Abstract
The rise of inexpensive, user-friendly cameras and editing software promises to revolutionize data collection with minimal disturbance to marine mammals. Video sequences recorded by aerial drones and GoPro cameras provided close-up views and unique perspectives of humpback whales engulfing juvenile salmon at or just below the water surface in Southeast Alaska and Prince William Sound. Although humpback feeding is famous for its flexibility, several stereotyped events were noted in the 47 lunges we analyzed. Engulfment was rapid (mean 2.07 s), and the entrance through which the tongue inverts into the ventral pouch was seen as water rushes in. Cranial elevation was a major contributor to gape, and pouch contraction sometimes began before full gape closure, with reverberating waves indicating rebounding flow of water within the expanded pouch. Expulsion of filtered water began with a small splash at the anterior of the mouth, followed by sustained excurrent flow in the mouth’s central or posterior regions. Apart from a splash of rebounding water, water within the mouth was surprisingly turbulence-free during engulfment, but submersion of the whale’s head created visible surface whirlpools and vortices which may aggregate prey for subsequent engulfment.
Keywords: mysticete, rorqual, humpback whale, Megaptera novaeangliae, feeding, anatomy, baleen, tongue
The resourceful and highly acrobatic foraging behaviors of humpback whales (Megaptera novaeangliae) are widely known, both during typical surface and subsurface lunge feeding (Hain et al. 1981; Weinrich et al. 1992; Goldbogen et al. 2008; Hazen et al. 2009; Friedlaender et al. 2009, 2013; Ware et al. 2010; Simon et al. 2012) and particularly when using bubbles to corral prey into densely packed balls for more efficient engulfment (Gormley 1983, Leighton et al. 2007, Wiley et al. 2011). Bubble-related behaviors have frequently been observed and recorded, especially in the coastal waters of Southeast Alaska (Jurasz and Jurasz 1979, D’Vincent et al. 1985, Winn and Winn 1985), where humpback whales have recently learned to capture juvenile salmon released from hatchery sites (Chenoweth et al. 2017).
The study of feeding in humpback whales and other mysticetes has been revolutionized by digital tagging (Goldbogen et al. 2013, Goldbogen and Meir 2014, Kirchner et al. 2018). Small, unobtrusive devices temporarily affixed to a whale’s body by suction cups measure a whale’s acceleration, depth, and body position/motion in various directions while cameras record close-up features. Together these instruments provide precisely quantified data revealing a whale’s movements as it approaches and ingests prey, as well as its activities between feeding bouts. A flurry of biologging studies continues to reveal intricate details of ecology, physiology, morphology, biomechanics, and behavior in humpback and other whale species (Goldbogen et al. 2006, 2007; Simon et al. 2012; Cade et al. 2016). Unfortunately, some of the most relevant phenomena of whale feeding—indeed, the most pertinent aspects of filtration, including directions, quantities, and timing of intraoral current flow and their relation to intraoral morphology—can be surmised only indirectly given the inability, at present, of placing a tag and recording directly within a whale’s mouth. Nonetheless, tag data or calculations from related mathematical modeling have substantially clarified diverse intraoral events (Goldbogen et al. 2012, 2015, 2017a, b). Although video footage from whale-mounted cameras reveals valuable findings directly from foraging animals, often at depths or positions otherwise unavailable to human researchers (Cade et al. 2016, Goldbogen et al. 2017a), they are somewhat constrained by the limited vantage points they provide from their affixed location on the backs or flanks of whales. Importantly, these cameras can only provide views within the mouth or show a whole whale interacting with its environment when a second whale serendipitously enters the frame of a tagged whale (Segre et al. 2017).
Fortunately, just as digital tags have gained new capabilities while decreasing in size and cost (Goldbogen et al. 2013, 2017a; Goldbogen and Meir 2014), other recent technological advances have made it easier to capture and analyze video recordings of marine mammal behavior (Nowacek et al. 2001, 2016; Anderson and Gaston 2013; Hunt et al. 2013; Karnowski et al. 2016; Pirotta et al. 2017, 2019; Johnston 2019), and particularly of humpback whales (Ware et al. 2006, Christiansen et al. 2016a, Kirchner et al. 2018). Simple, user-friendly, and inexpensive video cameras and editing software enable researchers to document feeding and other activities (Letessier et al. 2015, Raoult et al. 2016, Brooks 2017). Advantages include great clarity due to high pixel resolution, zoom lenses, and swivel capability, and the ability to record video in previously limited settings, such as underwater or from high vantage points, as with unmanned aerial systems (UAS, commonly called drones). Aerial and underwater drones can be remotely operated or autonomous (Schiffman 2014). Other miniature, highly mobile video cameras, such as GoPro cameras, can be quickly affixed to people and poles or other objects (Raoult et al. 2016).
There are several obvious advantages to collecting data with drones and GoPro cameras. First, these tools can generally be set up and used by a single operator, or at most by just a few people. Second, these tools can be used proficiently with minimal training. Third, reliable data can be obtained and immediately analyzed with little or no postcollection data processing. Fourth and perhaps most important, drone and GoPro camera systems can be used under a wide range of field conditions, even those that would normally prohibit traditional vessel-based observation, although they are generally unsuitable for use in high wind speeds or water currents, or for conditions with low visibility in air or water.
These new technologies are rapidly changing the study of wildlife (Bevan et al. 2016, Rümmler et al. 2016, Schofield et al. 2017, Sykora-Bodie et al. 2017, Rees et al. 2018, Rieucau et al. 2018, Weimerskirch et al. 2018, Verfuss et al. 2019), especially in marine settings where observation had been mostly limited to vessel-based or airborne observers. Because new camera systems allow more rapid (near instantaneous) changes in orientation than most boats and manned aircraft can achieve, animals can be followed and stable vantage points maintained or switched very quickly (Hodgson et al. 2013, 2017; Goebel et al. 2015; Koski et al. 2015; Durban et al. 2016; Fiori et al. 2017; Johnston et al. 2017; Krause et al. 2017; Torres 2017; Burnett et al. 2018; Torres et al. 2018). Not only have these video recording systems become less expensive and more readily available (Goldbogen and Meir 2014, Nowacek et al. 2016, Dawson et al. 2017), they are also less noisy and less dangerous to operate (Christiansen et al. 2016b), so that they pose fewer risks to animals and likely cause fewer changes in wild animals’ natural behavior (Ditmer 2015 et al., Dominguez-Sanchez et al. 2018). Nonetheless, risks remain when these systems are used in close proximity to marine mammals or other threatened wildlife (Pomeroy et al. 2015, Hodgson and Koh 2016, Smith et al. 2016, Sullivan and Torres 2018). As is often the case, technology can evolve faster than rules governing its use (Vas et al. 2015).
During long-term studies of humpback whale foraging behavior and ecology, we analyzed video sequences of surface and near-surface lunge feeding. Many observed behaviors (and much video footage) of our study involved humpback whales feeding on juvenile salmon immediately after the fish were released from net pens at hatcheries located in coastal fjords of Southeast Alaska and Prince William Sound.
Our findings encompass diverse yet interrelated aspects of humpback whale anatomy and biomechanics with behavioral ecology, many of which we presume apply to feeding in other rorquals (Balaenopteridae). This makes it difficult to organize them into discrete categories, although all findings focus more on the morphology than the behavior of feeding. Close-up drone and GoPro videos reveal hidden details when played back at real-time speed or frame-by-frame. Thus the “new views” of this paper’s title include both novel vantage points affording new visual perspectives as well as new insights about humpback (and possibly general rorqual) feeding ecology, oral morphology, and biomechanics.
Methods
We analyzed 36 min of video footage that included 47 humpback whale engulfment events. In nearshore waters of the Gulf of Alaska during 2014–2018, footage was captured with three sources: hand-held cameras, a drone, and a GoPro Hero5 Black camera affixed to a 3.5 m pole. Videos were captured near hatchery release sites and whales were likely feeding on yearling or young-of-the-year coho salmon or young-of-the-year chum salmon recently released from the hatcheries, although whales could have been feeding on some wild fish as well.
In Southeast Alaska we recorded feeding bouts of at least five individual humpback whales (average body length in North Pacific 12.3 m; Nichol and Heise 1992) engaged in bubble net-related or other lunge feeding at or just below the surface within small fjord inlets around Baranof Island in the eastern Gulf of Alaska, in water ~40 m deep. Typically these involved feeding on schools of small (4–20 g, or 4–14 cm) yearling coho and young-of-the-year chum salmon just released from floating net pens (Chenoweth et al. 2017). Floating walkway platforms around net pens provide a unique and exceptionally close-up vantage point, typically directly above and within a circular bubble net/ring released by the whales to corral prey.
