Abstract
Anosmia has been recognized as a prevalent and early symptom by many COVID-19 patients. However, most researchers have recorded smell dysfunction solely as present or absent and based on subjective evaluation by patients. We described the results of 57 consecutive COVID-19 patients seen at FIOCRUZ, Rio de Janeiro, Brazil, from April to May 2020. Data about the presence of smell loss, the onset of smell loss and other COVID-19 symptoms such as ageusia and nasal congestion or rhinorrhea were recorded. All patients at the initial consultation and 34 healthy controls underwent the Q-SIT, which is a quick disposable three-item smell identification test, by a trained physician. We compared three groups: healthy controls, COVID+ patients with reported smell loss (COVID w/ SL) and COVID+ patients without smell loss (COVID+ w/o SL). The mean age of patients was 41.4 years (SD ± 10.4), and 54.4% were women. Smell loss was reported by 40.4% of COVID-19 patients. We observed a gradual effect with higher Q-SIT scores in healthy controls, followed by COVID+ w/o SL and COVID+ w/ SL (medians = 3, 2 and 0; respectively, p < 0.001). Anosmia or severe microsmia (Q-SIT≤1) was present in 11.1% (CI: 3.1%–26.1%) of controls, 32.4% (CI: 17.4%–50.5%) of COVID-19 w/o SL and 87% (CI: 66.4%–97.2%) of COVID+ w/ SL (p < 0.001). This study provides evidence that olfactory dysfunction in COVID-19 is common and more prevalent than what is perceived by patients. Q-SIT is a quick and reliable screening test for the detection of smell dysfunction during the pandemics.
Keywords: Olfaction disorders, COVID-19, Central nervous system viral diseases
Highlights
-
•
Smell dysfunction is frequent in COVID-19 patients.
-
•
Subtle olfactory dysfunction is frequently not perceived by patients when inquired during medical visits.
-
•
Q-SIT is a quick and reliable screening test for the detection of smell dysfunction during the pandemics.
1. Introduction
Olfactory dysfunction is common during and after viral infections. Among 354 patients presenting to a smell disorder clinic, 22% had a viral infection as etiology [1]. During the COVID-19 pandemic, anosmia has been recognized as a prevalent and early symptom by many patients. In a recent study, the prevalence of anosmia was 56%, being the first symptom in 10% of patients [2]. However, most researchers have recorded smell dysfunction in COVID-19 solely as present or absent and based on subjective evaluation by patients [[3], [4], [5]]. Herein, we evaluate olfactory dysfunction in COVID-19 patients using a quick, accessible, and disposable three-item smell identification test.
2. Materials and methods
Case series of 57 consecutive patients seen at the National Institute of Infectious Diseases Evandro Chagas (INI), FIOCRUZ, Rio de Janeiro, Brazil, from April to May 2020 with a diagnosis of COVID-19 diagnosed by SARS-CoV-2 RNA RT-qPCR in nasal and oropharyngeal swabs (Biomanguinhos kit (E + P1), FIOCRUZ, Brazil).
Data about the presence of smell loss, the onset of smell loss and other COVID-19 symptoms, and nasal congestion or rhinorrhea were recorded prospectively during the clinical visit. All patients at the initial consultation and 34 healthy controls underwent the Q-SIT, which is a disposable three-item smell identification test, by a trained physician. Detailed information about the test is provided elsewhere [6], but, briefly, it consists of individual and disposable tear-out cards, each of which contains three microencapsulated odorant strips (chocolate, banana, and smoke). For each odor, the patient was asked to choose among four possible alternatives or declare None/other. The final score was 0–3 based on the number of correct answers. Microsmia was defined as a lessened ability to smell [7].
Statistical analysis was performed using the statistical software R 3.6.0. Participants' baseline characteristics were summarized using means (±standard deviation-SD) for continuous variables and counts and percentages for categorical variables. We compared three groups: healthy controls, COVID+ patients with reported smell loss (COVID w/ SL) and COVID+ patients without smell loss (COVID+ w/o SL) by Pearson Chi-Square to identify an association with categorical variables, and Mann-Whitney and Kruskal-Wallis with post hoc tests for continuous values. Statistical significance was assumed for a p-value <0.05. Due to the criticism of use of p-value, mainly in small samples, we added 95% confidence intervals (CI) for the percentages.
