Graphical abstract
Keywords: Systemic infections, Sepsis treatment, Nanotechnology, Drug delivery systems
Abbreviations: A, alginate; ABZ-SO and CUR, microemulsion whit albendazole sulfuroxide and curcumin; ACV, acyclovir; AFM, atomic force microscopy; AgNPs, silver nanoparticles; AMB-NPs, amphotericin B polymeric nanoparticle with Dibloco copolymer D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly (ε-caprolactone glycolide) succinate; AMB/AmB, amphotericin B; Amp, ampicillin; AMPs, antimicrobial peptides; AUNC-L-Amp, lysozyme capped gold nanoclusters with β-lactam; AV, aloe vera; AZM, azithromycin; BBB, blood-brain barrier; BMS, β-methasone hemisuccinate; BNZ-nps, polymeric nanoparticles with benznidazole; BNZ, benznidazole; BRB, blood-retinal barrier; CeO2, cerium oxide; CFU, colony forming units; CLN, nanoemulsion carried with cefuroxime; CM-SH, cecropin melittin with cysteine; CNPs-KAg, killed SwIAV H1N2 (δ-lineage) antigens (KAg) were encapsulated in chitosan polymer-based nanoparticles; CNPs, hitosan nanoparticles; CNS, central nervous system; CS-ALG, chitosan-alginate nanoparticles; CUR, curcumin; DCP, dicetylphosphate; DHA, docosahexaenoic acid; DLS, dynamic light scattering; DM, diabetes mellitus; DODAC, double-chain cationic surfactant; DPPC, 1,2-dipalmitoyl-sn-glycerol-3-phosphocholine; EDX, energy-dispersive X-ray spectroscopy; ELISA, enzyme linked immunosorbent assay; Esc-Fluo-NPs, rhodamine-labeled NPs loaded with Esc(1-21); EXAFS, extended X-ray absorption fine structure; FE-SEM, field emission scanning electronic microscopy; FTIR, fourier-transform infrared spectroscopy; G, dendrimer generation; G2-S16, polyanionic carbosilane dendrimer; H5N, avian influenza virus; HBL, Hydrophilic–Lipophilic Balance; HBV, chronic hepatitis B; HCV, hepatite C virus; HIA, haemagglutination inhibition assay; HIV-1, human immunodeficiency virus type 1; HIV, human Immunodeficiency virus; HPH, high pressure homogenization; HS 15, macrogol hydroxystearate 15; HSK, herpes simplex keratitis; HSV-2, herpes simplex type 2; HSV, herpes simplex virus; ICAM-1, intercellular adhesion molecule-1; ICP-MS, inductively coupled plasma-mass spectrometry; ICU, intensive Care Units; IDV, Indinavir; IN, Intranasally; IP, Intraperitoneal; ITZ ME, Intranasal Delivery of Itraconazole in Microemulsion; IVM CS-ALG, chitosan-alginate nanoparticles with ivermectin; IVM, Ivermectin; KAg, killed SwIAV H1N2 (δ-lineage) antigens; LF-IDV-NEs, nanoemulsion carried with indinavir, treated with lactoferrin; LPS, lipopolysaccharide; LVCZ, voriconazole incorporated into liposomal structure; ME-AmB, Amphotericin B Incorporated in Microemulsion; ME, Microemulsion; MNPs, metallic nanoparticles; MODS, multiple organ dysfunction syndrome; MPS, methylprednisolone sodium hemisuccinate; MRSA, methicillin resistant Staphylococcus aureus; MTB, Mycobacterium tuberculosis; MTX NE, nanoemulsion containing with methotrexate; N-LCT, Indigenous Natural Lipophile; Nan Osorb-ARM, Solid Microemulsion Preconcentrates With Artemether; NE, Nanoemulsion; NE02, nanoemulsion containing CpG oligonucleotide; NEG, Microemulsion in Sitogel; NLC, Nanostructured lipid carriers; nm, nanometer; NS3, non-structural protein 3; NSP, nanoscale silicate platelet; O/W, oil in water; P188, Lutrol® F-68; PAMAM, poly(amidoamine) dendrimer; PCL, Poly-ɛ-Caprolactone; PDI, polydispersity index; PE-PEG5K, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine; PEG 400, low molecular weight polyethylene glycol; PEG-lipid, unmodified liposome containing PEG; PEG, polyethylene glycol; Pep4, peptide of a chlamydial glycolipid antigen-Peptide 4; PEPEG2K, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt; PGA, glycolic acid; pH, hydrogen potential; PLA, poly (lactic acid); PLGA, Poly (lactide-co-glycolide); PLGA PNs, polymeric nanoparticle of poly (lactide-co-glycolide) with amphotericin B; PLGA-TPGS, dibloco copolymer D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly (ε-caprolactone glycolide) succinate; PLL, poly(propylenimine); PNs/NPs, polymeric nanoparticles; PPI, poly(propylene imine); PTM, platensimycin; PVA, Polyvinyl alcohol; Rif NE, nanoemulsion with rifampicin; SEM, Scanning electron microscopy; SFX, Sparfloxacin; siRNA, small interfering RNA; SLN, lolid lipid nanoparticles; SMEDDS, Self-Microemulsifying Drug Delivery Systems; SMIX, mixture of tween 20 and carbitol; SPC, soy phosphatidylcholine; SQVM, saquinavir; SwIAV H1N2, Influenza A virus; TAC, Tacrolimus; TEM, Transmission electron microscope; TGFBIp, factor β-induced protein; TLR9 / CpG - ODN 1826, class B oligonucleotide murine TLR9 agonist Topiramate PMS; TPM NE, nanoemulsion carried with topiramate; USA, United States of America; UV–Vis, ultraviolet–visible spectroscopy; VCZ, voriconazole; W / O, water in oil; W2, chloroquine resistant P. falciparum strain; WGA-Lip, WGA-modified liposome; WGA, wheat germ agglutinin; WHO, World Health Organization; WI-26-VA4, human lung fibroblast cell line; XPS, X-ray photoelectron spectroscopy; XRD, X-Ray diffraction; y3-PLGA/S + T NPs, polymeric nanoparticle of poly (lactide-co-glycolide) with Sparfloxacin and tacrolimus and conjugated with y3 peptide; ZP, Zeta potential Analysis
Abstract
Systemic infections is one of the major causes of mortality worldwide, and a shortage of drug approaches applied for the rapid and necessary treatment contribute to increase the levels of death in affected patients. Several drug delivery systems based in nanotechnology such as metallic nanoparticles, liposomes, nanoemulsion, microemulsion, polymeric nanoparticles, solid lipid nanoparticles, dendrimers, hydrogels and liquid crystals can contribute in the biological performance of active substances for the treatment of microbial diseases triggered by fungi, bacteria, virus and parasites. In the presentation of these statements, this review article present and demonstrate the effectiveness of these drug delivery systems for the treatment of systemic diseases caused by several microorganisms, through a review of studies on scientific literature worldwide that contributes to better information for the most diverse professionals from the areas of health sciences. The studies demonstrated that the drug delivery systems described can contribute to the therapeutic scenario of these diseases, being classified as safe, active platforms and with therapeutic versatility.
1. Introduction
The ability of microorganisms to trigger disease in humans is a global problem, and one that is of concern in all sectors of the Health Sciences. Around 15 million peoples are affected (>25%) of 57 million annual deaths worldwide are estimated to be related directly to infectious diseases (Morens, 2004).
The commensalism relationship between microorganisms and humans is known; however, it is known that most microorganisms have an opportunistic profile, in which, in situations where there is a deficit in the host's immune system, these organisms start to assume an aggressive character and extremely complex for the health patient (Fauci, 2001).
Most pathogenic and opportunistic microorganisms have the ability to cause simple infections, also known as superficial infections; however, according to the pathogenic profile of the microorganism, as well as the patient health status, these infections can reach deeper levels and aggressive at the systemic level, so they are known as systemic infections or sepsis (Angus et al., 2001, Fernández et al., 2017, Rello et al., 2017). Several diseases caused by microbial species are associated with severe acute episodes of sepsis especially in nosocomial environment (Mayr et al., 2014).
In nowadays, the antimicrobial therapy available for the treatment for this infections has some limitations as the high cost, intense harmful effects for the patients and specially the antibiotic resistance profile of the microorganism responsible for the infection (Marston et al., 2016, Ramos et al., 2018a).
Microbial resistance to the available antibiotics in clinical practice has become a significant problem, as microorganisms are acquiring the ability to resist drugs, making them a threat to public health (Bochud et al., 2001, Cushnie and Lamb, 2005). The genetic profile of the microorganisms associated with the infection is directly related with their resistance profile, due the spontaneous mutations and gene recombination for example. Additionally, the indiscriminate use of antibiotics contribute with the increase of this profile (Tacconelli et al., 2018).
Along the knowledge of the antimicrobial resistance as a worldwide problem, some strategies to improve the therapy and control the infectious diseases are employed as a new hope to improve the antimicrobial arsenal, as the use of nanotechnology tools to delivery available drugs with more safe, stability and promote a better targeting to the microorganism (Pelgrift and Friedman, 2013). Some nanotechnology-based drug delivery systems are appreciated in the scientific field of antimicrobial agents, due some important characteristics favorable to improve the therapy of microbial infections, as the ability to improve the solubility parameters of insoluble drugs, interaction between the nanotechnological system and microorganism, functionalized drug delivery promoting the differentiation of normal cells and microbial cells, diminution of side effects, low hepatotoxic effects and specially the possibility of the use of less drugs to promote the microbial inhibition (Bonifácio et al., 2014, Ramos et al., 2018b, Silva et al., 2014, Škalko-Basnet and Vanić, 2017).
Nanotechnology-based drug delivery systems can provide a new and safe platform for therapy in systemic microbial infections. Some nanotechnological systems can promote a better performance of active substances to be use aiming the enhancement of the therapy of systemic infections (Joshi and Müller, 2009, Zhang et al., 2018), such as metallic nanoparticles, liposomes, nanoemulsion, microemulsion, polymeric nanoparticles, solid lipid nanoparticles, dendrimers and others drug delivery systems (hydrogels and liquid crystals) that can contribute in the biological performance of active substances for the treatment of microbial diseases at systemic level, for different microorganisms. Thus, the aim of this review article is elucidate and shown important drug delivery systems based in nanotechnology for the treatment of systemic diseases caused by several infectious agents to demonstrate the applicability, versatility and potential performance for professionals in the health sciences.
2. General characteristics and updates of the microbial systemic infections
Systemic infections (infection at systemic level affecting blood, organs and all body) caused by microorganisms or also known as sepsis are classified as being a serious organic dysfunction that promotes a great risk of life to the affected patient. This dysfunction is caused by a host's unregulated response to infection by the microorganism. This type of infection is a major public health problem, affecting millions of people around the world each year and killing up to one in four (and often more) (Angus et al., 2001, Rhodes et al., 2017, Singer et al., 2016).
Although modern medicine relies on intelligent drugs for the control of infectious diseases, systemic infections, especially sepsis, are the main causes of mortality worldwide. Due to the aggressiveness of this type of infection, the diagnosis is often complicated and late, which creates difficulties in therapy due to the lack of information about the microorganism responsible for the onset of the disease, as well the susceptibility profile to the available antimicrobials (Hotchkiss and Karl, 2003, Pierrakos and Vincent, 2010). A study about the systemic infections incidence performed in 2014 showed that this kind of diseases are the principal death causes (300 cases per 100 000 population) in the United States of America (USA) and the most common cause of critically patients in intensive unit care at hospitals (Mayr et al., 2014). The global problem of sepsis is treated as priority in all countries, in this sense, a resolution was created in 2017 by the World Health Assembly and the World Health to improve the prevention, diagnosis, and management these important infection episodes (Konrad et al., 2017). The high costs attributed to the public economic governmental section are another important fact in the treatment of these diseases. In 2013, the sepsis treatment was the most expensive condition treated in USA hospitals, with a financial burden exceeding US$23 billion only in that year (Torio and Moore, 2016).
In all cases the systemic infections or sepsis diagnostic is confirmed after the observation of the microorganism presence and diagnose in several evaluated body samples, as blood, organs, dysfunctional hemostasis are disseminated intravascular coagulation and formation of massive edema due to loss of vascular integrity (Prescott and Angus, 2018, Russell and Kumar, 2015). Additionally, severe renal failure is commonly observed and also contributes to the mortality of late-phase sepsis (Angeli et al., 2016, Gaudet et al., 2019).
Normally, the signs and symptoms of systemic infections are extremely variable in each patient. A expressive influence as the microorganism virulence and burden of the pathogen, host susceptibility and the site of primary infection are associated with symptoms as fever, mental fog, temporary hypotension, decreasing urine amount, or unexplained thrombocytopenia and coma (Polat et al., 2017). As well as the brain is the site of primary infection, in which neurological symptoms are prominent, brain function is often deranged. The evidence of organ dysfunction or shock provides the necessary information to describe the severity, usually without contributing to knowledge about causation (Lever and Mackenzie, 2007).
According to the signs and symptoms, four different denominations can be attributed for sepsis clinical stages in the clinical practice: 1-Sepsis: presence of two or more symptoms as body temperature of > 38 °C or < 36 °C, heart rate more than > 90/min, tachypnea (respiratory frequency of > 20/min) or mechanical respiratory requirement and white blood cell count of > 12 × 109/L or < 4 × 109/L. 2- Severe sepsis: Sepsis-induced organ dysfunction or hypotension along with sepsis. 3-Septic shock: Severe sepsis along with arterial hypotension (systolic arterial pressure of < 90 mmHg or mean arterial blood pressure of < 65 mmHg). 4- Multiple Organ Dysfunction Syndrome (MODS): >2 organs affected due the infection (Polat et al., 2017).
In general these kind of infections can be caused by different microorganisms, as bacteria (Hato et al., 2019, Rangel-Frausto, 1999), fungi (Ashish Pratap Singh, 2019, Marena et al., 2020), virus (Azamfirei, 2020, Rollé et al., 2016) and parasites (Mcgwire and Satoskar, 2014) and the treatment of each type of infection is performed according the identification of the etiologic pathogen (Hotchkiss and Karl, 2003). Thought the etiologic pathogen is classified as the most prevalent specie identified by laboratorial methods, is common observe the presence of more than one microorganisms associated with the infections, as the presence of more than one type of bacteria, fungus, or the both types in the same time, thus, the therapy success is direct related with the use of a large action spectrum of antibiotics that can be active against different pathogens. This is one of the most problem associated with systemic treatment in hospitals worldwide (Bochud et al., 2001, Terblanche et al., 2007).
In general, the policy of the treatment for patients diagnosed with sepsis, septic shock, severe sepsis and MODS is based in an integrated approach of the combination of the correct diagnostic measures, a rapid initiation of appropriate antimicrobial therapy and supportive care. Antimicrobial therapy with chemotherapy drugs remains the primordial and the first act conducted by health professionals in diagnosed patients. Additionally, the drainage process of the abscesses and removal of infected foreign material or necrotic tissues are also important to the therapy performance aiming the patient recovery (Bochud et al., 2001). The evaluation provide by a laboratorial test is primordially requested to help in the diagnose, aiming to distinguish the suspected infection from other conditions and evaluate the blood oxygenation, the acid–base balance and monitor the organ function for MODS detection. Additionally, hematological, biochemical and microbiological test are essential for a complete and confinable diagnose (Rello et al., 2017).
Important research updates are described in the scientific literature showing the progress and actual outcomes of systemic infections caused by different microorganisms(Rello et al., 2017). Bacteremia is the denomination to describe systemic infections caused by bacterial strains. Some important bacterial species are directly associated in cases of sepsis, septic shock, severe sepsis and MODS (Hato et al., 2019).
In general, Gram positive and Gram negative bacteria are able to start the infection and promote the evolution for a more complicate case, as the sepsis. However, the fact that gram-positive bacteria are better suited to invade tissues than Gram-negative bacteria is known (Minasyan, 2017, Sriskandan and Cohen, 1999). The reason is based in the fact that the lack of endotoxin in the outer cell wall is compensated for by the presence of exposed peptidoglycan and a range of other toxic secreted products. Cell wall components of Gram-positive bacteria may signal via the same receptor as Gram-negative endotoxin (Bassetti et al., 2011).
Infections due to Gram-positive bacteria continue to be one of the leading causes of morbidity and mortality in the Intensive Care Units (ICU) at hospitals worldwide. Several reports have shown an increase in antimicrobial resistance among this type of bacteria, as example is highlighted the species belonging of Staphyloccucus genus (Bassetti et al., 2011). The methicillin-resistant Staphylococcus aureus (MRSA) is one of the most important pathogen associated with invasive bacterial infections, their isolation is associated to high levels of mortality incidence in several countries (Gonzalez et al., 2005, Raghuram et al., 2017, Sa’adu et al., 2019). Streptococcus pneumoniae is another Gram-positive bacteria highly isolated in systemic infection cases, being the lungs as principal affected organ in the host, specially neonates (Hoffman et al., 2003, Malhotra et al., 2012).
Although Gram-positive bacterial species are the most associated in systemic infections, Gram-negative are also observed. Escherichia coli is a bacteria associated whit urinary infections specially. Normally is presented as a normal pathogen in casual urinary infections; however, in some cases, the disease can evolve to more complex cases, as gastrointestinal infections and sepsis(Jauréguy et al., 2007, Mendoza-Palomar et al., 2017). Another important gram-negative bacteria associated with sepsis episode in ICU is the specie Klebsiella pneumoniae, usually is associated with cases of urinary tract infections, pneumonia, and other infections as ocular, liver and others in hospitalized persons whose immunity is compromised by underlying diseases, such as diabetes mellitus (Fang et al., 2007, Lee et al., 2007, Saleem et al., 2013).
Systemic infections triggered by fungal species are aggressive pathological states and lack of therapeutic approaches, and this is mainly due to the rapid expansion of the resistance profile attributed to related species, as well as to the reappearance of strains with intrinsic resistance profile used in practice clinic, as well as some of the members of the Candida genus (Pea and Lewis, 2018). Some species directly are associated with expressive episodes of mortality and morbidly all over the world (Delaloye and Calandra, 2014). This genus is constituted by more than 200 species, in which the C. albicans was during long time described as the more aggressive and prevalent specie involved in cases of superficial (Mayer et al., 2013, Sobel, 2016) and systemic infections (Ahmadi et al., 2019, Mundt et al., 2016, Naglik et al., 2019). However, other species known as non-albicans species has become as expressive problem in cases of candidemia, due the intrinsic resistance profile attributed to available antibiotics in the clinical practice (Jabeen et al., 2019, Lopes Colombo et al., 1999, Miceli et al., 2011).
An important Candida specie is in evidence in the last few years. The specie C. auris was initially of the external auditory canal of a Japanese patient in 2009. Since that, infections caused by this pathogen have become a major threat to public health worldwide (Jeffery-Smith et al., 2018, Satoh et al., 2009). Besides Japan, reports of the isolation of C. auris in patients diagnosed with candidemia have been reported in South Korea, India, Pakistan, Kuwait, Israel, Oman, South Africa, Colombia, Venezuela, the United States, Canada and Europe, including United Kingdom, Norway, Germany and Spain (Jeffery-Smith et al., 2018). In addition, recent studies showed an increase in the geographical scope of spread of this type of infection in Latin America (de Cássia Orlandi et al., 2018, Prakash et al., 2016).
C. auris has been highlighted in the medical field in recent years due to its high virulence, its active role in the establishment of candidemia and its direct relationship with the increase in mortality rates from systemic infections acquired in a nosocomial environment (Chowdhary et al., 2017). The lack of understanding of the mechanisms of progression and resistance of this specie, combined with the lack of specific knowledge on the part of health professionals in the hospital environment, often leads to an erroneous diagnosis, classifying it as another species of the Candida genus, which makes therapy unfeasible, in which reflect directly reflecting on failures in the therapeutic process and enhancing infectious outbreaks in the hospital environment, further increasing mortality rates (Das et al., 2018).
Although bacterial or fungal species are responsible to the infections and are commonly attributed as the cause systemic infections conditions (sepsis), the diseases can also be attributed to presence and viral manifestation. The viral sepsis are regarded as virus-induced direct tissue or cell damage as the influenza virus-induced or pulmonary epithelial damage, that can instead of systemic infestation caused by virus presence (Lin et al., 2018).
Basically, any virus infection can progress and cause viral sepsis in susceptible populations. The specie Herpes simplex virus (HSV) and also enteroviruses are the most common viral causes of sepsis worldwide specially in neonatal patients (Pinninti and Kimberlin, 2018), in addition, the enteroviruses and human parechoviruses are the most common causes of systemic infections in young (Wolthers et al., 2008).
Other viral infections are responsible for important infectious manifestation in all age groups. They are one of the major causes of severe infections and deaths among children younger than 5 years of age, older adults, pregnant and immunosuppressed individuals (Lin et al., 2018). Specially in tropical country as Brazil, the dengue viruses and Chikungunya viruses are a leading cause of sepsis in all age groups (de Cavalcanti et al., 2017, Kumar Sharma et al., 2018).
The infections caused by virus are most of time difficult to treat and diagnose. In nowadays, the world is in complete alert due the pandemic risk of infections caused by a novel virus infection. The current outbreak of the novel coronavirus SARS- Covid 19 (coronavirus disease 2019; previously 2019- nCoV) was initiated in Hubei Province - Republic of China, and since that was spread to many other countries as Italy, USA, Brazil and others (Velavan and Meyer, 2020). On 30 of January 2020, the World Health Organization (WHO) Emergency Committee declared a global health emergency based on growing case notification rates in China and other countries. The number of suspected infections and diagnosed patients all over the world growing every day and also the deaths rate is changing daily (Azamfirei, 2020). The systemic infection caused by this virus is an alarm to all Health authorities, due the uncommon profile of virulence and specially to the rapid diseases progress and difficulties in the therapy(Chang et al., 2020).
Parasitosis are infectious disease that affects animals and humans, occur in tropical and subtropical regions, with poor sanitation as the main factor responsible for ease of transmission. Therefore, it is estimated that around 2 billion people worldwide are infected with parasites, mainly in regions with poor sanitation (Kappagoda et al., 2011, Nutman, 2017, Schär et al., 2013). Parasitic infections can be caused by protozoa and helminths, being responsible for more superficial or deep infections, reaching the systemic level. Organs, such as heart, liver, and lung are the most affected by systemic manifestations (Franco-Paredes et al., 2007, Hidron et al., 2010, Nunes et al., 2017).
Among the main parasitic infections of the bloodstream, we can mention leishmaniasis, Chagas disease, malaria, toxoplasmosis, barbesiose and among others. Leishmaniasis, a disease transmitted by blood-sucking sandflies, where parasites infect macrophages and spread. The consequence of parasitic spread is the development of visceral, cutaneous and mucous leishmaniasis, which result from the replication of the parasite in macrophages (Herwaldt, 1999, Mcgwire and Satoskar, 2014).
Malaria, a systemic infection caused by the parasite of the genus Plasmodium sp, is one of the main parasitic manifestations at the systemic level. The main target is red blood cells and causes haemolytic anemia, also, pulmonary and cerebral changes are also symptoms of malaria. Malaria kills almost 1 million people and causes almost 300 million symptomatic diseases annually, being classified as a worldwide public health problem (Kappagoda et al., 2011, Luzolo and Ngoyi, 2019).
Chagas disease is transmitted by the vector Triatoma and its infection is caused by the presence of the protozoan Trypanosoma cruzi in the bloodstream. It is estimated that about 8 million people worldwide are infected. Having cardiac anomaly as the main clinical manifestation, Chagas disease is considered one of the main systemic parasitic diseases (Andrade et al., 2014).
Finally, many of the parasitic infections are neglected and the difficulty of treatment and diagnosis is very common when it comes to these anomalies. In addition, the number of incidences increases each year, with thousands of deaths every year. Therefore, alternative mechanisms of treatment with new therapeutic and diagnostic approaches need to be studied (Hefnawy et al., 2017).
In general, all types of systemic infections have great difficulties in therapy and maintenance of the health and well-being of the affected patient. The therapies currently available have some limitations such as high cost, high side effects and mainly difficulty in reaching the microorganisms responsible for the infectious condition without affecting other healthy cells in the body(Rhodes et al., 2017).
Thus, nanotechnological drug delivery tools appear as a new hope for the treatment of these patients, providing modern, stable and mainly biocompatible drug delivery systems.
3. Pharmaceutical nanotechnology
Nanotechnology is related to the characterization, production and applications of structures, devices and systems, controlling the shape and size on a nanometric scale. This science is based on the creation and use of materials and devices at the level of molecules and atoms, turning to the design, synthesis, creation, manipulation and application of materials on a nanometric scale. It is becoming increasingly important in fields such as agriculture, engineering, construction, microelectronics and healthcare, etc. In particular, the eyes of science have turned to the application of nanotechnology in the field of health care in order to promote the optimization of diagnoses and therapies. The use of structured materials at the nanoscale are the main members of nanomedicine, a science that remains in constant evolution, and generates satisfactory impacts on the world economy of the pharmaceutical industries (Fachinetti et al., 2018, Hasan et al., 2016, Rizzello et al., 2013a).
In general, all sectors of the Health Sciences are based on the use of pharmacologically active agents (prophylactic and / or therapeutic use) to provide safe bases for the management or reversal of the course of different types of diseases. Notably, it is common to note that several pharmacologically active agents in in vitro biological assays do not effectively perform their pharmacological profile in the body, and this reason is attributed to several factors, such as the route of administration, drug interactions, genetic predisposition, among others (Tibbitt et al., 2016).
Pharmaceutical nanotechnology is classified as an important tool present in the numerous applications of working with compounds in order of nanometers. Pharmaceutical Sciences relies on the use of this tool to discover and optimize the biological potential of active compounds, based on promising drug delivery systems that promote indisputable benefits to bioactive compounds and, as a consequence, generate greater performance (Pelgrift and Friedman, 2013).
One of the major problems related to the loss of pharmacological activity of active compounds is caused by their insolubility and mainly in the route of administration which the drug is intended to be used in the therapy. In addition, factors related to pharmacokinetics, absorption, distribution, metabolism, duration of therapeutic effect, excretion and toxicity are the main problems encountered by pharmaceutical researchers as new therapeutic molecules are discovered, which creates the need to complement and optimize delivery, in order to protect the active ingredients from factors such as early degradation and inactivation of them, as these factors directly affect the safety and efficacy of drugs making therapy unfeasible (Bonifácio et al., 2014).
The structures that make up the drug delivery systems have the necessary characteristics for these controlled delivery systems to be effective, since there is some freedom in the choice of constituents, which allows the active ingredients to be delivered in an appropriate place, keeping them concentration of them at adequate levels for long periods, in addition to promoting the prevention of degradation. These nanostructures allow even greater encapsulation and controlled release efficiency compared to conventional encapsulation systems, in addition to being small enough to be injected directly into the circulatory system and offering the possibility of administration by other routes such as pulmonary, nasal, transcutaneous and oral (Pinto Reis et al., 2006, Rizzello et al., 2013b).
Among the types of nanostructures used by the pharmaceutical industry for the encapsulation of assets, liposomes, polymeric nanoparticles, cyclodextrins and lipid nanoparticles stand out. In addition, nanostructured systems such as micro and nanoemulsion, liquid crystals, among others, are excellent options for incorporating insoluble drugs or with a low selectivity standard. Also listed as nanoparticles in which the assets can be associated are known as metallic type, fullerenes, dendrimers or carbon nanotubes (Aparecido et al., 2019, Bonifácio et al., 2014, Ramos et al., 2018a, Silva et al., 2014).
In the scenario of infectious diseases, the use of nanostructured drug delivery systems behaves as a great ally against several types of microbial infections, and is currently classified as one of the main options for delivery of antimicrobial agents. The use of these structures can direct therapeutic agents to the site of infection, so that more appropriate doses of drug are administered at the infected site, overcoming the existing resistance mechanisms, and with less adverse effects on the patient (Leid et al., 2012).
The use of this tool in medicine goes far beyond the creation of innovative drug delivery systems. In addition to promoting the release of bioactive compounds, the systems are used as sources of heat, light or other substances strategically synthesized in order to reach specific targets, such as the case of cancer cells (Barkalina et al., 2014, Bharali et al., 2011). The versatility of this tool allows particles in nano order to be designed to attract diseased cells, which allows for direct treatment of them, keeping healthy cells safe (Hasan et al., 2016).
Actually, some important drug discovery delivery systems are avaible in the clinical practice for the treatment of microbial infections. Table 1 summarizes the main characteristics of some nanostructured drug delivery systems used nowadays in the clinical practice.
Table 1.
Nano-drug delivery systems available in the clinical practice for systemic microbial infections treatment.
| Clinical product | System | Year of clinical approval | Drug | Administration | System composition | Proportion | Dose | Objective/indication |
|---|---|---|---|---|---|---|---|---|
| Abelcet® | L | 1996 | Amphotericin B | I.V | Dimyristoyl phosphatidylcholine and dimyristoyl phosphatidylglycerol | 7/3 M | 5 mg/Kg/day | Invasive severe fungal infections |
| Ambisome® | L | 1997 | Amphotericin B | I.V | Hydrogenated soy phosphatidylcholine, cholesterol, distearoylphosphatidylglycerol and AmB | 2/1/0.8/0.4 M | 3 mg/Kg/day | Fungal infections |
| Amphotec® | L | 1996 | Amphotericin B | I.V | Cholesteryl sulphate:Amphotericin B | 1/1 M | 1 at 5 3 mg/Kg/day | Several fungal infections |
| Epaxal® | L | 1993 | Inactivated hepatitis A virus | I.M | 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine and dioleoylphosphatidylcholine | 25/75 M | 0.25 mL | Hepatitis A |
| Inflexal® V | L | 1997 | Inactivated hemaglutinine of Influenza virus strains A and B | I.M | 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine and dioleoylphosphatidylcholine | 25/75 M | Single full of 0.5 mL or two dose pf 0.25 mL | Influenza A and B |
| NB-001 | N | – | Oil-in-water emulsion | TP | Soybean oil, water, ethanol, edetate disodium dihydrate Tween® 20 cetylpyridinium chloride | – | NB-001 (0.3%) | Recurrent Herpes Labialis |
N: Nanoemulsion.; L: Liposome.; I.V: Intravenous.; I.M: Intramuscular.; TP: Topical.; M: Molar.
Some systems such as amikacin-loaded liposomes have been evaluated in many clinical studies. A double-blind, phase 2, randomized study to assess the efficacy, safety and tolerability of a daily dose of 590 mg of amikacin versus placebo for 84 days was explored in individuals with lung infection to treat non-tuberculous Mycobacteria in a regimen multiple drug stability. Another phase 2 study that investigated the efficacy, safety and long-term tolerance of a daily dose of 560 mg of inhaled and amikacin-loaded liposome, was administered over 18 months, in patients with cystic fibrosis with chronic infections caused by Pseudomonas Aeruginosa. Therefore, we can verify that clinical studies using innovative systems are showing great promise, as described in Table 2 . These data were collected at United States National Library of Medicine (clinicaltrials.gov) at July 2020.
