795 | 12.03 ± 0.09 | 0.009233 | 13.89 |
| P. mirabilis | 15.07 ± 0.70 | 0.0147 | 11.03 ± 0.43 | 0.01611 | 26.80 |
| P. aeruginosa | 11.03 ± 0.29 | 0.0161 | 9.03 ± 0.15 | 0.01968 | 18.13 |
| B. cereus | 12.07 ± 0.35 | 0.0184 | 11.07 ± 0.12 | 0.01405 | 8.29 |
| E. coli | 12.93 ± 0.24 | 0.01202 | 10.03 ± 0.15 | 0.01771 | 22.43 |
4. Conclusion
Food products become unsuitable for consumption after spoilage by microorganisms. Silver nanoparticles are used widely in food-packaging industries to prevent food-spoilage. In the present study, the catkin extract of P. longum was used for the optimized synthesis of AgNPs. The synthesized nanoparticles were characterized using UV, FTIR, TEM, XPS, GC-MS and XRD. Since, this study reports the antibacterial activity of the biosynthesized AgNPs against seven different food-borne gram-negative and gram-positive microorganisms, this study seems significant for the antibacterial testing of nanoparticles intended for antimicrobial applications. All the tested microorganisms were susceptible to the synthesized nanoparticles even after three months of synthesis and storage. The catkin-derived nanoparticles are economical and have an additional benefit of the natural capping agents compared to the nanoparticles synthesized using glucose. Hence, this rapid, eco-friendly and cost-effective method for the synthesis of AgNPs could be exploited for the development of beneficial medical appliances in food-packaging industries as evidenced by conventional disk diffusion and mathematical approaches.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Natural Science Foundation of Jiangsu Province (SBK2020041671), the Foundation of Wu's Medical School Project (SYSD2017202), Jiangsu Branch of China Academy of Chinese Medical Sciences (FY201803, Nanjing 210028, China), Project of Kunshan science and Technology Bureau (KS1905), Kunshan Social Development of Science and Technology Special Project (KS1740).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioorg.2020.104230.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 2.Singhal G., Bhavesh R., Kasariya K., Sharma A.R., Singh R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanopart. Res. 2011;13:2981–2988. [Google Scholar]
- 3.Huq M. Green Synthesis of silver nanoparticles using pseudoduganella eburnea MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int. J. Mol. Sci. 2020;21:1510. doi: 10.3390/ijms21041510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya D., Gupta R.K. Nanotechnology and potential of microorganisms. Crit. Rev. Biotechnol. 2005;25:199–204. doi: 10.1080/07388550500361994. [DOI] [PubMed] [Google Scholar]
- 5.Yu D.-G. Formation of colloidal silver nanoparticles stabilized by Na+–poly (γ-glutamic acid)–silver nitrate complex via chemical reduction process. Colloids Surf., B. 2007;59:171–178. doi: 10.1016/j.colsurfb.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y.-C., Lin L.-H. New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochem. Commun. 2004;6:1163–1168. [Google Scholar]
- 7.Mallick K., Witcomb M., Scurrell M. Polymer stabilized silver nanoparticles: a photochemical synthesis route. J. Mater. Sci. 2004;39:4459–4463. [Google Scholar]
- 8.Sivalingam P., Antony J.J., Siva D., Achiraman S., Anbarasu K. Mangrove Streptomyces sp. BDUKAS10 as nanofactory for fabrication of bactericidal silver nanoparticles. Colloids Surf., B. 2012;98:12–17. doi: 10.1016/j.colsurfb.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Senthilkumar N., Nandhakumar E., Priya P., Soni D., Vimalan M., Potheher I.V. Synthesis of ZnO nanoparticles using leaf extract of Tectona grandis (L.) and their anti-bacterial, anti-arthritic, anti-oxidant and in vitro cytotoxicity activities. New J. Chem. 2017;41:10347–10356. [Google Scholar]
- 10.Ganapathy M., Senthilkumar N., Vimalan M., Jeysekaran R., Potheher I.V. Studies on optical and electrical properties of green synthesized TiO2@ Ag core-shell nanocomposite material. Mater. Res. Express. 2018;5 [Google Scholar]
- 11.Jayapriya M., Dhanasekaran D., Arulmozhi M., Nandhakumar E., Senthilkumar N., Sureshkumar K. Green synthesis of silver nanoparticles using Piper longum catkin extract irradiated by sunlight: antibacterial and catalytic activity. Res. Chem. Intermed. 2019;45:3617–3631. [Google Scholar]