This study was approved by the local ethical committee at INI/FIOCRUZ
3. Results
The clinical characteristics of our sample are shown in Table 1 . There were 57 patients with a mean age of 41.4 years (SD ± 10.4), and 54.4% were women, while the 36 healthy controls had a mean age of 37.2 years (SD ± 9.6) and 52.8% were women. No differences were observed between these two groups concerning gender (p = 0.88) or age (p = 0.07).
Table 1.
Clinical characteristics of controls, COVID+ patients without smell loss, and COVID+ patients with smell loss.
| Controls (36) | COVID+ w/o SL (34) | COVID+ w/ SL (23) | p value | |
|---|---|---|---|---|
| Female gender (%) | 19 (52.8) | 15 (44.1) | 16 (69.6) | 0.166 |
| Mean age ± SD (years) | 37.1 ± 9.6 | 42.5 ± 11.4 | 39.7 ± 8.6 | 0.129 |
| Median duration of symptoms in days (IQR) | N/A | 3 (3) | 4 (4) | 0.56 |
| Ageusia (%) | N/A | 0 (0%) | 4 (17.4) | 0.02 |
| Nasal congestion (%) | N/A | 18 (52.9) | 13 (56.5) | 0.79 |
| Past respiratory diseases (%) | N/A | 3 (8.8) | 2 (8.7) | 0.98 |
| Median Q-SIT (IQR) | 3 (1) | 2 (2) | 0 (1) | <0.001 |
| Anosmia or severe microsmia (%) | 4 (11.1) | 11 (32.4) | 20 (87) | <0.001 |
COVID+ w/o SL: COVID+ patients with reported smell loss; COVID+ w/ SL: COVID+ patients without smell loss; SD: standard deviation; IQR: interquartile range.
The median time from onset of symptoms to the medical evaluation was four days (1–19 days), and 23 of them (40.4%) reported smell loss. In 17 of these (73.9%), smell loss was the first symptom or was noticed during the first day of the disease. Ageusia was reported by only 5/57 (8.7%) patients. Congestion or rhinorrhea occurred in 28 out of 57 patients (49.1%), and no difference was observed between COVID-19 patients with (12/23) and without (16/34) smell dysfunction (p = 0.79). Five COVID-19 patients had previous respiratory disorders (two with rhinitis, two with asthma, and one with chronic sinusitis). All but two patients had mild disease and were treated on an outpatient basis. Two patients were admitted to ICU and died from respiratory complications related to COVID-19.
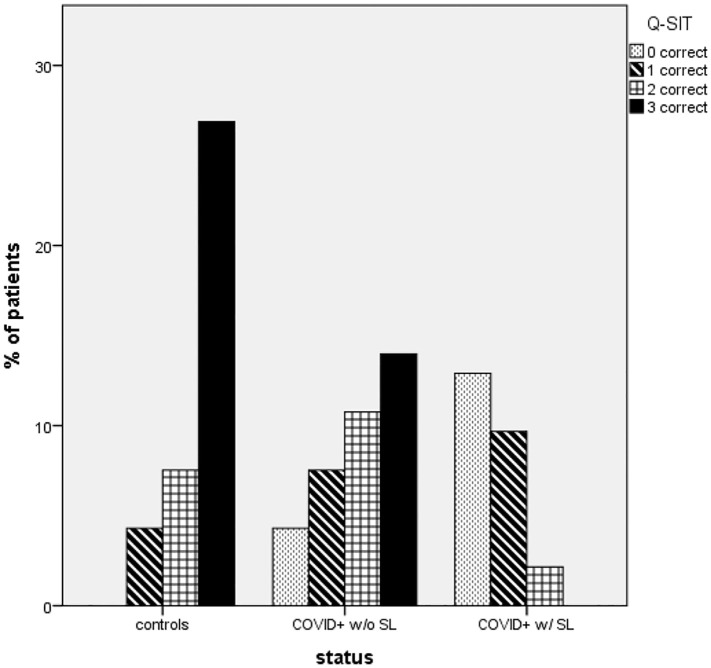
The results of Q-SIT in the three different groups are shown in Fig. 1 and Table 2 . We observed a gradual effect with higher Q-SIT scores in healthy controls, followed by COVID+ w/o SL and COVID+ w/ SL (medians = 3, 2, and 0; respectively, p < 0.001). Most COVID+ w/SL missed all answers in Q-SIT (score 0).