Table 2.
Nanosystems applied to systemic microbial infections treatment in different clinical stages.
| Drug | Clinical Trial | Indication | Trial Phase | Intervention treatment |
|---|---|---|---|---|
| Amikacin | Liposomal Amikacin for Inhalation (LAI) for Nontuberculous Mycobacteria | Mycobacterium Infections, Nontuberculous | Phase 2 | Ciprofloxacin dispersion for inhalation (Liquid mixture of liposomally encapsulated and un encapsulated ciprofloxacin) Placebo: Liquid formulation of empty liposomes. |
| Amikacin | Study to Evaluate Ecacy of inhaled amikacin loaded-liposome combined with multi-drug regimen, Compared to Multi-drug Regimen Alone (CONVERT) | Mycobacterium Infections, Nontuberculous | Phase 3 | Liposomal Amikacin for Inhalation, 590 m.g |
| Amphotericine B | Ambisome® Preemptive Treatment of Multiple Candida Colonization in Sepsis Patients (AMBIDEX) | Candida | Phase 4 | Amphotericine in liposome (Ambisome®); 2 IV infusions separated by one week 10 mg/kg per injection. |
| Micafungin And Amphotericin B | Micafungin Versus AmBisome in Invasive Candidiasis and Candidemia | Candidiasis | Phase 3 | Micafungin IV; Liposomal Amphotericin B IV. |
| Amphotericine B | CRITIC - Treatment of Candidemia and Invasive Candidiasis (CRITIC) | Candidemia Invasive Candidiasis | Phase 4 | AmBisome® 2 mg/kg/day in a unique daily IV administration. |
| Voriconazole; Amphotericin B; Fluconazole | A Clinical Study Intended To Compare Treatment With Voriconazole To Treatment With Amphotericin Followed By Fluconazole In Patients With Candidemia, A Serious Fungus Infection Of The Blood. | Candidiasis | Phase 3 | VFEND® I.V., Oral; Conventional amphotericin B; Diflucan IV, oral. |
Based on the countless benefits presented by pharmaceutical nanotechnology, below are presented the main drug delivery systems used in the treatment of systemic infections, as well some important scientific results that used pharmaceutical nanotechnology as a tool for the treatment of these infections.
3.1. Metallic nanoparticles (MNPs)
Metallic nanoparticles (MNPs) are clusters containing from a few to millions of atoms, MNPs exhibit fascinating properties that are quite different from those of individual atoms or bulk materials. MNPs have a size between 1 and 100 nm, may be smaller than the organic nanoparticles, but have a higher encapsulation efficiency (Mahajan et al., 2012).
The main characteristics of MNPs are large surface area to volume ratio as compared to the bulk equivalents, large surface energies, the transition between molecular and metallic states providing specific electronic structure (local density), plasmon excitation, quantum confinement, short range ordering, increased number of kinks, a large number of low coordination sites such as corners and edges, having a large number of ˝danglingbonds˝ and consequently specific and chemical properties and the ability to store excess electrons (Mahajan et al., 2012).
The synthesis of MNPs can be performed by “bottom-up” and “top-down” approaches. The first refers to the aggregation atom by atom, in which the particle size gradually increased, providing uniform characteristics combined with fewer defects. This technique, also called “constructive approach” is the most commonly used when a high homogeneity of the material is necessary (Khan et al., 2017). The second is an approach “destructive” which occurs by reduction of large portions of material into small particles using a variety of techniques such as milling, spray, laser and electro-explosion (Khan et al., 2017). The most used routes for the synthesis of MNPs, using the bottom-up approach, are the chemical, biological and green synthesis pathways (Barbosa et al., 2019).
The chemical route is the most used today, and is even applied to industrial methods of nanoparticle production because of its simplicity, reproducibility, low cost and high yield (Banach and Pulit-Prociak, 2016). The process is based on the reduction of metal ions to zero oxidation state, using a metal precursor and reducing agents, as well as surfactants to stop the reaction and stabilize colloidal suspension nanoparticles (Marcato et al., 2015). Biological synthesis uses fungi, yeast, bacteria, enzymes and algae to form nanoparticles in extra or intracellular medium (Banach and Pulit-Prociak, 2016). Extracellular quinone and the enzyme reductase present in organisms are responsible for the synthesis, that is, only microorganisms that have these substances are able to synthesize nanoparticles (Marcato et al., 2015). The process is advantageous because it consumes low amount of energy and reagents, besides being environmentally friendly (Kuppusamy et al., 2016). In this approach, the addition of stabilizing agents is not necessary, since the proteins produced by the microorganism act to maintain the suspension in colloidal form (Marcato et al., 2015).
MNPs can be characterized by ultraviolet–visible spectroscopy (UV–Vis), Fourier-transform infrared spectroscopy (FTIR), Transmission electron microscope (TEM), Scanning electron microscopy (SEM), Atomic force microscopy (AFM), X-Ray diffraction (XRD), Energy-dispersive X-ray spectroscopy (EDX), Extended X-ray absorption fine structure (EXAFS) and X-ray photoelectron spectroscopy (XPS) (Venkatesh, 2018).
The advantages of the use of MNPs are attributed to the enhancement of the Rayleigh scattering, surface enhanced Raman scattering, strong plasma absorption, biological system imaging and determine chemical information on metallic nanoscale substrate. While the disadvantages are particles instability, impurity, immunogenic, toxicity issues and difficulty in synthesis (Venkatesh, 2018).
Colloidal solutions closely depend on the interaction between particles and medium to remain stable. In the solution there may be the union of nanoparticles forming clusters, either by action of interferents in the solution or by gravity. When the clusters come together and the density increases coagulation occurs, an irreversible process. The DVLO theory (referring to the initials of its creators, Derjaguin, Verway, Landau, and Overbeek), which deals with colloidal stability, proposes that an energy barrier resulting from repulsive forces prevent two particles from approaching and coming together. However, if the particle has enough energy to break this barrier the attractive force will pull them and put them in irreversible contact. The forces that influence these processes can be: electrostatic, electromagnetic and/or Van der Waals forces. For solution stabilization there are two basic mechanisms: Electrostatic stabilization: the surface of nanoparticles have charges that will interact with themselves or with the medium charges, they can be attracted or repelled; Steric stabilization: passivating ligands such as polymers, molecules and organic complexes that prevent particles from approaching (Ramos et al., 2018a, Venkatesh, 2018).
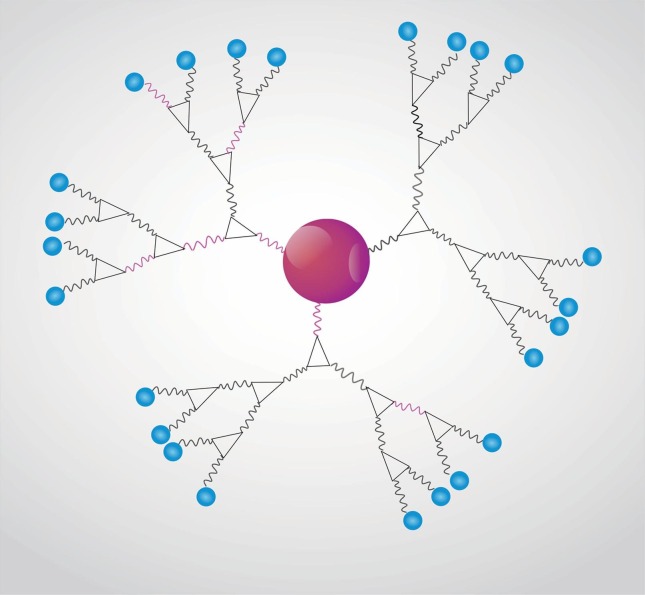
Fig. 1 shows sterically stabilized metallic nanoparticles (MNPs) with polymers on their surface.
Fig. 1.
Representative scheme of a colloidal solution of sterically stabilized metallic nanoparticles with polymers on the nanoparticle surface.
The toxicity of metallic nanoparticles for humans is still unknown, there are still many studies in vitro and the big problem is that its bioaccumulation occurs in tissues and organs (Yao et al., 2019). With regard to immunogenicity, there are few studies in the literature relating the physical-chemical properties of metallic nanoparticles and their potential effects on the immune system in order to have a more precise understanding of activity structure, more studies are needed (Engin and Hayes, 2018).
A property of these nanoparticles, MNPs, that may be advantageous in treating systemic infections is their size, generally smaller than 20 nm, so that they circulate easily in the bloodstream and are easily eliminated by renal excretion (Mahajan et al., 2012).
Selvaraj et al. (2015) investigated if cerium oxide (CeO2) nanoparticles (NPs) could be used for treatment of severe sepsis. The CeO2 NPs were obtained by NanoScale Active with 99% purity. The spherical shape was observed for CeO2 NPs by TEM and AFM and the mean hydrodynamic diameter obtained was 140 ± 53 nm by dynamics light scattering (DLS). The effect of CeO2 NPs on growth on the Gram-negative Escherichia coli (ATCC 35150) and the Gram-positive Staphylococcus aureus (ATCC 29213) was determined by disc diffusion and the authors observed that CeO2 NPs inhibited E. coli growth and colony-forming units in concentrations of 50 or 100 mg mL−1. Polymicrobial sepsis was induced in male sprague–dawley rats weighing 300 to 350 g by intraperitoneal injection of cecal material (400 mg kg−1 in 5 mL kg−1 of 5% sterile dextrose water) obtained from uninfected rats. The animals were divided into four groups: vehicle control group (group 1), CeO2 NPs treatment with a dose of 3.5 mg kg−1/intravenous (group 2), cecal inoculate group (group 3) and cecal inoculation with 3.5 mg kg−1 of CeO2 NPs (treatment group–group 4). The group 3showed several signs of septic shock and the group 2 that received a dose of 3.5 mg kg−1 intravenous had a 48 h survival increase from 20 to 90%. The authors observed that CeO2 NPs treatment decreased sepsis-induced mortality and organ damage. The treatment also decreased the serum IL-6 levels at 6 h, as well as blood urea nitrogen and inflammatory markers. CeO2 NPs were still able decreased Lipopolysaccharide-induced cytokine release and p65-nuclear factor kB (NF-κB) activation in cultured RAW264.7 cells. The findings of this study indicate that CeO2 NPs may be useful for the treatment of sepsis. Given the data obtained in this study, it can be stated that CeO2 NPs can be used to treat sepsis.
A study if the gold nanoparticles (AuNPs) could attenuate bacterial sepsis was performed by Taratummarat et al. (2018). AuNPs were synthesized following the standard by Chen et al. (2013) using citrate-reduction method. AuNPs were characterized with spherical shape and average size of 21.3 ± 0.7 nm by SEM, and the size was confirmed by UV–Vis. The authors studied the administration of AuNPs with antibiotics (imipenem/cilastatin) in a mouse model of bacterial sepsis, cecal ligation and puncture (CLP). CLP was performed as described in literature (Leelahavanichkul et al., 2014). After surgery and 6 h after, the animals received an analgesic drug (tramadol) for analgesia at 10 mg kg−1 (in 0.2 mL of normal saline solution - NSS) intraperitoneally and an antibiotic (imipenem/cilastatin) was administered subcutaneously in the dose 14 mg kg−1 also in 0.2 mL of NSS. The analgesic and antibiotic were administered of 3–5 days once a day. To assess the survival of mice after surgery, the animals received AuNPs in the doses 3.925, 7.85, 15.7 and 31.4 μg/gram body weight also diluted in 0.2 mL of NSS and it was administered through the tail vein at post-operation. The control group received only analgesic and antibiotic. For bacterial analysis was collected whole blood from animals. At a dose of 7.85 μg/gram body weight the AuNPs improved survival of mice, blood bacterial (E. coli was analysed) burdens, but, only after 4 h incubation, ie, a weak antibiotic effect nanoparticle. Levels of blood urea nitrogen (BUN) and serum creatinine (Scr) evaluate the kidney function, and in this study AuNPs improved this function when compared to the control group, because the levels of BUN ad Scr were reduced. And the liver injury was evaluated by alanine transaminase (ALT) which also improved. The inflammatory cytokines determined were TNF-α, IL-6, IL-1β and IL-10, and data showed an improvement in the levels of them. AuNPs reduced M1 macrophages, but increased M2 macrophages. Analyzing the data obtained, it can be stated that AuNPs can be used as adjuvant with an appropriate antibiotic, as they were able to attenuate sepsis.
Chen and Xu (2018) used eco-friendly method (Camellia sinens leaves extract extract) to synthesize CeO2 CSNPs. The techniques used for characterization were UV–Vis, FTIR, XRD, SEM, EDX and TEM. The formation of MNPs was observed by UV–Vis with a high absorbance and intense surface plasmon resonance (SPR) at 285 nm. FTIR analysis revealed that phytochemicals (proteins, phenolic compounds, alkaloids and terpenes of the plant extracts) are very frequently found in association with NPs, acting as capping agent in nanoparticles synthesis. The EDX analysis confirmed the purity of CeO2 CSNPs. CeO2 CSNPs are well arranged and has spherical shape by SEM. The TEM image showed an almost hexagonal shape with slight variations in thickness, given in accordance with SEM. In this work the authors used the model of sepsis induced by lipopolysaccharide (LPS) to male Sprague Dawley rats. LPS when injected intravenously will induce the sepsis symptoms in animals and the parameters are monitored. The dose of 0.5 mg kg−1 of CeO2 CSNPs was intravenously administered to each animal and it was found that the death rate was reduced from 73% to 10%, the respiratory rate and blood pressure also were checked and they were decreased. The body temperature was maintained. The parameters related to hepatic damage, serum cytocines/chemokines, and swelling indicators were reduced, which shows that the rats recovered from sepsis. The results showed that CeO2 CSNPs can be applied as curative agent for hepatic sepsis.
Antimicrobial peptides (AMPs) conjugated with gold nanoparticles (AuNPs) have been studied by Rai et al. (2016) for a systemic infection model. The formulation consists of Au core and a hydrophilic cationic peptide shell, that is cecropin melittin with cysteine (CM-SH) as AMPs, then called, CM-SH-AuNPs. The authors obtained nanoparticles with a diameter of 14 nm and with positively charged +28 ± 2 mV, the conjugation of the peptide was confirmed by the FTIR technique by the presence of the amide-I band at 1646 cm−1. Cecal ligation and puncture (CLP) mouse model of experimental sepsis were studied by authors according to the literature (Rittirsch et al., 2009), CM-SH-AuNPs were injected intraperitoneally at a total dose of 1 mg per animal, and they observed that this sample reduced the bacteria concentration 2 logs in the bloodstream when compared with animals treated only with AuNPs.
El Din et al. (2016) studied silver nanoparticles (AgNPs) combined with visible blue light as potential antimicrobial agent against resistant pathogens. AgNPs were obtained acoording to the literature (Métraux and Mirkin, 2005), the antimicrobial activity alone AgNPs against Pseudomonas aeruginosa was determined, the minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) obtained were 8 mg mL−1, respectively. The effects of the AgNPs in combination with blue light at 460 nm and 250 mW for 2 h were analyzed by TEM and the authors observed that the combination was able to lyse most cells at the end of the experiment, thus, as it was able to prevent colonization and sepsis burn wounds in mice.
Kalita et al. (2018) studied a hybrid fabricated through surface functionalization of lysozyme capped gold nanoclusters and β-lactam antibiotic ampicillin (AUNC-L-Amp). The ampicillin (Amp) conjugation was confirmed by absorption peak in UV–Vis spectra in 343 nm. Transmission electron microscopy (TEM) analysis was performed and showed that AUNC-L-Amp that nanoparticle are solid, spherical and ultra-small, with the diameter ranging from 1 to 5 nm, while, the measure by dynamic light scattering (DLS) was 11 nm, zeta potential was 26.7 mV. Hemolytic potential and the cytocompatibility were analysed and the sample (AUNC-L-Amp) exhibited negligible hemolysis at 0.01–0.1 mg mL−1 and for the MTT assay shows higher viability (80.7%) at up 250 µg mL−1 against L929 cells compared to free-amp (78.4%). The authors also studied the efficacy of AUNC-L-Amp in a systemic Methicillin Resistant Staphylococcus aureus (MRSA) murine infection model, an intraperitoneal injection MRSA culture was given to Swiss albino male mice, after infection, the animals were treated with intraperitoneal injection of 25 mg kg−1 of AUNC-L-Amp, hybrid fabricated was able eliminates the systemic MRSA infection.
SPIONs (Superparamagnetic iron oxide (γ-Fe2O3) nanoparticles) was provided by Professor Ning Gús team of Southeast University, China, and the nanoparticles were studied in a murine model of LPS-induced sepsis by Xu et al. (2019b). SPIONs were characterized by TEM and the results show that the nanoparticle has average core size 6.5 nm, while by the technique of DLS was 50 ± 2.2 nm. The mice received a 5 mg kg−1 intraperitoneal dose of lipopolysaccharide (LPS) and after 4 h they were treated with SPIONs at a dose of 4 mg Kg−1 intravenously, and after 24 h they were euthanized and evaluated. The authors observed that SPIONs are able to block the absorption of inflammatory cells in the liver, as well as increased levels of interleukin-10 (IL-10) in hepatic macrophages, and SPIONs were also able to revoke LPS-induced sepsis in mice showing that the defensive effect is dependent on IL-10+ macrophages.
Table 3 summarizes the results of several studies related to the application of MNPs applied to the treatment of systemic infections.
Table 3.
Metallic Nanoparticles applied to the treatment of systemic infections.
| Formulation name | Active Principle | Composition | Therapeutic target | Route of administration | Reference |
|---|---|---|---|---|---|
| CeO2 NPs | CeO2 | CeO2 | Polymicrobial sepsis | Intravenous | Selvaraj et al., 2015 |
| CM-SH-AuNPs | Au | Au, cecropin, melittin and cysteine | Polymicrobial sepsis | Intraperitoneal | Rai et al., 2016 |
| AuNPs | Au | Au | Polymicrobial sepsis | Intravenous | Taratummarat et al., 2018 |
| CeO2 CSNPs | CeO2 | CeO2 | lipopolysaccharide (LPS) induced sepsis | Intravenous | Chen and Xu, 2018 |
| AgNPs | Ag | Ag | Sepsis by P. aeruginosa | Topical | El Din et al., 2016 |
| AUNC-L-Amp | Au | Au | Systemic MRSA | Intraperitoneal | Kalita et al., 2018 |
| SPIONs | γ-Fe2O3 | γ-Fe2O3 | Lipopolysaccharide (LPS) induced sepsis | Intravenous | Xu et al., 2019a, Xu et al., 2019b |
CeO2 NPs: cerium oxide nanoparticles; CeO2: cerium oxide; AuNPs: gold nanoparticles; Au: gold; CeO2 CSNPs: cerium oxide nanoparticles from Camellia sinens; CM-SH: cecropin melittin with cysteine; LPS: lipopolysaccharide; AgNPs: silver nanoparticles; Ag: silver; AUNC-L-Amp: lysozyme capped gold nanoclusters with β-lactam antibiotic ampicillin MRSA: Methicillin Resistant Staphylococcus aureus.
By analyzing the presented research works it is possible to conclude that MNPs are able to attenuate sepsis, as well as improve its symptoms, improve survival rate, among others, however, studies in the literature are scarce and recent.
3.2. Liposomes
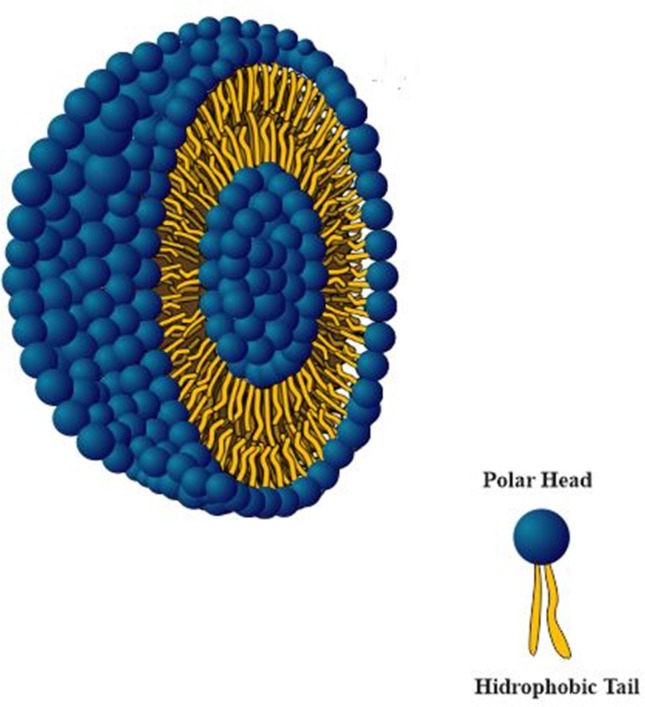
Liposomes emerged in the early 1960s and were used in plasma membrane model studies, as they are composed of lipid bilayers separated by an aqueous content. Liposomes are characterized as colloidal dispersions composed mainly of phospholipids that form lipid bilayers in aqueous medium, which, in turn, tend to group together forming vesicles, incorporating part of the medium in which they are inserted (Torchilin, 2005). This is a thermodynamically favorable formation, reinforced by hydrogen bonds, van der Waals forces and other electrostatic interactions (Pattni et al., 2015). Due to its structural properties, such as submicrometric or nanometric size, high biodegradability, low toxicity, ability to cross membranes and compatibility with hydrophilic and hydrophobic drugs, the use of this type of drug delivery system is highly appreciated by several health areas (Rideau et al., 2018). Fig. 2 represent the liposome morphology and internal and external interface.
Fig. 2.
Representative scheme of the external and internal interface structure of a liposome.
Some of the first demonstrations of improving in vivo activity in animal models of drugs encapsulated in liposomes demonstrated the ability of these structures to optimize cancer therapy. Since then, new research using liposomes as structures capable of improving therapeutic performance has been evidenced (Allen and Cullis, 2013, Dinardo and Perl, 2019).
This system allows the prolonged release of the drug, reducing side effects and increasing its bioavailability (Allen and Cullis, 2013). The stability of liposomes can be affected by chemical, physical and biological processes and may have a short half-life and several parameters influence their instability, including the size of the vesicles, the nature of the lipid components, the electrical charge on the surface, extravasation of its content over time, recognition by the complement system and by macrophages, which influences the elimination of liposomes from the circulation (Li et al., 2017).
Another advantage attributed to liposomal systems in relation to other encapsulation strategies is the use of biocompatible and biodegradable raw materials, which reduces the effects of toxicity and improves their interaction with different application sites (Pattni et al., 2015). The excellent applicability of this type of drug delivery system comes from the ability to trap the bioactive agents of interest in the internal or external region, which are released after the interaction of the lipid layers with the target site, such as bacteria, fungi and cells (Sharma et al., 2018).
According some important reports published in the scientific literature, liposomes do not have immunogenic activity, being considered as promising vaccine vehicles (Batista et al., 2007, van Rooijen and van Nieuwmegen, 1983). The cytotoxic activity of liposomes is mainly explained to the components, such as surfactants, present in its formulation. These components have an affinity for the lipids present in the cell membrane, exerting cytotoxic activity. Also, articles highlight the importance of the liposomal system for antitumor use, mainly due to the electrostatic attraction between liposomes and the cell membrane (de Lima Luna et al., 2016, Pereira et al., 2016).
The versatility of incorporating bioactive principles with different solubility profiles is one of the main benefits attributed to liposomes. Based on this property, the use of different drugs with different solubility profiles in the same liposome has been observed in recent years, which is known as a co-encapsulation technique (Park et al., 2012). The use of this technique has already provided great advances in areas such as microbiology (Halwani et al., 2008, Wang et al., 2018), oncology (Przespolewski et al., 2018) cardiology (Chen et al., 2012) and food (Tavano et al., 2014) due the ability to use two drugs with different mechanisms of action, which combined with the properties biocompatibility with cell membranes attributed to liposomes, start to exert a direct action and with low toxic effects to the organism. Allied to the biocompatible profile, it is worth noting that lesser amounts of drugs can be used (Al-Jamal and Kostarelos, 2011).
The versatility of these nanotechnological drug delivery systems is appreciated in the pharmaceutical area, as in antibiotics research. Thus, research groups employ liposomes in important studies aiming to control several microorganisms associated with systemic infections.
Hardeep et al. (2017) evaluated the efficiency of Influenza immunization by modified liposomes carrying the sublingual Toll-like receptor-4 agonist CRX-601. Three types of liposomes were produced and characterized as: Unmodified liposomes containing of 40 mg / mL phospholipid, 10 mg/mL cholesterol and 2 mg/mL CRX-601, phospholipid-PEG modified liposomes composed of 1.5 to 25 mol% 1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (PEPEG2K) and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (PE-PEG5K); modified liposomes containing 5, 15 and 25 mol% of Pluronics L64, F68 and F127; and chitosan modified liposomes consisting of chitosan glycol trimethyl ammonium iodide and mixed with CRX-601 liposomes in varying proportions. The test was performed by assessing influenza specific antibody responses determined by two independent immunoassays, the influenza haemagglutination inhibition assay (HIA) and the enzyme linked immunosorbent assay (ELISA) and using an in vivo murine model. The data obtained showed that phospholipid-PEG conjugated liposomes presented more effective results when compared with Pluronic copolymers in the generation of stable and neutral surface liposomes. Immunization was more efficient for CRX-601 adjuvant-bearing liposomes. In addition, chitosan-containing liposomes showed a more efficient immune response to the virus. Finally, the results provide important information on the administration of liposome-modified sublingual vaccines to improve immunization against influenza virus.
Polymyxin B-related colistin is an antibacterial drug of the bactericidal peptide group against many gram-negative bacilli, but its cytotoxicity rate makes its use limited (Sarkar et al., 2007). Aiming to control at these limitations, Li et al. (2016) encapsulated colistin into liposomes and evaluated the systemic availability and microbial action against E. coli. The difficulty of colistin permeability was also taken into consideration in this work. To improve permeability, liposomes were modified with sodium cholesteryl sulfate (Chol-SO4-) to improve colistin charge by increasing the electrostatic attraction of colistin with the microbial phospholipid bilayer. Two types of liposomes were developed: Chol-SO4- (CCL) colistin retained liposomes and Chol-SO4-/ colistin (CCCL) coated liposome. The results showed that both liposomes increased colistin concentration in the blood, showing efficiency in locating infectious targets and prolonging colistin time in the bloodstream.
Meers et al. (2008) evaluated the action of liposomal amikacin in patients with chronic pulmonary infection against inhaled Pseudomonas aeruginosa. Flowering-labelled liposomes consisted of 2 g lipid, 2: 1 by weight 1,2-dipalmitoyl-sn-glycerol-3-phosphocholine (DPPC) and cholesterol. Liposomal amikacin, on the other hand, presented the same constituents with a difference only in the concentration scale and with a variation of 20 to 75 mg/mL of amikacin. For antimicrobial analysis, groups of female rats with intratracheal P. aeruginosa infection were used, followed by inhalation of liposomal amikacin. The data showed a better action of liposomal amikacin when compared with the conventional drug. Thus, the incorporation of amikacin into liposomes inhaled may represent an alternative for the treatment of chronic pulmonary infections against P. aeruginosa.
Carvalheiro et al. (2015) evaluated the action of dinitroaniline (TFL-A), an important drug to treat diseases caused by parasites of the genus Leishmania, incorporated in liposomal system. Liposomes consisted of various lipid components such as PC F1 and F2, DOPG, DOPC and DPPG and TFL A (335–450 µg / mL) dissolved in chloroform. For in vivo testing, groups of Visceral Leishmaniasis mice followed by treatment with intraperitoneal liposomal TFL A were used. The results showed that liposomal TFL A reduced amastigote loads in the spleen by up to 97%. Thus, drug release systems assist in the treatment of parasitic diseases caused by Leishmania.
Guo et al. (2014) investigated the activity of a liposome-encapsulated glucocorticosteroid, β-metasone hemisuccinate (nSSL-BMS) against the parasite Plasmodium berghei. The liposomes were composed of HSPC / cholesterol / PEG-DSPE at 55: 40: 5 M ratio with 250 mM calcium hydration, 82.2 ± 0.73 nm and drug composition molar ratio of 0.17 ± 0.06. Female C57BL mice treated with 20 mg / kg nSSL-BMS were used for the assays. The results showed that the encapsulated drug provided a significant reduction in the proinflammatory response that would cause cerebral malaria. In addition, it avoids immunopathological effects, assisting in antiplasmodial treatment.
In order to evaluate new therapies for cerebral malaria, Waknine-Grinberg et al. (2013) also used the liposomal system as a controlled and selective system against P. berghei in an experimental mouse model under the same conditions of liposome preparation and administration approach addressed by Guo et al. (2014). The difference is the incorporation of two drugs, β-methasone hemisuccinate (BMS) and methylprednisolone sodium hemisuccinate (MPS) administered at 5, 10 or 20 mg / kg free or incorporated. The data proved effective for treating cerebral malaria by eliminating parasites and preventing long-term cognitive impairment.
With the emergence of penicillin-resistant bacterial strains in 1941, the need for the production of a drug that would attack the resistant penicillase producing strains, known as β-lactamases, became of great importance. Then, in 1960, methicillin emerged as an antimicrobial alternative; however, the emergence of methicillin-resistant Staphylococcus aureus (MRSA) was reported in the same year. Today, MRSA is the most worrying nosocomial bacterial pathogen in the clinical area (Stryjewski and Chambers, 2008).
MRSA is more resistant to vancomycin, considered the gold standard of treatment against this microorganism. For effective treatment, Sande et al. (2012) investigated the action of vancomycin incorporated into a liposomal structure against MRSA. Dicetylphosphate (DCP) liposomes had as components DSPC, DCP, and cholesterol in a 7: 2: 1 M ratio (71.5 mg DSPC, 14.1 mg DCP, and 5 mg cholesterol). Unincorporated vancomycin 50 mg / kg and vancomycin DCP were administered by intraperitoneal route in mice. Results showed that the efficacy of incorporated vancomycin compared to free vancomycin was improved. In conclusion, encapsulation of the antibiotic in the liposomal system may improve vancomycin anti-Staphylococcal efficacy against systemic infections.