The 2017 GoPro-recorded MP4 sequences were shot with the camera mounted on a pole held straight up or at an angle over the water, so as to be as close as possible to the feeding whales without disturbing them (often within 2–4 m; NOAA NMFS permits 14122, 18529), and to record their feeding behaviors in multiple views. It is important to note that the whales approached the observers who recorded whale behaviors while the observers were standing on the floating net-pen walkways; there was no need for people to approach whales. In this fortuitous setting (with floating net pens above 25-m-deep water) a pole-mounted camera is quieter than a drone and easier to control and move rapidly into proper position, including underwater (see Video S1 for this study and from Chenoweth et al. 2017).
In Prince William Sound in 2016 and 2017, underwater and drone videos were obtained of humpback whales feeding, presumably on young-of-the-year chum salmon and pink salmon recently released from hatcheries. Drone video (DJI Phantom 3 Professional, shooting 23 frames/s at 8,300 kbps and 1,280 × 720 pixel resolution) was recorded in May 2017 near Evans Island, above the Armin F. Koernig Hatchery at Port San Juan, after a release of chum salmon. Underwater video was recorded in April 2016 with a GoPro Hero4 Silver at Wally Noerenberg hatchery and release site in Lake Bay on the southern end of Esther Island in Prince William Sound after a release of pink salmon. The underwater videos display direct anterior views (i.e., looking into the mouth) prior to engulfment (Fig. 1), continuing to full lateral and ventral views as whales turn and sweep by the camera (Fig. 2). Underwater footage provided valuable information but poor water clarity limited the image resolution. Water in these protected inlets has limited visibility (1–4 m; better on sunny days) but few or no surface waves except for small, wind-driven ripples (capillary waves), promoting excellent aerial views of whales and their feeding behavior. These opportunistic videos were donated to our study by the Prince William Sound Aquaculture Corporation.
Figure 1.
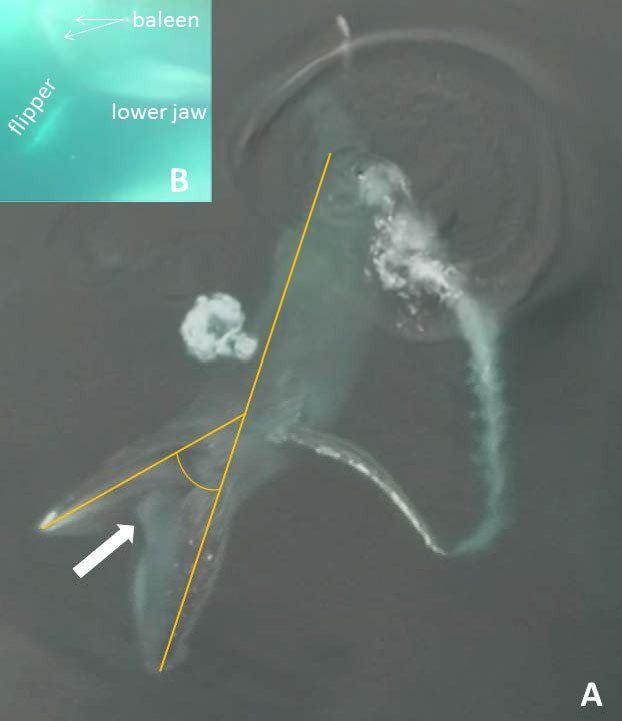
Aerial view (A) showing measurement of gape angle (here 44º) prior to engulfment and mandibular depression, with left flipper sweeping anteriorly and retracted tongue (arrow) just before it inverts and translocates into inflated ventral pouch. Inset (B) shows underwater head-on view of approaching whale in GoPro video frame just after tongue inversion (start of engulfment).
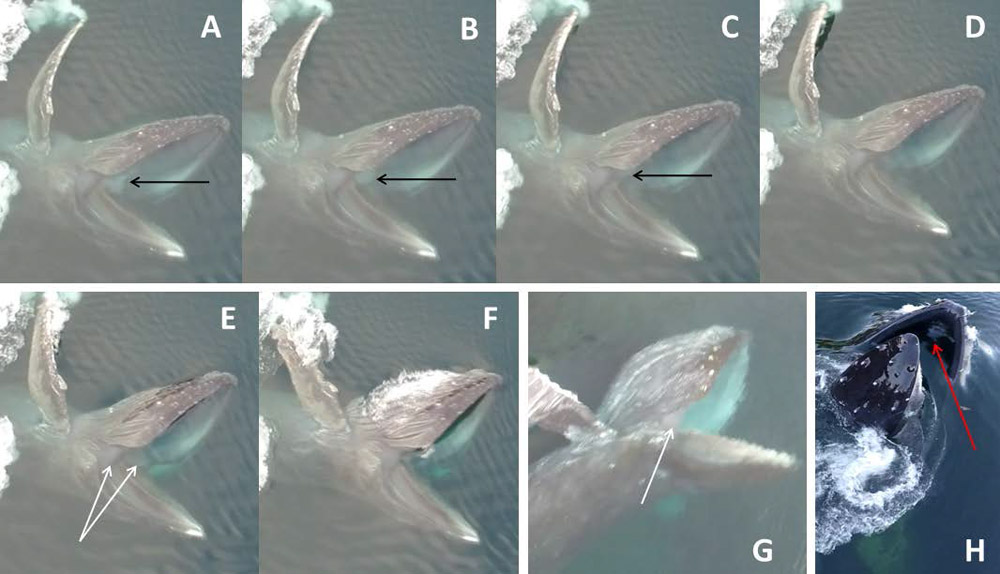
Figure 2.
Expulsion of engulfed water usually begins with contraction of posterior-most ventral groove blubber (VGB) musculature, resulting in water-filled expansion mainly of the anterior of the pouch, as at the start of this sequence (frames A→F = 3.38 s). Toward the end of this sequence the pouch’s expanded posterior region (arrows) probably represents mainly engulfed prey, although there may also be more water still to be expelled.
Video sequences were analyzed with VLC Media Player 3.0.3. Because the GoPro camera has a wide-angle fisheye lens that can distort images, postproduction lens correction was used to create a linear field of view. The rolling shutter of GoPro and other CMOS-based cameras can cause blurring if the camera is unsteady; postproduction image correction via GoPro Studio v.2.5.7 software can improve clarity and contrast. Frame-by-frame video analysis was also done using GoPro Studio, with digitization of landmarks and kinematic analysis using Tracker v.4.92 and angle measurement via MB-Ruler 5.3.
Whales were observed/recorded swimming and feeding in many different body positions and rotatory orientations, including lateral (side) swimming and partially or wholly inverted (upside-down) body posture (Kot et al. 2014), with frequent but not exclusive use of bubble netting and engulfment upward through or parallel to the sea surface. Because the 47 engulfment events we recorded involved differing body positions, some sequences yielded less information than others and could not be used in all analyses. Also, we hesitate to use the term “lunge feeding” (Pivorunas 1979, Werth 2000, Goldbogen 2010) for all sequences; about 64% (30 of 47) of sequences demonstrate “classic” rapid rorqual lunges, whereas the rest involve humpback whales gently rising through the surface (~1.0–<2.0 m/s swim speed, estimated by body length as determined when in proximity to objects of known proportions), following a similarly slow approach—possibly to avoid alarming or otherwise scattering aggregated prey.
Results
Among the highlights of our analysis (elaborated upon in correspondingly numbered sections that follow):
Cranial elevation (up to 54º) is a major contributor to gape.
The entrance through which the tongue inverts into the ventral pouch (Lambertsen 1983, Werth et al. 2018) can be seen.
Engulfment is rapid (mean 2.07 s, n = 24).
Expulsion of filtered water lasts 10 times longer than engulfment (mean 22.3 s, n = 24).
Pouch contraction (and some expulsion of filtered water) begins before full gape closure.
Reverberating waves along the gular region indicate rebounding water within the pouch.
Expelled water begins with a small splash at the anterior of the mouth, followed by more sustained flow in the mouth’s central or posterior regions.
Apart from a splash of rebounding water, water within the mouth is surprisingly turbulence-free during engulfment.
