The present pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has resulted in a number of neurological problems whose exact frequency and type are being defined. Critically ill patients often develop encephalopathy of multifactorial origin.
We here report a case of severe encephalopathy secondary to non-convulsive status epilepticus (NCSE) in a patient with coronavirus disease 2019 (COVID-19) infection.
A 62-year-old male, with left eye amblyopia since childhood and no other relevant medical history, was admitted to the Emergency Department in March 2020 due to upper respiratory tract symptoms and fever for eight days before admission. Given the epidemiological background of COVID-19 pandemics, a nasopharyngeal reverse transcriptase polymerase chain reaction (RT – PCR) test for SARS-Cov-2 was performed with positive results. A chest X-ray showed bilateral interstitial pneumonia. ECG and corrected QT interval were normal. He had hypoxemia with an oxygen saturation (SaO2) of 89%.
Following admission, hydroxychloroquine (200 mg twice-daily) was administered for seven days and Lopinavir-Ritonavir (200 mg/50 mg twice-daily) for 15 days. However, blood tests showed a progressive inflammatory response (PCR 309 mg/dL, LDH 613 U/L, ferritin 5700 ng/mL, D-Dimer 9550 ng/mL) and he developed a severe respiratory distress syndrome on the 2nd day of admission, needing an oxygen reservoir bag to maintain acceptable oxygen saturation (91%). Dexamethasone (20 mg daily for five days) and Tocilizumab (600 mg) were administered. His condition progressively improved and respiratory stability was achieved and maintained thereafter.
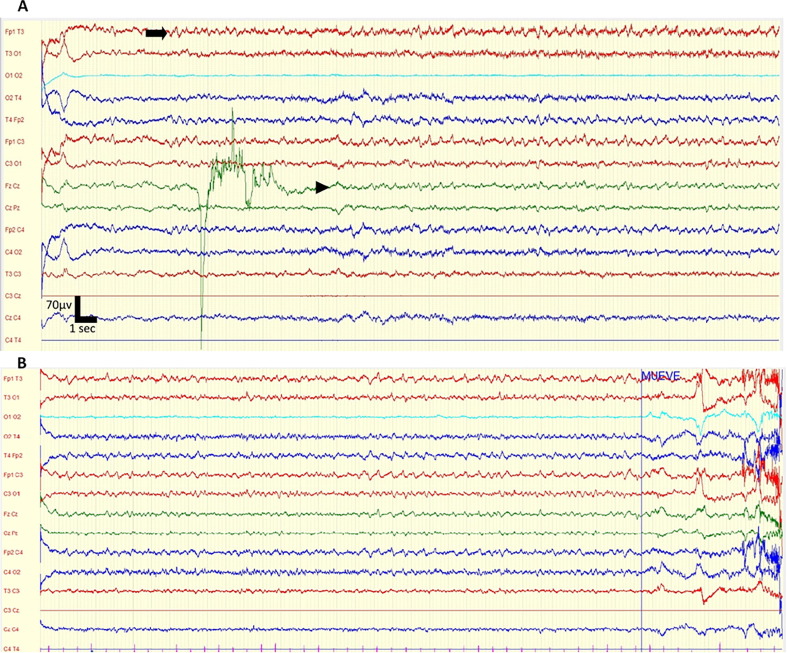
On the 8th day of admission, while in a stable respiratory condition, he became suddenly confused and disorientated, with bradyphrenia, ideomotor apraxia, and bilateral Babinski signs. Brain CT scan was normal. Blood chemistry, blood cell count, and an extensive autoimmune work-up that included antinuclear, anti-thyroid, onconeural (Hu, Yo, Ri, CV2, Amphiphysin, Ma, Recoverin, SOX1, Titin, Zic4, GAD 65, Tr) and membrane (GABA B, NMDA; CASPR2, LGI1, AMPA 1 and 2, DPPX) antibodies were all normal or negative. A lumbar puncture showed a clear cerebrospinal fluid (CSF) with slightly increased protein (49 mg/dL), no cells and normal glucose. RT-PCR for SARS-Cov-2 in the CSF was negative. Due to technical issues, an EEG could not be performed until 5 days later, and during that period his respiratory situation remained stable and his neurological condition unchanged. An EEG recording for 30 min in supine position revealed the presence of continuous, abrupt and rhythmic sharply-contoured delta waves at 2–2.5 Hz., bilaterally, more evident in the fronto-temporal regions. There was a temporal-spatial evolution towards central and posterior electrodes (Fig. 1 ). We did not use benzodiazepines during the EEG so as to minimize the time of examination in a COVID-19 area. Due to the SARS-CoV-2 patient’s condition, an old EEG machine had to be used, which prevented us from changing montages or obtaining best-quality images.
Fig. 1.
Electroencephalogram findings. (A) Electroencephalogram of the patient on day 13 showing continuous, bilateral discharges of delta, sharply-contoured waves, more evident in fronto-temporal areas (arrow) spreading to central and posterior electrodes (arrowhead). Settings: gain 70 microvolts/cm, LFF 0.53 Hz, HFF 70 Hz, notch on. Time base 40 s. (B) Continuation of the EEG recording showing the persistent discharges.