Meng et al. (2016) evaluated the activity of clarithromycin incorporated into two different liposomes. In this study, the authors used wheat germ agglutinin (WGA), a cytinvasive and cytoadhesive lectin, for liposomal functionalization. WGA-modified liposome (WGA-Lip) and unmodified liposome (PEG-Lipid) were composed of soybean phosphatidylcholine, cholesterol, cholesteryl 3β-N-dimethylamino-carbamate hydrochloride and clarithromycin. The administration was performed by intraperitoneal injection of 10 mg / kg clarithromycin in a male and female CD-1 ICR rat model. The data showed the efficiency of WGA-Lip with clarithromycin for the smaller amount of MRSA colonies found in kidney and spleen when compared to unincorporated clarithromycin. In addition, intracellular MRSA localization was higher in macrophages in contact with WGA-Lip than PEG-Lip. Thus, the liposomal system has benefits for the treatment of infectious diseases caused by systemic MRSA, besides increasing the immune response of macrophages against the pathogen.
Veloso et al. (2018) studied the antifungal action of voriconazole (VCZ) incorporated into liposomal structure (LVCZ) against Candida albicans and Aspergillus sp. The liposomes were composed of soybean phosphatidylcholine, cholesterol, VCZ and alpha tocopherol dissolved in chloroform. An intravenous injection of 10 mg / kg LVCZ was administered to rats. The results showed that LVCZ presented a more efficient systemic antifungal activity when compared to free VCZ. In addition, incorporation promoted better drug protection against biological degradation, leading to a safer and more effective therapeutic platform against systemic fungal infections.
Table 4 summarizes the results of several studies related to the application liposomes in the treatment of systemic infections.
Table 4.
Liposomes applied to the treatment of systemic infections.
| Formulation | Active principle | Composition | Therapeutic target | Route of administration | Reference |
|---|---|---|---|---|---|
| Unmodified liposomes, Phospholipid-PEG modified liposomes, Pluronic modified liposomes and Chitosan coated liposomes | synthetic toll like receptor-4 agonist, CRX-601 | Unmodified liposomes : 2 mg/mL CRX- 601, 10 mg/mL cholesterol, and 40 mg/mL of phospholipid Phospholipid-PEG modified liposomes: 1.5 and 25 mol% PEPEG2K or PE-PEG5K Pluronic modified liposomes: 5, 15 and 25 mol% of Pluronics L64, F68 and F127 Chitosan coated liposomes: chitosan glycol trimethyl ammonium iodide with CRX-601 liposomes at varying ratios |
Influenza virus | sublingual | Hardeep et al., 2017 |
| CCL and CCCL | Colistin |
CCL: 8 mg colistina, 1 mL distilled water (ddH2O), 200 mg Lipoid ®S75 and Chol-SO4 CCCL: 8.0 mg colistina, outer layer: DSPC, DOPE, PEG-PE and CH (3:3:2:4 or 4:3:1:4) inner layer: DODAP and DOPE (9:1) |
E. coli | Intravenous | Li et al., 2016 |
| Fluorescently labelled liposomes and Liposomal amikacin | Amikacin | 0.2 wt% of [diI(3), 2 g of lipid 2:1 (DPPC and cholesterol, respectively) | P. aeruginosa | Inhaled | Meers et al., 2008 |
| TFL Lipossome | dinitroaniline (TFL-A) | lipidic components: PC F1 and F2, DOPG, DOPC and dppg (10 µmol/ mL) and TFL (335–450 µg/mL) | Leishmania infantum | intraperitoneal | Carvalheiro et al., 2015 |
| nSSL-BMS | glucocorticosteroid, β-methasone hemisuccinate | HSPC/cholesterol/PEG-DSPE at a mole ratio of 55 : 40 : 5 hydrated with 250 mM calcium acetate | Plasmodium berghei | Intravenous | Guo et al., 2014 |
| nSSL- MPS and nSSL - BMS | glucocorticoid prodrugs methylprednisolone hemisuccinate and β-methasone hemisuccinate | HSPC/cholesterol/PEG-DSPE at a mole ratio of 55 : 40 | Plasmodium berghei | Intravenous | Waknine-Grinberg et al., 2013 |
| DCP Vancomycin | Vancomycin | DSPC, DCP and cholesterol in a molar ratio of 7:2:1 (71.5 mg of DSPC, 14.1 mg of DCP and 5 mg of cholesterol) | Staphylococcus aureus (MRSA) | Intravenous | Sande et al., 2012 |
| WGA-Lip PEG-Lip |
Clarithromycin | PC, cholesterol, DC-Chol and clarithromycin | Staphylococcus aureus (MRSA) | Intravenous | Meng et al., 2016 |
| LVCZ | Voriconazole | PC, cholesterol, VCZ, and alpha-tocopherol, (1.0:0.5:0.11:0.05 M ratio) | Candida albicans and Aspergillus sp. | Intravenous | Veloso et al., 2018 |
1,2-distearoyl-snglycero-3-phosphoethanolamine sodium salt (PE-PEG2K) and N1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, sodium salt (PE-PEG5K), sodium cholesteryl sulphate (Chol-SO4-) distilled water (ddH2O), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2- distearoyl-sn-glycero-3-phosphoethanolamine-N [methoxy(polyethylene glycol)-2000] (mPEG-PE), 1,2-dioleoyl-3-dimethylammonium-propane (DODAP), and cholesterol (CH), 1,10 -dioctadecyl-3,3,30,30 -tetramethylindocarbocyanine perchlorate [diI(3), egg phosphatidylcholine (PC F1), addition of PG promotes good incorporation to PC (PC F2), dioleoylphosphatidylcholine (DOPC), dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylglycerol (DPPG), Hydrogenated soybean phosphatidylcholine (HSPC), distearoyl-snglycero-3-phospho-ethanolamine sodium salt (DSPE), Dicethylphosphate (DCP), Cholesteryl 3β-N-di-methyl-amino-ethyl-car bamate hydrochloride (DC-Chol) and soybean phosphatidylcholine (PC).
3.3. Microemulsion (ME)
The term microemulsion (ME) was first used by TP Hoar and JH Shulman, professors of chemistry at the University of Cambridge, in 1943, who today have become a promising alternative in the scientific field, being targeted by many researchers in the field to improve permeability and penetration of new drugs, and is considered a technological hope for improving the permeation of hydrophobic and hydrophilic drugs (Callender et al., 2017, Franklyne et al., 2016, Lawrence and Rees, 2012, Oliveira et al., 2015).
MEs are metastable colloidal droplet systems that exhibit a wide variety of structures, which involve the formation of one, two or three phases in equilibrium. Each of these phases may have very different types of nanoscale morphology of very different geometries which are, for example, cylindrical, plane-like, sponge-like structures, crystalline liquids or spherical hexagons depending on the proportions of the phase components formed by a liquid system dispersed within another immiscible liquid. ME are characterized as thermodynamically stable system with nanoscale droplets that form in the internal phase by two immiscible liquids (either water-on- oil W / O or oil-on-water - O / W) with very small droplets (Anton and Vandamme, 2011, Dong et al., 2011, Ghosh et al., 2013) with addition of the surfactant, the hydrophilic portions of the molecule orientate with the aqueous phase molecules and the hydrophobic portions of the molecule orientate with the oil phase molecules. As a result, the overall interfacial tension is reduced. Without a surfactant, it is almost impossible to sufficiently stabilize this system. Where very low interfacial stresses are desired, co-surfactants may be added. Co-surfactants act in concert with surfactants to further reduce interfacial tension and introduce an element of flexibility into the interfacial film. This allows the system to adapt a wider range of curvature values over a wide range of droplet formation conditions (Callender et al., 2017).
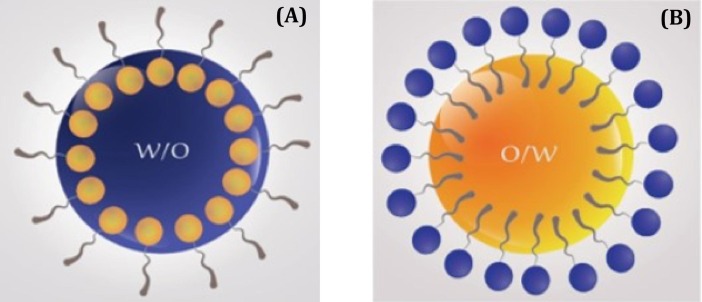
Fig. 3 represent the droplets present in water-on-oil (W / O) oil-on-water – (O / W) ME.
Fig. 3.
Schematic representation of droplets presented in water-on-oil (A) and oil-on-water (B) microemulsion.
The high solubilization of drug molecules makes ME potential antimicrobial agents, improving the bioavailability of substances, decreasing surface tension and resulting in high absorption and permeation because it is a small drop (Bonifácio et al., 2015, Franklyne et al., 2016). This activity of ME is due to the reduced droplet size and increased droplet surface area available to interact with microorganisms. Diluting with water leads to considerable structural and morphological changes in ME droplets and influences their efficiency against microorganisms (Ghosh et al., 2013).
In addition to the possible administration of the drug in liquid form and contributing to a faster absorption and avoiding disintegration, the ME acts in the optimization of the hydrophilic and hydrophobic drug solubility, protecting it against the environment, release control, long shelf life and facilitating the incorporation of poorly soluble drugs. However, although there are numerous advantages, ME have certain disadvantages such as high cost, difficulty in extending processes.
The main components of MSs are surfactants and co-surfactants. These components have cytotoxic effects when evaluated and this is due to the action against the lipid layer present in the cell membrane. It is believed that the cytotoxic effects are due to the presence of these components, mainly due to the lipid action and the difficulty in exchanging substances essential for cell survival. These cytotoxic effects can contribute to the evaluation of antitumor and antimicrobial action. However, cytotoxic effects have also been observed in normal cells (Arechabala et al., 1999, Gundogdu et al., 2013, Sieniawska et al., 2019).
Emulsions, especially ME, have recently been studied for future carriers with potential adjuvant vaccine candidates. Recent studies show that emulsions administered orally or intranasal showed improvements in the systemic immune response, for example, vaccines containing emulsions against hemorrhagic septicaemia in cattle showed significant results in the protection of the animal and that could be used as a control against the effects of septicaemia (Leclercq et al., 2011, Verma and Jaiswal, 1997). The high solubilization capacity, bioavailability and thermodynamic stability, make MEs a system of great importance in the development of adjuvant formulations and immunogenic protection, helping in the local and systemic immune response (Leclercq et al., 2011).
Interest in these versatile carriers is increasing and their applications have been diversified across multiple administration routes, in addition to the oral route. Thus, a very important route of administration of ME is systemically(Acharya et al., 2013). Smaller droplets have a better ability to pass through small capillaries and reach the deepest target sites in the body, as well as avoiding the rapid clearance produced by the host's natural defense mechanisms, and may aid in better action by the high drug delivery in the action site, due do not pass in the digestive and hepatic environment. Moreover, many factors that hinder the action of the drug, such as pH, solubility, enzymatic action, temperature, among others, are reduced or even avoided by the use of systemic ME (Nirmala et al., 2013).
Nasal cavity are another administration route to ME is due the large surface area of the nasal cavity and the relatively high blood flow enable good absorption and avoid elimination of the first hepatic passage, as well as rapid onset and ease of administration. However, mucociliary clearance is the main barrier to drug delivery via this route. In short, ME have been shown to be a promising strategy for improving the bioavailability of poorly tissue-permeable drugs (Hosny and Hassan, 2014). Lee et al. (2016) developed a ME formulation as one of the colloidal systems for intranasal delivery of itraconazole (ITZ ME) against human rhinovirus. Released amounts of ITZ ME were significantly increased compared to the drug withdrawal group. In particular, the ITZ ME group had lower levels of inflammatory markers in the lung compared to the drug-only suspension group after intranasal administration in the human rhinovirus serotype 1B (HRV1B) infected mouse model. According to the author, ITZ ME release was significantly improved compared to drug withdrawal group.
The efficacy of amphotericin B (AmB) for leishmania therapy has been established for several decades. However, the drug has a very high rate and toxicity, especially renal and hepatic, because of its non-selectivity, besides presenting high cost. In addition, some cases of microbial resistance, fungi and parasites have emerged, making the development of new therapeutic alternatives essential. Aiming at AmB's acquired resistance and its high cost, Rochelle do Vale Morais et al. (2018) evaluated the activity of AmB incorporated in ME (ME-AmB) and data showed that ME-AmB, although not statistically different from AmB free, showed a successful action in the treatment of Leishmania donovani in the Balb / c rat model. Evaluations showed good efficacy and low toxicity.
Brime et al. (2004) evaluated the activity of amphotericin B incorporated into a ME containing lecithin aiming the treatment of systemic infections caused by C. albicans in immunocompetent and neutropenic mice. The ME was composed of isopropyl myristate (IPM), Brij® 96, lecithin and water, followed by incorporation of amphotericin B (M AmB) by phase inversion temperature. The physical analysis of the droplets showed an average hydrodynamic size of 45 nm. The groups of mice were treated with ME AmB with intravenous administration at periods of 24, 48 and 72 h after fungal infection. The results showed that ME AmB promoted mortality reduction and fungal load in affected organs. The group of mice infected with ME AmB treatment was able to circumvent the infection, reducing mortality and fungal load, resulting in an improvement in the action of the antibiotic. This improvement in action was observed in groups of mice with immunocompetence and neutropenics. Finally, the authors conclude that the administration of ME AmB in non-toxic doses and with good efficacy and stability, being an important delivery system for AmB.
Hosny and Hassan (2014) analyzed the activity of saquinavir misilate, a protease inhibitor of Acquired Immunodeficiency Virus type 1 (HIV 1), incorporated in a nasal ME in situgel (NEG) in groups of rabbits. According to the authors, the drug has low solubility, high rate of first-pass metabolism, low permeability and absorption by the gastrointestinal system. However, ME could improve drug efficacy. The authors used several constituents of oil phase, surfactants and cosurfactants, where the system selecting for the incorporation of saquinavir misylate was the system containing Labrafac Pg as an oil phase, Labrasol as a surfactant and transcutol Hp as a cosurfactant. According to the results, the droplets showed considerable uniformity with a size ranging from 25 to 61 nm, with promising results of permeability and bioavailability in in vivo tests with rabbits. The concentration of the drug incorporated in NEG was higher when compared to the same in the free form. Finally, the authors report that the developed system presents an important route of transport of antiviral medication by increasing the permeability in nasal tissue and greater systemic bioavailability.
The study provide by Shinde et al. (2015) observed that ME containing albendazole sulfuroxide and curcumin (ABZ-SO and CUR) improved therapeutic efficacy against Taenia solium Neurocysticercosis (NCC). In this paper, the self also points out that containing docosahexaenoic acid (DHA) ME, provides an even greater benefit, confirming that DHA in ME plays a crucial role in improving transmission from the nose to the brain. Degeneration and breakdown of the cyst wall in the wall, vacuolization in the cyst parenchyma, destruction and disintegration of the integumentary wall were the main changes caused by the treatment, resulting in increased efficacy.
Joshi et al. (2008) developed a microemulsion preconcentrate based on Artemether solubility (NanOsorb-ARM) for oral administration and treatment against malaria, caused by Plasmodium berghei. The experiments were carried out with male Swiss albino mice infected intraperitoneally and treated by oral gavage. For the production of the system, an oil phase composed of Capmul® MCM, surfactant Gelucire 44 / 14® + Labrasol® and diluted in water was used. Finally, the system was characterized, obtaining a size of 183 nm. According to the results, the system without the antimalarial showed interesting activity against infection. The authors suggest that components of the formulation may exert activity against the parasite. NanOsorb-ARM was able to decrease the parasitic load in the bloodstream and a significantly better antimalarial action when compared to the other treated groups In addition, the toxicity tests showed that the system containing the antimalarial drug presented less toxicity when compared to the drug alone, being considered safer and more effective.
Rastogi et al. (2017) developed microemulsions containing specific T4 bacteriophages against Escherichia coli. The methodology used mice infected with E. coli systemically and treated with ME containing bacteriophages by transdermal administration. The system was composed of glyceryl monooleate, oleic acid, ethyl oleate or isopropyl myristate as an oil phase, Tween 20®, Labrasol® or Span 20® with HCO-40® as surfactants, and Transcutol® as a co-surfactant. The results showed that the system showed uniformity and a size ranging from 150 to 320 nm and with excellent skin permeability. According to the results, the group treated with bacteriophages showed longer survival. Furthermore, histological tests showed the efficiency of treatment and the safety of the system against infection. In conclusion, ME was considered a promising delivery system and indicated for the treatment of systemic infections caused by E. coli, mainly due to the increase in the amount of T4 bacteriophages at the infection site. The stability was considered ideal, as the ME remained stable in all temperature changes, however, the ability of the bacteriophage to reach and cause cell lysis was impaired at temperatures above 45 °C. Finally, adverse effects are observed in the group that did not receive the treatment, with increased inflammatory response, the appearance of inflammatory markers (interleucin 6, for example) and damage to the intestinal wall.
Griffin and O’Driscoll (2006) evaluated the antiretroviral activity of saquinavir, a drug that acts on lipophilic inhibition of HIV protease, incorporated in ME. In this work, the authors observed the potential of the system in the directed intestinal lymphatic transport of saquinavir in rats and by intraduodenal administration. The ME were developed containing oleic acid as an oil phase, a co-surfactant with low hydrophilic-hydrophobic balance (Plurol Oleique, HLB 10), a high hydrophilic-hydrophobic balance surfactant (Labrasol HLB 14) and water as an aqueous phase. MEs had an average hydrodynamic size of approximately 130 nm and good uniformity. According to the results, the MEs provided a greater amount of saquinavir in the lymphatic system when compared to other systems developed by the authors, presenting a dosage level three times a day. In addition, the system had a systemic bioavailability of 8.5%. Finally, MEs can be considered as an important transport system for antiretroviral treatment, mainly due to the considerable increase in drug concentration in the lymphatic system.
Mandawgade et al. (2008) evaluated antimalarial activity through self-microemulsifying drug delivery systems (SMEDDS) composed of a natural lipophile of indigenous origin (N-LCT), β –Artemether. Swiss rats were infected intraperitoneally with Plasmodium berghei and treated by oral gavage. ME was composed of N-LCT / Capryol 90® with Plurol oleic® as an oil phase, Cremophor EL® or Tween 80® as a surfactant and Gelucire 44/14® as a co-surfactant. The results showed that the SMEDDS had an average hydrodynamic size equivalent to 100 nm, which is characteristic of ME. In addition, improvement in treatment was observed in the group treated with SMEDDS when compared to the group treated with Larither® or with β –Artemether solubilized with oily phase or surfactants. SMEDDS contributed to better absorption, high uptake by blood cells and resulted in better treatment efficiency against malaria. In conclusion, the developed system can be used in the future as a great alternative for delivering safe medication for antiparasitic treatment.
Table 5 summarizes the results of several studies related to the application of ME in the treatment of systemic infections.
Table 5.
Microemulsions applied to the treatment of systemic microbial infections.
| Formulation | Active principle | Composition | Therapeutic target | Route of administration | Reference |
|---|---|---|---|---|---|
| ITZ ME | Itraconazole | Cremophor EL and Solutol HS15, Benzyl Alcohol and Water | Rhinovirus Infection | Intranasal | Lee et al., 2016 |
| ME- AmB | Amphotericin B | Phosphate buffer pH 7.4, Tween ® 80, Lipoid ® S100Miglyol ® 812 | Leishmania donovani infection | Intravenous | Rochelle do Vale Morais et al., 2018 |
| M AmB | Amphotericin B | Isopropyl myristate (IPM)/Brij® 96 V/lecithin/water | C. albicans | intravenous | Brime et al., 2004 |
| NEG | Saquinavir mesylate | Labrafac PG, Labrasol, Transcutol Hp and water | HIV tipo 1 | Intranasal | Hosny and Hassan, 2014 |
| DHA ME of ABZ-SO and CUR | Albendazole Sulfoxide and Curcumin | 60% tween 80: ethanol, 30% water | Taenia solium infection | Intravenous | Shinde et al., 2015 |
| NanOsorb-ARM | Artemether | Oil phase: Capmul MCM®, Surfactant: Gelucire, 44/14® Labrasol® and and water | Plasmodium berghei | Oral gavage | Joshi et al., 2008 |
| ME | Bacteriophage T4 | Oil phase: Tween 20®, Labrasol®, or Span 20®, Surfactant: HCO-40® (1:1 ratio), Co-surfactant: Transcutol® | Escherichia coli | Transdermal | Rasgoti et al., 2017 |
| Microemulsion | Saquinavir | Oleic acid, Plurol HLB 10, Labrasol HLB 14 and Water | HIV | intraduodenally | Griffin and O’Driscoll, 2006 |
| SMEDDS | β-Artemether | Oil phase: N-LCT, Capryol 90 ,Plurol Oleique CC 497, Surfactante: Cremophor EL and Tween 80, Co-surfactant: Gelucire 44/14 | Plasmodium berghei | oral gavage | Mandawgade et al., 2008 |
ITZ: Microemulsion with Itraconazole; ME-AmB: Microemulsion with Amphotericin B; IPM: isopropyl myristate; M AmB: oil-in-water lecithin-based microemulsion whit AmB; NEG: Nanozized Microemulsion gel; HIV type 1: Human Immunodeficiency virus.; DHA ME of ABZ-SO and CUR: Microemulsion of docosahexaenoic acid containing albendazole sulfuroxide and Curcumin; SDC: sodium deoxycholate.; HBL: hydrophilic–lipophilic balance.; SMEDDS: self-microemulsifying drug delivery systems.; N-LCT: indigenous natural lipophile.; NanOsorb-ARM: Solid microemulsion preconcentrates with Artemether.
3.4. Nanoemulsion (NE)
Nanoemulsions (NE) are systems made up of immiscible liquids, water and oil, with droplets of less than 500 nm and which stabilize by the aid of appropriate surfactants into various dosage forms such as liquids, creams, sprays, gels, aerosols, foams, and may be administered by equally varied routes as topical, oral, intravenous, intranasal, pulmonary and ocular (Singh et al., 2017). The main components of nanoemulsion are oil, surfactants and aqueous phase, However, different than microemulsion systems, in order to make emulsion formation possible it is necessary to use the energy (Jaiswal et al., 2015), in which is the main characteristic to differ this system of microemulsion.
The energy required to produce an NE can be generated by a mechanical device (high energy emulsification) or the chemical potential of the components, usually surfactants (low energy emulsification). Although there are a number of processes for high energy emulsification, ie high pressure homogenization (HPH), ultrasound and microfluidization, probe sonication is one of the most commonly used high energy homogenization methods for synthesis (Ghaderi et al., 2017, Hörmann and Zimmer, 2016).
Surfactants exhibit amphiphilic characteristics that stabilize NE which reduce the tension between immiscible liquids and prevents aggregation between droplets. They tend to adsorb rapidly at the oil water interface and provide steric or electrostatic or double electrostatic stabilization (Hwang et al., 2013). Therefore, they play important roles in the formation of NE, reducing interfacial tension, and reducing the stress needed to break a droplet, preventing the coalescence of newly formed droplets (Tadros et al., 2004).
Droplet diameters generally reach < 500 nm and have lighter or more hazy characteristics that differ from the milky white color associated with the emulsion. NE, although having the same droplet size range as microemulsions, the structures and long-term thermodynamic stability are very different (Singh et al., 2017).
Fig. 4 represent the water-on-oil (W / O) or oil-on-water (O / W) droplets that compose the NE systems.
Fig. 4.
Schematic representation of droplets presented in and oil-on-water (A) water-on-oil (B) nanoemulsion formulation.
NEs have been shown to have broad antimicrobial activity against bacteria, encapsulated viruses and fungi at concentrations that are harmless to in vivo assays. When NE function by fusion with lipid bilayers of cell membranes, the energy stored in the oil emulsion and surfactant is released and destabilizes the lipid membrane of bacteria; justifying antimicrobial activity, in addition, allowing broad spectrum activities and limiting the ability to generate resistance. These characteristics make NE an innovative and plausible candidate for the treatment of infectious diseases (Hwang et al., 2013).
The main advantages of NE are related to the fast absorption by internalization in the enterocyte, improvement of drug solubility and its bioavailability, solubilization of hydrophobic or hydrophilic components, smaller droplet size and imminent kinetic stability against sedimentation, flocculation and coalescence and resisting to the denaturation process, due the brownian motion of the droplets outweighs the force of gravity. Moreover, due to the small particle size and lower surface tension between the oil and the aqueous phase, it hardly allows agglomeration or precipitation, contributing to the reduction of the possibility of sedimentation formation. NE assists in the permeability of biological membranes by reversibly altering the cell arrangement and improving interaction with cells after solubilization of the lipid barrier or fusion of the lipid double layer interface with the cell wall. Finally, it is a very important transport mechanism in selectively targeting drugs to the desired site (Rai et al., 2018).
The disadvantages of NE are mainly related to the inability of NE to solubilize high melting substances, low energy prepared NEs often require large amounts of surfactants to stabilize the droplets, therefore, the use of high concentration of surfactants may lead to fluidization in the membrane, discarding its internal use. In addition, the surfactant can be toxic to cells. The price effectiveness of NE manufacturing is also an issue that needs to be addressed in advance, as expensive instruments are often involved (Singh et al., 2017).
In relation to the immunogenic effects of nanoemulsions, it should be noted that the vaccine aims to promote the development of long-lasting protective immune responses. This objective is achieved, since specific B and T memory cells are stimulated, as well as certain circulating antibodies. This purpose was commonly achieved through parenteral application of attenuated viruses, however, the risk of reversion of the microorganism to a pathogenic state is not ruled out (O’Hagan and Valiante, 2003, Valiante et al., 2003).
Endowed with greater security, the purified antigens as well as the epitopes synthesized to measure, have great difficulty in generating efficient immune responses. Adjuvants such as naonemulsions emerge in the vaccination scenario, arousing interest in view of the benefits provided by this system, such as increased thermostability, increased immunogenic response, increased immunity availability of the antigen, among others (Kramer et al., 2018). When the adjuvant is co-administered together with antigens, they interact as immunomodulators and / or immunostimulators, becoming immunologically promising (Csaba et al., 2009).
As in the microemulsions, the cytotoxic profile of nanoemulsions is attributed to surfactants in the composition. These effects can contribute to the evaluation of antitumor and antimicrobial action. However, effects have also been observed in normal cells (Arechabala et al., 1999, Gundogdu et al., 2013, Sieniawska et al., 2019).
In recent years, NE have attracted great interest from researchers with regard to controlled drug delivery to target organs and tissues. target, in addition, may provide increased stability and bioavailability (Khalkhali et al., 2019).
Although there is great therapeutic diversity, few drugs achieve clinical success due to their low bioavailability and specificity in relation to the target site. Given this, nanotechnology not only has great potential to improve biopharmaceutical action, but also enables the delivery of bioactive molecules to the desired site of action (Rai et al., 2018).
NE have shown great versatility in their applications, ranging from topical to systemic, and may be administered orally, parenterally or nasally, and have been used to combat infectious diseases caused by viruses, bacteria, fungi and other diseases as infections caused by parasites (Choudhury et al., 2017).
An example to be highlighted is pharmacological therapy for the treatment of tuberculosis (TB), which, due to the low bioavailability of oral medications, is unsatisfactory. In this sense, NE is considered a favorable alternative to increase the bioavailability of oral drugs in order to increase therapeutic efficacy. In addition, anti-TB drug-loaded NE can easily overcome biological barriers and reach systemic circulation, thereby lowering the burden of MTB. Added to this is the lipid nature of the system, which enables drug delivery to lymph nodes, enhancing bioavailability and consequently reducing the frequency of dosages (Patil et al., 2018).
In the study by Karami et al. (2019) demonstrated that Indinavir (IDV), an antiretroviral protease inhibitor used in the treatment of HIV patients, has limited cerebral permeability due to efflux exerted by P-glycoprotein, which acts on the blood–brain barrier thus hindering the passage of the drug and permanence in the target site. In this sense it was synthesized NE loaded with IDV (0.2%), oleic acid (0.32% w / v), α-tocopherol (0.25% w / v), was added to olive oil (7, 45% w / v) and polysorbate 80 (4% w / v). The oily phase was added to the aqueous phase, and finally glycerol (2.25% w / v), lactoferrin-treated (LF-IDV-NEs) was added to increase brain permeability as well as its permanence in the target organ. The authors showed that after the components were incorporated into the system, there was a significant increase in brain permeability as well as the permanence of the drug in brain tissue.
Regarding the class of antiretroviral drugs, it is worth mentioning saquinavir mesylate (SQVM), which is a protease inhibitor drug, but little soluble widely used as antiretroviral drug, with oral bioavailability of about 4%. Aiming at improving bioavailability, Mahajan et al. (2014) developed a NE composed of 86% water, 6% capmul MCM oil, 6% tween and 2% PEG 400 as surfactants and co-surfactant respectively. The drug-loaded NE was adapted for intranasal administration to achieve central nervous system (CNS) targeting for the treatment of neuro-AIDS. ESSV with NE had a significant effect on measured brain concentrations. Gamma scintigraphy of rat brain has conclusively demonstrated greater CNS drug transport following intranasal administration with NE. The NE droplet size, which was smaller than 200 nm, can directly cross the blood–brain barrier, thus increasing the CNS drug concentration.
Infectious diseases affecting the CNS such as meningitis (meningeal and subarachnoid space inflammation, which may involve the cerebral cortex and parenchyma), require delivery of the drug directly to the target organ for therapy (van de Beek et al., 2016). Harun et al. (2018) synthesized a CLN containing cefuroxime, a drug that has difficulty permeating the blood–brain barrier (BBB) due to its limited fat solubility. The CLN consisted of an oil phase prepared with cefuroxime (0.3%, w / w) mixture of oil (10%, w / w) and lecithin (3%, w / w) as surfactant. Then Tween 80 (0.8% w / w) was added. The aqueous phase was composed of glycerol (2.3%, w / w) and sodium oleate (0.1%, w / w), the oil phase was added dropwise to the oil phase, for homogenization the High-pressure method. The results obtained were promising with regard to drug delivery to the target site, as the CLN formulation proved viable for the parenteral (intravenous) route of administration and demonstrated good physicochemical pharmacokinetics stability when compared to cefuroxime.
Although current antivirals may maintain viral suppression and decrease liver complications, patients with chronic hepatitis B (HBV) do not respond well to usual drug therapy due to lymphatic system input to HBV virus, even if there are concentrations Appropriate drug delivery in the systemic current viral particles remain viable in the lymphatic system (Gane, 2017).
Xu et al. (2019a) developed an NE consisting of isopropyl myristate, phosphatidylcholine, propylene glycol and distilled water (27.3: 31.8: 31.8: 9.1, w / w), in which baicalin was first dissolved in phosphatidylcholine and propylene glycol and subsequently mixed with isopropyl myristate (IPM), and finally a certain amount of distilled water was added. The constituents were submitted to homogenization, at the end a fixed concentration NE of 9.5 mg / mL was obtained. The authors evaluated the potential of baicalin-containing NE for absorption of the lymphatic system and found that baicalin NE showed an increase in bioavailability and increased the concentration of baicalin at the target site for about 12 h. The authors concluded that prolonged release of the drug will aid in viral suppression. Thus, the results obtained in this study indicate that baicalin NE (A / O) may be a promising delivery and release system for clinical application against HBV infection.