Submersion of the whale’s head creates visible surface whirlpools and vortices.
Although humpback whales rotate into varied body orientations when engulfing prey, there is little variation in the timing or kinematics of engulfment or expulsion by body position.
1. Gape Angle and Cranial Elevation
We recorded widely varying foraging behaviors including presence or absence of bubble netting, rapid lunges with forward locomotion but no change in depth, slow or quick vertical ascent from directly below the surface with no horizontal movement, and engulfment in varied body orientations. Despite this variation, our recorded engulfment sequences display remarkably stereotyped events. Total gape angle (i.e., between upper and lower jaws, including both cranial elevation and mandibular depression) averaged 82.5º (SD = 5.89, range 70º–94º, n = 20). Previous studies (Arnold et al. 2005, Goldbogen et al. 2017b) mentioned the role of cranial elevation in rorqual feeding but perhaps underestimated its ubiquity and contribution to gape. Our kinematic analysis revealed substantial cranial elevation (mean 39.5º, SD = 8.5, range 24º–54º, n = 20).
2. Tongue Inversion and Water Entry into the Ventral Pouch
Lambertsen (1983) initially proposed, based on ideas of Pivorunas (1979), the anatomical mechanism whereby the vast quantity of prey-laden water engulfed by rorquals (Fig. 2) fills a massive, balloon-like pouch temporarily created by inversion of the tongue and invaginated oral floor into an intermuscular gular space: the ventral (oral) pouch, AKA cavum ventrale. This space extends from the oral (buccal) cavity to the umbilicus and is externally demarcated by the accordion-like throat pleats and associated ventral groove blubber (VGB) and its musculature (Shadwick et al. 2013). Lambertsen (1983) demonstrated the tongue inversion phenomenon via post mortem manipulation of a suspended minke whale (Balaenoptera acutorostrata) body: viz., suturing the esophagus and filling the mouth with flowing water from a hose. Although this process is externally observable in vivo via extensive gular expansion, actual infolding and translocation of the inverted tongue and influx of water into the opening of the pouch have not heretofore been seen—and precise anatomical details remain vague—because these actions occur rapidly and on the floor of the mouth, in a location briefly visible only from within or directly above the oral cavity. We have captured what we believe to be the first in vivo views recording the moments of tongue inversion and water flow into the rapidly filling ventral pouch.
Lingual inversion was recorded in dorsal (Fig. 3) and lateral view (Fig. 4), whereas the opening into the ventral pouch (created during lingual inversion), and water flow into this opening, can be seen only from directly above the whale’s open gape in the early phase of engulfment (Fig. 3). As the grayish tongue folds inward and the oral floor slides backward into the expanding pouch during rapid water influx, a dark opening is briefly visible (Fig. 3), for only about 0.2 s, after which the oral floor drops away and all that can be seen in the open mouth is deep (>3 m, based on water clarity), prey-laden seawater. Seen from above, the initial opening is a dark, oval-shaped hole, longer anteroposteriorly than mediolaterally, before it almost immediately (in about 0.05 s) becomes a long slit about 1.5–2.5 × 0.5 m. (These measurements refer to the fleeting moment before the rapidly sliding oral floor gives way and drops out of sight.) The briefly observable dark, midline opening in the ventral oral floor is surrounded by lighter white tissue of the tongue on both sides (Fig. 3). This white tissue lies an estimated >1–1.5 m away from the dark lips at the dorsum of the mandibles; it is distinguishable from the light gray baleen plates and the expansive gray connective tissue at the angle of the jaws (which is farther posteriorly and darker in coloration), and this white tissue does not represent the rotating mandibles themselves.
Figure 3.

Outlined arrows show patent opening from buccal cavity into filling ventral pouch created by infolding of tongue (black arrows) in these dorsal views of whales ascending within circular bubble nets. Red arc indicates extent of lower jaws (i.e., boundary of the buccal cavity); note also juvenile salmon being engulfed and swept into expanding pouch, and slight lateral (outward) bending of the tips of baleen plates.
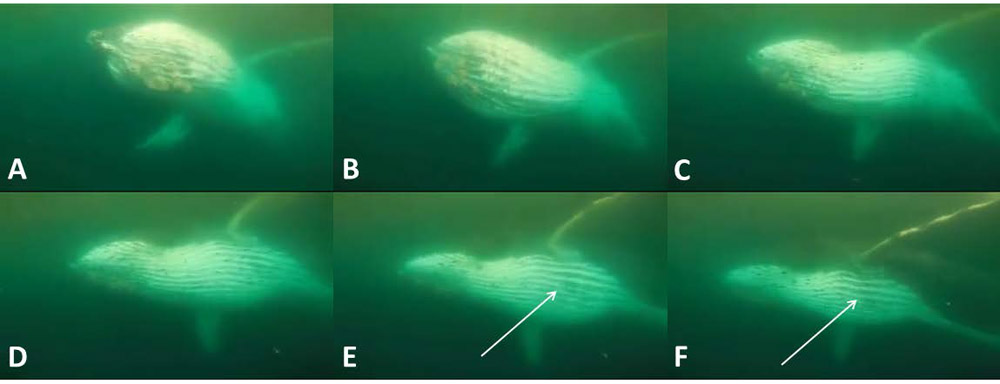
Figure 4.
Interior anatomical features (i.e., within the humpback mouth) are observable during these dorsolateral views of engulfment. In sequence A→F (total 0.24 s), the infolding tongue is visible in frames A-C (black arrows) just before it inverts and disappears into the expanded ventral pouch; white arrows indicate mandibular tendons, best seen with full pouch inflation (photo G, of the same whale during a different engulfment event). Red arrow in frame H (different whale during different engulfment event) indicates genioglossal tubercle at origin, by mandibular symphysis, of genioglossus tongue muscle.
As water streams through this channel—due to the combined influence of gravity and the ram mechanism provided by the whale’s forward locomotion—the ventral pouch is filled like a water balloon. Kinematic analysis confirms that fish within the oral cavity move downward and toward the opening (caudoventrally, relative to the whale), presumably caught in the powerful current flowing into the expanding pouch. In our video sequences, this motion is somewhat difficult to discern (looking down into murky green water from the camera’s vantage point several meters above the surface), but water/prey influx is clearly detectable via displacement of individual fish. As the pouch fully inflates, the oral floor tissue is deeper (i.e., farther from the surface of the water) and instantly disappears from view. Then gape closes as the depressed lower jaws are elevated and the elevated upper jaw is depressed. The true muscular “tongue proper” (aside from a flaccid, floppy mass on the oral floor) can best be seen in lateral view (Figs. 1A, 4), although the genioglossal tubercle, immediately posterior to the mandibular symphysis, is evident in several recorded sequences (described below).
3. Oral Extension and Anatomy (Mandibular Tendons, Genioglossal Tubercle, Baleen)
At the moment of peak gape (Fig. 4), a whitish gray band can be seen between the upper and lower jaws at the angle of the mouth. This band presumably shows stretched tendons of jaw adductor musculature (mainly m. temporalis, it appears, but also possibly m. masseter), which controls gape closure as well as mandibular rotation (Lambertsen et al. 1995).
Another briefly glimpsed “internal” anatomical structure that is momentarily seen but readily identifiable is the prominent tubercle of the m. genioglossus, the large muscle that originates along the mandibular symphysis (Fig. 4H) and controls tongue protraction to its normal position (Werth and Ito 2017). This tubercle is visible just as the mouth opens (when the tongue is in its resting position, prior to inversion), but it can no longer be seen as gape increases to >~30º and the oral floor drops away. We noticed nothing notable (that has not previously been reported) about the baleen filtering apparatus, with the sole exception being that the most distal (= ventral) tips of the plates bend laterally (labially) during engulfment (Fig. 3). Externally visible features associated with engulfment, including the Y-shaped fibrocartilage just under the mandibles at the front of the pouch (Pivorunas 1977), can also be seen in several of our recorded sequences.
4. Timing of Engulfment and Expulsion
Engulfment occurs rapidly, with a mean time of 2.07 s (SD 0.19, range 1.72–2.48, n = 24), measured from the start of water influx and gular expansion (even before peak gape) until the pouch is fully expanded. Expulsion (purging) of engulfed water—at which time filtration occurs, separating retained prey from expelled water—is also a rapid event, occurring in ~20 s (mean 22.3 s, SD = 5.15, range 14.2–36.1, n = 24). Depending on the whale’s orientation and depth it is often easy to visualize excurrent water flow (from bubbles, streams, and surface disruption) during expulsion; whales expelled water with the head above or underwater and the body partially or completely on its side (90º rotation) or in normal, dorsal-up position. Specific observations about degrees and directions of excurrent flow are outlined below.