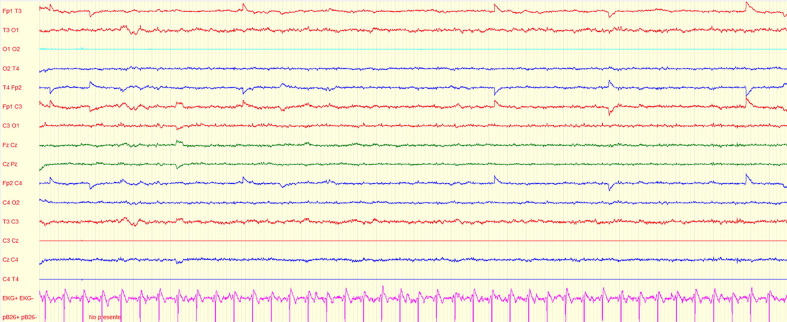
With the diagnosis of NCSE, the patient was transferred to the ICU and received levetiracetam (1500 mg every 12 h) and valproic acid (400 mg every six hours) with cessation of his seizures and improvement of the paroxysmal activity on an EEG performed after 48 h (Fig. 2 ).
Fig. 2.
EEG after 48 h of antiepileptic therapy. Electroencephalogram of the patient showing an EEG considered as normal without discharges. Settings: gain 70 microvolts/cm, LFF 0.53 Hz, HFF 70 Hz, notch on. Time base 40 s.
His neurological examination normalized. Cranial MRI on day 21 showed focal leptomeningeal enhancement in the right parietal peri-Rolandic sulci consistent with findings described in severely-ill COVID-19 patients (Helms et al., 2020).
With a stable respiratory condition and normal neurological exam, the patient was discharged on day 21 on antiepileptic therapy and has not had seizure recurrence to date. An outpatient EEG performed on day 35 was normal and brain MRI on day 45 showed the resolution of the meningeal enhancement.
Discussion
We here report a severe encephalopathy secondary to NCSE associated with SARS-CoV-2 infection.
Several limitations, inherent to the contagiousness of this infection, were present for carrying out an optimal EEG examination and included the use of an old EEG machine specifically dedicated to infected patients and the brief time of examination in a COVID-19 area. Despite this, the presence of continuous rhythmic epileptiform discharges with spatial evolution could be registered. These findings, together with a prompt and significant clinical and EEG improvement after antiepileptic therapy initiation, support the diagnosis of NCSE, fulfilling the Salzburg criteria.
The patient had no history of seizures and there was no other evident etiology for them except for the viral infection. A similar situation has been described in influenza patients, where otherwise healthy adults develop seizures during Influenza A and B; there may be an associated encephalopathy resembling that of our patient, with neuroimaging being normal and seizures tending to disappear after a few weeks (Ruisanchez-Nieva et al., 2017).
Several mechanisms may have played a role in the establishment of the NCSE in this patient. Direct viral damage is unlikely in view of the absence of viral RNA in the CSF (negative RT-PCR for SARS-CoV-2) with a normal CSF cell count and a mild protein increase that could be explained by the status epilepticus itself. Critically ill patients are prone to develop status epilepticus. However, the neurological disturbances developed when the patient’s respiratory condition was stable, which argues against the severity of his condition playing a fundamental role at that point. The timing of presentation, 8 days after admission, suggests a para-infectious mechanism involving the central nervous system.
Previous reports have described a variety of neurological disorders in COVID-19 patients. In a retrospective study of 214 hospitalized patients with COVID-19, 78 (36.4%) had neurologic manifestations, but only one patient (0.5%) had a tonic-clonic seizure (Mao et al., 2020). Encephalopathy of multifactorial origin has been described in COVID-19, particularly in severely-ill patients admitted to the ICU; in seven of those patients, RT-PCR was performed in the CSF with negative results in all samples (Helms et al., 2020).
In a multicenter retrospective study carried out in China on 304 patients with COVID-19 of whom 108 had a severe condition, neither acute symptomatic seizures nor status epilepticus was observed (Lu et al., 2020). A recent report described a status epilepticus in a COVID-19 patient without evidence of meningoencephalitis or structural lesion (Balloy et al., 2020).
In summary, it is extremely important to consider the possibility of non-convulsive status epilepticus in patients with COVID-19 and encephalopathy to avoid missing a treatable disorder. Performing necessary ancillary tests such as EEG for establishing the diagnosis in contagious COVID-19 patients makes this situation particularly challenging (Gélisse et al., 2020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Balloy G., Leclair-Visonneau L., Pereon Y., Magot A., Peyre A., Mahe P.J. Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020;131(8):2059–2061. doi: 10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gélisse P., Rossetti A.O., Genton P., Crespel A., Kaplan P.W. How to carry out and interpret EEG recordings in COVID-19 patients in ICU? Clin Neurophysiol. 2020;131:2023–2031. doi: 10.1016/j.clinph.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Xiong W., Liu D., Liu J., Yang D., Li N. New onset acute symptomatic seizure and risk factors in Corona Virus Disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisanchez-Nieva A., Martinez-Arroyo A., Gomez-Beldarrain M., Bocos Portillo J., Garcia-Monco J.C. Influenza-associated seizures in healthy adults: Report of 3 cases. Epilepsy Behav Case Rep. 2017;8:12–13. doi: 10.1016/j.ebcr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]