In an attempt to contain this type of infection Shukla et al. (2014), demonstrated in their study that Turmeric NE (CUR) provides advantageous properties such as increased release and targeting of the active ingredient in target tissues and organs, increased therapeutic efficacy, reduced dose numbers, and decreased the side effects. NE was formulated using the high energy method, the oil phase was constituted Tween 80, tocol acetate, in which 12.5 mg of CUR was dissolved, finally soybean phosphatidylcholine (SPC) was added. In the oil phase glycerol (5% w / v) was added as dropwise, and the mixture was sonicated. The present study revealed that CUR NE improved plasma concentration as well as tissue distribution leading to reduced lung and liver injury.
The study performed by Lin et al. (2012) aimed to synthesize a W/ O nanoemulsion of chitosan and heparin for oral administration in order to carry Amoxicillin and deliver it to the target organ for the treatment of infections originating from H. pylori. The system was consisted of 4 mL of water containing chitosan (0.6 mg / mL) and heparin (0.2 mg / mL) with the aqueous phase, the oil phase was composed of paraffin oil, as a surfactant Span20 was used and Tween20 and finally, amoxicillin was added. The results obtained by the authors demonstrate that the nanoemulsion could control the release of amoxicillin in a dissolution medium in the simulated gastrointestinal environment. Chitosan / heparin nanoemulsion particles loaded with amoxicillin delivered to the H. pylori infection site potentiated the inhibition of the microorganism growth compared to amoxicillin alone. Results obtained from in vivo tests by means of a clearance test indicated that the chitosan / heparin nanoemulsion carried with amoxicillin showed a more concise clearance effect for H. pylori in mice with gastric infection induced when compared to amoxicillin alone.
Henostroza et al. (2020) developed a cationic nanoemulsion incorporated with rifampicin, for ophthalmic administration. The rifampicin nanoemulsion (Rif-NE) was synthesized by the high pressure homogenization technique. The oil phase consisting of rifampicin (0.1% w / w) and oleic acid (1.0% w / w), and the aqueous phase composed of surfactant and Milli-Q water were heated separately to 70 ± 5° C. magnetically stirred at 200 rpm for 30 min. The aqueous phase was dispersed in the oil phase with magnetic stirring at 800 rpm for 1 min. The mixture was then dispersed by Ultra-Turrax at 10,000 rpm for 5 min; the newly formed emulsion was subjected to a high pressure homogenizer.The authors conclude that cationic nanoemulsions (Rif NE) did not present good mucoadhesiveness to the target organ, which reflects in the decrease in instillations. This fact can provide better patient adherence to treatment, promoting a reduction in the rate of retreatments ocular tuberculosis.
Mahboobian et al. (2020) developed ten different thermo-sensitive in situ thermo-sensitive nanoemulsions in gel carried with acyclovir (ACV) in order to assess the permeation capacity as well as the eye irritability generated by eye pathway infections in models of ex vivo. The NE2b was chosen as being ideal, it consisted of an aqueous phase composed of purified water, triacetin was used as the oil phase, Poloxamer 407 and Poloxamer188 as surfactants and Transcutol P® acting as co-surfactant and finally LCA was added. The authors conclude after the results obtained that NEs of thermosensitive gel in situ containing ACV, demonstrate great potential for permeation of the drug through the cornea in a bovine animal model. In addition, in vivo and ex vivo irritation tests have indicated that this drug delivery system can be considered safe for ophthalmic application, being promising for topical use in ophthalmic disease therapy, such as HSK.
The study provide by Silva et al. (2020) developed an O / W nanoemulsion for oral administration containing the antimalarial thiazoline. The system consists of thiazoline, a water-soluble substance, little aqueous hydrophobic, oleic acid (solubilizing agent), paraben solution (methylparaben and propylparaben in propylene glycol) (preservative) and butylhydroxyanisole (antioxidant), as an oily phase; and Solutol HS 15 (surfactant) and ultrapure water, as an aqueous phase. Solutol HS15 (Macrogol hydroxystearate 15) was added to the formulation,.. The phases were then homogenized by extrusion using syringes, and finally the nanoemulsion was subjected to the sonication process. The results showed that the system was able to release the active ingredient thiazoline in neutral and acidic media in a controlled and prolonged showed in vitro antimalarial activity due to its low IC 50 (1.32 µM) against the P. falciparum strain resistant to chloroquine (W2) and high selectivity index for WI-26-VA4 (12.50), indicating that it provoked toxicity in a selective way against the microorganism, preserving healthy cells. In the in vivo model, however, the formulation did not increase the survival time of infected animals, this factor may be due to the short half-life of thiazoline. In contrast, the thiazoline nanoemulsion showed a good performance when compared to unloaded thiazoline.
Wang et al. (2020) developed a nanoemulsion containing oligonucleotide (NE02) to investigate the effects against the avian influenza virus (H5N1) in animal models. The authors reported that NE02 containing the oligonucleotide, generated rapid antibody responses, which may be accompanied by an increase in the responses of IFN-γ and IL-17 and the negative regulation of IL-5. The authors conclude that the intramuscular application of rH5 with NE02 adjuvant generates efficient immune responses against the H5 antigen and promotes protection for animals against H5. In addition, the combination of NE02 with CpG increased immune responses, thus, the results indicate that the combination of adjuvants can be promising to improve immune responses to H5 and promote more effective protection against infection by avian influenza.
Table 6 summarizes the results of several studies related to the application of NE in the treatment of systemic infections.
Table 6.
Nanoemulsions applied to the treatment of systemic infections.
| Formulation | Drug | Composition | Therapeutic target | Route of administration | Reference |
|---|---|---|---|---|---|
| SQVM NE | Saquinavir mesylate | Water, Camul MCM (4–8%), Tween 80 (6–15,75%) e PEG 400 (2–5,25%) | Neuro-AIDS | Intravenous | Mahajan et al., 2014 |
| Lf-IDV-NEs | Indinavir and Lactoferrin | IDV (0.2%), oleic acid (0.32% w / v), α-tocopherol (0.25% w / v), was added to olive oil (7.45% w / v) | HIV | Intravenous | Karami et al., 2019 |
| CUR NE | Curcumin | CUR (95%) Purity, SPC, Tween 80, tocol acetate | Sepsis | Intravenous | Shukla et al., 2014 |
| CLN | Cefuroxime | Cefuroxime (0.3%, w / w) mixture of oil (10%, w / w) and lecithin (3%, w / w), Tween 80 (0.8%, w / w), glycerol (2, 3% w / w) and sodium oleate (0.1%) | Meningitis | Intravenous | Harun et al., 2018 |
| Baicalin NE | Baicalin | Isopropyl myristate phosphatidylcholine, propylene glycol, distilled water (27.3: 31.8: 31.8: 9.1 (w / w) | HBV | Oral | Xu et al., 2019a, Xu et al., 2019b |
| NE | Amoxicilin | Water, (4 mL) with chitosan (0.6 mg / ml) and heparin (0.2 mg / ml), oily paraffin oil phase (80 mL) containing 1.2 mL of a Span20: Tween20 in a proportion 75:25. | Úlcer | Oral | Lin et al., 2012 |
| NE | Thiazoline | oleic acid (2.75 g), paraben in a 6% (w / v) solution of methylparaben and 3% (w / v) of propylparaben in propylene glycol (0.22 g), butylhydroxyanisole (0.001 g) 0, Solutol HS 15 (1.5 g) as an organic phase plus ultrapure water (5.97 g) that made up the aqueous phase. | Malária | Oral | Silva et al., 2020 |
| NE02 | Oligonucleotide CpG |
Double-chain cationic surfactant (DODAC), class B oligonucleotide - murine TLR9 agonist (TLR9 / CpG - ODN 1826), sterile water | H5N1 | Intramuscular | WANG et al., 2020 |
| Rif NE | Rifampicin | rifampicin (0.1% w / w), oleic acid (1.0% w / w), polysorbate 80 (0.9% w / w) Mili-Q water |
Ocular tuberculosis | eyepiece | Henostroza et al., 2020 |
| NE2b | acyclovir | Water (w / w), ACV 0.25% w / w, polaxamer 407 15.3% (w / w), polaxamer 188 1.33% (w / w) Transcutol®P (w / w), Triacetin (w / w) | Herpes | eyepiece | Mahboobian et al., 2020 |
NE (Nanoemulsion); SQVM NE (Nanoemulon carried with siquinavir mesylate); Lf-IDV-NEs (idinavir and lactoferrin nanoemulsion); CUR NE (Curcumin Nanoemulsion); CLN (cefuroxime nanoemulsion); Baicalin NE (baicalin nanoemulsion; HBV (chronic hepatitis B); HIV (human immunodeficiency virus); Neuro AIDS (acquired immunodeficiency syndrome); NE2b (acyclovir nanoemulsion); NE02 (nanoemulsion) containing oligonucleotide CpG); Rif NE (Ripamficin Nanoemulsion).
3.5. Dendrimers
Dendrimers were designed in 1980s and presented structures that are defined as starburst polymers, cascade molecules, or tree-like (Janaszewska et al., 2019, Kambhampati et al., 2015). Overall, as opposed to traditional polymers, the dendrimers exhibit three main characteristics: (1) functional core to enclose the branches, (2) repeated branches and (3) surface multivalent groups (Cheng et al., 2008, Da Silva Santos et al., 2016, Dias et al., 2020, Tomalia, 2005). Fig. 5 presents the general structure of dendrimers.
Fig. 5.
General structure of dendrimers.
The dendrimers synthesis occurs by divergent and convergent methods. The choice of the method depends on the developed structure and production (Cheng et al., 2008). The divergent method was firstly described by Tomália et al. (1985) to synthetize poly(amidoamine) (PAMAM) dendrimer and this approach consist of expansion radially from the core to surface part. Dendrimer generation (G) is called to each stage of radial expansion, what involves increase of molecular weight and number of surface termini, with either 16, 32 or 64 surface groups in core G2, G3, or G4 dendrimers (Hsu et al., 2017). Unlike divergent, the convergent method developed by Hawker and Frechet, 1990, starts from the surface to core to synthetize the dendrimers (Mendes et al., 2017). The characterization of dendrimers consist of spectroscopic and spectrometric methods, scattering techniques, microscopy, chromatography, electrophoretic techniques and others (García-Gallego et al., 2017).
The most common dendrimers are represented by PAMAM, poly(propylenimine) (PLL) and poly(propylene imine) (PPI) due to their biomedical applications (Hsu et al., 2017). Each dendrimer is more suitable for specific target, since it present peculiar characteristics. For instance, PAMAM dendrimer exhibit ethylenediamine core with amine groups in the branches, what can be employed to load bioactive compounds, because of its properties, such as, biocompatible and hydrophilic (Mhlwatika and Aderibigbe, 2018). To gene therapy, PLL dendrimer is mostly used, due to efficient uptake by cells, once is water soluble, increasing the stability of DNA in solvents (Choi et al., 1999, Patil et al., 2011). PPI dendrimer, as well as, PAMAM dendrimer show cationic group on dendrimer surface, and can be used for drug delivery and diagnosis (Sharma et al., 2017, Xiong et al., 2016, Ziemba et al., 2011).
The consistently increase of generation number is responsible by the size of dendrimers, for instance the size of PAMAM dendrimers can present nanoscale size ranging from 1.5 to 13.5 nm (Kesharwani et al., 2014, Yellepeddi and Ghandehari, 2019). Dendrimers have been studied due to their immense potential to drug delivery, because of several advantages, such as, enhancing solubility and stability, improved bioavailability, controlled delivery, and, specifically, the dendrimers show antimicrobial activity, being relevant in the treatment of infectious diseases (Chauhan, 2018, Kesharwani et al., 2014). The therapy against infectious diseases is difficult since many factors can be interfere in the efficiency of the treatment, such as low drug bioavailability, drug interactions and, mainly, drug resistance, which decreases the options of treatment (Scire et al., 2019, Scorciapino et al., 2017).
The antimicrobial activity of dendrimer has been described due to its own antibacterial action or because of the combination with existing antibacterial agents, incorporated in the its structure(Kamaruzzaman et al., 2019). The general antibacterial effect of dendrimers occurs because of the bacterial membrane disturb by formation of small holes, leading to cell death, showing antibacterial action mode on permeability cellular once there is an interaction with bacterial lipid layer (Chen and Cooper, 2002, Winnicka et al., 2013).
Several studies have been showed the antibacterial action of dendrimers. A study showed antibacterial activity with synergistic effect between modified dendrimer and levofloxacin against Escherichia coli, Proteus hauseri and Staphylococcus aureus, what is possible reduce the levofloxacin dosage (Wrońska et al., 2019). Another work evaluated the interaction of cationic dendrimers with bacteria, as a promising antibacterial action due to clustering formation and membrane disruption (Leire et al., 2016).
A study showed the antibacterial activity of lipidated peptide dendrimers (TNS18) against multidrug-resistant bacteria, such as Gram-negative strains of Pseudomonas aeruginosa and Acinetobacter baumannii and Gram-positive strain of methicillin-resistant Staphylococcus aureus (MRSA). The inclusion of fatty acid chain to the dendrimer core promoted bacteria membrane disruption, once the lipidated dendrimer can develops in contact with bacteria membrane, allowing fast bacterial death. These data are important since showed that TNS18 has large antibacterial spectrum against relevant strains which are present in systemic infections, in addition in vivo assay exhibited that TSN18 is effective against multidrug-resistant clinical isolates of Acinetobacter baumannii and Escherichia coli (Siriwardena et al., 2018).
A recent work showed that PAMAM dendrimer improved the pharmacokinetic property of platensimycin (PTM), an inhibitor of bacterial fatty acid synthases, which presents in vivo inhibitory activity against MRSA. The authors suggest that the loaded PTM in the PAMAM (PAMAM/PTM) dendrimer increased the circulation half-life of PTM, enhancing the level of PTM in mouse plasma, and consequently promoted effective in vitro and in vivo anti-staphylococcal activity. The use of dendrimer improved the antibacterial action of PTM, probably due to interactions between bacteria (negatively charged) and PAMAM/PTM (positively charged), in addition to improve the penetration of PTM through the bacterial membranes (Liu et al., 2020).
Another recent study described that the conjugation of a peptide to PAMAM dendrimers shows relevant efficacy against sepsis, once PAMAM dendrimer improved of bioavailability of the peptide, which inhibits the acetylation of transforming growth factor β-induced protein (TGFBIp), what is related as a therapeutic target for sepsis. For human TGFBIp secretion is necessary its acetylation to regulate septic inflammatory response, thus, in this study, the authors developed a dendrimer-based nanostructure that has effects on human TGFBIp acetylation. In addition, a new nanomaterial increased the survival rate in a mouse model and the secretion of endotoxin human TGFBIp caused by infection was inhibited (Lee et al., 2020).
Gao et al. (2020) showed that azithromycin (AZM) conjugated to PAMAM dendrimer has effective in vitro and in vivo antimicrobial action against Pseudomonas aeruginosa biofilm, which is responsible by chronic lung infection. The use of dendrimer demonstrated many beneficials such as, maintained the AZM inside of biofilms, improved the penetration and permeabilization of the bacterial membrane. Moreover, in in vivo assay was verified reduction of bacterial burden and also decreasing of the inflammation, what is relevant considering that the chronic lung infection is the main reason of mortality in patients with cystic fibrosis.
Winnicka et al. (2012) described the solubility and antifungal activity of ketoconazole hydrogel in combination with PAMAM-NH2 and PAMAM-OH dendrimers generation 2 and generation 3 against C. albicans, C. krusei, C. parapsilosis, C. glabrata and C. dubliniensiss. The MIC values of combinations (0.064 to 0.008 µg/mL) were decreased or kept when compared to ketoconazole, showing the PAMAM dendrimers suitable to drug delivery in topical fungal infections.
Nishikawa et al. (2002)verified the intravenous administration of carbosilane dendrimer carrying trisaccharides of globotriaosyl ceramide in mice against infections by Stx-producing E. coli O157:H7. The study showed that the intravenous administration of dendrimer promoted the inhibition of the bacterial toxin incorporation into cells, reduction of fatal brain injury and protection of the mice from challenge with a fatal dose of E. coli O157:H7. Another work exhibited effective antibacterial action against multidrug resistant bacteria Pseudomonas aeruginosa and Acinetobacter baumannii by intraperitoneal administration of peptide dendrimer in mice and the peptide dendrimers did not show toxicity (Siriwardena et al., 2018).
Another study showed promising antiviral action of dendrimer against HIV-1 infection, whereby intravenous administrations of dendrimer-siRNA in mice promoted complete inhibition of HIV-1 titers and protection against viral induced CD4 + T-cell depletion. Besides, it was observed that the dendrimer-dsiRNAs did not exhibit any toxicity. Thus, the combination of dendrimers with siRNAs can be a promising strategy for treatment of HIV-1 infection (Zhou et al., 2011).
Date from work developed by Guerrero-Beltrán et al. (2020) indicated that G2-S16 polyanionic carbosilane dendrimer presents relevant in vitro and in vivo inhibitory activity against herpes simplex type 2 (HSV-2) infection, with complete inhibition of HSV-2 (>90%). Moreover, the G2-S16 provided protection to mice vaginal microbiome, once the composition of vaginal microbiome was similar to healthy mice.
The work developed by Jain et al. (2015) demonstrated that intraperitoneal administration of amphotericin-B loaded in the PPI dendrimers in mice presented effective antiparasitic activity against Leishmania donovani and reduction of toxicity (hemolytic and cytotoxicity).
A study demonstrated that folate-PAMAM dendrimer is effective to deliver drugs to reduce infection and associated inflammation in Chlamydia-induced reactive arthritis by intravenous administration of dendrimer in mouse, due to its potential conjugation characteristic, what encourage studies involving other microorganisms (Benchaala et al., 2014).
The attributes of dendrimers involve parameters such as, biopermeable, non-toxic, what make dendrimers able to stay in blood circulation until the target specific. However, the high cytotoxicity presented by some kinds of dendrimers is a limited condition to their use(Janaszewska et al., 2019). The cytotoxicity of dendrimers is related to functional surface groups (number and character). Cationic dendrimers are considered the most toxic dendrimers due to their interaction (positively charged) with negatively charged cell membranes, showing in vitro and in vivo toxicity. In this sense, the reduction of cytotoxicity of dendrimers is obtained by alteration of the surface of the dendrimer to negatively charged or by surface functionalization using polyethylene glycol (PEG) (Janaszewska et al., 2019, Labieniec-Watala and Watala, 2015).
The dendrimers can interact with nucleic acid and the number of dendrimer-DNA contact can increase, as far as, the generation number of dendrimer occurs. This action is an important dendrimers’s ability to use it as vectors for gene transfection, however, the interactions with nucleic acid are not selective, which can cause genotoxicity (Malik et al., 2000, Ziemba et al., 2012).
A work investigated the genotoxicity and cytotoxicity of PPI dendrimer (4th generation) and the authors suggested that PPI dendrimer has considerable interaction with DNA, due to its positive surface charge and possible DNA single-strand breaks and/or the formation of abasic sites (Ziemba et al., 2012). This fact can also be explained by dendrimer’s ability to generate intracellular reactive oxygen species, causing DNA damage and DNA single-strand breaks (Mukherjee et al., 2010).
The immunogenic dendrimers G2′s ability was demonstrated by Javadi et al. (Mukherjee et al., 2010) which the authors described for the first time the enhance immune responses against hepatite C virus (HCV) infection in mice model, once the dendrimer G2 was conjugated to non-structural protein 3 (NS3 HCV), which is engaged in HCV replication and translation, inducing strongly TCD8 + response. Another work showed the potential of dendrimer-conjugated vaccine to prevent infection by Chlamydia trachomatis vaginal in mouse using subcutaneous administration. The results demonstrated that dendrimer-conjugated decreased the infection burden and improved the Pep4 immunogenicity, suggesting the dendrimer-conjugated as a promising vaccines candidate to prevent infectious diseases (Ganda et al., 2017).
The ability of dendrimers in to be used with several administration routes such as oral, transdermal, ocular, transmucosal, intravenous and topical routes is an advantage as promising drug delivery system (Mignani et al., 2013), what it permits the application of dendrimers to delivery of antibiotics to control of infectious diseases.
Table 7 summarizes the results of several studies related to the application of dendrimers in the treatment of systemic infections.
Table 7.
Dendrimers applied to the treatment of systemic infections.
| Formulation | Composition | Therapeutic target | Route of administration | Reference |
|---|---|---|---|---|
| Carbosilane dendrimer | Carbosilane dendrimer + trisaccharides of globotriaosyl ceramide | Shiga toxin producing Escherichia coli O157:H7 | Intravenous | Nishikawa et al., 2002 |
| Cationic PAMAM dendrimers | dendrimer-siRNA | HIV-1 | Intravenous | Zhou et al., 2011 |
| PPI dendrimer | MdPPI dendrimer + amphotericin-B | Leishmania donovani | Intraperitoneal | Jain et al., 2015 |
| Dendrimer TNS18 | peptide dendrimers | Pseudomonas aeruginosa; Acinetobacter baumannii | Intraperitoneal | Siriwardena et al., 2018 |
| Folate-PAMAM dendrimer | PAMAM + folate + Cy5.5 conjugate | Chlamydia trachomatis | Intravenous | Benchaala et al., 2014 |
| PAMAM dendrimer (G4OH) | PAMAM dendrimer + immunogenic peptide Pep4 | Chlamydia trachomatis | Subcutaneous | Ganda et al., 2017 |
| Lipidated peptide dendrimers (TNS18) | Lipidated peptide dendrimers | Pseudomonas aeruginosa; Acinetobacter baumannii; methicillin-resistant Staphylococcus aureus Escherichia coli | Intraperitoneal | Siriwardena et al., 2018 |
| PAMAM dendrimer | PAMAM + platensimycin | methicillin-resistant Staphylococcus aureus | Intraperitoneal | Liu et al., 2020 |
| PAMAM dendrimers | peptide dendrimers | factor β-induced protein | Intravenous | Lee et al., 2020 |
| G2-S16 polyanionic carbosilane dendrimer | G2-S16 polyanionic carbosilane dendrimer | herpes simplex type 2 | Intravaginal | Guerrero-Beltrán et al., 2020 |
| PAMAM dendrimer | PAMAM dendrimer+azithromycin | Pseudomonas aeruginosa | Intratracheal | Gao et al., 2020 |
PAMAM: poly (amidoamine); PPI: poly (propylene imine); TNS18: peptide dendrimer; Cy5.5: used as the near-IR imaging agent; siRNA: small interfering RNA; (G4OH): dendrimer generation 4; Pep4: peptide of a chlamydial glycolipid antigen-Peptide 4; HIV-1: human immunodeficiency virus; G2-S16: polyanionic carbosilane dendrimer.
3.6. Solid lipid nanoparticles and nanostructured lipid carriers
Solid lipid nanoparticles (SLN) began to be studied in the 1990s, so a new generation of this group has emerged in recent years as nanostructured lipid carriers. In general, they can be effective systems for treating bacterial and fungal infections. The large growth of this system by the pharmaceutical industry as it provides greater drug solubility, prolonged release, low toxicity and greater protection from drug degradation (Aljuffali et al., 2015, Rigon et al., 2018, Rigon et al., 2016, Selvamuthukumar and Velmurugan, 2012).
Nanostructured lipid carriers (NLC) differ from SLN in that they have two types of both solid and liquid lipids in their composition, liquid lipid generally up to 30%, to help prevent rigid matrix formation, thus enabling greater drug encapsulation and stability in the system, as there are various imperfections in the matrix, the surfactants used in these systems depend not only on the properties of the particles to be suspended, but also on the physical principles and route of administration (Fachinetti et al., 2018, Müller et al., 2002, Škalko-Basnet and Vanić, 2017).
The lipid composition and preparation technique can obtain three types: Type I - nanostructured lipid carriers which are imperfect which have a single matrix formation and can accommodate the amorphous; Type II - drug which when lipid mixing occurs does not recrystallize after cooling, obtaining solid particles containing multiple amorphous; Type III - structures where the drug solubilizes more in liquid lipid than solid, forming within the solid lipid matrix nanocompartments of oil (Fang et al., 2008, Jaiswal et al., 2016, Üner and Yener, 2007). Fig. 6 is presented the structure of these carriers.
Fig. 6.
Schematic representation of solid lipid nanoparticles in three different types: Type 1 (low oil), type 2 (amorphous) and type 3 (high oil).
Thus, these systems can be prepared by various methods such as solvent evaporation, microemulsion, sonication, high hot shear speed, homogenization and high pressure, among others (Jaiswal et al., 2016). In the solvent evaporation method two hot and cold processes can occur. The compound is dissolved or dispersed in the melted lipid before being applied to the homogenizer in both processes. High pressure (100–2000 bar) moves fluid into the narrow space of the homogenizer, resulting in small particle sizes. This method has advantages for large scale production, absence of organic solvents and stability (Al Haj et al., 2008, Naseri et al., 2015).
Microemulsion method consists of melting the lipid matrix and adding a surfactant and water until a region of microemulsion is observed. When we disperse this microemulsion in cold water under agitation the particles precipitate and according to literature data these particles can reach gauge scale sizes without energy use (Naseri et al., 2015, Obeidat et al., 2010).
The sonication method generates a strong mechanical ultrasound vibration and this generates bubbles that over time break violently, generating energy and releasing hydroxyl radicals. This turbulence and high velocity make the oil phase to be dispersed in the aqueous phase, being advantageous for its easy production (Sharma et al., 2009).
The high-speed hot shear methodology means heating the lipid phase and the surfactant at the same temperature, pouring the oil phase into the aqueous phase and stirring (Ultra-Turrax) at a set speed and time, forming a preemulsion, where it will occur a solidification of solid lipid under agitation (Jaiswal et al., 2016).
The high pressure homogenization method consists of heating the lipid phase above the melting point of the lipids, the aqueous phase with the surfactant at the same temperature, and pouring the lipid phase into the aqueous to form a pre-emulsion, providing a high speed of agitation. Nanoparticle formation occurs by propelling the pre-emulsion through a narrow cavity at various high pressure cycles. This technique has the advantage of scaling (Sánchez-López et al., 2017, Schäfer-Korting et al., 2007, Teeranachaideekul et al., 2007).
The characterization of this system can be performed by several methodologies, such as determination of the average hydrodynamic diameter and polydispersity index (PDI), zeta potential analysis (ZP) and AFM. Raza et al. (2013) showed antimicrobial activity on the skin with NLC. The average size of the NLC was approximately 80 nm. The efficacy of drug encapsulation was 79% NLC. The system showed a MIC for P. acnes (62.5 g / ml). This study observed that NLC even at low drug doses increased antimicrobial activity relative to free drug and commercial gel (Aljuffali et al., 2015, Raza et al., 2013).
Üstündaǧ-Okur et al. (2014) conducted a study with drug-borne NLC and prepared by microemulsion or high-pressure homogenizer methods for treating bacterial keratitis. The size was 254 nm ± 4.3. Permeation performed on rabbit cornea in Franz diffusion cells showed that drug containing systems were more significant than commercial solution. Microbiological studies showed antibacterial activity against E. coli and S. aureus. NLC offer a promising strategy for eye distribution.
NLC are used to mix solid and liquid lipids and, due to differences in structure, they do not fit together very well to form a perfect crystal. Thus, this arrangement creates many imperfections in the matrix, leading to the accommodation of more drug in the molecular form (Domingo and Saurina, 2012). They can be reproduced on an industrial scale easily and inexpensively, meeting the needs of the pharmaceutical and cosmetic industries, thus promoting a promising business future. However, NLC have many advantages such as using a low surfactant or co-surfactant concentration, the matrices can incorporate a large amount of lipophilic drugs, controlling release, low toxicity or immunogenicity, degradation, and side effects can be reduced, improving stability chemical physic characteristics.
Important studies in the scientific literature suggest that nanostructured lipid carriers can increase the dissolution of drugs and decrease toxicity, thus improving bioavailability and, therefore, these systems show to be very promising (Elmowafy et al., 2018, Jansook et al., 2019, Ling et al., 2019, Nordin et al., 2018, Souto et al., 2011, Yang et al., 2014). According to these advantages of NLC, in recent decades they have obtained excellent results and several applications, such as oral, topical, parenteral and ocular administration (Müller et al., 2002, Sato et al., 2017). This system can lead to some disadvantages such as irritative action by sensitivity to some surfactants, application and efficiency in the case of proteins and peptides (Jaiswal et al., 2016, Schäfer-Korting et al., 2007).
According to studies by Moreno-Sastre et al. (2016), NLC with tobramycin have been developed for Pseudomonas aeruginosa infection treatment. The NLC had an average diameter of 250 nm, encapsulation about 93%, sustained release profile and showed activity against the clinical P. aeruginosa strain. In the in vivo experiment they were administered intratracheally, and the system showed a wide distribution of the systems in the lungs. The results suggest that tobramycin-encapsulated NLC may be an encouraging alternative to therapies (Moreno-Sastre et al., 2016).
Gaba et al. (2015) incorporated terbinafine into NLC aiming the treatment of fungal infections. An average diameter of 128 nm was observed, a release of 92.60 ± 0.87% in 24 h, showing to be superior to the commercial formulation (69.41%). Pharmacodynamic studies also show a reduction in fungal burden of about 95.58 CFU with carriers and 1558 CFU with the commercial formulation, so NLC reduced the fungal load in a shorter time compared to the commercial formulation.
Studies by Kelidari et al. (2017), found a growing number of invasive fungal infections. Therefore, an alternative was to develop fluconazole-encapsulated NLC. These formulations had spherical shape, mean diameter of 126 nm, encapsulation efficiency of 93% and a controlled release of the drug. The use of NLC showed a greater decrease in the minimum inhibitory concentration against C. albicans, C. glabata and C. parapsilosis strains. This system may be a strategy for improving the antifungal activity of fluconazole against these Candida strains.
Khalil (2014) showed that NLC containing nystatin to increase its antifungal activity. It found that the particle was stable with a mean diameter of 141 nm, an encapsulation efficiency of 92%, zeta potential of −30 mV and in microbiological studies the NLC had the lowest 1.5 CFU / mL compared to commercial drug (3.5 CFU / mL). It can be concluded that nystatin in NLC has better physical stability, high encapsulation efficiency and offers effective skin treatment against fungal infections.