5. Timing of Gape Closure and VGB Contraction
In about 65% of recorded engulfments (17 of 26), gape begins closing before the pouch is fully expanded. Our video sequences reveal that contraction of VGB musculature (to expel engulfed water from the mouth) typically and unexpectedly begins well before full gape closure (mean 0.34 s, SD = 0.06, range 0.24–0.49, n = 17).
6. Reverberating Waves Rebounding Through Filled Ventral Pouch
It is often possible (in about 40% or 10 of 26 recorded engulfment events where the expanded gular region is fully or largely visible) to see one or more waves moving rapidly over the external surface of the VGB and inflated oral pouch (Fig. 5). In most instances, a single wave is visible; this wave appears to represent completed filling of the pouch, as the wave originates at the posterior-most point of the pouch and “bounces” (reflects) anteriorly, rebounding toward the mouth opening just as gape begins to close and expulsion begins. This would be a simple phenomenon resulting from reversing longitudinal fluid flow, as inflowing water “hits” the posterior wall of the fully expanded pouch and reverses course. This splash can be seen from inside an open whale mouth (Fig. 5C).
Figure 5.
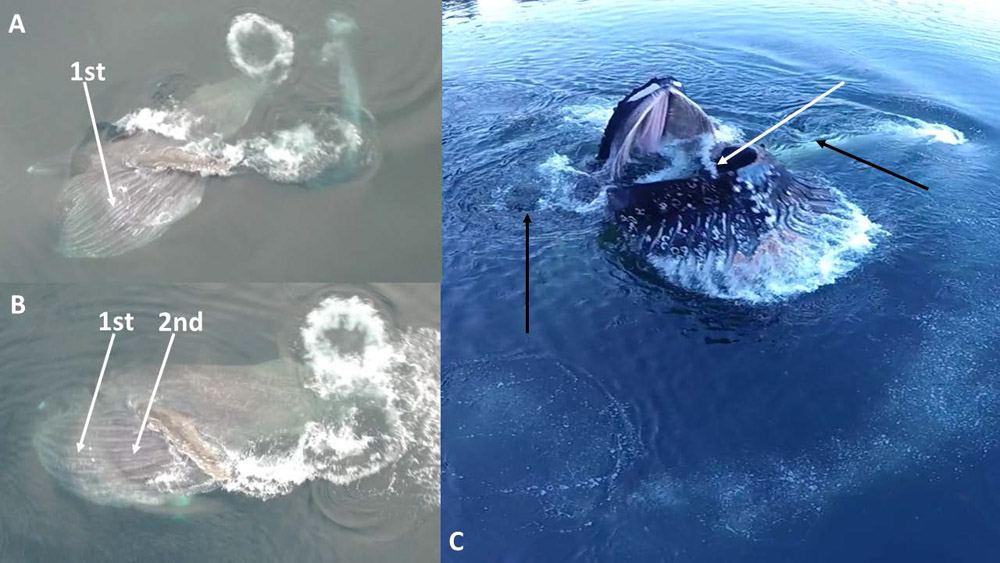
Rebounding waves (white arrows) can be seen reverberating through the expanded ventral pouch, initially (A) after the pouch is fully filled and water reverses flow as it “bounces” off the filled chamber’s posterior limit, then (B, 0.12 s later) as the first wave travels anteriorly (toward the mouth) over the still-opened pouch, a second wave arises posteriorly, most likely from contracting VGB musculature. Occasionally (C) a sharp splash of water can be seen emerging from the open mouth, above and distinct from the volume of water filling the pouch; this splash probably arises via rebounding wave energy. Also note (black arrows in C) paired, oppositely flowing whirlpools created by the whale’s (and flippers’) rapid descent after rising up to engulf prey in the middle of a bubble net the whale created.
7. Excurrent Flow of Engulfed Then Expelled Water
The location/direction of excurrent flow (due to postengulfment expulsion/purging during filtration) varies according to a whale’s body orientation, forward swimming speed, and whether the head is at, above, or below the sea surface, and—if above—whether the head is pointed upward (Fig. 6) or held parallel to the surface (Fig. 7). Thus unlike other aspects of engulfment, where there was little observed variation, there was far less stereotypy of excurrent flow.
Figure 6.
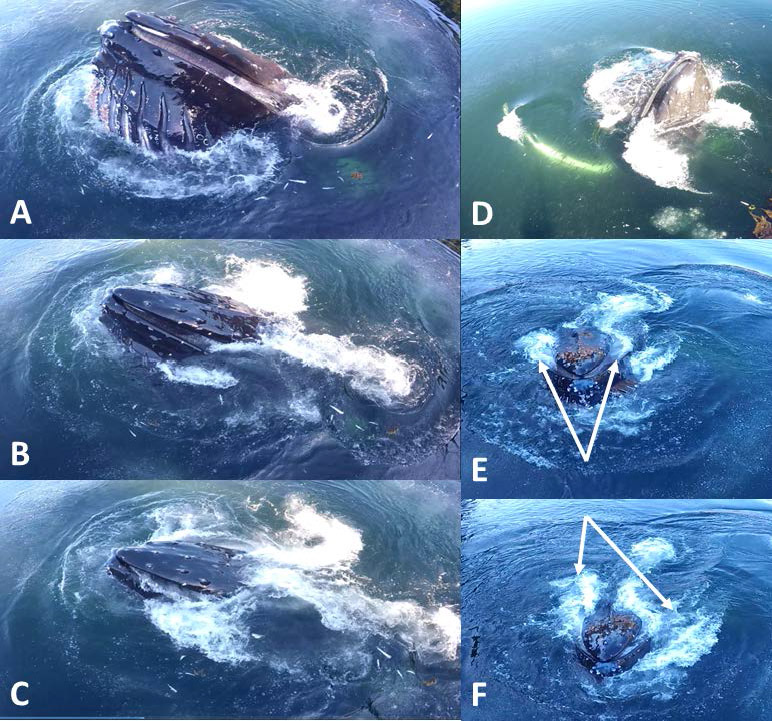
Expulsion of filtered water in whales slowly rising vertically through the water surface is complicated by the fact that flow is initially or mainly governed by gravity, particularly if the whale is wholly vertical (D). Otherwise the excurrent flow begins posteriorly before being overtaken by stronger flow at the center of the mouth (sequence A→C = 1.34 s total). However, this central expulsion (arrows in E) is itself replaced by sustained expulsion from the posterior of the mouth (F, 0.66 s after E).
Figure 7.

For a lunge-feeding humpback whale (i.e., with rapid horizontal locomotion), excurrent flow of filtered (purged) water initially (A) appears at the anterior of the mouth (perhaps from a rebounding wave within the ventral pouch), then almost immediately via a second distinct flow at the posterior end of the mouth. The posterior flow becomes larger (frame B, 0.07 s after A) as the anterior flow dissipates and disappears (C; different engulfment event). The posterior expulsion often decreases (black arrow in frame D, 0.18 s after C) and a large, sustained (10-20 s) flow emerges from the center of the mouth and baleen apparatus (white arrow).
If the head is pointed upward (Fig. 6), water first exits through the posterior portion of the baleen racks, probably assisted by gravity, then in most cases after a slight (0.4–0.5 s) delay a second surge of water abruptly flows through the anterior-most baleen plates. This break in water flow, in which water does not flow gradually from the posterior toward the anterior of the mouth but indeed skips the middle baleen plates as it takes a sharp jump and surges directly to the anterior of the racks, is highly suggestive of a rebound phenomenon. If the whale surfaces with its head pointed straight upward, two major excurrent flows can be seen pouring out of each angle of the mouth well before gape closure (presumably due to gravity’s influence).
However, with whales swimming forward at or just below the sea surface prior to and during engulfment (in all body orientations; Fig. 7), the first flow of excurrent water is not observed at the angle of the mouth, but instead close to the anterior of the mouth—whether from simple reversed/rebounded flow or contraction of VGB musculature and return of the tongue to its original position, it is impossible to determine from our video sequences. In such cases, a second, larger excurrent flow carrying most (perhaps 60%–80%) of the expelled water is later visible (from bubbles or streaming water; Fig. 7) more posteriorly. In summary, the portion of the baleen racks that commence the filtration process (Werth 2013) seems to depend on a whale’s orientation, forward locomotion, and related aspects of engulfment, particularly its position above or below the water’s surface.