- 12.Nandhakumar E., Priya P., Rajeswari R., Aravindhan V., Sasikumar A., Senthilkumar N. Studies on structural, optical and thermal properties of Fe 3 O 4 (NR)/ZrO 2 CSNCs synthesized via green approach for photodegradation of dyes. Res. Chem. Intermed. 2019;45:2657–2671. [Google Scholar]
- 13.Senthilkumar N., Aravindhan V., Ruckmani K., Potheher I.V. Coriandrum sativum mediated synthesis of silver nanoparticles and evaluation of their biological characteristics. Mater. Res. Express. 2018;5 [Google Scholar]
- 14.Srinivasa Reddy P., Jamil K., Madhusudhan P., Anjani G., Das B. Antibacterial activity of isolates from Piper longum and Taxus baccata. Pharm. Biol. 2001;39:236–238. [Google Scholar]
- 15.Yadav V., Krishnan A., Vohora D. A systematic review on Piper longum L.: Bridging traditional knowledge and pharmacological evidence for future translational research. J. Ethnopharmacol. 2020;247:112255. doi: 10.1016/j.jep.2019.112255. [DOI] [PubMed] [Google Scholar]
- 16.Kiran G., Karthik L., Shree Devi M.S., Sathiyarajeswaran P., Kanakavalli K., Kumar K.M. In Silico computational screening of Kabasura Kudineer - Official Siddha Formulation and JACOM against SARS-CoV-2 spike protein. J Ayurveda Integr. Med. 2020;S0975–9476(20):30024–30033. doi: 10.1016/j.jaim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamouda R.A., Hussein M.H., Abo-elmagd R.A., Bawazir S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019;9:1–17. doi: 10.1038/s41598-019-49444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John M.S., Nagoth J.A., Ramasamy K.P., Mancini A., Giuli G., Natalello A. Synthesis of Bioactive Silver Nanoparticles by a Pseudomonas Strain Associated with the Antarctic Psychrophilic Protozoon Euplotes focardii. Mar. Drugs. 2020;18:38. doi: 10.3390/md18010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erol K., Bolat M., Tatar D., Nigiz C., Köse D.A. Synthesis, characterization and antibacterial application of silver nanoparticle embedded composite cryogels. J. Mol. Struct. 2020;1200 [Google Scholar]
- 20.Tran D.N., Vu N.N., Nhan T., Bich N.T.T., Quang M.L., To N.B. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater. Res. Express. 2020 [Google Scholar]
- 21.Balraj B., Senthilkumar N., Potheher I.V., Arulmozhi M. Characterization, antibacterial, anti-arthritic and in-vitro cytotoxic potentials of biosynthesized Magnesium Oxide nanomaterial. Mater. Sci. Eng., B. 2018;231:121–127. [Google Scholar]
- 22.Zachar O. Formulations for COVID-19 early stage treatment via silver nanoparticles inhalation delivery at home and hospital. ScienceOpen Preprints. 2020 [Google Scholar]
- 23.Huang W., Yan M., Duan H., Bi Y., Cheng X., Yu H. Synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against Setosphaeria turcica. Journal of Nanomaterials. 2020;2020 [Google Scholar]
- 24.Devagi P., Suresh T.C., Sandhiya R.V., Sairandhry M., Bharathi S., Velmurugan P. Actinobacterial-mediated fabrication of silver nanoparticles and their broad spectrum antibacterial activity against clinical pathogens. J. Nanosci. Nanotechnol. 2020;20:2902–2910. doi: 10.1166/jnn.2020.17440. [DOI] [PubMed] [Google Scholar]
- 25.Khandel P., Shahi S.K., Soni D.K., Yadaw R.K., Kanwar L. Alpinia calcarata: potential source for the fabrication of bioactive silver nanoparticles. Nano Convergence. 2018;5:37. doi: 10.1186/s40580-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carbone M., Donia D.T., Sabbatella G., Antiochia R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. – Sci. 2016;28:273–279. [Google Scholar]
- 27.Simbine E.O., Rodrigues L.C., Lapa-Guimaraes J., Kamimura E.S., Corassin C.H., Oliveira C.A.F.d. Application of silver nanoparticles in food packages: a review. Food Sci. Technol. 2019;39:793–802. [Google Scholar]
- 28.Kraśniewska K., Galus S., Gniewosz M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications-a review. Int. J. Mol. Sci. 2020;21:698. doi: 10.3390/ijms21030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavakoli H., Rastegar H., Taherian M., Samadi M., Rostami H. The effect of nano-silver packaging in increasing the shelf life of nuts: an in vitro model. Ital. J. Food Saf. 2017;6:6874. doi: 10.4081/ijfs.2017.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M. Jayapriya, D. Dharumadurai, M. Arulmozhi, E. Nandhakumar, N C S, K Sureshkumar, Green synthesis of silver nanoparticles using Piper longum catkin extract irradiated by sunlight: antibacterial and catalytic activity, Research on Chemical Intermediates 2019.