Fu et al. (2017) observed that amphotericin B-loaded and nanostructured lipid carriers and chitosan-modified (AmB-CH-NLC) potential, for fungal keratitis. The characterization of the AmB-CH-NLC, showed a size of 185 nm, 27 mV zeta potential module, encapsulation efficiency about 90% and a controlled release of amphotericin B. In vivo eye pharmacokinetic studies showed better bioavailability and eye penetration studies in rabbit cornea obtained promising results and further noted that the formulation does not cause eye irritation. It can be concluded that nanostructured lipid carriers are an effective drug delivery system and can provide extra-ocular medication.
Garcia-Orue et al. (2016) developed a LL37 loaded nanostructured lipid carriers (NLC-LL37) to aid in the healing of chronic wounds. The NLC-LL37 obtained a size of 270 nm, and 26 mV zeta potential module, 96% encapsulation efficiency, cytotoxicity tests on fibroblasts where there was no inhibition in cell viability. Antimicrobial tests showed that NLC-LL37 has activity against E. coli and an in vivo wound model was evaluated, where it showed that healing improved significantly with NLC-LL37 when compared to the peptide alone. The authors concluded that NLC-LL37 has a very promising potential to chronic wounds treatment.
Mendes et al. (2013) developed a miconazole-loaded nanostructured lipid carriers (NLC) to improve their therapeutic activity. The formulation showed a droplet size of 200 nm, a low polydispersity (<0.3), good physical stability and high encapsulation efficiency being greater than 87%. The NLC incorporated with hydrogel, obtained a more controlled release of miconazole, when compared with a commercial oral gel, suggesting a better antifungal activity against Candida albicans. Therefore, he observed that the dose of miconazole administered with the NLC could be less than the commercial one and obtain a therapeutic effect as good as a commercial formulation.
Studies by Song et al. (2015) developed Rifampicin-loaded Mannosylated Cationic Nanostructured Lipid Carriers (RFP-Man-NLC) to deliver rifampicin to alveolar macrophages. The systems were characterized by a particle size of 160 nm, a polydispersity index of minus 0.3, a zeta potential module of 30 mV and the encapsulation efficiency of approximately 90%. In cellular studies he observed that the RFP-Man-NLC achieved a greater targeting capacity in the lungs than those available on the market. Thus, it is concluded that the RFP-Man-NLC can establish a strategy for greater selectivity in alveolar macrophages.
According to studies by Moreno-Sastre et al. (2016) Tobramycin-loaded nanostructured lipid carriers (Tb-NLC) was developed to combat infection by Pseudomonas aeruginosa, as it is among the pathogens that most affects patients with cystic fibrosis. Thus, the characterization of the systems showed a size of 250 nm, an encapsulation efficiency of 93% and a controlled release. Tb-NLC showed antimicrobial activity against Pseudomonas aeruginosa and did not affect cell viability. The in vivo experiments showed a wide distribution of the system in the lungs, suggesting this very promising system for therapies against cystic fibrosis.
Üstündaʇ-Okur et al. (2015) develop an ofloxacin-loaded nanostructured lipid carriers (OFX-NLC) for the treatment of bacterial keratitis. The system was characterized with a size of 153 nm, polydispersity 0.188 and zeta potential module 24 mV and a release around 86%. In the in vivo studies, rabbits were infected with Staphylococcus aureus and observed a pre-ocular OFX-NLC retention increased by 24 h and a Cmax six times higher than the commercial one. Therefore, the authors suggest that structured systems may be strategies for treating bacterial keratitis.
In the study of Bolla et al. (2019), the furosemide-silver complex was incorporated into solid lipid nanoparticles (SLN-Ag-FSE) and the antibacterial activity was investigated. The characterization tests show that the size, polydispersity index and zeta potential were 129.8 nm; 0.114 ± 0.033; −23.9 ± 3.62 mV, respectively. The nanoparticles showed encapsulation efficiency of 93%, a sustained release of 96 h and an increase of 2 and 4 times the activity against Pseudomonas aeruginosa and Staphylococcus aureus, respectively. Suggesting that SLN-Ag-FSE is a promising antimicrobial agent against bacterial infection.
Carneiro et al. (2019) showed the potential of nanostructured lipid carriers loaded with rapamycin and functionalized with peptide (NP-pRIF) for tuberculosis treatment. A characterization of this type selected for a particle size of 285 nm, polydispersity of 0.18, a potential modulus of 22 mV, an encapsulation efficiency of 81% and a selected selection profile. In cellular assays, functional nanoparticles were more internalized in macrophages than nanoparticles with rifampicin alone. Thus, the authors suggest that this change can be effective and promising against tuberculosis and allow less side effects (Carneiro et al., 2019).
Table 8 summarizes the results of several studies related to the application of SLN and NLC in the treatment of systemic infections.
Table 8.
Solid lipid nanoparticles and NLC applied to the treatment of systemic infections.
| Available in the clinical practice for systemic micr | Available in the clinical practice for systemic micr | Available in the clinical practice for systemic micr | Available in the clinical practice for systemic micr | Available in the clinical practice for systemic micr | Available in the clinical practice for systemic micr |
|---|---|---|---|---|---|
| Nanostructured lipid carriers | Ofloxacin | oleic acid; Tween 80; benzoyl polyoxyl-8 glycerides; chitosan oligosaccharide lactate; water. |
E. coli S. aureus |
ocular | Üstündaǧ-Okur et al., 2014 |
| Nanocolloidal Carriers | Isotretinoin | Phospholipon 90 G; butylated hydroxytoluene; Tween 80; benzoyl polyoxyl-8 glycerides; Isopropyl Myristate; water. | P. acnes | Skin | Raza et al., 2013 |
| nanostructured lipid carriers | tobramycin | glyceryl palmitostearate; benzoyl polyoxyl-8 glycerides; miglyol ® 812; Tween 80; Poloxamer 188; water. | P. aeruginosa | intratracheal | Moreno-Sastre et al., 2016 |
| Nanostructured lipid carrier | terbinafine hydrochloride | Glyceryl Monostearate; Labrasol; Pluronic F-127; water. | C. albicans | topical | Gaba et al., 2015 |
| nanostructured lipid carrier | fluconazole | benzoyl polyoxyl-8 glycerides; stearic acid; oleic acid; Tween 80; water. |
C. albicans C. glabrata C. parapsilosis |
topical | Kelidari et al., 2017 |
| Nanostructured Lipid Carriers | Nystatin | Miglyol; Compritol; Poloxamer 188; water. | C.albicans Aspergillus spp. | topical | Khalil, 2014 |
| Nanostructured lipid carriers | Amphotericin B | Compritol® 888; lecithin; soybean oil; quitosana; poloxamer 188; water. | Candida, Curvularis Aspergillus | topical | Fu et al., 2017 |
| Nanostructured lipid carriers | Peptide LL37 | Poloxamer 188; Tween 80; Precirol® 5; Miglyol® 812; Water. | E. coli | topical | Garcia-Orue et al., 2016 |
| Nanostructured lipid carriers | Miconazole | Gelucire®; Miglyol®; Tween® 80; Gelling PFC®; Glycerin; Benzalkonium chloride; Water. | C. albicans. | topical | Mendes et al., 2013 |
| Nanostructured lipid carriers | tobramycin | Precirol® ATO; Compritol® 888; Miglyol®812; Tween® 80; Poloxamer 188; Water. | P. aeruginosa | intratracheally | Moreno-Sastre et al., 2016 |
| Nanostructured lipid carriers | Rifampicin | Ovolecithin; Medium chain triglyceride; Octadecylamine; Dimethyldioctadecylammonium bromide; Water. | M. tuberculosis | intratracheally | Song et al., 2015 |
| Nanostructured lipid carriers | ofloxacin | Compritol®; oleic acid; Tween® 80; glycerin; water. | S. aureus | topical | Üstündaʇ-Okur et al., 2015 |
| Solid lipid nanoparticles | furosemide-silver | Glycerol monostearate; Poloxamer 188; sucrose; water | P. aeruginosa, S. aureus | topical | Bolla, et al., 2019 |
| Nanostructured lipid carriers | Rifampicin | Acid stearic; oleic acid; phospholipon 80H; tween 80; water | M. tuberculosis | Pulmonary | Carneiro et al., 2019 |
3.7. Polymeric nanoparticles
Polymeric nanoparticles (PNs) represent an area of application of controlled release technology that has been in clinical use for over 25 years for local and systemic drug administration. They are classified into nanometric structures ranging from 10 to 1000 nm, providing physicochemical properties that make them important release systems such as small size, large surface area and various surface charge characteristics. In this context, PNs attract interest due to their easily adjustable properties such as size, composition and functionalization of their surface (de Freitas et al., 2016, Markwalter et al., 2019, Moinard-Chécot et al., 2008, Troiano et al., 2016).
The term polymeric nanoparticle includes two types, nanocapsules and nanospheres, which differ according to composition and structural organization. Nanocapsules consist of a polymeric shell arranged around a nucleus, which may be oily or not, and the active principle may be dissolved in this nucleus and / or adsorbed to the polymeric wall. On the other hand, the nanospheres comprise a polymeric matrix, in which the active compounds and the polymer are uniformly dispersed, and do not have oil in their composition (Fig. 7 ). Thus, considering the encapsulation mechanisms, drug molecules may be trapped within, dispersed in the polymer matrix or adsorbed to the surface of nanoparticles (Nagavarma et al., 2012).
Fig. 7.
Schematic representation of polymeric nanoparticles.
A variety of polymers has been used in the preparation of PNs, being biocompatible and biodegradable and may be of natural or synthetic origin. Chitosan, albumin, alginate, hyaluronic acid, collagen and gelatin are the most commonly used natural polymers. Similarly, glycolic acid (PGA), poly-ɛ-Caprolactone (PCL), poly (lactic acid) (PLA) are some of the widely used synthetic polymers (Mahmoudi Saber, 2019, Mansoor et al., 2019).
Surfactants are used to prevent aggregation of PN after preparation and during storage, promoting physical and / or chemical stabilization. The most commonly used are nonionic high hydrophilic polysorbates (Tween®) and poloxamers (Pluronic®) (Bouchemal et al., 2004). While choosing the organic solvent, account should be taken of the lower toxicity and ability to solubilize the active ingredient and the polymer. In addition, the method must be taken into account as there are those that use water miscible organic solvents. Among the most used are acetone, ethanol and ethyl acetate (Pinto Reis et al., 2006).
Among the many methods for the synthesis of PNs, the choice of method is due to the necessary requirements for its application, as well as the characteristics of the incorporated active principle (hydrophobic and hydrophilic molecules). Thus, the choice of the appropriate method is essential to obtain PNs with desired properties, being divided into two groups: from polymerization of monomers and from preformed polymers (Crucho and Barros, 2017, Urrejola and Liliam et al., 2018).
In the polymerization method, the monomers are polymerized to form the encapsulating polymer in two ways: using emulsion polymerization or interfacial polymerization techniques. However, some drawbacks have been reported, limiting the use of polymerization methods for PNs synthesis. Thus, the preparation using preformed polymers is more easily controllable, higher yielding and can be performed by solvent emulsification-evaporation, solvent displacement or nanoprecipitation, solvent emulsification-diffusion or salting-out (Crucho and Barros, 2017, Souto et al., 2012).
In choosing the ideal preparation method, one should take into consideration the physicochemical characteristics of the active ingredient chosen for incorporation, minimizing its loss and ensuring its pharmacological activity.
As characterization of the PNs, size exclusion chromatography techniques are used, which provide the polymer weight and weight distribution after the preparation of the PNs, DLS, low angle X-ray scattering, and microscopy techniques as scanning electron microscopy (SEM), TEM and AFM (Guterres et al., 2007, Kirby and Hasselbrink, 2004, Schaffazick and Guterres, 2003).
The use of PNs as drug delivery systems are significant due to the many characteristics presented by polymers, such as simplicity of synthesis, controllable molecular weight, low cost, environmental responsibility, as well as biocompatibility and biodegradability. PNs should be non-toxic, non-immunogenic, have sufficient amounts and release the active ingredient at the optimal dose. It has been shown that polymer-drug conjugates can improve blood residence time and reduce side effects (Alexis et al., 2008, Li et al., 2018).
The use of biodegradable polymers is of great interest, as it reduces the toxicity of the system. NP studies have shown the non-toxicity of the system (Kuskov et al., 2016, Osorio et al., 2016, Sanoj Rejinold et al., 2011). However, it is worth emphasizing the importance of studying the entire composition of the system as well as the constituents separately (Grabowski et al., 2015).
Advantages of applying PNs as a carrier of molecules include the possibility of modulating the diameter and characteristics of PNs, which, having a small diameter, allow intravenous administration, as well as other routes such as oral, nasal, intraocular and topical, high stability, long storage stability and increased biocompatibility and biodegradability(Ramos et al., 2018b). However, there are some limitations due mainly to the products being aqueous colloidal suspensions as aggregation of nanoparticles can occur in the medium, resulting in the formation of precipitates, problems of chemical stability of the polymer, the active ingredient or other raw materials, and premature release of the active substance. Thus, poor physicochemical stability, as a function of time, is a limitation for the various applications of aqueous nanoparticle suspensions, which can be minimized by drying the suspensions through operations such as freeze drying or spray drying (Mora-Huertas et al., 2010, Pinto Reis et al., 2006, Saez et al., 2000).
Amphotericin B (AmB) is a broad-spectrum antifungal, but it has clinical toxicities and low solubility, limiting the broad application of AmB in clinical practice. Thus, Tang et al. (2014) developed Dibloco copolymer D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly (ε-caprolactone glycolide) succinate (PLGA-TPGS) PNs (AMB-PNs). AMB-PNs were prepared by the modified nanoprecipitation method and were evaluated against a Candida albicans infection model. The therapeutic efficacy of AMB-loaded PNs was evaluated by comparing C. albicans infected mice with and without intravenous therapy. The results showed a significant reduction in CFU of different organs, mainly kidney and spleen, after AmB-NP therapy. Regarding mortality of C. albicans infected mice without AmB therapy was 100% after 10 days, while the survival rate of C. albicans infected mice treated with free AMB and AMB-PNs was 30.0 and 80.0%, respectively. These results suggest that AMB-PNs had better free AmB activity, also suggesting that the frequency of antifungal therapy is lower for AMB-PNs than for free AMB, when therapeutic efficacy is comparable.
Van De Ven et al. (2012) describe the development of poly (D, L-lactide-co-glycolide) (PLGA) and amphotericin B (AmB) nanoparticles by the solvent displacement method (nanoprecipitation). In vitro tests were performed on Candida albicans, Aspergillus fumigatus and Trichophyton rubrum. However, in vivo efficacy was evaluated against Aspergillus fumigatus, and compared to Fungizone and AmBisome. A female mouse model was used, which was infected through the lateral tail vein, and then received intraperitoneal (PI) treatment. PLGA PNs have been shown to be about twice as active compared to AmBisome, representing a powerful and economical alternative to Fungizone and AmBisome.
Casciaro et al. (2019) developed poly (lactide-co-glycolide) (PLGA) PNs for pulmonary release of antimicrobial peptides (AMPs) (Esc peptide). The PNs were prepared by the solvent diffusion-emulsion technique, using methylene chloride and ethanol as solvent. A single intratracheal administration of Esc-peptide-loaded nanoparticles in a mouse model with P. aeruginosa lung infection resulted in a reduction of 3 logarithms of pulmonary bacteria up to 6 h. Overall, the results reveal the potential of PLGA nanoparticles as a reliable system for delivering AMPs to the lungs.
PNs can also be used to transmit vaccine antigens, as in the case of the work by Dhakal et al. (2018). Chitosan nanoparticles (CNPs) were prepared to incorporate the killed SwIAV H1N2 (δ-lineage) antigens (KAg) to obtain CNPs-KAg. The system was developed using the ionic gelation method in 4.0% aqueous acetic acid solution. A vaccination model was carried out on crossbred piglets, which have the absence of antibodies and colostrum. Two doses of vaccination were performed intranasally (IN) and the system developed showed an increase in the levels of IgA antibodies, in addition to the systemic response of IgG antibodies. In addition to the other trials, the study suggests the study of future comparative studies of CNPs-KAg and commercial vaccines.
Yang et al. (2020) developed PLGA PNs using the emulsification-solvent evaporation technique. The system was designed to encapsulate an antibiotic, Sparfloxacin (SFX) and an immunosuppressant, Tacrolimus (TAC). In addition, the NPs were conjugated to the y3 peptide, y3-PLGA/S + T PNs, to target the intercellular adhesion molecule-1 (ICAM-1), present on the surface of inflammatory endothelial cells. A model of acute pulmonary infection was performed in mice by P. aeruginosa through the intratracheal route. Free SFX solution, free TAC, free SFX + free TAC, conjugated and unconjugated NPs were administered intravenously. The NPs conjugated with y3 peptide demonstrated that the targeting to ICAM-1 allowed a greater bacterial inhibition, confirming a better delivery of antibiotics. In addition, a minor inflammatory response has been reported, which may be related to decreased bacterial concentration. Thus, the study brings the use of PN functionalization as an alternative for the treatment of sepsis.
In the work of Scalise et al. (2016) the PNs the Lutrol® F-68 (P188) were prepared by the solvent diffusion method, better known as nanoprecipitation, using ethanol as an organic solvent. The objective was to encapsulate Benznidazole (BNZ) (BNZ-nps) to evaluate the activity of this system in the treatment of chagas disease. A model of acute infection of Trypanosoma cruzi Nicaragua was evaluated in mice, through the intraperitoneal introduction of trypomastigotes inoculum. Doses of 10, 25 and 50 mg/kg/day were administered with BNZ-nps via oral gavage. It was possible to observe the dose-dependent antiparasitic effect, as the two highest concentrations allowed 100% survival in 50 days, while at the concentration of 10 mg/kg/day a 70% survival rate was acquired after 38 days of experiment. Thus, BNZ-nps can be considered an option for the treatment of Chagas disease.
Abdelkader et al. (2017) developed chitosan nanoparticles by the method of ionic gelation for encapsulation of Meropenem, a broad-spectrum beta-lactam antibiotic. In vivo biological activity was evaluated in a model of systemic infection by Klebsiella pneumoniae sensitive to meropenem. The bacterial inoculum, as well as the treatments, were injected intraperitoneally. The survival rate of untreated rats treated with meropenem solution and meropenem nanoparticles was analyzed. The developed system allowed a 100% survival in 48 h, indicating a potentiation of the antibiotic, since the same in solution allowed a survival of only 30%. In addition, it was observed that the nanoparticles allowed to prolong the bactericidal activity of meropenem, which is suggested by the presence of chitosan. Thus, chitosan nanoparticles can be an alternative for encapsulation of meropenem with potential treatment for systemic infections.
Analyzing antifilarial activity, Ali et al. (2013), evaluated the activity of ivermectin (IVM) encapsulated in chitosan-alginate nanoparticles (CS-ALG). The IVM CS-ALG nanoparticles were developed using the complex coacervation method. A host parasite model was performed, using Mastomys coucha, where they were infected and treated subcutaneously. Microfilarial load was assessed through systemic circulation. The IVM CS-ALG showed potent, allowing total elimination on day 60. Maintaining until the end of the experiment. The study is a biocompatible and safe system that allows an improvement in ivermectin activity in antifilarial treatment.
Table 9 summarizes the results of several studies related to the application PNs in the treatment of systemic infections.
Table 9.
Polymeric nanoparticles applied to the treatment of systemic infections.
| Formulation | Active principle | Composition | Therapeutic target | Route of administration | Reference |
|---|---|---|---|---|---|
| AMB- PNs | Amphotericin B (AmB) | Diblock copolymer D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly(ε-caprolactone-ran-glycolide) (PLGA-TPGS) | Candida albicans | intravenous | Tang et al., 2014 |
| PLGA PNs | Amphotericin B (AmB) | poly (D, L-lactide-co-glycolide) (PLGA), AmB, DMSO or DMSO/acetone (1:1), PVA (stabilizing solution) | Aspergillus fumigatus | intraperitoneal | Van de Ven et al., 2012 |
| Esc_ Fluo- PNs | peptide Esc | poly (D, L-lactide-co-glycolide) (PLGA, peptide Esc, methylene chloride, ethanol | Pseudomonas aeruginosa | intratracheal | Casciaro et al., 2019 |
| CNPs-KAg | SwIAV H1N2 antigens | Chitosan, SwIAV H1N2, acid acetic, | Virulent SwIAV | intranasally | Dhakal et al. 2018 |
| y3-PLGA/S + T NPs | Sparfloxacin and Tacrolimus | PLGA, dichoromethane (DCM), Bovine serum albumin, y3 peptide | Pseudomonas aeruginosa | intratracheal | Yang et al. 2020 |
| BNZ-nps | Benznidazole | Lutrol® F-68 (P188), Benznidazole, ethanol | Trypanosoma cruzi | oral | Scalise et al. 2016 |
| Meropenem-loaded nanoparticles | Meropenem | Chitosan, acetic acid, Meropenem | Klebsiella pneumoniae | intraperitoneally | Abdelkader et al. 2017 |
| IVM CS-ALG | Ivermectin | Chitosan, alginate, acetic acid solution | Brugia malayi | subcutaneously | Ali et al., 2013 |
PNs: Polymeric nanoparticles; AMB/AmB: amphotericin B; AMB-NPs - Amphotericin B polymeric nanoparticle with Dibloco copolymer D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly (ε-caprolactone glycolide) succinate; PLGA-TPGS: Diblock copolymer D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly(ε-caprolactone-ran-glycolide); PLGA: poly(d, l-lactide-co-glycolide); PLGA PNs – Polymeric nanoparticle of poly (lactide-co-glycolide) with amphotericin B; DMSO: dimethyl sulfoxide; PVA: polyvinyl alcohol; Esc_ Fluo-NPs: rhodamine-labeled NPs loaded with Esc(1–21); BNZ: Benznidazole; BNZ-nps: polymeric nanoparticles with benznidazole; IVM CS-ALG: chitosan-alginate nanoparticles with ivermectin; CNPs-KAg: - Killed SwIAV H1N2 (δ-lineage) antigens were encapsulated in chitosan polymer-based nanoparticle; SwIAV H1N2: Influenza A virus; y3-PLGA/S + T NPs – Polymeric nanoparticle of poly (lactide-co-glycolide) with Sparfloxacin and tacrolimus and conjugated with y3 peptide; DCM: dichoromethane; P188: Lutrol® F-68.
3.8. Hydrogels
Some important routes of drug administration act as a barrier to the entry of drugs into the body, limiting the absorption of drugs. This is due to the physiological defense mechanisms, which eliminate any exogenous substance; consequently, drastically decreasing the period of permanence of the medication at the absorption site (Komati et al., 2019). Thus, the mucoadhesion mechanism is an efficient strategy to prolong the duration of the medication in contact with the absorption site. This is the main objective of hydrogels. In addition, the drug delivery system known as hydrogels, also allows for the controlled administration of drugs in local and systemic administration. Thus, this medication administration system is an efficient alternative in the administration of medications for mucus-producing routes, such as the nasal, vaginal, buccal and ocular routes (Collado-González et al., 2019).
The search for alternative routes of drug administration is becoming increasingly important; because the limitations of conventional drug delivery routes lead to the loss of most of the drugs administered. Not to mention the increased cost of treatment. The most important factor is the failure of treatment, due to the low plasma concentrations of the drug that do not reach the therapeutic dose. In the case of systemic infection, this is an extremely important factor, since a lower concentration that minimum inhibitory concentration of the drug, generate resistance to the pathogen and cause intractable complications (Ways et al., 2018). With the advent of mucoadhesive systems such as hydrogels, it became possible to use alternative routes for administering medications, such as nasal, vaginal, oral and others (Laffleur, 2014).
The hydrogels must be capable of transporting both hydrophilic and lipophilic drugs, in addition to delaying the action of enzymes of administration pathway and increase drug absorption effect. When this system prioritize systemic action, should to have Ability to induce or potentiate the endocytosis (Laffleur, 2014).
As any mucoadhesive system, hydrogels can be classified according to their surface load, and can be cationic, anionic, non-ionic. The cationic systems are able to interact with the mucus surface, due to its positive charge in physiological pH. In this environment there are electrostatic interactions between the surface of the system and the amino group in the mucous layer. In anionic systems, however, there is bind of van der Waals, hydrogen bind and hydrophobic interactions between the mucoadhesive system and a mucosal surface, these interactions being regulated by the ionic charge and the pH of the medium. Non-ionic systems are less common than those mentioned earlier due to weak and less effective interactions (Hombach and Bernkop-Schnurch, 2010).
In general, hydrogels have several advantages, among them, the biocompatible potential. Hydrogels have several biomedical applications due to their flexibility characteristics that can absorb large amounts of water, saline and body fluids; in addition to the low chronic or acute inflammatory potential, low immunogenic and mutagenic potential; in addition to tolerable levels of cytotoxicity (Nawaz et al., 2018).
Currently, several techniques are used to characterize hydrogels, such as spectroscopic and spectrometric techniques, scattering techniques, microscopy, chromatography, electrophoretic techniques; in addition to techniques to verify the bioadhesion directly accessible through the chosen administration (Collado-González et al., 2019).
Listed below are some studies that relate the use of hydrogels systems to administer medications for the treatment of systemic infections.
Pereira et al. (2013) described the developed and characterization of mucoadhesive hydrogel thermo-responsive consisting of polaxamer 407, Carbopol 934P for the delivery of propolis in the treatment of vulvovaginal candidiasis. The authors related the characterization of mucoadhesive systems according to hardness, compressibility, adhesiveness, elasticity, cohesion, mucoadhesion, rheology and antimicrobial activity. The antimicrobial activity tests were performed against Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis and Saccharomyces cerevisiae. The mucoadhesive system developed showed activity against all tested yeasts. According to the authors the results obtained, they demonstrated the development of a useful platform in the case of drug delivery in the treatment of candidiasis, and still suggest results worthy of clinical evaluation.
Grolman et al. (2019) develop an agarose hydrogel with high doses of cyclocycline or gentamicin to treat wound infections by controlling systemic infections. According to the authors of article, the hydrogel is an attractive system for the topical administration of antimicrobials; since antibiotic therapy administered by the conventional pathways is limited due to its low reach in the lesions. Especially burns and wounds where the microcirculation of tissues is interrupted. The authors state that the developed agarose hydrogel is very promising, as it allows application at the site of the lesions, good biodegradation and adequate mechanical, chemical and biological resistance. In addition, the hydrogel created a moist environment, benefiting the healing of the lesion. The results obtained demonstrated that the ideal concentration of agarose in the hydrogel was 0.5%, allowing good viscosity and adequate pH. The tests showed that the antibiotics remain stable in the hydrogel for 7 days. Still the in vivo test indicated that the hydrogel with minocycline decreases the depth of the lesion, the amount of the number of bacteria; and even more, the same efficiency as the silver sulfadiazine cream available on the market.
Gustafson et al. (2016) developed a hydrogel matrix consisting of oligo (poly (ethylene glycol) fumarate) / sodium methacrylate for the delivery of vancomycin for surgical applications. The hydrogel matrix was efficiently loaded with vancomycin and according to the authors the kinetics of drug release and dependent on the negative charge of the copolymer. The release results showed that in the first 6 h the release reached 33.7% and at the end of 24 h 80% of the initial vancomycin had been released. Antimicrobial tests showed methicillin-resistant growth inhibition of methicillin-resistant Staphylococcus aureus. Finally, the authors concluded that hydrogels are suitable platforms for the prophylaxis of surgical site infections.
The development of injectable mucoadhesive systems is the way to make possible the use of these systems in the treatment of sistemic infections. Liang et al. (2019) develop injectable hydrogels with tissue adhesiveness and responsive to pH. According to the authors, these hydrogens have good injectability, gelation time, morphology and satisfactory rheological characteristics. In the drug release assay, the system developed a pH-responsive release. Also, the hydrogels described have a good antibacterial activity against E. coli and S. aureus for delivery amoxicillin. According to the authors, all the results obtained suggest that adhesive and injectable hydrogels are good candidates for a drug delivery platform.
Johnson et al. (2018) designed an injectable PEG hydrogel to deliver lysostaphin to treat Staphylococcus aureus infections in bone fractures. Knowing that the lysostaphin have bacteriolytic and anti-Staphylococcal activity and taking into account that the commonly are responsible orthopedic implants for systemic bacterial infections; the PEG hydrogel with concertation of lysostaphin is a promising platform for prophylaxis of infections in cases of orthopedic surgery. The hydrogel guaranteed the stability of the enzyme, maintaining antibiotic activity, exhibiting an improved antibiofilm activity in relation to the soluble lysostaffin. The hydrogels eliminated infections by S. aureus, results observed murine model of fracture of the femur. Inflammatory response tests also showed superior results in the profile of cytokine secretion in lesions treated with hydrogel. Still, the authors state that fractures exposed to infections and treated with hydrogel were completely healed in 5 weeks, observing bone formation and equivalent properties to uninfected fractures. Hydrogels containing lysostaffin completely eliminated infection by methicillin-resistant S.aureus. Finally, the authors state that the results presented show that the hydrogel eradicated, orthopedic infections and simultaneously helped in bone repair and constitution
Although in the vast majority of cases of systemic infections the etiologic agent is bacterium; it is necessary to mention that viruses are also a major concern when it comes to systemic infection. Among the many viral agents, the Herpes Simplex virus is highly incident in human infections. Jain et al. (2016) developed a system for administering acyclovir, composed of cholestyramine resin, sodium alginate and Carbopol with gastro-mucoadhesive characteristics. The system developed with the aim of being used in the treatment of the herpes simplex virus. According to the authors, the characterization of the system was carried out using techniques such as scanning electron microscopy, differential scanning calorimetry and infrared spectroscopy by Fourier. The characterization results showed the formation of the nanostructured system through interactions of the polyelectrolytic complex. Acyclovir release studies have also revealed that the system is able to control release due to the formation of a drug-resin complex. The authors claim that the developed system is effective for the delivery of acyclovir and other antivirals and can be a useful system in the treatment of herpes simplex
Table 10 summarizes the results of several studies related to the application of hydrogels in the treatment of systemic infections.
Table 10.