8. Wave Propagation or Other Water Motion Around and Within Mouth
It is striking how calm and smooth the water level in the mouth is during engulfment relative to the water outside the mouth (Fig. 8), which often shows waves or chop due to wind or the motion of the whale (particularly its emergence above the surface). This is true no matter the whale’s body orientation; even whales turned nearly upside-down show calm water in the mouth (Fig. 8A). In many recorded sequences there are no turbulent, disruptive currents within or immediately adjacent to the mouth.
Figure 8.
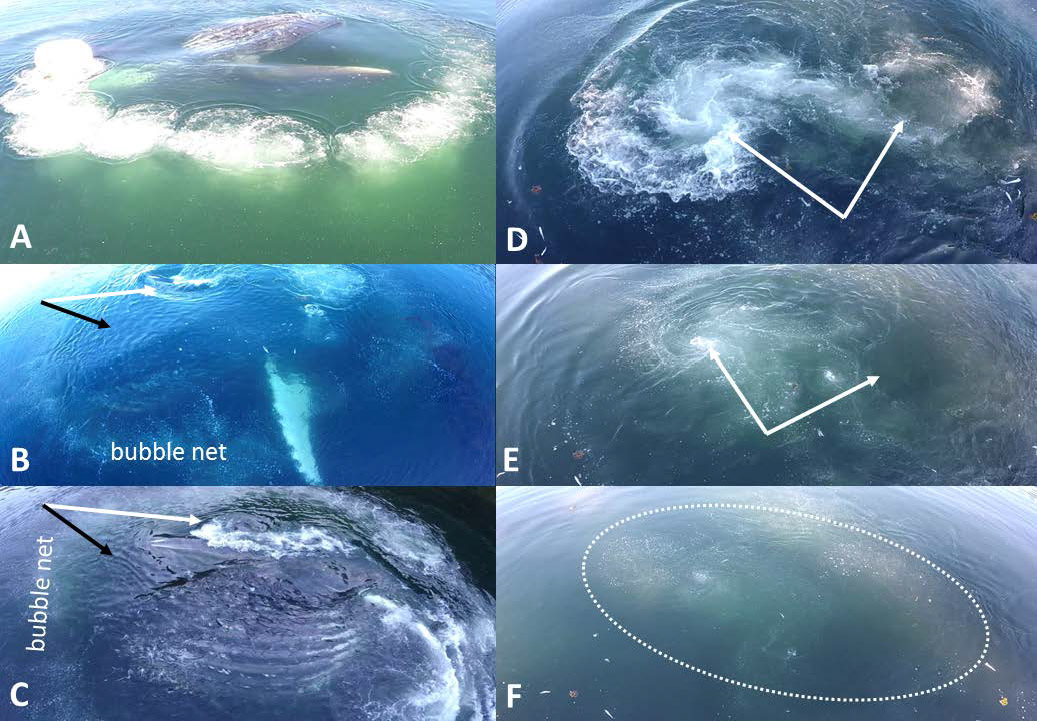
Apart from the dynamic flows of engulfment and expulsion, water within the mouth is often calm, with no surface waves, even in postengulfment gape closure as in the inverted whale in frame A closing its mouth around fish within a bubble ring. In B and C (separate feeding events), water within the mouth shows only slight wind-generated capillary waves (black arrows) despite surface waves created by motion/depression of the upper jaw (white arrows). In sequence D→F (total 24.36 s) a whale has disappeared below the surface after engulfment, leaving two whirlpools on the surface (connected by a circulating underwater vortex). The vortex generates tiny bubbles (not directly produced by the whale) which rise to the surface and may continue to aggregate prey; some small fish are also shown with bubbles in the dotted oval.
9. Vortical Flow from Head Depression
In multiple sequences (distant aerial plus close-up GoPro), large (2–4 m diameter) whirlpools are visible on the water’s surface at the point where the head, following engulfment and water expulsion, sinks underwater (Fig. 5C, 8D-F). In multiple instances a pair of whirlpools swiftly rotating in opposite directions (clockwise and counterclockwise, presumably linked by a submerged semitoroidal vortex) are clearly evident, persisting for >30 s. These whirlpools apparently form from vortices shed as the depressed head entrains air from above the surface into a collapsing bowl-like cavity.
10. Influence of Body Position on Engulfment
Earlier we alluded to the stereotyped nature of humpback whale feeding in our recorded sequences: despite a wide range of body positions/orientation, swim speeds, and behaviors (especially flipper motion), there is little variation in kinematic events of water engulfment and expulsion, especially in terms of timing. Minor exceptions found in whales rotated from the normal dorsal-up orientation include slightly lower gape angles and cranial elevations. As noted above, excurrent flow also varies with body position: water normally exits at the angle of the mouth (instead of anteriorly or all along the jaws) when a whale surfaces from straight below and rises in a chin-up posture. The opening to the oral pouch can be seen only when looking straight down into the mouth, but VGB contractions and waves rebounding along the pouch are best observed in rotated whales.
Discussion
The speed of the engulfment events analyzed here (mean 2.07 ± 0.19 s) is in line with previously published data that used biologging tags to measure (Goldbogen et al. 2008, Simon et al. 2012) or mathematical modeling to estimate (Potvin et al. 2010) engulfment timing in humpback whale lunge feeding. Cade et al. (2016) reported similar (2.0 ± 0.5 s) tag-based engulfment times for humpbacks engulfing krill, but longer (4.8 ± 3.0 s) tag-based times for humpbacks engulfing fish. Based on our finding that the mouth often (in 65% of recorded engulfments) begins closing before full pouch expansion, we conclude that the pouch does not always completely expand prior to initiation of gape closure; this has been previously suggested (Arnold et al. 2005). The reason for this is uncertain. It may be that earlier gape closure (before peak pouch expansion) helps to (1) minimize drag, (2) maintain forward momentum, (3) control water influx, and (4) filter/retain elusive prey before they have an opportunity to swim from the engulfed water mass. Alternatively, the whales recorded for our study may have engulfed nonmaximal volumes. This would minimize filtering time, expend less energy, and allow for more engulfments overall. As long as nonmaximal engulfment could still capture the bulk of a whale’s intended schooling prey, this would prove beneficial.
Cranial elevation appears to peak before maximal gape (Fig. 1), possibly because the water mass entering the oral pouch or the influence of fluking or pectoral braking (Edel and Winn 1978, Fish and Battle 1995) generate forces that pull the head downward, thereby lessening cranial elevation during peak gape. With regard to tongue inversion and water entry into the pouch, some of our recorded sequences involved inverted or otherwise rotated lunging whales, where the expanding pouch must be filled solely by ram-driven engulfment forces (Goldbogen et al. 2006, 2007) with no assist from gravity.
It is unlikely that the externally visible reverberations along the inflated pouch of postengulfment whales are powered by VGB musculature or otherwise represent an active process, although this phenomenon might also be attributed to passive elastic rebound from stretchy tissues within the VGB (Orton and Brodie 1987, Shadwick et al. 2013). Propagation of such elastic waves is most likely when two waves are evident (Fig. 5B), the first probably stemming from simple reversed flow and the second (which also originates posteriorly but reaches the mouth later) probably due to highly elastic tissues that permit remarkably rapid and extensive gular expansion. However, most of these reverberations are unlikely to be caused by tissue elasticity because maximum tissue deformation (i.e., strain) during engulfment, as determined by Shadwick et al. (2013), are within the compliant, near zero-stress range of the VGB stress-strain curve measured by Orton and Brodie (1987). Based on studies by Potvin et al. (2009, 2010, 2012), larger (15+ m) rorquals may have insufficient VGB-induced elastic potential energy to generate the kinetic energy needed to expel engulfed water masses, so a combination of elastic rebound of the VGB tissue and passive rebounding (reflected) flow of engulfed water may both aid excurrent (filtering) flow in large whales.
The externally visible pouch reverberations and the rebounding flow they represent may aid humpback (and other rorqual) whales in expelling large volumes of engulfed water, but their consequences for oral filtration are unclear. Provided this unsteady flow affects baleen porosity, the reverberations may have an adverse impact; alternatively, they might speed up or otherwise aid filtration, but our study found no evidence to support these speculative inferences.