- 31.Antony J.J., Sithika M.A.A., Joseph T.A., Suriyakalaa U., Sankarganesh A., Siva D. In vivo antitumor activity of biosynthesized silver nanoparticles using Ficus religiosa as a nanofactory in DAL induced mice model. Colloids Surf., B. 2013;108:185–190. doi: 10.1016/j.colsurfb.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 32.Antony J.J., Sivalingam P., Siva D., Kamalakkannan S., Anbarasu K., Sukirtha R. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf., B. 2011;88:134–140. doi: 10.1016/j.colsurfb.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Khan Z.A., Siddiqui M.F., Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics (Basel) 2019;9:49. doi: 10.3390/diagnostics9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gopinath V., MubarakAli D., Priyadarshini S., Priyadharsshini N.M., Thajuddin N., Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf., B. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Rajeshkumar S., Menon S., Kumar S.V., Tambuwala M.M., Bakshi H.A., Mehta M. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol., B. 2019;197 doi: 10.1016/j.jphotobiol.2019.111531. [DOI] [PubMed] [Google Scholar]
- 36.Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12:788–800. [Google Scholar]
- 37.Raghunandan D., Mahesh B.D., Basavaraja S., Balaji S., Manjunath S., Venkataraman A. Microwave-assisted rapid extracellular synthesis of stable bio-functionalized silver nanoparticles from guava (Psidium guajava) leaf extract. J. Nanopart. Res. 2011;13:2021–2028. [Google Scholar]
- 38.Stebounova L.V., Guio E., Grassian V.H. Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation, and dissolution. J. Nanopart. Res. 2011;13:233–244. [Google Scholar]
- 39.Dzido G., Jarzębski A.B. Fabrication of silver nanoparticles in a continuous flow, low temperature microwave-assisted polyol process. J. Nanopart. Res. 2011;13:2533–2541. [Google Scholar]
- 40.Zaheer Z. Silver nanoparticles to self-assembled films: green synthesis and characterization. Colloids Surf., B. 2012;90:48–52. doi: 10.1016/j.colsurfb.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 41.Mock J., Barbic M., Smith D., Schultz D., Schultz S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002;116:6755–6759. [Google Scholar]
- 42.Vijayaraghavan K., Nalini S.K., Prakash N.U., Madhankumar D. One step green synthesis of silver nano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloids Surf., B. 2012;94:114–117. doi: 10.1016/j.colsurfb.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Suriyakalaa U., Antony J.J., Suganya S., Siva D., Sukirtha R., Kamalakkannan S. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf., B. 2013;102:189–194. doi: 10.1016/j.colsurfb.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 44.Sekar C., Suguna K. Effect of H3PO4 reactant and NaF additive on the crystallization and properties of brushite. Adv. Mater. Lett. 2011;2:227–232. [Google Scholar]
- 45.Kong J., Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophy. Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 46.Macdonald I., Smith W. Orientation of cytochrome c adsorbed on a citrate-reduced silver colloid surface. Langmuir. 1996;12:706–713. [Google Scholar]
- 47.Chen Z., Paley D.W., Wei L., Weisman A.L., Friesner R.A., Nuckolls C. Multicolor live-cell chemical imaging by isotopically edited alkyne vibrational palette. J. Am. Chem. Soc. 2014;136:8027–8033. doi: 10.1021/ja502706q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma G., Nam J.-S., Sharma A.R., Lee S.-S. Antimicrobial potential of silver nanoparticles synthesized using medicinal herb Coptidis rhizome. Molecules. 2018;23:2268. doi: 10.3390/molecules23092268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shahverdi A.R., Minaeian S., Shahverdi H.R., Jamalifar H., Nohi A.-A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Process Biochem. 2007;42:919–923. [Google Scholar]
- 50.Khandel P., Yadaw R.K., Soni D.K., Kanwar L., Shahi S.K. Biogenesis of metal nanoparticles and their pharmacological applications: present status and application prospects. J. Nanostruct. Chem. 2018;8:217–254. [Google Scholar]