Hydrogels applied to the treatment of systemic infections.
| Formulation | Drug | Composition | Therapeutic target | Route of administration | Reference |
|---|---|---|---|---|---|
| Mucoadhesive thermo-responsive systems | Propolis | Polaxamer 407 + Carbopol 934P | Candida spp | vaginal | Pereira et al., 2013 |
| Hydrogel | Cyclocyclin/gentamicin | Agarose | Staphylococcus aureus | Topical | Grolman et al., 2019 |
| Hydrogel | Vancomycin | Oligo(poly(ethylene glycol)fumarate/sodium metacrylate | Staphylococcus aureus | Topical | Gustafson et al.,2016 |
| Injectable mucoadhesive hydrogel | Amoxicilin | Chitosan-grafted-dihydrocaffeic acid + oxidized pullulan |
Staphylococcus aureus Escherichia coli |
Intravenous | Liang et al., 2018 |
| Injetable hydrogel | Lisostaffin | Polyethylene glycol | Staphylococcus aureus | Intravenous | Johnson et al.,2018 |
| Gastro-mucoadhesive hydrogel | Acyclovir | Cholestyramine resin/sodium alginate/carbopol | Herpes Simplex virus | Oral | Jain et al.,2015 |
3.9. Liquid crystals
Liquid crystal systems are amphiphilic systems, used to deliver drugs, proteins, peptides and nucleic acids. These systems are classified according to the complexity of their mesophases (Ubbink et al., 2008). The lamellar mesophase is a mesophase with a lower level of organization, usually systems consisting of lamellar mesophase are completely liquid and fluid and do not have mucoadhesive characteristics. The hexagonal mesophase has an intermediate level of organization, with mucoadhesive characteristics and medium viscosity. Finally, the cubic mesophase the mesophase with a higher level of complexity and organization with extremely low fluidity and high viscosity, consequently high mucoadhesion potential. Due the intrinsic characteristics as hexagonal and cubic, this mesophases are the most used for drug delivery for your ability to sustain and control the release of a wide range of drugs (Mohammady et al., 2009). Usually, the hexagonal and cubic mesophases consist of polar lipids in aqueous solution. These lipids must have a water absorption spontaneously forming a network in which they are incorporated or drugs (Spicer, 2005). Currently, these mesophases are being widely used for drug delivery by alternative routes of administration such as buccal, pulmonary, nasal, rectal and vaginal. In addition, the liquid crystals have advantages due to their low cytotoxicity, high biocompatibility and biodegradable characteristics (Guo et al., 2010, Ramos et al., 2016, dos Ramos et al., 2015, Silva et al., 2014).
Although the liquid crystals meets all the needs for a drug delivery system for the treatment of systemic infections and is possibly a promising platform for the treatment of these infections; the data available in the literature concerning this type drug administration system for systemic infection are still extremely scarce. Making clear the need for studies on these points.
Listed below are some studies that relate the use of the mucoadhesive system for delivering drugs for treating local infections, however such pathogens in other cases they are primarily responsible for systemic infections.
Carvalho et al. (2013) created a low-viscosity liquid crystal precursor with lyotropic behavior for the administration of zidovudine in nasal administration. A physical-chemical characterization was performed using rheology techniques where the system shows the behavior of Newtonian flow, polarized light microscopy that showed the isotropic characteristics, mucoadhesion tests was that was performed exhibiting adequate mucoadhesiveness for nasal administration. For in vivo tests, plasma zidovudine concentration was quantificated by HPLC after intranasal administration, showing a process of rapid absorption through the nasal mucosa; since the nasal region is highly vascularized and the absorptive capacity is extremely high. According to the authors or precursor system of liquid crystal administered via nasal route is a promising tool for the systemic administration of zidovudine and other antiretroviral drugs in the treatment of HIV.
Pisano et al. (2019) developed a liquid crystal platform with the objective of controlled release of ciprofloxacin for the treatment of infections of the female reproductive tract. Second, the authors the liquid crystal platform containing ciprofloxacin, have increased bactericidal efficacy and reduced cellular toxicity. In addition, the liquid crystal platform offers a slow drug release profile, eliminating a need for countless applications allowing for prolonged treatment. Also, the liquid crystal platform has low cytotoxicity for cervical epithelial (HeLA) or endomic (HEC1A) cells. The authors state that the use of liquid crystal with ciprofloxacin in the treatment of cells infected with Escherichia coli, is viable alternative to reduce bacterial. The results obtained suggest that this ciprofloxacin delivery platform can be used to improve the effectiveness of the conventional antibiotic therapies
Miyashiro et al. (2020) developed a precursor of liquid crystal for the delivery of fluconazole in the treatment of oral candidiasis. The liquid crystal precursor was constituted by surfactant, oily phase (trans-resveratrol) and polymer dispersion. Second, the authors the system was able to transit from microemulsion to crystal liquids when in contact with the aqueous solution, still with a presence of sodium alginate was capable which increased the mucoadhesion strength of the system. Microbiological tests in the presence of Candida albicans showed positive antimicrobial effect.
Table 11 summarizes the results of several studies related to the application of liquid crystals as potential platforms in the treatment of systemic infections.
Table 11.
Liquid crystals as a potential drug delivery system for systemic infections treatment.
| Formulation | Drug | Composition | Therapeutic target | Route of administration | References |
|---|---|---|---|---|---|
| Liquid crystal | Zidovudine | PPG-5-CETETH-20/oleic acid | Human immunodeficiency virus | Nasal | Carvalho et al., 2013 |
| Liquid crystal | Ciprofloxacin | Monomuls®90-018 | Escherichia coli | Vaginal | Pisano et al.,2019 |
| Precursor of liquid crystal | Fluconazole | PPG-5-CETETH-20/trans-resveratrol/sodium alginate | Candida albicans | Oral | Miyashiro et al., 2020 |
PPG-5-CETETH-20: Procetyl®AWS.
4. Future directions
Responsible for a serious problem of public health worldwide, the pandemic caused by the new Coronavirus, SARS COV 2 (COVID-19), causes great scientific interest in the development of new therapies against infection. Nano interventions are being developed with the objective to be used as promising antivirals.
Innovative strategies, such as nanocarriers, that work by the drug directing to the target site in a safer and more effective way, blocking the initial interactions of the viral peak glycoprotein with the receptors on the host cell surface and interrupting the construction of new virions in the cells, inhibiting viral spread. These nanocarriers offer greater safety and better efficiency in crossing biological barriers and reaching therapeutic concentrations in protected viral reservoirs (Chauhan et al., 2020)
All intelligent drug delivery and transport systems, such as liposomes, microemulsion, metallic nanoparticles, dendrimers, nanoemulsion and others, can be applied in preclinical and clinical studies in the treatment and prevention of SARS COV 2, mainly due to the ease of combination hydrophilic and hydrophobic drugs (Chauhan et al., 2020, Gadde, 2015, Hu et al., 2011).
Other nanotechnology tests are the production of nanocarriers containing antigens, to be used as vaccines. These nanocarriers provide a higher rate of immunization, improve the delivery and defense of the antigen in the organism. Administered orally or intravenously, these systems have the advantage in their size (nanometers), since microorganisms, such as virus, have the size in nanoscale (Park et al., 2013)
5. Final considerations
The actual scenario of microbial systemic infections is big lead at science to explore new alternatives to compose the antimicrobial arsenal with new strategies to control and treatment of ill patients. According to the scientific reports presented in this review article, it may be concluded that the application of nanotechnology in drug delivery systems has enormous potential and can be considered as an effective alternative for the improvement of systemic infections treatment. The ability exerted by the presented systems in the microbial inhibition is a promising characteristic as it allows the use of drugs available in clinical practice in a more efficient manner that guarantees overcoming of the constraints related to the bioavailability of the antimicrobials. Among drug delivery systems for systemic microbial infections treatment, we reported metallic nanoparticles (MNPs), liposomes, microemulsion, nanoemulsion, dendrimers, solid lipid nanoparticles, nanostructured lipid carriers, polymeric nanoparticles, hydrogels and liquid crystals, systems with different compositions and completely different physical–chemical characteristics. However, systems that can be considered the most promising are those having in their composition biocompatible lipids with transition temperature relatively low so that one can have a better fluidity of the formulation and potentially better interaction with the membrane of the microorganism, and consequently better activity, and being the biocompatible material, the possibility of toxicity to humans is reduced, that is, with these characteristics there is a highly selective formulation for the pathogen.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank São Paulo Research Foundation - FAPESP, Brazil (grant #2018/23442-2). This work was financed in part by the “Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior-Brazil” (CAPES)-Finance code-001. This study is part of the National Institute of Science and Technology in Pharmaceutical Nanotechnology: a transdisciplinary approach INCT-NANOFARMA, which is supported by São Paulo Research Foundation (FAPESP, Brazil) Grant #2014/50928-2, and by “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq, Brazil) Grant # 465687/2014-8.
References
- Abdelkader A., El-Mokhtar M.A., Abdelkader O., Hamad M.A., Elsabahy M., El-Gazayerly O.N. Ultrahigh antibacterial efficacy of meropenem-loaded chitosan nanoparticles in a septic animal model. Carbohydr. Polym. 2017;174:1041–1050. doi: 10.1016/j.carbpol.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Acharya S.P., Pundarikakshudu K., Panchal A., Lalwani A. Preparation and evaluation of transnasal microemulsion of carbamazepine. Asian J. Pharm. Sci. 2013;8:64–70. doi: 10.1016/j.ajps.2013.07.008. [DOI] [Google Scholar]
- Ahmadi N., Ahmadi A., Kheirali E., Hossein Yadegari M., Bayat M., Shajiei A., Amini A.A., Ashrafi S., Abolhassani M., Faezi S., Yazdanparast S.A., Mahdavi M. Systemic infection with Candida albicans in breast tumor bearing mice: Cytokines dysregulation and induction of regulatory T cells. J. Mycol. Med. 2019;29:49–55. doi: 10.1016/j.mycmed.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Al-Jamal W.T., Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011;44:1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- Al Haj N.A., Abdullah R., Ibrahim S., Bustamam A. Tamoxifen drug loading solid lipid nanoparticles prepared by hot high pressure homogenization techniques. Am. J. Pharmacol. Toxicol. 2008;3:219–224. doi: 10.3844/ajptsp.2008.219.224. [DOI] [Google Scholar]
- Alexis F., Pridgen E., Molnar L.K., Farokhzad O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Afzal M., Verma M., Misra-Bhattacharya S., Ahmad F.J., Dinda A.K. Improved antifilarial activity of ivermectin in chitosan-alginate nanoparticles against human lymphatic filarial parasite, Brugia malayi. Parasitol. Res. 2013;112:2933–2943. doi: 10.1007/s00436-013-3466-4. [DOI] [PubMed] [Google Scholar]
- Aljuffali I., Huang C.-H., Fang J.-Y., et al. Nanomedical strategies for targeting skin microbiomes. Curr. Drug Metab. 2015;16:255–271. doi: 10.2174/1389200216666150812124923. [DOI] [PubMed] [Google Scholar]
- Allen T.M., Cullis P.R. Liposomal drug delivery systems : From concept to clinical applications ☆. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Andrade D.V., Gollob K.J., Dutra W.O. Acute chagas disease: new global challenges for an old neglected disease. PLoS Negl. Trop. Dis. 2014;8:1–10. doi: 10.1371/journal.pntd.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli P., Tonon M., Pilutti C., Morando F., Piano S. Sepsis-induced acute kidney injury in patients with cirrhosis. Hepatol. Int. 2016;10:115–123. doi: 10.1007/s12072-015-9641-1. [DOI] [PubMed] [Google Scholar]
- Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Anton N., Vandamme T.F. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm. Res. 2011;28:978–985. doi: 10.1007/s11095-010-0309-1. [DOI] [PubMed] [Google Scholar]
- Aparecido, M., Bento, P., Toledo, L.G. De, Oda, F.B., Cristiane, I., Campaner, L., Gonzaga, A., Teresa, M., Almeida, G. De, Pavan, F.R., Chorilli, M., Bauab, T.M., 2019. Intravaginal Delivery of Syngonanthus nitens (Bong .) Ruhland Fraction Based on a Nanoemulsion System Applied to Vulvovaginal Candidiasis Treatment. https://doi.org/10.1166/jbn.2019.2750. [DOI] [PubMed]
- Arechabala B., Coiffard C., Rivalland P., Coiffard L.J.M., De Roeck-Holtzhauer Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J. Appl. Toxicol. 1999;19:163–165. doi: 10.1002/(SICI)1099-1263(199905/06)19:3<163::AID-JAT561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ashish Pratap Singh, P.A., 2019. Evaluation of bacterial & fungal sepsis in neonates. J. Adv. Med. Dent Sci. Res. 7, 179–182. https://doi.org/10.21276/jamdsr.
- Azamfirei R. The 2019 novel coronavirus: a crown jewel of pandemics? J. Crit. Care Med. 2020;6:3–4. doi: 10.2478/jccm-2020-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banach, M., Pulit-Prociak, J., 2016. Synthesis, characteristics, and biocidal activity of silver nanoparticles, Fabrication and Self-Assembly of Nanobiomaterials: Applications of Nanobiomaterials. https://doi.org/10.1016/B978-0-323-41533-0.00012-X.
- Barbosa, V.T., Souza, J.K.C., Alvino, V., Meneghetti, M.R., Florez-Rodriguez, P., P., Moreira, R.E., Paulino, G.V.B., Landell, M.F., Basílio-Júnior, I.D., Nascimento, T.G. do, Grillo, L.A.M., Dornelas, C.B., Barbosa, V.T., Souza, oyelanne K.C., Alvino, V., Mario R. Meneghetti, P.P.F.-R., Moreira, R.E., Paulino, G.V.B., Melissa F. Landell4, Irinaldo D. Basílio-Júnior1, Ticiano G. do Nascimento1, Luciano A. M. Grillo1, C.B.D., 2019. Biogenic synthesis of silver nanoparticles using Brazilian propolis. Biotechnol. Prog. 1–27. https://doi.org/10.1002/btpr. [DOI] [PubMed]
- Barkalina N., Charalambous C., Jones C., Coward K. Nanotechnology in reproductive medicine: Emerging applications of nanomaterials. Nanomedicine Nanotechnology. Biol. Med. 2014;10:e921–e938. doi: 10.1016/j.nano.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Bassetti M., Ginocchio F., Giacobbe D.R. New approaches for empiric therapy in Gram-positive sepsis. Minerva Anestesiol. 2011;77:821–827. [PubMed] [Google Scholar]
- Batista C.M., De Carvalho C.M.B., Magalhães N.S.S. Liposomes and their therapeutic: State of art applications. Rev. Bras. Ciencias Farm. J. Pharm. Sci. 2007;43:167–179. doi: 10.1590/S1516-93322007000200003. [DOI] [Google Scholar]
- Benchaala I., Mishra M.K., Wykes S.M., Hali M., Kannan R.M., Whittum-Hudson J.A. Folate-functionalized dendrimers for targeting Chlamydia-infected tissues in a mouse model of reactive arthritis. Int. J. Pharm. 2014;466:258–265. doi: 10.1016/j.ijpharm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Bharali D.J., Siddiqui I.A., Adhami V.M., Chamcheu J.C., Aldahmash A.M., Mukhtar H., Mousa S.A. Nanoparticle delivery of natural products in the prevention and treatment of cancers: current status and future prospects. Cancers (Basel). 2011;3:4024–4045. doi: 10.3390/cancers3044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochud P.Y., Glauser M.P., Calandra T. Antibiotics in sepsis. Intensive Care Med. 2001;27:33–48. doi: 10.1097/ccm.0000000000002390. [DOI] [PubMed] [Google Scholar]
- Bolla P.K., Kalhapure R.S., Rodriguez V.A., Ramos D.V., Dahl A., Renukuntla J. Preparation of solid lipid nanoparticles of furosemide-silver complex and evaluation of antibacterial activity. J. Drug Deliv. Sci. Technol. 2019;49:6–13. doi: 10.1016/j.jddst.2018.10.035. [DOI] [Google Scholar]
- Bonifácio B.V., dos Santos Ramos M.A., da Silva P.B., Negri K.M.S., de Oliveira Lopes É., de Souza L.P., Vilegas W., Pavan F.R., Chorilli M., Bauab T.M. Nanostructured lipid system as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int. J. Nanomed. 2015;10:5081–5092. doi: 10.2147/IJN.S79684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifácio B.V., Silva P.B., Ramos M.A.S., Negri K.M.S., Bauab T.M., Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int. J. Nanomed. 2014;9:1. doi: 10.2147/IJN.S52634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchemal K., Briançon S., Perrier E., Fessi H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int. J. Pharm. 2004;280:241–251. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Brime B., Molero G., Frutos P., Frutos G. Comparative therapeutic efficacy of a novel lyophilized amphotericin B lecithin-based oil-water microemulsion and deoxycholate-amphotericin B in immunocompetent and neutropenic mice infected with Candida albicans. Eur. J. Pharm. Sci. 2004;22:451–458. doi: 10.1016/j.ejps.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Callender S.P., Mathews J.A., Kobernyk K., Wettig S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017;526:425–442. doi: 10.1016/j.ijpharm.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Carneiro, S.P., Carvalho, K.V., de Oliveira Aguiar Soares, R.D., Carneiro, C.M., de Andrade, M.H.G., Duarte, R.S., dos Santos, O.D.H., 2019. Functionalized rifampicin-loaded nanostructured lipid carriers enhance macrophages uptake and antimycobacterial activity. Colloids Surfaces B Biointerfaces 175, 306–313. https://doi.org/10.1016/j.colsurfb.2018.12.003. [DOI] [PubMed]
- Carvalheiro M., Esteves M.A., Santos-Mateus D., Lopes R.M., Rodrigues M.A., Eleutério C.V., Scoulica E., Santos-Gomes G., Cruz M.E.M. Hemisynthetic trifluralin analogues incorporated in liposomes for the treatment of leishmanial infections. Eur. J. Pharm. Biopharm. 2015;93:346–352. doi: 10.1016/j.ejpb.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Carvalho F.C., Campos M.L., Peccinini R.G., Gremião M.P.D. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur. J. Pharm. Biopharm. 2013;84:219–227. doi: 10.1016/j.ejpb.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Casciaro B., D’Angelo I., Zhang X., Loffredo M.R., Conte G., Cappiello F., Quaglia F., Di Y.P.P., Ungaro F., Mangoni M.L. Poly(lactide- co-glycolide) nanoparticles for prolonged therapeutic efficacy of Esculentin-1a-derived antimicrobial peptides against pseudomonas aeruginosa lung infection. in vitro and in vivo studies. Biomacromolecules. 2019;20:1876–1888. doi: 10.1021/acs.biomac.8b01829. [DOI] [PubMed] [Google Scholar]
- Cavalcanti, L.P. de G., Freitas, A.R.R., Brasil, P., da Cunha, R.V., 2017. Surveillance of deaths caused by arboviruses in Brazil: From dengue to Chikungunya. Mem. Inst. Oswaldo Cruz 112, 583–585. https://doi.org/10.1590/0074-02760160537. [DOI] [PMC free article] [PubMed]
- Chang L., Yan Y., Wang L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus. Med. Rev. J. 2020;XX:1–6. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A.S. Dendrimers for drug delivery. Molecules. 2018;23 doi: 10.3390/molecules23040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano. 2020 doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- Chen C.Z., Cooper S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/S0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- Chen G., Xu Y. Biosynthesis of cerium oxide nanoparticles and their effect on lipopolysaccharide (LPS) induced sepsis mortality and associated hepatic dysfunction in male Sprague Dawley rats. Mater. Sci. Eng. C. 2018;83:148–153. doi: 10.1016/j.msec.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Chen H., Dorrigan A., Saad S., Hare D.J., Cortie M.B., Valenzuela S.M. In Vivo Study of Spherical Gold Nanoparticles: Inflammatory Effects and Distribution in Mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Wu, Q., Zhang, Z., Yuan, L., Liu, X., Zhou, L., 2012. Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics 5972–5987. https://doi.org/10.3390/molecules17055972. [DOI] [PMC free article] [PubMed]
- Cheng Y., Xu Z., Ma M., Xu T. Dendrimers as drug carriers: Applications in different routes of drug administration. J. Pharm. Sci. 2008;97:123–143. doi: 10.1002/jps.21079. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Lee E.J., Choi Y.H., Jeong Y.J., Park J.S. Poly(ethylene glycol)-block-poly(L-lysine) dendrimer: Novel linear polymer/dendrimer block copolymer forming a spherical water-soluble polyionic complex with DNA. Bioconjug. Chem. 1999;10:62–65. doi: 10.1021/bc9800668. [DOI] [PubMed] [Google Scholar]
- Choudhury H., Gorain B., Chatterjee B., Mandal U.K., Sengupta P., Tekade R.K. Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr. Pharm. Des. 2017;23:2504–2531. doi: 10.2174/1381612822666161201143600. [DOI] [PubMed] [Google Scholar]
- Chowdhary A., Sharma C., Meis J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13:1–10. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-González, González Espinosa, Goycoolea, 2019. Interaction Between Chitosan and Mucin: Fundamentals and Applications. Biomimetics 4, 32. https://doi.org/10.3390/biomimetics4020032. [DOI] [PMC free article] [PubMed]
- Crucho C.I.C., Barros M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C. 2017;80:771–784. doi: 10.1016/j.msec.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Csaba N., Garcia-Fuentes M., Alonso M.J. Nanoparticles for nasal vaccination. Adv. Drug Deliv. Rev. 2009;61:140–157. doi: 10.1016/j.addr.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva Santos S., Ferreira E.I., Giarolla J. Dendrimer prodrugs. Molecules. 2016;21 doi: 10.3390/molecules21060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Rai G., Tigga R.A., Srivastava S., Singh P.K., Sharma R., Datt S., Singh N.P., Dar S.A. Candida auris in critically ill patients: Emerging threat in intensive care unit of hospitals. J. Mycol. Med. 2018;28:514–518. doi: 10.1016/j.mycmed.2018.06.005. [DOI] [PubMed] [Google Scholar]
- David, M., Morens, G.K.F., A.S.F., 2004. The challenge of emerging and tropical infectious diseases. Mol. Microbiol. 430, 8–11.
- de Cássia Orlandi Sardi, J., Silva, D.R., Soares Mendes-Giannini, M.J., Rosalen, P.L., 2018. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb. Pathog. 125, 116–121. https://doi.org/10.1016/j.micpath.2018.09.014. [DOI] [PubMed]
- de Freitas L.M., Calixto G.M.F., Chorilli M., Giusti J.S.M., Bagnato V.S., Soukos N.S., Amiji M.M., Fontana C.R. Polymeric nanoparticle-based photodynamic therapy for chronic periodontitis in Vivo. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima Luna A.C., Viegas Saraiva G.K., Ribeiro Filho O.M., Chierice G.O., Neto S.C., Cuccovia I.M., Maria D.A. Potential antitumor activity of novel DODAC/PHO-S liposomes. Int. J. Nanomed. 2016;11:1577–1591. doi: 10.2147/IJN.S90850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye J., Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:154–162. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal S., Renu S., Ghimire S., Lakshmanappa Y.S., Hogshead B.T., Feliciano-Ruiz N., Lu F., HogenEsch H., Krakowka S., Lee C.W., Renukaradhya G.J. Mucosal immunity and protective efficacy of intranasal inactivated influenza vaccine is improved by chitosan nanoparticle delivery in pigs. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A.P., da Silva Santos S., da Silva J.V., Parise-Filho R., Igne Ferreira E., Seoud O. El, Giarolla J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020;573:118814. doi: 10.1016/j.ijpharm.2019.118814. [DOI] [PubMed] [Google Scholar]
- Dinardo, C.D., Perl, A.E., 2019. Advances in patient care through increasingly individualized therapy 16, 73–74. [DOI] [PMC free article] [PubMed]
- Domingo C., Saurina J. An overview of the analytical characterization of nanostructured drug delivery systems: Towards green and sustainable pharmaceuticals: A review. Anal. Chim. Acta. 2012;744:8–22. doi: 10.1016/j.aca.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Dong X., Ke X., Liao Z. The microstructure characterization of meloxicam microemulsion and its influence on the solubilization capacity. Drug Dev. Ind. Pharm. 2011;37:894–900. doi: 10.3109/03639045.2010.548067. [DOI] [PubMed] [Google Scholar]
- El Din S.N., El-Tayeb T.A., Abou-Aisha K., El-Azizi M. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int. J. Nanomedicine. 2016;11:1749–1758. doi: 10.2147/IJN.S102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmowafy M., Shalaby K., Badran M.M., Ali H.M., Abdel-Bakky M.S., Ibrahim H.M. Multifunctional carbamazepine loaded nanostructured lipid carrier (NLC) formulation. Int. J. Pharm. 2018;550:359–371. doi: 10.1016/j.ijpharm.2018.08.062. [DOI] [PubMed] [Google Scholar]
- Engin A.B., Hayes A.W. The impact of immunotoxicity in evaluation of the nanomaterials safety. Toxicol. Res. Appl. 2018;2:1–9. doi: 10.1177/2397847318755579. [DOI] [Google Scholar]
- Fachinetti N., Rigon R.B., Eloy J.O., Sato M.R., dos Santos K.C., Chorilli M. Comparative study of glyceryl behenate or polyoxyethylene 40 stearate-based lipid carriers for trans-resveratrol delivery: development, characterization and evaluation of the in vitro tyrosinase inhibition. AAPS PharmSciTech. 2018;19:1401–1409. doi: 10.1208/s12249-018-0961-z. [DOI] [PubMed] [Google Scholar]
- Fang C.-T., Lai S.-Y., Yi W.-C., Hsueh P.-R., Liu K.-L., Chang S.-C. Klebsiella pneumoniae Genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin. Infect. Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- Fang J.Y., Fang C.L., Liu C.H., Su Y.H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur. J. Pharm. Biopharm. 2008;70:633–640. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Fauci A.S. Infectious diseases: considerations for the 21st Century. Clin. Infect. Dis. 2001;32:675–685. doi: 10.1086/319235. [DOI] [PubMed] [Google Scholar]
- Fernández, J., Acevedo, J., Weist, R., Gustot, T., Amoros, A., Deulofeu, C., Reverter, E., Martínez, J., Saliba, F., Jalan, R., Welzel, T., Pavesi, M., Hernández-Tejero, M., Ginès, P., Arroyo, V., 2017. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut gutjnl-2017-314240. https://doi.org/10.1136/gutjnl-2017-314240. [DOI] [PubMed]
- Franco-Paredes C., Nadine R., Méndez J., Folch E., Rodríguez-Morales A.J., Santos J.I., Hurst J.W. Cardiac manifestations of parasitic infections Part 1: overview and immunopathogenesis. Clin. Cardiol. 2007;30:326–330. doi: 10.1002/clc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklyne J.S., Mukherjee A., Chandrasekaran N. Essential oil micro- and nanoemulsions: promising roles in antimicrobial therapy targeting human pathogens. Lett. Appl. Microbiol. 2016;63:322–334. doi: 10.1111/lam.12631. [DOI] [PubMed] [Google Scholar]
- Fu T., Yi J., Lv S., Zhang B. Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J. Liposome Res. 2017;27:228–233. doi: 10.1080/08982104.2016.1224899. [DOI] [PubMed] [Google Scholar]
- Gaba B., Fazil M., Khan S., Ali A., Baboota S., Ali J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Fac. Pharmacy, Cairo Univ. 2015;53:147–159. doi: 10.1016/j.bfopcu.2015.10.001. [DOI] [Google Scholar]
- Gadde S. Multi-drug delivery nanocarriers for combination therapy. Medchemcomm. 2015;6:1916–1929. doi: 10.1039/c5md00365b. [DOI] [Google Scholar]
- Ganda I.S., Zhong Q., Hali M., Albuquerque R.L.C., Padilha F.F., da Rocha S.R.P., Whittum-Hudson J.A. Dendrimer-conjugated peptide vaccine enhances clearance of Chlamydia trachomatis genital infection. Int. J. Pharm. 2017;527:79–91. doi: 10.1016/j.ijpharm.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane E.J. Future anti-HBV strategies. Liver Int. 2017;37:40–44. doi: 10.1111/liv.13304. [DOI] [PubMed] [Google Scholar]
- Gao Y., Wang J., Chai M., Li X., Deng Y., Jin Q., Ji J. Size and charge adaptive clustered nanoparticles targeting the biofilm microenvironment for chronic lung infection management. ACS Nano. 2020;14:5686–5699. doi: 10.1021/acsnano.0c00269. [DOI] [PubMed] [Google Scholar]
- García-Gallego S., Franci G., Falanga A., Gómez R., Folliero V., Galdiero S., De La Mata F.J., Galdiero M. Function oriented molecular design: Dendrimers as novel antimicrobials. Molecules. 2017;22:1–29. doi: 10.3390/molecules22101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Orue I., Gainza G., Girbau C., Alonso R., Aguirre J.J., Pedraz J.L., Igartua M., Hernandez R.M. LL37 loaded nanostructured lipid carriers (NLC): A new strategy for the topical treatment of chronic wounds. Eur. J. Pharm. Biopharm. 2016;108:310–316. doi: 10.1016/j.ejpb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Gaudet A., Parmentier E., De Freitas Caires N., Portier L., Dubucquoi S., Poissy J., Duburcq T., Hureau M., Lassalle P., Mathieu D. Impact of acute renal failure on plasmatic levels of cleaved endocan. Crit. Care. 2019;23:4–6. doi: 10.1186/s13054-019-2349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi L., Moghimi R., Aliahmadi A., McClements D.J., Rafati H. Development of antimicrobial nanoemulsion-based delivery systems against selected pathogenic bacteria using a thymol rich Thymus daenensis essential oil Lida. J. Appl. Microbiol. 2017;123:832–840. doi: 10.1111/ijlh.12426. [DOI] [PubMed] [Google Scholar]
- Ghosh V., Saranya S., Mukherjee A., Chandrasekaran N. Antibacterial microemulsion prevents sepsis and triggers healing of wound in wistar rats. Colloids Surfaces B Biointerfaces. 2013;105:152–157. doi: 10.1016/j.colsurfb.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Gonzalez B.E., Martinez-Aguilar G., Hulten K.G., Hammerman W.A., Coss-Bu J., Avalos-Mishaan A., Mason E.O., Kaplan S.L. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115:642–648. doi: 10.1542/peds.2004-2300. [DOI] [PubMed] [Google Scholar]
- Grabowski N., Hillaireau H., Vergnaud J., Tsapis N., Pallardy M., Kerdine-Römer S., Fattal E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int. J. Pharm. 2015;482:75–83. doi: 10.1016/j.ijpharm.2014.11.042. [DOI] [PubMed] [Google Scholar]
- Griffin B.T., O’Driscoll C.M. A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J. Pharm. Pharmacol. 2006;58:917–925. doi: 10.1211/jpp.58.7.0006. [DOI] [PubMed] [Google Scholar]
- Grolman J.M., Singh M., Mooney D.J., Eriksson E., Nuutila K. Antibiotic-containing agarose hydrogel for wound and burn care. J. Burn Care Res. 2019;40:900–906. doi: 10.1093/jbcr/irz113. [DOI] [PubMed] [Google Scholar]
- Guerrero-Beltrán C., Garcia-Heredia I., Ceña-Diez R., Rodriguez-Izquierdo I., Serramía M.J., Martinez-Hernandez F., Lluesma-Gomez M., Martinez-Garcia M., Muñoz-Fernández M.Á. Cationic dendrimer g2–s16 inhibits herpes simplex type 2 infection and protects mice vaginal microbiome. Pharmaceutics. 2020;12:1–14. doi: 10.3390/pharmaceutics12060515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogdu E., Karasulu H.Y., Koksal C., Karasulu E. The novel oral imatinib microemulsions: Physical properties, cytotoxicity activities and improved Caco-2 cell permeability. J. Microencapsul. 2013;30:132–142. doi: 10.3109/02652048.2012.704952. [DOI] [PubMed] [Google Scholar]
- Guo C., Wang J., Cao F., Lee R.J., Zhai G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today. 2010;15:1032–1040. doi: 10.1016/j.drudis.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Guo J., Waknine-Grinberg J.H., Mitchell A.J., Barenholz Y., Golenser J. Reduction of experimental cerebral malaria and its related proinflammatory responses by the novel liposome-based β -methasone nanodrug. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/292471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson C.T., Boakye-Agyeman F., Brinkman C.L., Reid J.M., Patel R., Bajzer Z., Dadsetan M., Yaszemski M.J. Controlled delivery of vancomycin via charged hydrogels. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0146401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterres, S.S., Alves, M.P., Pohlmann, A.R., 2007. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2, 117739280700200. https://doi.org/10.1177/117739280700200002. [PMC free article] [PubMed]
- Halwani M., Yebio B., Suntres Z.E., Alipour M., Azghani A.O., Omri A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008;62:1291–1297. doi: 10.1093/jac/dkn422. [DOI] [PubMed] [Google Scholar]
- Hardeep, S. Oberoi, Yvonne, M. Yorgensen, Audrey, Morasse, Jay, T. Evans, D.J.B., 2017. PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination. J Control Release 176, 139–148. https://doi.org/10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed]
- Harun S.N., Nordin S.A., Gani S.S.A., Shamsuddin A.F., Basri M., Basri H. Bin. Development of nanoemulsion for efficient brain parenteral delivery of cefuroxime: Designs, characterizations, and pharmacokinetics. Int. J. Nanomedicine. 2018;13:2571–2584. doi: 10.2147/IJN.S151788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, M. et al., 2016. Nanotechnology drug delivery system: tools in advance pharmaceutical & human health care.