It was easy to observe excurrent flow of filtered water exiting the mouth when whales were at or just above the surface (Fig. 6), but streams of expelled bubbles could be seen in whales below the surface (Fig. 7). Unfortunately, we cannot tell from our results if or how excurrent flow might differ in subsurface expulsion, although it does appear to be at least partly affected by gravity when a whale’s head is out of water (Fig. 6).
Other flow phenomena may also be noteworthy, including the calmness of water around and within the mouth immediately prior to and during engulfment (Fig. 8). This is potentially important given that nonlaminar flow might scatter or disperse prey items directly, or startle or alarm prey (as by vibrations or currents) and thereby indirectly lead to decreased prey density (Werth 2012). This is not mere speculation: video sequences provided direct evidence of preengulfment bubble netting and post-engulfment whirlpools and vortices (created by rapid head/body depression through the air-water interface at the surface) resulting in more tightly aggregated prey (Fig. 8), although it is uncertain whether this response was active (from fish swimming away from the phenomena) or passive, with currents alone aggregating the prey. Although our video analysis suggests such hydrodynamic effects, further study is needed to resolve the precise mechanisms involved, and to determine their relative importance.
Vortical flows from head depression are potentially significant in that they presumably occur to substantial depths, perhaps ~3–5 m, and hence might further aggregate small schooling prey, either passively, by creating a low-pressure zone in which prey become concentrated (as in the humpback feeding described by Hays et al. 1985), or due to prey items’ active behavioral response to moving water currents (Werth 2012). We observed humpbacks repeatedly engulfing prey in the same approximate surface location (within ~4 m) after short intervals (<30 s), and it is possible that hydrodynamic effects of previous engulfments might aid further feeding bouts. It is possible that subsurface vortical flow continues to aggregate prey for future ingestion, especially in the case of small or weakly swimming prey. It must be noted that all of our recorded engulfments were at or just below the surface. An obvious benefit of tags affixed to whales is the possibility of recording data (including video) from depths where cameras attached to drones or held by observers cannot penetrate.
With regard to overall body position and orientation, humpback whale feeding is famous for its behavioral flexibility (e.g., McMillan et al. 2018). There are many humpback foraging behaviors that were not observed in our study. Nonetheless we have found—throughout all the sequences we analyzed, and relative to other published accounts of humpback feeding behavior (Jurasz and Jurasz 1979; Hain et al. 1981; D’Vincent et al. 1985; Hays et al. 1985; Goldbogen et al. 2008; Friedlaender et al. 2009, 2013; Hazen et al. 2009; Ware et al. 2010; Simon et al. 2012; Kirchner et al. 2018)—certain common aspects that appear to be largely or entirely invariant. These include high (>80°) gape angle with a large contribution from cranial elevation, rapid (within 2 s) filling of the oral (ventral) pouch, and initial expulsion of excurrent water from the anterior-most baleen (unless the whale’s head is raised at an angle above the water surface, when water pours out closer to the angle of the mouth).
From this kinematic conservatism, we conclude that mechanisms of engulfment, including precise timing of events and involvement of anatomical structures, are physically constrained relative to other aspects of feeding. Although humpback whale foraging is remarkable among cetaceans for encompassing a greatly varied behavioral repertoire, the morphology and biomechanics of feeding are likely curbed by brute limitations of tissues and the environment.
Supplementary Material
Video S1. Representative video clips showing different views of humpback whale prey engulfment.
Acknowledgments
We thank the Northern Southeast Regional Aquaculture Association for assistance and access to their facilities. Geoff Clark and the Prince William Sound Aquaculture Corporation provided drone and GoPro footage of their facilities captured by Klint Hischke and Dan Orlando. We are grateful for field support provided by the Alaska Whale Foundation and to Mark Kelley Photography which generously donated a percentage from book sales to support this study. We thank scientists who have aided our understanding of rorqual engulfment, especially Rick Lambertsen, Jeremy Goldbogen, Bob Shadwick, Jean Potvin, Nick Pyenson, Brian Kot, Haruka Ito, and Frank Fish. Research in Southeast Alaska was authorized under NOAA Fisheries permits 14122 and 18529. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers UL1GM118991, TL4GM118992, or RL5GM118990. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Alexander J. Werth, Department of Biology, Hampden-Sydney College, Hampden-Sydney, Virginia 23943, U.S.A..
Madison M. Kosma, College of Fisheries and Ocean Sciences, University of Alaska Fairbanks, Juneau, Alaska 99801, U.S.A.
Ellen M. Chenoweth, College of Natural Science and Mathematics, University of Alaska Fairbanks, Sitka, Alaska 99835, U.S.A.
Janice M. Straley, Department of Natural Sciences, University of Alaska Southeast, Sitka, Alaska 99835, U.S.A.
Literature Cited
- Anderson K, and Gaston KJ. 2013. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Frontiers in Ecology and the Environment 11(3):138–146. [Google Scholar]
- Arnold PW, Birtles RA, Sobtzick S, Matthews M and Dunstan A. 2005. Gulping behaviour in rorqual whales: Underwater observations and functional interpretation. Memoirs of the Queensland Museum 51:309–332. [Google Scholar]
- Bevan E, Wibbels T, Navarro E, et al. 2016. Using unmanned aerial vehicle (UAVs) technology for locating, identifying, and monitoring courtship and mating behavior in the green sea turtle (Chelonia mydas). Herpetological Review 47:27–32. [Google Scholar]
- Brooks D 2017. Field biology shows that GoPro cameras aren’t just for snowboarding daredevils. Concord Monitor May 9, 2017:12. [Google Scholar]
- Burnett JD, Lemos L, Barlow DR, Wing MG, Chandler TE and Torres LG. 2018. Estimating morphometric attributes of baleen whales with photogrammetry from small UAS: A case study with blue and gray whales. Marine Mammal Science 35:108–139. [Google Scholar]
- Cade DE, Friedlaender AS, Calambokidis J and Goldbogen JA. 2016. Kinematic diversity in rorqual whale feeding mechanisms. Current Biology 26:2617–2624. [DOI] [PubMed] [Google Scholar]
- Chenoweth EM, Straley JM, McPhee MV, Atkinson S and Reifenstuhl S. 2017. Humpback whales feed on hatchery-released juvenile salmon. Royal Society Open Science 4(7):170180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen F, Dujon AM, Sprogis KR, Arnould JPY and Bejder LL. 2016a. Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 7(10):e01468. [Google Scholar]
- Christiansen F, Rojano-Doñate L, Madsen PT and Bejder L. 2016b. Noise levels of multi-rotor unmanned aerial vehicles with implications for potential underwater impacts on marine mammals. Frontiers in Marine Science 3:277. [Google Scholar]
- Dawson SM, Bowman MH, Leunissen E and Sirguey P. 2017. Inexpensive aerial photogrammetry for studies of whales and large marine animals. Frontiers in Marine Science 4:366. [Google Scholar]
- Ditmer MA, Vincent JB, Werden LK, et al. 2015. Bears show a physiological but limited behavioral response to unmanned aerial vehicles. Current Biology 25:2278–2283. [DOI] [PubMed] [Google Scholar]
- Domínguez-Sánchez CA, Acevedo-Whitehouse KA and Gendron D. 2018. Effect of drone-based blow sampling on blue whale (Balaenoptera musculus) behavior. Marine Mammal Science 34:841–850. [Google Scholar]
- Durban JW, Moore MJ, Chiang G, et al. 2016. Photogrammetry of blue whales with an unmanned hexacopter. Marine Mammal Science 32:1510–1515. [Google Scholar]