- 51.Burlacu E., Tanase C., Coman N.-A., Berta L. A review of bark-extract-mediated green synthesis of metallic nanoparticles and their applications. Molecules (Basel, Switzerland) 2019;24:4354. doi: 10.3390/molecules24234354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salari S., Esmaeilzadeh Bahabadi S., Samzadeh-Kermani A., Yosefzaei F. In-vitro evaluation of antioxidant and antibacterial potential of greensynthesized silver nanoparticles using prosopis farcta fruit extract. Iran J. Pharm. Res. 2019;18:430–455. [PMC free article] [PubMed] [Google Scholar]
- 53.El-Seedi H.R., El-Shabasy R.M., Khalifa S.A., Saeed A., Shah A., Shah R. Metal nanoparticles fabricated by green chemistry using natural extracts: biosynthesis, mechanisms, and applications. RSC Adv. 2019;9:24539–24559. doi: 10.1039/c9ra02225b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17:1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sathishkumar G., Gobinath C., Karpagam K., Hemamalini V., Premkumar K., Sivaramakrishnan S. Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf., B. 2012;95:235–240. doi: 10.1016/j.colsurfb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Anandalakshmi K., Venugobal J., Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016;6:399–408. [Google Scholar]
- 57.Jyoti K., Baunthiyal M., Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016;9:217–227. [Google Scholar]
- 58.Elamawi R.M., Al-Harbi R.E., Hendi A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control. 2018;28:28. [Google Scholar]
- 59.Salvadori M.R., Ando R.A., Nascimento C.A.O., Corrêa B. Dead biomass of Amazon yeast: A new insight into bioremediation and recovery of silver by intracellular synthesis of nanoparticles. J. Environ. Sci. Health, Part A. 2017;52:1112–1120. doi: 10.1080/10934529.2017.1340754. [DOI] [PubMed] [Google Scholar]
- 60.Uznanski P., Zakrzewska J., Favier F., Kazmierski S., Bryszewska E. Synthesis and characterization of silver nanoparticles from (bis)alkylamine silver carboxylate precursors. J. Nanopart Res. 2017;19:121. doi: 10.1007/s11051-017-3827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasil’kov A.Y., Dovnar R.I., Smotryn S.M., Iaskevich N.N., Naumkin A.V. Plasmon resonance of silver nanoparticles as a method of increasing their antibacterial action. Antibiotics. 2018;7:80. doi: 10.3390/antibiotics7030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruíz-Baltazar Ád.J., Reyes-López S.Y., Mondragón-Sánchez Md.L., Estevez M., Hernández-Martinez A.R., Pérez R. Biosynthesis of Ag nanoparticles using Cynara cardunculus leaf extract: Evaluation of their antibacterial and electrochemical activity. Results Phys. 2018;11:1142–1149. [Google Scholar]
- 63.Li S., Shen Y., Xie A., Yu X., Qiu L., Zhang L. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9:852–858. [Google Scholar]
- 64.Theivasanthi T., Alagar M. Nano sized copper particles by electrolytic synthesis and characterizations. International Journal of Physical Sciences. 2011;6:3662–3671. [Google Scholar]
- 65.Suvith V., Philip D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;118:526–532. doi: 10.1016/j.saa.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Kumari D., Bansal H. Benzohydrazides: As potential bio-active agents. Pharma Innov J. 2018;7:543–550. [Google Scholar]
- 67.Otto M., Wynands B., Lenzen C., Filbig M., Blank L.M., Wierckx N. Rational engineering of L-phenylalanine accumulation in Pseudomonas taiwanensis to enable high-yield production of trans-cinnamate. Front. Bioeng. Biotechnol. 2019;7:312. doi: 10.3389/fbioe.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chowdhury N., Al Hasan A., Tareq F.S., Ahsan M., Azam A.Z. 4-Hydroxy-trans-cinnamate Derivatives and Triterpene from Barleria cristata. Dhaka University Journal of Pharmaceutical Sciences. 2013;12:143–145. [Google Scholar]
- 69.Huang Q.S., Zhu Y.J., Li H.L., Zhuang J.X., Zhang C.L., Zhou J.J. Inhibitory effects of methyl trans-cinnamate on mushroom tyrosinase and its antimicrobial activities. J Agric Food Chem. 2009;57:2565–2569. doi: 10.1021/jf8036227. [DOI] [PubMed] [Google Scholar]
- 70.Anwar A., Siddiqui R., Shah M.R., Khan N.A. Gold nanoparticle-conjugated cinnamic acid exhibits antiacanthamoebic and antibacterial properties. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00630-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pu Z.-H., Zhang Y.-Q., Yin Z.-Q., Jiao X., Jia R.-Y., Yang L. Antibacterial activity of 9-octadecanoic acid-hexadecanoic acid-tetrahydrofuran-3, 4-diyl ester from neem oil. Agricult. Sci. China. 2010;9:1236–1240. [Google Scholar]