- Hato T., Maier B., Syed F., Myslinski J., Zollman A., Plotkin Z., Eadon M.T., Dagher P.C. Bacterial sepsis triggers an antiviral response that causes translation shutdown. J. Clin. Invest. 2019;129:296–309. doi: 10.1172/JCI123284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefnawy A., Berg M., Dujardin J.C., De Muylder G. Exploiting knowledge on leishmania drug resistance to support the quest for new drugs. Trends Parasitol. 2017;33:162–174. doi: 10.1016/j.pt.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Henostroza M.A.B., Curo Melo K.J., Nishitani Yukuyama M., Löbenberg R., Araci Bou-Chacra N. Cationic rifampicin nanoemulsion for the treatment of ocular tuberculosis. Colloids Surfaces A Physicochem. Eng. Asp. 2020;597 doi: 10.1016/j.colsurfa.2020.124755. [DOI] [Google Scholar]
- Herwaldt B.L. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- Hidron A., Vogenthaler N., Santos-Preciado J.I., Rodriguez-Morales A.J., Franco-Paredes C., Rassi A. Cardiac involvement with parasitic infections. Clin. Microbiol. Rev. 2010;23:324–349. doi: 10.1128/CMR.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J.A., Mason E.O., Schutze G.E., Tan T.Q., Barson W.J., Givner L.B., Wald E.R., Bradley J.S., Yogev R., Kaplan S.L. Streptococcus pneumoniae Infections in the Neonate. Pediatrics. 2003;112:1095–1102. doi: 10.1542/peds.112.5.1095. [DOI] [PubMed] [Google Scholar]
- Hombach J., Bernkop-Schnurch A. Mucoadhesive drug delivery systems. Handbook Exp. Pharmacol. 2010:251–266. doi: 10.1007/978-3-642-00477-3. [DOI] [PubMed] [Google Scholar]
- Hörmann K., Zimmer A. Drug delivery and drug targeting with parenteral lipid nanoemulsions - A review. J. Control. Release. 2016;223:85–98. doi: 10.1016/j.jconrel.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Hosny K.M., Hassan A.H. Intranasal in situ gel loaded with saquinavir mesylate nanosized microemulsion: Preparation, characterization, and in vivo evaluation. Int. J. Pharm. 2014;475:191–197. doi: 10.1016/j.ijpharm.2014.08.064. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Hsu H.J., Bugno J., Lee S.R., Hong S. Dendrimer-based nanocarriers: a versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:1–21. doi: 10.1002/wnan.1409. [DOI] [PubMed] [Google Scholar]
- Hu Q.Da., Fan H., Ping Y., Liang W.Q., Tang G.P., Li J. Cationic supramolecular nanoparticles for co-delivery of gene and anticancer drug. Chem. Commun. 2011;47:5572–5574. doi: 10.1039/c1cc10721f. [DOI] [PubMed] [Google Scholar]
- Hwang Y.Y., Ramalingam K., Bienek D.R., Lee V., You T., Alvarez R. Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant acinetobacter baumannii. Antimicrob. Agents Chemother. 2013;57:3568–3575. doi: 10.1128/AAC.02109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabeen G., Naz S.A., Jabeen N., Shafique M., Sharafat S., Baig S., Nazeer S. Non-albicans Candida species: Emergence of neglected pathogens among population of Karachi. Pak. J. Pharm. Sci. 2019;32:1185–1192. [PubMed] [Google Scholar]
- Jain K., Verma A.K., Mishra P.R., Jain N.K. Characterization and evaluation of amphotericin B loaded MDP conjugated poly(propylene imine) dendrimers. Nanomed. Nanotechnol. Biol. Med. 2015;11:705–713. doi: 10.1016/j.nano.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Jain S.K., Kumar Ajay, Kumar Amrish, Pandey A.N., Rajpoot K. Development and in vitro characterization of a multiparticulate delivery system for acyclovir-resinate complex. Artif. Cells, Nanomedicine Biotechnol. 2016;44:1266–1275. doi: 10.3109/21691401.2015.1024841. [DOI] [PubMed] [Google Scholar]
- Jaiswal M., Dudhe R., Sharma P.K. Nanoemulsion: an advanced mode of drug delivery system. 3. Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal P., Gidwani B., Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells, Nanomedicine Biotechnol. 2016;44:27–40. doi: 10.3109/21691401.2014.909822. [DOI] [PubMed] [Google Scholar]
- Janaszewska A., Lazniewska J., Trzepiński P., Marcinkowska M., Klajnert-Maculewicz B. Cytotoxicity of dendrimers. Biomolecules. 2019;9:1–23. doi: 10.3390/biom9080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansook P., Fülöp Z., Ritthidej G.C. Amphotericin B loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): physicochemical and solid-solution state characterizations. Drug Dev. Ind. Pharm. 2019;45:560–567. doi: 10.1080/03639045.2019.1569023. [DOI] [PubMed] [Google Scholar]
- Jauréguy, F., Carbonnelle, E., Bonacorsi, S., Clec’h, C., Casassus, P., Bingen, E., Picard, B., Nassif, X., Lortholary, O., 2007. Host and bacterial determinants of initial severity and outcome of Escherichia coli sepsis. Clin. Microbiol. Infect. 13, 854–862. https://doi.org/10.1111/j.1469-0691.2007.01775.x. [DOI] [PubMed]
- Jeffery-Smith A., Taori S.K., Schelenz S., Jeffery K., Johnson E.M., Borman A., Manuel R., Browna C.S. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2018;31:1–18. doi: 10.1128/CMR.00029-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.T., Wroe J.A., Agarwal R., Martin K.E., Guldberg R.E., Donlan R.M., Westblade L.F., García A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E4960–E4969. doi: 10.1073/pnas.1801013115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M., Pathak S., Sharma S., Patravale V. Solid microemulsion preconcentrate (NanOsorb) of artemether for effective treatment of malaria. Int. J. Pharm. 2008;362:172–178. doi: 10.1016/j.ijpharm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Joshi M.D., Müller R.H. Lipid nanoparticles for parenteral delivery of actives. Eur. J. Pharm. Biopharm. 2009;71:161–172. doi: 10.1016/j.ejpb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kalita S., Kandimalla R., Bhowal A.C., Kotoky J., Kundu S. Functionalization of β-lactam antibiotic on lysozyme capped gold nanoclusters retrogress MRSA and its persisters following awakening. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-22736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaruzzaman N.F., Tan L.P., Hamdan R.H., Choong S.S., Wong W.K., Gibson A.J., Chivu A., De Fatima Pina M. Antimicrobial polymers: The potential replacement of existing antibiotics? Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20112747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhampati S.P., Clunies-Ross A.J.M., Bhutto I., Mishra M.K., Edwards M., McLeod D.S., Kannan R.M., Lutty G. Systemic and intravitreal delivery of dendrimers to activated microglia/macrophage in ischemia/reperfusion mouse retina. Investig. Ophthalmol. Vis. Sci. 2015;56:4413–4424. doi: 10.1167/iovs.14-16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappagoda S., Singh U., Blackburn B.G. Antiparasitic therapy. Mayo Clin. Proc. 2011;86:561–583. doi: 10.4065/mcp.2011.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami Z., Saghatchi Zanjani M.R., Rezaee S., Rostamizadeh K., Hamidi M. Neuropharmacokinetic evaluation of lactoferrin-treated indinavir-loaded nanoemulsions: remarkable brain delivery enhancement. Drug Dev. Ind. Pharm. 2019;45:736–744. doi: 10.1080/03639045.2019.1569039. [DOI] [PubMed] [Google Scholar]
- Kelidari H.R., Moazeni M., Babaei R., Saeedi M., Akbari J., Parkoohi P.I., Nabili M., Gohar A.A., Morteza-Semnani K., Nokhodchi A. Improved yeast delivery of fluconazole with a nanostructured lipid carrier system. Biomed. Pharmacother. 2017;89:83–88. doi: 10.1016/j.biopha.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Kesharwani P., Jain K., Jain N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014;39:268–307. doi: 10.1016/j.progpolymsci.2013.07.005. [DOI] [Google Scholar]
- Khalil R. Formulation and characterization of nystatin-loaded nanostructured lipid carriers for topical delivery against cutaneous candidiasis. Br. J. Pharm. Res. 2014;4:490–512. doi: 10.9734/bjpr/2014/7055. [DOI] [Google Scholar]
- Khalkhali M., Mohammadinejad S., Khoeini F., Rostamizadeh K. Vesicle-like structure of lipid-based nanoparticles as drug delivery system revealed by molecular dynamics simulations. Int. J. Pharm. 2019:173–181. doi: 10.1016/j.ijpharm.2019.01.036. [DOI] [PubMed] [Google Scholar]
- Khan Ibrahim, Saeed K., Khan Idrees. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 2017;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Kirby B.J., Hasselbrink E.F. Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis. 2004;25:187–202. doi: 10.1002/elps.200305754. [DOI] [PubMed] [Google Scholar]
- Komati S., Swain S., Rao M.E.B., Jena B.R., Dasi V. Mucoadhesive multiparticulate drug delivery systems: An extensive review of patents. Adv. Pharm. Bull. 2019;9:521–538. doi: 10.15171/apb.2019.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad, Reinhart, Ron, Daniels, Niranjan, Kissoon, Flavia, R. Machado, Raymond, D. Schachter, Simon, Finfer, M., 2017. Recognizing Sepsis as a Global Health Priority — A. N. Engl. J. Med. 28, 1–4. [DOI] [PubMed]
- Kramer R.M., Archer M.C., Orr M.T., Dubois Cauwelaert N., Beebe E.A., Huang P.W.D., Dowling Q.M., Schwartz A.M., Fedor D.M., Vedvick T.S., Fox C.B. Development of a thermostable nanoemulsion adjuvanted vaccine against tuberculosis using a design-of-experiments approach. Int. J. Nanomedicine. 2018;13:3689–3711. doi: 10.2147/IJN.S159839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Sharma P., Kumar M., Bhandari N., Kushwaha A. Severe sepsis and septic shock associated with chikungunya fever in an adolescent. J. Trop. Pediatr. 2018;64:557–559. doi: 10.1093/tropej/fmx107. [DOI] [PubMed] [Google Scholar]
- Kuppusamy P., Yusoff M.M., Maniam G.P., Govindan N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications – An updated report. Saudi Pharm. J. 2016;24:473–484. doi: 10.1016/j.jsps.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuskov A.N., Kulikov P.P., Shtilman M.I., Rakitskii V.N., Tsatsakis A.M. Amphiphilic poly-N-vynilpyrrolidone nanoparticles: Cytotoxicity and acute toxicity study. Food Chem. Toxicol. 2016;96:273–279. doi: 10.1016/j.fct.2016.08.017. [DOI] [PubMed] [Google Scholar]
- Labieniec-Watala M., Watala C. PAMAM dendrimers: Destined for success or doomed to fail? Plain and modified PAMAM dendrimers in the context of biomedical applications. J. Pharm. Sci. 2015;104:2–14. doi: 10.1002/jps.24222. [DOI] [PubMed] [Google Scholar]
- Laffleur F. Mucoadhesive polymers for buccal drug delivery. Drug Dev. Ind. Pharm. 2014;40:591–598. doi: 10.3109/03639045.2014.892959. [DOI] [PubMed] [Google Scholar]
- Lawrence M.J., Rees G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012;64:175–193. doi: 10.1016/j.addr.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Leclercq S.Y., Dos Santos R.M.M., MacEdo L.B., Campos P.C., Ferreira T.C., De Almeida J.G., Seniuk J.G.T., Serakides R., Silva-Cunha A., Fialho S.L. Evaluation of water-in-oil-in-water multiple emulsion and microemulsion as potential adjuvants for immunization with rabies antigen. Eur. J. Pharm. Sci. 2011;43:378–385. doi: 10.1016/j.ejps.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Lee J.J., Shim A., Jeong J.Y., Lee S.Y., Ko H.J., Cho H.J. Development of intranasal nanovehicles of itraconazole and their immunological activities for the therapy of rhinovirus infection. Colloids Surfaces B Biointerfaces. 2016;143:336–341. doi: 10.1016/j.colsurfb.2016.03.050. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Cha S.I., Kim C.H., Park J.Y., Jung T.H., Jeon K.N., Kim G.W. Septic pulmonary embolism in Korea: Microbiology, clinicoradiologic features, and treatment outcome. J. Infect. 2007;54:230–234. doi: 10.1016/j.jinf.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Lee W., Park E.J., Kwon O.K., Kim H., Yoo Y., Kim S.W., Seo Y.K., Kim I.S., Na D.H., Bae J.S. Dual peptide-dendrimer conjugate inhibits acetylation of transforming growth factor β-induced protein and improves survival in sepsis. Biomaterials. 2020;246:120000. doi: 10.1016/j.biomaterials.2020.120000. [DOI] [PubMed] [Google Scholar]
- Leelahavanichkul A., Souza A.C.P., Street J.M., Hsu V., Tsuji T., Doi K., Li L., Hu X., Zhou H., Kumar P., Schnermann J., Star R.A., Yuen P.S.T. Comparison of serum creatinine and serum cystatin C as biomarkers to detect Sepsis-Induced acute kidney injury and to predict mortality in CD-1 mice. Am. J. Physiol. - Ren. Physiol. 2014;307:F939–F948. doi: 10.1152/ajprenal.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid J.G., Ditto A.J., Knapp A., Shah P.N., Wright B.D., Blust R., Christensen L., Clemons C.B., Wilber J.P., Young G.W., Kang A.G., Panzner M.J., Cannon C.L., Yun Y.H., Youngs W.J., Seckinger N.M., Cope E.K. In vitro antimicrobial studies of silver carbene complexes: Activity of free and nanoparticle carbene formulations against clinical isolates of pathogenic bacteria. J. Antimicrob. Chemother. 2012;67:138–148. doi: 10.1093/jac/dkr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leire E., Amaral S.P., Louzao I., Winzer K., Alexander C., Fernandez-Megia E., Fernandez-Trillo F. Dendrimer mediated clustering of bacteria: Improved aggregation and evaluation of bacterial response and viability. Biomater. Sci. 2016;4:998–1006. doi: 10.1039/c6bm00079g. [DOI] [PubMed] [Google Scholar]
- Lever A., Mackenzie I. Sepsis: Definition, epidemiology, and diagnosis. Br. Med. J. 2007;335:879–883. doi: 10.1136/bmj.39346.495880.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Cai T., Huang Y., Xia X., Cole S., Cai Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials. 2017;7:122. doi: 10.3390/nano7060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang L., Tang C., Zhang E., Ding L., Yang L. Preparation and characterisation of the colistin-entrapped liposome driven by electrostatic interaction for intravenous administration. J. Microencapsul. 2016;33:427–437. doi: 10.1080/02652048.2016.1205153. [DOI] [PubMed] [Google Scholar]
- Li Y., Thambi T., Lee D.S. Co-Delivery of Drugs and Genes Using Polymeric Nanoparticles for Synergistic Cancer Therapeutic Effects. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201700886. [DOI] [PubMed] [Google Scholar]
- Liang Y., Zhao X., Ma P.X., Guo B., Du Y., Han X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019;536:224–234. doi: 10.1016/j.jcis.2018.10.056. [DOI] [PubMed] [Google Scholar]
- Lin G.L., McGinley J.P., Drysdale S.B., Pollard A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018;9:2147. doi: 10.3389/fimmu.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.H., Chiou S.F., Lai C.H., Tsai S.C., Chou C.W., Peng S.F., He Z.S. Formulation and evaluation of water-in-oil amoxicillin-loaded nanoemulsions using for Helicobacter pylori eradication. Process Biochem. 2012;47:1469–1478. doi: 10.1016/j.procbio.2012.05.019. [DOI] [Google Scholar]
- Ling J.T.S., Roberts C.J., Billa N. Antifungal and Mucoadhesive Properties of an Orally Administered Chitosan-Coated Amphotericin B Nanostructured Lipid Carrier (NLC) AAPS PharmSciTech. 2019;20:1–11. doi: 10.1208/s12249-019-1346-7. [DOI] [PubMed] [Google Scholar]
- Liu, X., Wang, Z., Feng, X., Bai, E., Xiong, Y., Zhu, X., Shen, B., Duan, Y., Huang, Y., 2020. Platensimycin-Encapsulated Poly(lactic- co-glycolic acid) and Poly(amidoamine) Dendrimers Nanoparticles with Enhanced Anti-Staphylococcal Activity in Vivo. Bioconjug. Chem. https://doi.org/10.1021/acs.bioconjchem.0c00121. [DOI] [PubMed]
- Lopes Colombo A., Nucci M., Salomão R., Branchini M.L.M., Richtmann R., Derossi A., Wey S.B. High rate of non-albicans candidemia in Brazilian tertiary care hospitals. Diagn. Microbiol. Infect. Dis. 1999;34:281–286. doi: 10.1016/S0732-8893(99)00042-5. [DOI] [PubMed] [Google Scholar]
- Luzolo A.L., Ngoyi D.M. Cerebral malaria. Brain Res. Bull. 2019;145:53–58. doi: 10.1016/j.brainresbull.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Mahajan H.S., Mahajan M.S., Nerkar P.P., Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21:148–154. doi: 10.3109/10717544.2013.838014. [DOI] [PubMed] [Google Scholar]
- Mahajan S.D., Aalinkeel R., Law W.C., Reynolds J.L., Nair B.B., Sykes D.E., Yong K.T., Roy I., Prasad P.N., Schwartz S.A. Anti-HIV-1 nanotherapeutics: Promises and challenges for the future. Int. J. Nanomedicine. 2012;7:5301–5314. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboobian M.M., Mohammadi M., Mansouri Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J. Drug Deliv. Sci. Technol. 2020;55:101400. doi: 10.1016/j.jddst.2019.101400. [DOI] [Google Scholar]
- Mahmoudi Saber M. Strategies for surface modification of gelatin-based nanoparticles. Colloids Surfaces B Biointerfaces. 2019;183:110407. doi: 10.1016/j.colsurfb.2019.110407. [DOI] [PubMed] [Google Scholar]
- Malhotra A., Hunt R.W., Doherty R.R. Streptococcus pneumoniae sepsis in the newborn. J. Paediatr. Child Health. 2012;48 doi: 10.1111/j.1440-1754.2010.01929.x. [DOI] [PubMed] [Google Scholar]
- Malik N., Wiwattanapatapee R., Klopsch R., Lorenz K., Frey H., Weener J.W., Meijer E.W., Paulus W., Duncan R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release. 2000;65:133–148. doi: 10.1016/S0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- Mandawgade S.D., Sharma S., Pathak S., Patravale V.B. Development of SMEDDS using natural lipophile: Application to β-Artemether delivery. Int. J. Pharm. 2008;362:179–183. doi: 10.1016/j.ijpharm.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Mansoor S., Kondiah P.P.D., Choonara Y.E., Pillay V. Polymer-based nanoparticle strategies for insulin delivery. Polymers (Basel). 2019;11 doi: 10.3390/polym11091380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcato P.D., De Paula L.B., Melo P.S., Ferreira I.R., Almeida A.B.A., Torsoni A.S., Alves O.L. In vivo evaluation of complex biogenic silver nanoparticle and enoxaparin in wound healing. J. Nanomater. 2015;2015 doi: 10.1155/2015/439820. [DOI] [Google Scholar]
- Marena G.D., dos Santos Ramos M.A., Bauab T.M., Chorilli M. Biological Properties and Analytical Methods for Micafungin: A Critical Review. Crit. Rev. Anal. Chem. 2020:1–17. doi: 10.1080/10408347.2020.1726726. [DOI] [PubMed] [Google Scholar]
- Markwalter, C.E., Pagels, R.F., Wilson, B.K., Ristroph, K.D., Prud’homme, R.K., 2019. Flash nanoprecipitation for the encapsulation of hydrophobic and hydrophilic compounds in polymeric nanoparticles. J. Vis. Exp. 2019, 1–13. https://doi.org/10.3791/58757. [DOI] [PubMed]
- Marston H.D., Dixon D.M., Knisely J.M., Palmore T.N., Fauci A.S. Antimicrobial resistance. JAMA - J. Am. Med. Assoc. 2016;316:1193–1204. doi: 10.1001/jama.2016.11764. [DOI] [PubMed] [Google Scholar]
- Mayer F.L., Wilson D., Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr F.B., Yende S., Angus D.C. Epidemiology of severe sepsis. Virulence. 2014;5:1–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgwire B.S., Satoskar A.R. Leishmaniasis: Clinical syndromes and treatment. QJM An Int. J. Med. 2014;107:7–14. doi: 10.1093/qjmed/hct116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers P., Neville M., Malinin V., Scotto A.W., Sardaryan G., Kurumunda R., Mackinson C., James G., Fisher S., Perkins W.R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 2008;61:859–868. doi: 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- Mendes A.I., Silva A.C., Catita J.A.M., Cerqueira F., Gabriel C., Lopes C.M. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: Improving antifungal activity. Colloids Surfaces B Biointerfaces. 2013;111:755–763. doi: 10.1016/j.colsurfb.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Mendes L.P., Pan J., Torchilin V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22:1–21. doi: 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Palomar N., Balasch-Carulla M., González-Di Lauro S., Céspedes M.C., Andreu A., Frick M.A., Linde M.Á., Soler-Palacin P. Escherichia coli early-onset sepsis: trends over two decades. Eur. J. Pediatr. 2017;176:1227–1234. doi: 10.1007/s00431-017-2975-z. [DOI] [PubMed] [Google Scholar]
- Meng Y., Hou X., Lei J., Chen M., Cong S., Zhang Y., Ding W., Li G., Li X. Multi-functional liposomes enhancing target and antibacterial immunity for antimicrobial and anti-biofilm against methicillin-resistant staphylococcus aureus. Pharm. Res. 2016;33:763–775. doi: 10.1007/s11095-015-1825-9. [DOI] [PubMed] [Google Scholar]
- Métraux G.S., Mirkin C.A. Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv. Mater. 2005;17:412–415. doi: 10.1002/adma.200401086. [DOI] [Google Scholar]
- Mhlwatika Z., Aderibigbe B.A. Application of dendrimers for the treatment of infectious diseases. Molecules. 2018;23 doi: 10.3390/molecules23092205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli M.H., Díaz J.A., Lee S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011;11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- Mignani S., El Kazzouli S., Bousmina M., Majoral J.P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013;65:1316–1330. doi: 10.1016/j.addr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Minasyan H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J. Crit. Care. 2017;40:229–242. doi: 10.1016/j.jcrc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Miyashiro C.A., Bernegossi J., Bonifácio B.V., de Toledo L.G., Ramos M.A.S., Bauab T.M., Chorilli M. Development and characterization of a novel liquid crystalline system containing sodium alginate for incorporation of trans-resveratrol intended for treatment of buccal candidiasis. Pharmazie. 2020;75:178–184. doi: 10.1691/ph.2020.9165. [DOI] [PubMed] [Google Scholar]
- Mohammady S.Z., Pouzot M., Mezzenga R. Oleoylethanolamide-based lyotropic liquid crystals as vehicles for delivery of amino acids in aqueous environment. Biophys. J. 2009;96:1537–1546. doi: 10.1016/j.bpj.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moinard-Chécot D., Chevalier Y., Briançon S., Beney L., Fessi H. Mechanism of nanocapsules formation by the emulsion-diffusion process. J. Colloid Interface Sci. 2008;317:458–468. doi: 10.1016/j.jcis.2007.09.081. [DOI] [PubMed] [Google Scholar]
- Mora-Huertas C.E., Fessi H., Elaissari A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010;385:113–142. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Moreno-Sastre M., Pastor M., Esquisabel A., Sans E., Viñas M., Fleischer A., Palomino E., Bachiller D., Pedraz J.L. Pulmonary delivery of tobramycin-loaded nanostructured lipid carriers for Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2016;498:263–273. doi: 10.1016/j.ijpharm.2015.12.028. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.P., Lyng F.M., Garcia A., Davoren M., Byrne H.J. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol. 2010;248:259–268. doi: 10.1016/j.taap.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Müller R.H., Radtke M., Wissing S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002;242:121–128. doi: 10.1016/S0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Mundt S., Basler M., Buerger S., Engler H., Groettrup M. Inhibiting the immunoproteasome exacerbates the pathogenesis of systemic Candida albicans infection in mice. Sci. Rep. 2016;6:1–15. doi: 10.1038/srep19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagavarma B.V.N., Yadav H.K.S., Ayaz A., Vasudha L.S., Shivakumar H.G. Different techniques for preparation of polymeric nanoparticles- A review. Asian J. Pharm. Clin. Res. 2012;5:16–23. [Google Scholar]
- Naglik J.R., Gaffen S.L., Hube B. Candidalysin: discovery and function in Candida albicans infections. Curr. Opin. Microbiol. 2019;52:100–109. doi: 10.1016/j.mib.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure preparation and application. Adv. Pharm. Bull. 2015;5:305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S., Khan S., Farooq U., Haider M.S., Ranjha N.M., Rasul A., Nawaz A., Arshad N., Hameed R. Biocompatible hydrogels for the controlled delivery of anti-hypertensive agent: Development, characterization and in vitro evaluation. Des. Monomers Polym. 2018;21:18–32. doi: 10.1080/15685551.2018.1445416. [DOI] [Google Scholar]
- Nirmala M.J., Mukherjee A., Chandrasekaran N. Improved efficacy of fluconazole against candidiasis using bio-based microemulsion technique. Biotechnol. Appl. Biochem. 2013;60:417–429. doi: 10.1002/bab.1116. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Matsuoka K., Kita E., Okabe N., Mizuguchi M., Hino K., Miyazawa S., Yamasaki C., Aoki J., Takashima S., Yamakawa Y., Nishijima M., Terunuma D., Kuzuhara H., Natori Y. A therapeutic agent with oriented carbohydrates for treatment of infections by Shiga toxin-producing Escherichia coli O157:H7. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7669–7674. doi: 10.1073/pnas.112058999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin N., Yeap S.K., Zamberi N.R., Abu N., Mohamad N.E., Rahman H.S., How C.W., Masarudin M.J., Abdullah R., Alitheen N.B. Characterization and toxicity of citral incorporated with nanostructured lipid carrier. PeerJ. 2018;2018:1–19. doi: 10.7717/peerj.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M.C.P., Guimarães M.H., Diamantino A.C., Gelape C.L., Ferrari T.C.A. Cardiac manifestations of parasitic diseases. Heart. 2017;103:651–658. doi: 10.1136/heartjnl-2016-309870. [DOI] [PubMed] [Google Scholar]
- Nutman T.B. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144:263–273. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan D.T., Valiante N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat W.M., Schwabe K., Müller R.H., Keck C.M. Preservation of nanostructured lipid carriers (NLC) Eur. J. Pharm. Biopharm. 2010;76:56–67. doi: 10.1016/j.ejpb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Oliveira M.B., Calixto G., Graminha M., Cerecetto H., González M., Chorilli M. Development, characterization, and in vitro biological performance of fluconazole-loaded microemulsions for the topical treatment of cutaneous leishmaniasis. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/396894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio R., Alfonso-Rodríguez C.A., Medina-Castillo A.L., Alaminos M., Toledano M. Bioactive polymeric nanoparticles for periodontal therapy. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0166217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Wrzesinski S.H., Stern E., Look M., Criscione J., Ragheb R., Jay S.M., Demento S.L., Agawu A., Licona Limon P., Ferrandino A.F., Gonzalez D., Habermann A., Flavell R.A., Fahmy T.M. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.-M., Lee S.J., Kim Y.S., Lee M.H., Cha G.S., Jung I.D., Kang T.H., Han H.D. Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw. 2013;13:177. doi: 10.4110/in.2013.13.5.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil K., Bagade S., Bonde S., Sharma S., Saraogi G. Recent therapeutic approaches for the management of tuberculosis: Challenges and opportunities. Biomed. Pharmacother. 2018;99:735–745. doi: 10.1016/j.biopha.2018.01.115. [DOI] [PubMed] [Google Scholar]
- Patil M.L., Zhang M., Minko T. Multifunctional triblock nanocarrier (PAMAM-PEG-PLL) for the efficient intracellular siRNA delivery and gene silencing. ACS Nano. 2011;5:1877–1887. doi: 10.1021/nn102711d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattni, B.S., Chupin, V. V, Torchilin, V.P., 2015. New developments in liposomal drug delivery. https://doi.org/10.1021/acs.chemrev.5b00046. [DOI] [PubMed]
- Pea F., Lewis R.E. Overview of antifungal dosing in invasive candidiasis. J. Antimicrob. Chemother. 2018;73:i33–i43. doi: 10.1093/jac/dkx447. [DOI] [PubMed] [Google Scholar]
- Pelgrift R.Y., Friedman A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Pereira R.R.D.A., Godoy J.S.R., Svidzinski T.I.S., Bruschi M.L. Preparation and characterization of mucoadhesive thermoresponsive systems containing propolis for the treatment of vulvovaginal candidiasis. J. Pharm. Sci. 2013;102:1222–1234. doi: 10.1002/jps. [DOI] [PubMed] [Google Scholar]
- Pereira S., Egbu R., Jannati G., Al-Jamal W.T. Docetaxel-loaded liposomes: The effect of lipid composition and purification on drug encapsulation and in vitro toxicity. Int. J. Pharm. 2016;514:150–159. doi: 10.1016/j.ijpharm.2016.06.057. [DOI] [PubMed] [Google Scholar]
- Pierrakos C., Vincent J.L. Sepsis biomarkers: A review. Crit. Care. 2010;14:1–18. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinninti S.G., Kimberlin D.W. Neonatal herpes simplex virus infections. Semin. Perinatol. 2018;42:168–175. doi: 10.1053/j.semperi.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Pinto Reis C., Neufeld R.J., Ribeiro A.J., Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine Nanotechnology. Biol. Med. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Pisano S., Giustiniani M., Francis L., Gonzalez D., Margarit L., Sheldon I.M., Paolino D., Fresta M., Conlan R.S., Healey G.D. Liquid crystal delivery of ciprofloxacin to treat infections of the female reproductive tract. Biomed. Microdevices. 2019;21 doi: 10.1007/s10544-019-0385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat G., Ugan R.A., Cadirci E., Halici Z. Sepsis ve septik şok: Mevcut tedavi stratejileri ve yeni yaklaşımlar. Eurasian J. Med. 2017;49:53–58. doi: 10.5152/eurasianjmed.2017.17062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A., Sharma C., Singh A., Kumar Singh P., Kumar A., Hagen F., Govender N.P., Colombo A.L., Meis J.F., Chowdhary A. Evidence of genotypic diversity among Candida auris isolates by multilocus sequence typing, matrix-assisted laser desorption ionization time-of-flight mass spectrometry and amplified fragment length polymorphism. Clin. Microbiol. Infect. 2016;22:277.e1–277.e9. doi: 10.1016/j.cmi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Prescott H.C., Angus D.C. Enhancing recovery from sepsis: A review. JAMA - J. Am. Med. Assoc. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przespolewski A., Szeles A., Wang E.S. Advances in immunotherapy for acute myeloid leukemia. Futur. Oncol. 2018;14:963–978. doi: 10.2217/fon-2017-0459. [DOI] [PubMed] [Google Scholar]
- Raghuram, A., Gnoni, M., Wiemken, T.L., Beavin, L.A., Arnold, F.W., Zervos, M.J., Kett, H., Jr, T.M.F., Stein, G.E., Ford, K.D., Ramirez, J.A., Peyrani, P., Hap, I., Group, S., 2017. Sepsis in Patients with Ventilator Associated Pneumonia due to Methicillin- Resistant Staphylococcus aureus: Incidence and Impact on Clinical out- comes. J. Respir. Infect. 1, 3–7. https://doi.org/10.18297/jri/vol1/iss3/3.