- D’Vincent CG, Nilson RM and Hanna RE. 1985. Vocalization and coordinated feeding behavior of the humpback whale in Southeastern Alaska. Scientific Reports of the Whales Research Institute, Tokyo: 36:41–47. [Google Scholar]
- Edel RK, and Winn HE. 1978. Observations on underwater locomotion and flipper movement of the humpback whale Megaptera novaeangliae. Marine Biology 48:279–287. [Google Scholar]
- Fiori L, Doshi A, Martinez E, Orams MB and Bollard-Breen B, B. 2017. The use of unmanned aerial systems in marine mammal research. Remote Sensing 9:543. [Google Scholar]
- Fish FE, and Battle JM. 1995. Hydrodynamic design of the humpback whale flipper. Journal of Morphology 225:51–60. [DOI] [PubMed] [Google Scholar]
- Friedlaender AS, Hazen EL, Nowacek DP, et al. 2009. Diel changes in humpback whale Megaptera novaeangliae feeding behavior in response to sand lance Ammodytes spp. behavior and distribution. Marine Ecology Progress Series 395:91–100. [Google Scholar]
- Friedlaender AS, Tyson RB, Stimpert AK, Read AJ and Nowacek DP. 2013. Extreme diel variation in the feeding behavior of humpback whales along the western Antarctic Peninsula during autumn. Marine Ecology Progress Series 494:281–89. [Google Scholar]
- Goebel M, Perryman WL, Hinke JT, Krause DJ, Hann NA, Gardner S and LeRoi DJ. 2015. A small unmanned aerial system for estimating abundance and size of Antarctic predators. Polar Biology 38:619–630. [Google Scholar]
- Goldbogen JA 2010. The ultimate mouthful: Lunge feeding in rorqual whales. American Scientist 98:124–131. [Google Scholar]
- Goldbogen JA, and Meir JU. 2014. The device that revolutionized marine organismal biology. Journal of Experimental Biology 217:167–168. [DOI] [PubMed] [Google Scholar]
- Goldbogen JA, Calambokidis J, Shadwick RE, Oleson EM, McDonald MA and Hildebrand JA. 2006. Kinematics of foraging dives and lunge-feeding in fin whales. Journal of Experimental Biology 209:1231–1244. [DOI] [PubMed] [Google Scholar]
- Goldbogen JA, Pyenson ND and Shadwick RE. 2007. Big gulps require high drag for fin whale lunge feeding. Marine Ecology Progress Series 349:289–301. [Google Scholar]
- Goldbogen JA, Calambokidis J, Croll DA, et al. 2008. Foraging behavior of humpback whales: Kinematic and respiratory patterns suggest a high cost for a lunge. Journal of Experimental Biology 211:3712–3719. [DOI] [PubMed] [Google Scholar]
- Goldbogen JA, Calambokidis J, Croll DA, et al. 2012. Scaling of lunge feeding performance in rorqual whales: Mass-specific energy expenditure increases with body size and progressively limits diving capacity. Functional Ecology 26:216–226. [Google Scholar]
- Goldbogen JA, Friedlaender AS, Calambokidis J, McKenna MF, Simon M and Nowacek DP. 2013. Integrative approaches to the study of baleen whale diving behavior, feeding performance, and foraging ecology. BioScience 63:90–100. [Google Scholar]
- Goldbogen JA, Shadwick RE, Lillie MA, Piscitelli MA, Potvin J, Pyenson ND and Vogl AW. 2015. Using morphology to infer physiology: Case studies on rorqual whales (Balaenopteridae). Canadian Journal of Zoology 93:687–700. [Google Scholar]
- Goldbogen JA, Cade DE, Boersma AT, et al. 2017a. Using digital tags with integrated video and inertial sensors to study moving morphology and associated function in large aquatic vertebrates. Anatomical Record 300:1935–1941. [DOI] [PubMed] [Google Scholar]
- Goldbogen JA, Cade D, Calambokidis JA, Friedlaender AS, Potvin J, Segre PS and Werth AJ. 2017b. How baleen whales feed: The biomechanics of engulfment and filtration. Annual Review of Marine Science 9:367–386. [DOI] [PubMed] [Google Scholar]
- Gormley G 1983. Hungry humpbacks forever blowing bubbles. Sea Frontiers 29(5):258–265. [Google Scholar]
- Hain JHW, Carter GR, Kraus SD, Mayo CA and Winn HE. 1981. Feeding behavior of the humpback whale, Megaptera novaeangliae, in the Western North Atlantic. Fishery Bulletin 80:259–268. [Google Scholar]
- Hays HE, Winn HE and Petricig R. 1985. Anomalous feeding behavior of a humpback whale. Journal of Mammalogy 66:819–821. [Google Scholar]
- Hazen EL, Friedlaender AS, Thompson MA, Ware CR, Weinrich MT, Halpin PN and Wiley DN. 2009. Fine-scale prey aggregations and foraging ecology of humpback whales Megaptera novaeangliae. Marine Ecology Progress Series 395:75–89. [Google Scholar]
- Hodgson JC, and Koh LP. 2016. Best practice for minimising unmanned aerial vehicle disturbance to wildlife in biological field research. Current Biology 26:R404–R405. [DOI] [PubMed] [Google Scholar]
- Hodgson A, Kelly N and Peel D. 2013. Unmanned aerial vehicles (UAVS) for surveying marine fauna: A dugong case study. PLoS ONE 8(11):e79556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A, Peel D and Kelly N. 2017. Unmanned aerial vehicles for surveying marine fauna: Assessing detection probability. Ecological Applications 27:1253–1267. [DOI] [PubMed] [Google Scholar]
- Hunt KE, Moore MJ, Rolland R, et al. 2013. Overcoming the challenges of studying conservation physiology in large whales: A review of available methods. Conservation Physiology 1:cot006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DW 2019. Unoccupied aircraft systems in marine science and conservation. Annual Review of Marine Science 11:439–463. [DOI] [PubMed] [Google Scholar]
- Johnston DW, Dale J, Murray KT, Josephson E, Newton EW and Wood S. 2017. Comparing occupied and unoccupied aircraft surveys of wildlife populations: Assessing the gray seal (Halichoerus grypus) breeding colony on Muskeget Island, USA. Journal of Unmanned Vehicle Systems 5:178–191. [Google Scholar]
- Jurasz CM, and Jurasz VP. 1979. Feeding modes of the humpback whale, Megaptera novaeangliae, in Southeast Alaska. Scientific Reports of the Whales Research Institute, Tokyo: 31:69–83. [Google Scholar]
- Karnowski J, Johnson C and Hutchins E. 2016. Automated video surveillance for the study of marine mammal behavior and cognition. Animal Behavior and Cognition 3:255–264. [Google Scholar]
- Kirchner T, Wiley DN, Hazen EL, Parks SE, Torres LG and Friedlaender AS. 2018. Hierarchical foraging movement of humpback whales relative to the structure of their prey. Marine Ecology Progress Series 607:237–250. [Google Scholar]
- Koski WR, Gamage G, Davis AR, Mathews T, LeBlanc B and Ferguson SH. 2015. Evaluation of UAS for photographic re-identification of bowhead whales, Balaena mysticetus. Journal of Unmanned Vehicle Systems 3:22–29. [Google Scholar]
- Kot BW, Sears R, Zbinden D, Borda E and Gordon MS. 2014. Rorqual whale (Balaenopteridae) surface lunge-feeding behaviors: Standardized classification, repertoire diversity, and evolutionary analyses. Marine Mammal Science 30:1335–1357. [Google Scholar]
- Krause DJ, Hinke JT, Perryman WL, Goebel ME and LeRoi DJ. 2017. An accurate and adaptable photogrammetric approach for estimating the mass and body condition of pinnipeds using an unmanned aerial system. PLoS ONE 12(11):e0187465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen RL 1983. Internal mechanism of rorqual feeding. Journal of Mammalogy 64:76–88. [Google Scholar]
- Lambertsen RL, Ulrich N and Straley J. 1995. Frontomandibular stay of Balaenopteridae: A mechanism for momentum recapture during feeding. Journal of Mammalogy 76:877–899. [Google Scholar]
- Leighton T, Finfer D, Grover E and White P. 2007. An acoustical hypothesis for the spiral bubble nets of humpback whales, and the implications for whale feeding. Acoustics Bulletin 2:17–21. [Google Scholar]
- Letessier TB, Juhel J-B, Vigliola L and Meeuwig JJ. 2015. Low-cost small action cameras in stereo generates accurate underwater measurements of fish. Journal of Experimental Marine Biology and Ecology 466:120–126. [Google Scholar]
- McMillan CJ, Towers JR and Hildering J. 2018. The innovation and diffusion of “trap-feeding,” a novel humpback whale foraging strategy. Marine Mammal Science 10.1111/mms.12557. [DOI] [Google Scholar]
- Nichol L, and Heise K. 1992. The historical occurrence of large whales of the Queen Charlotte Islands Prepared for South Moresby/Gwaii Haanas National Park Reserve, Canadian Parks Service; 68 pp. [Google Scholar]