- 72.Cartron M.L., England S.R., Chiriac A.I., Josten M., Turner R., Rauter Y. Bactericidal activity of the human skin fatty acid cis-6-hexadecanoic acid on Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58:3599–3609. doi: 10.1128/AAC.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aparna V., Dileep K.V., Mandal P.K., Karthe P., Sadasivan C., Haridas M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012;80:434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 74.Rahman M.M., Ahmad S.H., Mohamed M.T.M., Ab Rahman M.Z. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. ScientificWorldJournal. 2014;2014:635240. doi: 10.1155/2014/635240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Igwaran A., Iweriebor B.C., Okoh S.O., Nwodo U.U., Obi L.C., Okoh A.I. Chemical constituents, antibacterial and antioxidant properties of the essential oil flower of Tagetes minuta grown in Cala community Eastern Cape, South Africa. BMC Complement. Alternat. Med. 2017;17:351. doi: 10.1186/s12906-017-1861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gratzl G., Walkner S., Hild S., Hassel A.W., Weber H.K., Paulik C. Mechanistic approaches on the antibacterial activity of poly(acrylic acid) copolymers. Colloids Surf., B. 2015;126:98–105. doi: 10.1016/j.colsurfb.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 77.Gratzl G., Paulik C., Hild S., Guggenbichler J.P., Lackner M. Antimicrobial activity of poly(acrylic acid) block copolymers. Mater. Sci. Eng., C. 2014;38:94–100. doi: 10.1016/j.msec.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 78.Daood U., Matinlinna J.P., Pichika M.R., Mak K.-K., Nagendrababu V., Fawzy A.S. A quaternary ammonium silane antimicrobial triggers bacterial membrane and biofilm destruction. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-67616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samanta S., Agarwal S., Nair K.K., Harris R.A., Swart H. Biomolecular assisted synthesis and mechanism of silver and gold nanoparticles. Mater. Res. Express. 2019;6 [Google Scholar]
- 80.Rai M., Ingle A.P., Gupta I.R., Birla S.S., Yadav A.P., Abd-Elsalam K.A. Potential role of biological systems in formation of nanoparticles: mechanism of synthesis and biomedical applications. Curr. Nanosci. 2013;9:576–587. [Google Scholar]
- 81.Das R.K., Pachapur V.L., Lonappan L., Naghdi M., Pulicharla R., Maiti S. Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017;2:18. [Google Scholar]
- 82.Ahmad T. Reviewing the tannic acid mediated synthesis of metal nanoparticles. J. Nanotechnol. 2014;2014 [Google Scholar]
- 83.Velusamy P., Kumar G.V., Jeyanthi V., Das J., Pachaiappan R. Bio-inspired green nanoparticles: synthesis, mechanism, and antibacterial application. Toxicol. Res. 2016;32:95–102. doi: 10.5487/TR.2016.32.2.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nandhakumar E., Priya P., Selvakumar P., Vaishnavi E., Sasikumar A., Senthilkumar N. One step hydrothermal green approach of CuO/Ag nanocomposites: analysis of structural, biological activities. Mater. Res. Express. 2019;6 [Google Scholar]
- 85.Gong Z., Shi X., Bai F., He X., Zhang H., YuBin L. Characterization of a novel diarrheagenic strain of Proteus mirabilis associated with food poisoning in China. Front. Microbiol. 2019;10:2810. doi: 10.3389/fmicb.2019.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yazgan H., Ozogul Y., Kuley E. Antimicrobial influence of nanoemulsified lemon essential oil and pure lemon essential oil on food-borne pathogens and fish spoilage bacteria. Int. J. Food Microbiol. 2019;306 doi: 10.1016/j.ijfoodmicro.2019.108266. [DOI] [PubMed] [Google Scholar]
- 87.Mostafa A.A., Al-Askar A.A., Almaary K.S., Dawoud T.M., Sholkamy E.N., Bakri M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018;25:361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kıvanç S.A., Takım M., Kıvanç M., Güllülü G. Bacillus Spp. isolated from the conjunctiva and their potential antimicrobial activity against other eye pathogens. Afr. Health Sci. 2014;14:364–371. doi: 10.4314/ahs.v14i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Snafi A.E. Chemical constituents, pharmacological effects and therapeutic importance of Hibiscus rosa-sinensis – a review. J. Pharm. 2018;8:101–119. [Google Scholar]