- Rai A., Pinto S., Velho T., Ferreira A.F., Moita C., Evangelista M., Comune M., Rumbaugh K.P., Simões P.N., Moita L., Ferreira L. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials. 2016;85:99–110. doi: 10.1016/j.biomaterials.2016.01.051.This. [DOI] [PubMed] [Google Scholar]
- Rai V.K., Mishra N., Yadav K.S., Yadav N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- Ramos M.A., Toledo L.G., Calixto G.M.F., Bonifácio B.V., Araujo M.G.F., Santos L.C., Almeida M.T.G., Chorilli M., Bauab T.M. Syngonanthus nitens Bong. (Rhul.)-loaded nanostructured system for Vulvovaginal candidiasis treatment. Int. J. Mol. Sci. 2016;17:1–19. doi: 10.3390/ijms17081368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, M.A. dos S., Calixto, G., de Toledo, L.G., Bonifácio, B.V., do Ssantos, L.C., de Almeida, M.T.G., Chorilli, M., Bauab, T.M., 2015. Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei. Int. J. Nanomedicine 10, 7455–7466. https://doi.org/10.2147/IJN.S92638. [DOI] [PMC free article] [PubMed]
- Ramos M.A.S., Silva P.B., Spósito L., Toledo L.G., Bonifácio B.V., Rodero C.F., Santos K.C. Nanotechnology-based drug delivery systems for control of microbial biofilms : a review. Int. J. N. 2018:1179–1213. doi: 10.2147/IJN.S146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M.A.S., Silva P.B., Spósito L., Toledo L.G., Bonifácio B.V., Rodero C.F., Santos K.C., Chorilli M., Bauab T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomedicine. 2018;13:1179–1213. doi: 10.2147/IJN.S146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Frausto M.S. The epidemiology of bacterial sepsis. Infect. Dis. Clin. North Am. 1999;13:299–312. doi: 10.1016/S0891-5520(05)70076-3. [DOI] [PubMed] [Google Scholar]
- Rastogi V., Yadav P., Verma A., Pandit J.K. Ex vivo and in vivo evaluation of microemulsion based transdermal delivery of E. coli specific T4 bacteriophage: A rationale approach to treat bacterial infection. Eur. J. Pharm. Sci. 2017;107:168–182. doi: 10.1016/j.ejps.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Raza K., Singh B., Singla S., Wadhwa S., Garg B., Chhibber S., Katare O.P. Nanocolloidal carriers of isotretinoin: Antimicrobial activity against propionibacterium acnes and dermatokinetic modeling. Mol. Pharm. 2013;10:1958–1963. doi: 10.1021/mp300722f. [DOI] [PubMed] [Google Scholar]
- Rello J., Valenzuela-Sánchez F., Ruiz-Rodriguez M., Moyano S. Sepsis: A Review of Advances in Management. Adv. Ther. 2017;34:2393–2411. doi: 10.1007/s12325-017-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, A., Evans, L.E., Alhazzani, W., Levy, M.M., Antonelli, M., Ferrer, R., Kumar, A., Sevransky, J.E., Sprung, C.L., Nunnally, M.E., Rochwerg, B., Rubenfeld, G.D., Angus, D.C., Annane, D., Beale, R.J., Bellinghan, G.J., Bernard, G.R., Chiche, J.D., Coopersmith, C., De Backer, D.P., French, C.J., Fujishima, S., Gerlach, H., Hidalgo, J.L., Hollenberg, S.M., Jones, A.E., Karnad, D.R., Kleinpell, R.M., Koh, Y., Lisboa, T.C., Machado, F.R., Marini, J.J., Marshall, J.C., Mazuski, J.E., McIntyre, L.A., McLean, A.S., Mehta, S., Moreno, R.P., Myburgh, J., Navalesi, P., Nishida, O., Osborn, T.M., Perner, A., Plunkett, C.M., Ranieri, M., Schorr, C.A., Seckel, M.A., Seymour, C.W., Shieh, L., Shukri, K.A., Simpson, S.Q., Singer, M., Thompson, B.T., Townsend, S.R., Van der Poll, T., Vincent, J.L., Wiersinga, W.J., Zimmerman, J.L., Dellinger, R.P., 2017. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016, Intensive Care Medicine. Springer Berlin Heidelberg. https://doi.org/10.1007/s00134-017-4683-6.
- Rideau E., Dimova R., Schwille P., Wurm F.R., Landfester K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem. Soc. Rev. doi. 2018;10(1063/1):4892631. doi: 10.1039/c8cs00162f. [DOI] [PubMed] [Google Scholar]
- Rigon R.B., Fachinetti N., Severino P., Santana M.H.A., Chorilli M. Skin delivery and in vitro biological evaluation of trans-Resveratrol-Loaded solid lipid nanoparticles for skin disorder therapies. Molecules. 2016;21:1–14. doi: 10.3390/molecules21010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigon R.B., Gonçalez M.L., Severino P., Alves D.A., Santana M.H.A., Souto E.B., Chorilli M. Solid lipid nanoparticles optimized by 22 factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surfaces B Biointerfaces. 2018;171:501–505. doi: 10.1016/j.colsurfb.2018.07.065. [DOI] [PubMed] [Google Scholar]
- Rittirsch D., Huber-Lang M.S., Flierl M.A., Ward P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214.Immunodesign. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzello L., Cingolani R., Pompa P.P. Nanotechnology tools for antibacterial materials. Nanomedicine. 2013;8:807–821. doi: 10.2217/nnm.13.63. [DOI] [PubMed] [Google Scholar]
- Rizzello, L., Cingolani, R., Pompa, P.P., 2013b. Nanotechnology tools for antibacterial materials Review 8, 807–821. [DOI] [PubMed]
- Rochelle do Vale Morais, A., Silva, A.L., Cojean, S., Balaraman, K., Bories, C., Pomel, S., Barratt, G., do Egito, E.S.T., Loiseau, P.M., 2018. In-vitro and in-vivo antileishmanial activity of inexpensive Amphotericin B formulations: Heated Amphotericin B and Amphotericin B-loaded microemulsion. Exp. Parasitol. 192, 85–92. https://doi.org/10.1016/j.exppara.2018.07.017. [DOI] [PubMed]
- Rollé A., Schepers K., Cassadou S., Curlier E., Madeux B., Hermann-Storck C., Fabre I., Lamaury I., Tressières B., Thiery G., Hoen B. Severe sepsis and septic shock associated with chikungunya virus infection, Guadeloupe, 2014. Emerg. Infect. Dis. 2016;22:891–894. doi: 10.3201/eid2205.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.R.B., Kumar R. Early onset neonatal sepsis: Diagnostic dilemmas and practical management. Arch. Dis. Child. Fetal Neonatal Ed. 2015;100:F350–F354. doi: 10.1136/archdischild-2014-306193. [DOI] [PubMed] [Google Scholar]
- Sa’adu, L.O., Obasa, T.O., Saka, A.O., Saka, M.J., Nwabuisi, C., 2019. Multi-drug resistance in early and late onset neonatal sepsis in a tertiary hospital in Nigeria. Ann. African Med. Res. 2, 82–86. https://doi.org/10.4081/mi.2013.e4.
- Saez A., Guzmán M., Molpeceres J., Aberturas M.R. Freeze-drying of polycaprolactone and poly(D, L-lactic-glycolic) nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur. J. Pharm. Biopharm. 2000;50:379–387. doi: 10.1016/S0939-6411(00)00125-9. [DOI] [PubMed] [Google Scholar]
- Saleem A.F., Qamar F.N., Shahzad H., Qadir M., Zaidi A.K.M. Trends in antibiotic susceptibility and incidence of late-onset Klebsiella pneumoniae neonatal sepsis over a six-year period in a neonatal intensive care unit in Karachi. Pakistan. Int. J. Infect. Dis. 2013;17:e961–e965. doi: 10.1016/j.ijid.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Sánchez-López E., Espina M., Doktorovova S., Souto E.B., García M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye – Part II - Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017;110:58–69. doi: 10.1016/j.ejpb.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Sande L., Sanchez M., Montes J., Wolf A.J., Morgan M.A., Omri A., Liu G.Y. Liposomal encapsulation of vancomycin improves killing of methicillin-resistant Staphylococcus aureus in a murine infection model. J. Antimicrob. Chemother. 2012;67:2191–2194. doi: 10.1093/jac/dks212. [DOI] [PubMed] [Google Scholar]
- Sanoj Rejinold N., Muthunarayanan M., Divyarani V.V., Sreerekha P.R., Chennazhi K.P., Nair S.V., Tamura H., Jayakumar R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011;360:39–51. doi: 10.1016/j.jcis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Hermes DeSantis E.R., Kuper J. Resurgence of colistin use. Am. J. Heal. Pharm. 2007;64:2462–2466. doi: 10.2146/ajhp060501. [DOI] [PubMed] [Google Scholar]
- Sato M.R., Oshiro Junior J.A., Machado R.T.A., de Souza P.C., Campos D.L., Pavan F.R., da Silva P.B., Chorilli M. Nanostructured lipid carriers for incorporation of copper(II) complexes to be used against Mycobacterium tuberculosis. Drug Des. Devel. Ther. 2017;11:909–921. doi: 10.2147/DDDT.S127048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- Scalise M.L., Arrúa E.C., Rial M.S., Esteva M.I., Salomon C.J., Fichera L.E. Promising efficacy of benznidazole nanoparticles in acute trypanosoma cruzi murine model: In-vitro and in-vivo studies. Am. J. Trop. Med. Hyg. 2016;95:388–393. doi: 10.4269/ajtmh.15-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer-Korting M., Mehnert W., Korting H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007;59:427–443. doi: 10.1016/j.addr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Schaffazick, S.R., Guterres, S.S., 2003. Caracterização e estabilidade físico -química de sistemas poliméricos nanoparticulados para administração de fármacos 26, 726–737.
- Schär F., Trostdorf U., Giardina F., Khieu V., Muth S., Marti H., Vounatsou P., Odermatt P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013;7:1–18. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scire J., Hozé N., Uecker H. Aggressive or moderate drug therapy for infectious diseases? Trade-offs between different treatment goals at the individual and population levels. PLoS Comput. Biol. 2019 doi: 10.1371/journal.pcbi.1007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorciapino M.A., Serra I., Manzo G., Rinaldi A.C. Antimicrobial dendrimeric peptides: Structure, activity and new therapeutic applications. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamuthukumar S., Velmurugan R. Nanostructured Lipid Carriers: A potential drug carrier for cancer chemotherapy. Lipids Health Dis. 2012;11:1–8. doi: 10.1186/1476-511X-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V., Manne N.D.P.K., Arvapalli R., Rice K.M., Nandyala G., Fankenhanel E., Blough E.R. Effect of cerium oxide nanoparticles on sepsis induced mortality and NF-κB signaling in cultured macrophages. Nanomedicine. 2015;10:1275–1288. doi: 10.2217/nnm.14.205. [DOI] [PubMed] [Google Scholar]
- Sharma A.K., Gothwal A., Kesharwani P., Alsaab H., Iyer A.K., Gupta U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today. 2017;22:314–326. doi: 10.1016/j.drudis.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Sharma D., Ali A.A.E., Trivedi L.R. An updated review on: liposomes as drug delivery system. Pharmatutor. 2018;6:50. doi: 10.29161/PT.v6.i2.2018.50. [DOI] [Google Scholar]
- Sharma P., Ganta S., Denny W.A., Garg S. Formulation and pharmacokinetics of lipid nanoparticles of a chemically sensitive nitrogen mustard derivative: Chlorambucil. Int. J. Pharm. 2009;367:187–194. doi: 10.1016/j.ijpharm.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Shinde R.L., Bharkad G.P., Devarajan P.V. Intranasal microemulsion for targeted nose to brain delivery in neurocysticercosis: Role of docosahexaenoic acid. Eur. J. Pharm. Biopharm. 2015;96:363–379. doi: 10.1016/j.ejpb.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Shukla P., Dwivedi P., Gupta P.K., Mishra P.R. Optimization of novel tocopheryl acetate nanoemulsions for parenteral delivery of curcumin for therapeutic intervention of sepsis. Expert Opin. Drug Deliv. 2014;11:1697–1712. doi: 10.1517/17425247.2014.932769. [DOI] [PubMed] [Google Scholar]
- Sieniawska E., Świątek Ł., Wota M., Rajtar B., Polz-Dacewicz M. Microemulsions of essentials oils – Increase of solubility and antioxidant activity or cytotoxicity? Food Chem. Toxicol. 2019;129:115–124. doi: 10.1016/j.fct.2019.04.038. [DOI] [PubMed] [Google Scholar]
- Silva M.G.Da., Cardoso J.F., Perasoli F.B., Branquinho R.T., Mourão R.S., Tavares H.D.S., Xocaira M.L.C.T., Guimarães D.S.M., Viana G.H.R., Varotti F.D.P., Silva G.R.Da. Nanoemulsion composed of 10-(4,5-dihydrothiazol-2-yl)thio)decan-1-ol), a synthetic analog of 3-alkylpiridine marine alkaloid: development, characterization, and antimalarial activity. Eur. J. Pharm. Sci. 2020;151:105382. doi: 10.1016/j.ejps.2020.105382. [DOI] [PubMed] [Google Scholar]
- Silva P.B., Ramos M.A.S., Bonifácio B.V., Negri K.M.S., Sato M.R., Bauab T.M., Chorilli M. Nanotechnological Strategies for Vaginal Administration of Drugs—A Review. J. Biomed. Nanotechnol. 2014;10:2218–2243. doi: 10.1166/jbn.2014.1890. [DOI] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., Poll T. Der, Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA - J. Am. Med. Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Siriwardena T.N., Stach M., He R., Gan B.H., Javor S., Heitz M., Ma L., Cai X., Chen P., Wei D., Li H., Ma J., Köhler T., Van Delden C., Darbre T., Reymond J.L. Lipidated peptide dendrimers killing multidrug-resistant bacteria. J. Am. Chem. Soc. 2018;140:423–432. doi: 10.1021/jacs.7b11037. [DOI] [PubMed] [Google Scholar]
- Škalko-Basnet, N., Vanić, 2017. Lipid-based nanopharmaceuticals in antimicrobial therapy, functionalized nanomaterials for the management of microbial infection: a strategy to address microbial drug resistance. https://doi.org/10.1016/B978-0-323-41625-2.00005-3.
- Sobel J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016;214:15–21. doi: 10.1016/j.ajog.2015.06.067. [DOI] [PubMed] [Google Scholar]
- Song X., Lin Q., Guo L., Fu Y., Han J., Ke H., Sun X., Gong T., Zhang Z. Rifampicin loaded mannosylated cationic nanostructured lipid carriers for alveolar macrophage-specific delivery. Pharm. Res. 2015;32:1741–1751. doi: 10.1007/s11095-014-1572-3. [DOI] [PubMed] [Google Scholar]
- Souto E.B., Faculdade B., Ciências D., Pessoa U.F., Santana M.H.A., Pinho S.C. Quim. Nova. 2011;34:1762–1769. [Google Scholar]
- Souto E.B., Severino P., Santana M.H.A. Preparação de Nanopartículas Poliméricas a partir de Polímeros Pré-formados - Parte II. Polimeros. 2012;22:101–106. doi: 10.1590/S0104-14282012005000005. [DOI] [Google Scholar]
- Spicer P.T. Progress in liquid crystalline dispersions: Cubosomes. Curr. Opin. Colloid Interface Sci. 2005;10:274–279. doi: 10.1016/j.cocis.2005.09.004. [DOI] [Google Scholar]
- Sriskandan S., Cohen J. Gram-positive sepsis: Mechanisms and differences from gram-negative sepsis. Infect. Dis. Clin. North Am. 1999;13:397–412. doi: 10.1016/S0891-5520(05)70082-9. [DOI] [PubMed] [Google Scholar]
- Stryjewski M.E., Chambers H.F. Skin and soft tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008;46:368–377. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- Tacconelli E., Sifakis F., Harbarth S., Schrijver R., Mourik M. Van, Voss A., Sharland M. Personal View Surveillance for control of antimicrobial resistance. Lancet Infect. Dis. 2018;18:99–106. doi: 10.1016/S1473-3099(17)30485-1. [DOI] [PubMed] [Google Scholar]
- Tadros T., Izquierdo P., Esquena J., Solans C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Tang X., Zhu H., Sun L., Hou W., Cai S., Zhang R., Liu F. Enhanced antifungal effects of amphotericin B-TPGS-b-(PCL-ran-PGA) nanoparticles in vitro and in vivo. Int. J. Nanomedicine. 2014;9:5403–5413. doi: 10.2147/IJN.S71623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taratummarat S., Sangphech N., Vu C.T.B., Palaga T., Ondee T., Surawut S., Sereemaspun A., Ritprajak P., Leelahavanichkul A. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018;18:1–10. doi: 10.1186/s12866-018-1227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano L., Muzzalupo R., Picci N., De Cindio B. Co-encapsulation of lipophilic antioxidants into niosomal carriers: Percutaneous permeation studies for cosmeceutical applications. Colloids Surfaces B Biointerfaces. 2014;114:144–149. doi: 10.1016/j.colsurfb.2013.09.055. [DOI] [PubMed] [Google Scholar]
- Teeranachaideekul V., Souto E.B., Junyaprasert V.B., Müller R.H. Cetyl palmitate-based NLC for topical delivery of Coenzyme Q10 - Development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007;67:141–148. doi: 10.1016/j.ejpb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Terblanche M., Almog Y., Rosenson R.S., Smith T.S., Hackam D.G. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect. Dis. 2007;7:358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- Tibbitt M.W., Dahlman J.E., Langer R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016;138:704–717. doi: 10.1021/jacs.5b09974. [DOI] [PubMed] [Google Scholar]
- Tomalia D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005;30:294–324. doi: 10.1016/j.progpolymsci.2005.01.007. [DOI] [Google Scholar]
- Tomália D.A., Baker H., Dewald M., Kallos G., Martin S., Roeck J., Ryder J., Smith P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985 [Google Scholar]
- Torchilin, V.P., 2005. RECENT ADVANCES WITH LIPOSOMES AS PHARMACEUTICAL CARRIERS 4. https://doi.org/10.1038/nrd1632. [DOI] [PubMed]
- Torio C.M., Moore B.J. Statistical brief #204 national inpatient hospital costs: The most expensive conditions by payer, 2013. Hcup. 2016;204:1–15. doi: 10.1377/hlthaff.2015.1194.3. [DOI] [PubMed] [Google Scholar]
- Troiano G., Nolan J., Parsons D., Van Geen Hoven C., Zale S. A Quality by design approach to developing and manufacturing polymeric nanoparticle drug products. AAPS J. 2016;18:1354–1365. doi: 10.1208/s12248-016-9969-z. [DOI] [PubMed] [Google Scholar]
- Ubbink J., Burbidge A., Mezzenga R. Food structure and functionality: a soft matter perspective. Soft Matter. 2008;4:1569–1581. doi: 10.1039/b800106e. [DOI] [PubMed] [Google Scholar]
- Üner M., Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspective. Int. J. Nanomed. 2007;2:289–300. [PMC free article] [PubMed] [Google Scholar]
- Urrejola, M.C., Liliam, Soto, V., Zumarán, C.C., Juan, ;, Peñaloza, P., Álvarez, B., Fuentevilla, I., Haidar, Z.S., 2018. Sistemas de Nanopartículas Poliméricas II: Estructura, Métodos de Elaboración, Características, Propiedades, Biofuncionalización y Tecnologías de Auto-Ensamblaje Capa por Capa (Layer-by-Layer Self-Assembly) Polymeric Nanoparticle Systems: Structure, Elabo. Int. J. Morphol 36, 1463–1471.
- Üstündaǧ-Okur N., Gökçe E.H., Bozbiyik D.I., Eǧrilmez S., Özer Ö., Ertan G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur. J. Pharm. Sci. 2014;63:204–215. doi: 10.1016/j.ejps.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Üstündaʇ-Okur N., Gökçe E.H., Bozbiyik D.I., Eʇrilmez S., Ertan G., Özer Ö. Novel nanostructured lipid carrier-based inserts for controlled ocular drug delivery: Evaluation of corneal bioavailability and treatment efficacy in bacterial keratitis. Expert Opin. Drug Deliv. 2015;12:1791–1807. doi: 10.1517/17425247.2015.1059419. [DOI] [PubMed] [Google Scholar]
- Valiante N.M., O’Hagan D.T., Ulmer J.B. Innate immunity and biodefence vaccines. Cell. Microbiol. 2003;5:755–760. doi: 10.1046/j.1462-5822.2003.00318.x. [DOI] [PubMed] [Google Scholar]
- van de Beek D., Brouwer M., Hasbun R., Koedel U., Whitney C.G., Wijdicks E. Community-acquired bacterial meningitis. Nat. Rev. Dis. Prim. 2016;2:1–20. doi: 10.1038/nrdp.2016.74. [DOI] [PubMed] [Google Scholar]
- Van De Ven H., Paulussen C., Feijens P.B., Matheeussen A., Rombaut P., Kayaert P., Van Den Mooter G., Weyenberg W., Cos P., Maes L., Ludwig A. PLGA nanoparticles and nanosuspensions with amphotericin B: Potent in vitro and in vivo alternatives to Fungizone and Am Bisome. J. Control. Release. 2012;161:795–803. doi: 10.1016/j.jconrel.2012.05.037. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Use of liposomes as biodegradable and harmless adjuvants. Methods Enzymol. 1983;93:83–95. doi: 10.1016/S0076-6879(83)93036-7. [DOI] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. The Covid-19 epidemic. Trop. Med. Int. Health. 2020;00:19–21. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso D.F.M.C., Benedetti N.I.G.M., Avila R.I., Bastos T.S.A., Silva T.C., Silva M.R.R., Batista A.C., Valadares M.C., Lima E.M. Intravenous delivery of a liposomal formulation of voriconazole improves drug pharmacokinetics, tissue distribution, and enhances antifungal activity. Drug Deliv. 2018;25:1585–1594. doi: 10.1080/10717544.2018.1492046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh N. Metallic nanoparticle: a review. Biomed. J. Sci. Tech. Res. 2018;4:3765–3775. doi: 10.26717/bjstr.2018.04.0001011. [DOI] [Google Scholar]
- Verma R., Jaiswal T.N. Protection, humoral and cell-mediated immune responses in calves immunized with multiple emulsion haemorrhagic septicaemia vaccine. Vaccine. 1997;15:1254–1260. doi: 10.1016/S0264-410X(97)00025-X. [DOI] [PubMed] [Google Scholar]
- Waknine-Grinberg J.H., Even-Chen S., Avichzer J., Turjeman K., Bentura-Marciano A., Haynes R.K., Weiss L., Allon N., Ovadia H., Golenser J., Barenholz Y. Glucocorticosteroids in nano-sterically stabilized liposomes are efficacious for elimination of the acute symptoms of experimental cerebral malaria. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Yu, S., Lin, Y., Zou, P., Chai, G., Yu, H.H., Wickremasinghe, H., Shetty, N., Ling, J., Li, J., Zhou, Q. (Tony), 2018. Co-delivery of ciprofloxacin and colistin in liposomal formulations with enhanced in vitro antimicrobial activities against multidrug resistant pseudomonas aeruginosa. Pharm. Res. 35, 1–13. https://doi.org/10.1007/s11095-018-2464-8. [DOI] [PMC free article] [PubMed]
- Wang S.H., Chen J., Smith D., Cao Z., Acosta H., Fan Y., Ciotti S., Fattom A., Baker J. A novel combination of intramuscular vaccine adjuvants, nanoemulsion and CpG produces an effective immune response against influenza A virus. Vaccine. 2020;38:3537–3544. doi: 10.1016/j.vaccine.2020.03.026. [DOI] [PubMed] [Google Scholar]
- Ways T.M.M., Lau W.M., Khutoryanskiy V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers (Basel). 2018;10 doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnicka K., Wroblewska M., Wieczorek P., Sacha P.T., Tryniszewska E. Hydrogel of Ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules. 2012;17:4612–4624. doi: 10.3390/molecules17044612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnicka K., Wroblewska M., Wieczorek P., Sacha P.T., Tryniszewska E.A. The effect of PAMAM dendrimers on the antibacterial activity of antibiotics with different water solubility. Molecules. 2013;18:8607–8617. doi: 10.3390/molecules18078607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolthers K.C., Benschop K.S.M., Schinkel J., Molenkamp R., Bergevoet R.M., Spijkerman I.J.B., Kraakman H.C., Pajkrt D. Human Parechoviruses as an Important Viral Cause of Sepsislike Illness and Meningitis in Young Children. Clin. Infect. Dis. 2008;47:358–363. doi: 10.1086/589752. [DOI] [PubMed] [Google Scholar]
- Wrońska N., Majoral J.P., Appelhans D., Bryszewska M., Lisowska K. Synergistic effects of anionic/cationic dendrimers and levofloxacin on antibacterial activities. Molecules. 2019;24:1–11. doi: 10.3390/molecules24162894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Wang Y., Zhu J., He Y., Qu J., Effenberg C., Xia J., Appelhans D., Shi X. Gd-Chelated poly(propylene imine) dendrimers with densely organized maltose shells for enhanced MR imaging applications. Biomater. Sci. 2016;4:1622–1629. doi: 10.1039/c6bm00532b. [DOI] [PubMed] [Google Scholar]
- Xu Q., Zhou A., Wu H., Bi Y. Development and in vivo evaluation of baicalin loaded W/O nanoemulsion for lymphatic absorption. Pharm. Dev. Technol. 2019:1–32. doi: 10.1080/10837450.2019.1646757. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li Y., Liu X., Pan Y., Sun Z., Xue Y., Wang T., Dou H., Hou Y. SPIONs enhances IL-10-producing macrophages to relieve sepsis via CavI-NotchI/HESI-mediated autophagy. Int. J. Nanomed. 2019;14:6779–6797. doi: 10.2147/IJN.S215055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Corona, A., Schubert, B., Reeder, R., Henson, M.A., 2014. Journal of Colloid and Interface Science The effect of oil type on the aggregation stability of nanostructured lipid carriers 418, 261–272. [DOI] [PubMed]
- Yang Y., Ding Y., Fan B., Wang Y., Mao Z., Wang W., Wu J. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release. 2020;321:463–474. doi: 10.1016/j.jconrel.2020.02.030. [DOI] [PubMed] [Google Scholar]
- Yao Y., Zang Y., Qu J., Tang M., Zhang T. The toxicity of metallic nanoparticles on liver: the subcellular damages, mechanisms, and outcomes. Int. J. Nanomed. 2019;14:8787–8804. doi: 10.2147/IJN.S212907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellepeddi V.K., Ghandehari H. Pharmacokinetics of oral therapeutics delivered by dendrimer-based carriers. Expert Opin. Drug Deliv. 2019;16:1051–1061. doi: 10.1080/17425247.2019.1656607. [DOI] [PubMed] [Google Scholar]
- Zhang C.Y., Gao J., Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 2018;30:1–10. doi: 10.1002/adma.201803618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Neff C.P., Liu X., Zhang J., Li H., Smith D.D., Swiderski P., Aboellail T., Huang Y., Du Q., Liang Z., Peng L., Akkina R., Rossi J.J. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011;19:2228–2238. doi: 10.1038/mt.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba B., Janaszewska A., Ciepluch K., Krotewicz M., Fogel W.A., Appelhans D., Voit B., Bryszewska M., Klajnert B. In vivo toxicity of poly(propyleneimine) dendrimers. J. Biomed. Mater. Res. - Part A. 2011;99 A:261–268. doi: 10.1002/jbm.a.33196. [DOI] [PubMed] [Google Scholar]
- Ziemba B., Matuszko G., Appelhans D., Voit B., Bryszewska M., Klajnert B. Genotoxicity of poly(propylene imine) dendrimers. Biopolymers. 2012;97:642–648. doi: 10.1002/bip.22056. [DOI] [PubMed] [Google Scholar]