- Nowacek DP, Tyack PL and Wells RS. 2001. A platform for continuous behavioral and acoustic observation of free-ranging marine mammals: Overhead video combined with underwater audio. Marine Mammal Science 17:191–199. [Google Scholar]
- Nowacek DP, Christiansen F, Bejder L, Goldbogen JA and Friedlaender AS. 2016. Studying cetacean behaviour: New technological approaches and conservation applications. Animal Behavior 120:235–244. [Google Scholar]
- Orton LS, and Brodie PF. 1987. Engulfing mechanics of fin whales. Canadian Journal of Zoology 65:2898–2907. [Google Scholar]
- Pirotta V, Smith A, Ostrowski M, Russell D, Jonsen ID, Grech A and Harcourt R. 2017. An economical custom-built drone for assessing whale health. Frontiers in Marine Science 4:425. [Google Scholar]
- Pirotta E, Schwarz LK, Costa DP, Robinson PW and New L. 2019. Modeling the functional link between movement, feeding activity, and condition in a marine predator. Behavioral Ecology 30:434–445. [Google Scholar]
- Pivorunas A 1977. The fibrocartilage skeleton and related structures of the ventral pouch of balaenopterid whales. Journal of Morphology 151:299–313. [DOI] [PubMed] [Google Scholar]
- Pivorunas A 1979. The feeding mechanism of baleen whales. American Scientist 67:432–440. [Google Scholar]
- Pomeroy P, O’Connor L and Davies P. 2015. Assessing use of and reaction to unmanned aerial systems in gray and harbor seals during breeding and molt in the UK. Journal of Unmanned Vehicle Systems 3:102–113. [Google Scholar]
- Potvin J, Goldbogen JA and Shadwick RE. 2009. Passive versus active engulfment: Verdict from trajectory simulations of lunge-feeding fin whales Balaenoptera physalus. Journal of the Royal Society Interface 6:1005–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin J, Goldbogen JA and Shadwick RE. 2010. Scaling of lunge feeding in rorqual whales: An integrated model of engulfment duration. Journal of Theoretical Biology 267:437–453. [DOI] [PubMed] [Google Scholar]
- Potvin J, Goldbogen JA and Shadwick RE. 2012. Metabolic expenditures of lunge feeding rorquals across scale: Implications for the evolution of filter feeding and the limits to maximum body size. PLoS ONE 7(9):e44854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult V, David PA, Dupont SF, Mathewson CP, O’Neill SJ, Powell NN and Williamson JE. 2016. GoPros™ as an underwater photogrammetry tool for citizen science. PeerJ 4:e1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees AF, Avens L, Ballorain K, et al. 2018. The potential of unmanned aerial systems for sea turtle research and conservation: A review and future directions. Endangered Species Research 35:81–100. [Google Scholar]
- Rieucau G, Kiszka JJ, Castillo JC, Mourier J, Boswell KM and Heithaus MR. 2018. Using unmanned aerial vehicle (UAV) surveys and image analysis in the study of large surface-associated marine species: A case study on reef sharks Carcharhinus melanopterus shoaling behaviour. Journal of Fish Biology 93:119–127. [DOI] [PubMed] [Google Scholar]
- Rümmler M-C, Mustafa O, Maercker J, Peter UH and Esefeld J. 2016. Measuring the influence of unmanned aerial vehicles on Adélie penguins. Polar Biology 39:1329–1334. [Google Scholar]
- Schiffman R 2014. Drones flying high as new tool for field biologists. Science 344(6183):459. [DOI] [PubMed] [Google Scholar]
- Schofield G, Papafitsoros K, Haughey R and Katselidis K. 2017. Aerial and underwater surveys reveal temporal variation in cleaning-station use by sea turtles at a temperate breeding area. Marine Ecology Progress Series 575:153–164. [Google Scholar]
- Segre PS, Mduduzi S, Meÿer MA, Ken P and Goldbogen JA. 2017. A hydrodynamically active flipper-stroke in humpback whales. Current Biology 27:R636–R637. [DOI] [PubMed] [Google Scholar]
- Shadwick RE, Goldbogen JA, Potvin J, Pyenson ND and Vogl W. 2013. Novel muscle and connective tissue design enables high extensibility and controls engulfment volume in lunge-feeding rorqual whales. Journal of Experimental Biology 216:2691–2701. [DOI] [PubMed] [Google Scholar]
- Simon M, Johnson M and Madsen PT. 2012. Keeping momentum with a mouthful of water: Behavior and kinematics of humpback whale lunge feeding. Journal of Experimental Biology 215:3786–3798. [DOI] [PubMed] [Google Scholar]
- Smith CE, Sykora-Bodie ST, Bloodworth B, Pack SM, Spradlin TR and Leboeuf NR. 2016. Assessment of known impacts of unmanned aerial systems (UAS) on marine mammals: Data gaps and recommendations for researchers in the United States. Journal of Unmanned Vehicle Systems 4:31–44. [Google Scholar]
- Sullivan FA, and Torres LG. 2018. Assessment of vessel disturbance to gray whales to inform sustainable ecotourism. Journal of Wildlife Management 82:896–905. [Google Scholar]
- Sykora-Bodie ST, Bezy V, Johnston DW, Newton E and Lohmann KJ. 2017. Quantifying nearshore sea turtle densities: Applications of unmanned aerial systems for population assessments. Scientific Reports 7:17690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres LG 2017. A sense of scale: foraging cetaceans’ use of scale-dependent multimodal sensory systems. Marine Mammal Science 33:1170–1193. [Google Scholar]
- Torres LG, Nieukirk SL, Lemos L and Chandler TE. 2018. Drone up! Quantifying whale behavior from a new perspective improves observational capacity. Frontiers in Marine Science 5:319. [Google Scholar]
- Vas E, Lescroel A, Duriez O, Boguszewski G and Grémillet D. 2015. Approaching birds with drones: First experiments and ethical guidelines. Biology Letters 11:20140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfuss UK, Aniceto AS, Harris DV, et al. 2019. A review of unmanned vehicles for the detection and monitoring of marine fauna. Marine Pollution Bulletin 140:17–29. [DOI] [PubMed] [Google Scholar]
- Ware C, Arsenault R, Plumlee M and Wiley D. 2006. Visualizing the underwater behavior of humpback whales. IEEE Computer Graphics and Applications 26:14–18. [DOI] [PubMed] [Google Scholar]
- Ware C, Friedlaender AS and Nowacek DP. 2010. Shallow and deep lunge feeding of humpback whales in fjords of the West Antarctic Peninsula. Marine Mammal Science 27:587–605. [Google Scholar]
- Weimerskirch H, Prudor A and Schull Q. 2018. Flights of drones over sub-Antarctic seabirds show species- and status-specific behavioural and physiological responses. Polar Biology 41:259–266. [Google Scholar]
- Weinrich MT, Schilling MR and Belt CR. 1992. Evidence for acquisition of a novel feeding behavior: Lobtail feeding in humpback whales, Megaptera novaeangliae. Animal Behaviour 44:1059–1072. [Google Scholar]
- Werth AJ 2000. Feeding in marine mammals Pages 475–514 in Schwenk K, ed. Feeding: Form, function and evolution in tetrapod vertebrates. Academic Press, San Diego, CA. [Google Scholar]
- Werth AJ 2012. Hydrodynamic and sensory factors governing response of copepods to simulated predation by baleen whales. International Journal of Ecology 2018: Article ID 208913. 13 pp. [Google Scholar]
- Werth AJ 2013. Flow-dependent porosity of baleen from the bowhead whale (Balaena mysticetus). Journal of Experimental Biology 216:1152–1159. [DOI] [PubMed] [Google Scholar]
- Werth AJ, and Ito H. 2017. Sling, scoop, squirter: Anatomical features facilitating prey transport, concentration, and swallowing in rorqual whales (Mammalia: Mysticeti). Anatomical Record 300:2070–2086. [DOI] [PubMed] [Google Scholar]
- Werth AJ, Lillie MA, Piscitelli M, Vogl AW and Shadwick RE. 2018. Slick, stretchy fascia underlies sliding tongue of rorquals. The Anatomical Record 302:735–744. [DOI] [PubMed] [Google Scholar]
- Wiley D, Ware C, Bocconcelli A, Cholewiak DM, Friedlaender AS, Thompson M and Weinrich M. 2011. Underwater components of humpback whale bubble-net feeding behaviour. Behaviour 148:575–602. [Google Scholar]
- Winn LK, and Winn HE. 1985. Wings in the sea: The humpback whale. University Press of New England, Hanover, NH. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Representative video clips showing different views of humpback whale prey engulfment.