- 90.Lagadinou M., Onisor M.O., Rigas A., Musetescu D.-V., Gkentzi D., Assimakopoulos S.F. Antimicrobial properties on non-antibiotic drugs in the era of increased bacterial resistance. Antibiotics. 2020;9:107. doi: 10.3390/antibiotics9030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brehm-Stecher B.F., Johnson E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob Agents Chemother. 2003;47:3357–3360. doi: 10.1128/AAC.47.10.3357-3360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loo YY, Rukayadi Y, Nor-Khaizura M-A-R, Kuan CH, Chieng BW, Nishibuchi M, et al. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front Microbiol 2018;9:1555. [DOI] [PMC free article] [PubMed]
- 93.Rajeshkumar S., Malarkodi C. In vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioinorg. Chem. Appl. 2014;2014 doi: 10.1155/2014/581890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ya Qing, Cheng L., Li R., Liu G., Zhang Y., Tang X. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018;13:3311–3327. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Majoumouo M.S., Sibuyi N.R.S., Tincho M.B., Mbekou M., Boyom F.F., Meyer M. Enhanced anti-bacterial activity of biogenic silver nanoparticles synthesized from terminalia mantaly Extracts. Int. J. Nanomed. 2019;14:9031. doi: 10.2147/IJN.S223447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Al-Tememy T.M. Antibacterial activity of Piper cubeba Linn. fruit extracts against selected bacterial pathogens in Basrah City. Basrah Journal of. Vet. Res. 2013;12:142–151. [Google Scholar]
- 98.Elemike E.E., Fayemi O.E., Ekennia A.C., Onwudiwe D.C., Ebenso E.E. Silver nanoparticles mediated by costus afer leaf extract: synthesis, antibacterial, antioxidant and electrochemical properties. Molecules (Basel, Switzerland) 2017;22:701. doi: 10.3390/molecules22050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J.S., Kuk E., Yu K.N., Kim J.-H., Park S.J., Lee H.J. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Drogat N., Granet R., Sol V., Memmi A., Saad N., Koerkamp C.K. Antimicrobial silver nanoparticles generated on cellulose nanocrystals. J. Nanopart. Res. 2011;13:1557–1562. [Google Scholar]
- 101.Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bankier C., Matharu R., Cheong Y.-K., Ren G., Cloutman-Green E., Ciric L. Synergistic antibacterial effects of metallic nanoparticle combinations. Sci. Rep. 2019;9:1–8. doi: 10.1038/s41598-019-52473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aritonang HF, Koleangan H, Wuntu AD. Synthesis of Silver Nanoparticles Using Aqueous Extract of Medicinal Plants’(Impatiens balsamina and Lantana camara) Fresh Leaves and Analysis of Antimicrobial Activity. International journal of microbiology 2019;2019. [DOI] [PMC free article] [PubMed]
- 104.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 105.Ovais M., Khalil A.T., Raza A., Islam N.U., Ayaz M., Saravanan M. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl. Microbiol. Biotechnol. 2018;102:4393–4408. doi: 10.1007/s00253-018-8928-2. [DOI] [PubMed] [Google Scholar]
- 106.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 107.Yu-sen E.L., Vidic R.D., Stout J.E., McCartney C.A., Victor L.Y. Inactivation of Mycobacterium avium by copper and silver ions. Water Res. 1998;32:1997–2000. [Google Scholar]
- 108.Saravanan M., Arokiyaraj S., Lakshmi T., Pugazhendhi A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018;117:68–72. doi: 10.1016/j.micpath.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 109.Matsumura Y., Yoshikata K., Kunisaki S-i, Tsuchido T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003;69:4278–4281. doi: 10.1128/AEM.69.7.4278-4281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Durán N., Durán M., De Jesus M.B., Seabra A.B., Fávaro W.J., Nakazato G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016;12:789–799. doi: 10.1016/j.nano.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 111.Cramer D. Psychology Press; 1998. Fundamental statistics for social research: step-by-step calculations and computer techniques using SPSS for Windows. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.