Fig. 1.
Q-SIT results of controls and patients with COVID-19 COVID+.
Table 2.
Q-SIT in controls, COVID+ patients without smell loss, and COVID+ patients with smell loss.
| QSIT score | Controls N- % (95% CI) |
COVID+ w/o SL N- % (95% CI) |
COVID+ w/ SL N- % (95% CI) |
Total N- % (95% CI) |
|---|---|---|---|---|
| 0 correct | 0–0.0% (0.0%–9.8%) |
4–11.8% (3.3%–27.5%) |
12–52.2% (30.6%–73.2%) |
16–17.2% (10.2%–26.4%) |
| 1 correct | 4–11.1% (3.1%–26.1%) |
7–20.6% (8.7%–37.9%) |
9–39.1% (19.7%–61.5%) |
20–21.5% (13.7%–31.2%) |
| 2 correct | 7–19.4% (8.2%–36.0%) |
10–29.4% (15.1%–47.5%) |
2–8.7% (1.1%–28.0%) |
19–20.4% 12.8%–30.1% |
| 3 correct | 25–69.4% (51.9%–83.7%) |
13–38.2% (22.2%–56.4%) |
0–0.0% (0.0%–14.8%) |
38–40.9% (30.8%–51.5%) |
N: number of patients; % percentage; (CI) confidence interval; COVID+ w/o SL: COVID+ patients with reported smell loss; COVID+ w/ SL: COVID+ patients without smell loss.
If we compare the three groups regarding anosmia or severe microsmia (Q-SIT≤1), it was present in 11.1% (CI: 3.1%–26.1%) of controls, 32.4% (CI: 17.4%–50.5%) of COVID-19 w/o SL and 87% (CI: 66.4%–97.2%) of COVID+ w/ SL (p < 0.001).
4. Discussion
Olfaction dysfunction is associated with poor feeding, depression, as well as problems with hygiene and safety [8]. This study provides evidence that olfactory dysfunction in COVID-19 is common and more prevalent than what is perceived by patients when inquired about smell loss at clinical visits. The mechanisms implicated in frequent olfactory dysfunction in COVID-19 are not fully understood, but nasal epithelium cells are abundant in angiotensin-converting enzyme 2 receptor, which mediates SARS-CoV-2 entry into cells. Disruption of neuroepithelium secondary to inflammatory response may impair olfaction temporarily or permanently [9].
Besides anosmia, partial deficits (microsmia) were frequently and not recognized by many patients. Moein et al. recently described the results of UPSIT, a 40-odorant test in 60 patients during the recovery phase of the disease [10]. Anosmia was detected in 25% and severe microsmia in 33% of patients. Our results were similar, but patients were evaluated in the early phase of the disease using a three-odorant test, which is inexpensive, quick to apply (1 min), and reliable compared to UPSIT, especially for anosmia or severe microsmia. Q-SIT can be particularly useful during evaluation of COVID-19 patients when unnecessary exposition by heathy care personnel is desired. It is compact and can be brought into the examination room, and transmission of the SARS CoV-2 infection is prevented since each card is discarded after use. Patients with smell dysfunction detected by Q-SIT can be referred to further specialized evaluation and follow-up. Besides, since olfactory loss occurs early in the disease, a simple test can help in the detection of paucisymptomatic cases.
In conclusion, using a simple and objective instrument is possible to detect more subtle degrees of olfactory dysfunction in COVID-19 than what is subjectively reported by patients. Q-SIT is a quick and reliable screening test for the detection of smell dysfunction during the pandemics.
Acknowledgments
Acknowledgments
Dr. Alexandra Prufer Araújo for the donation of Q-SIT tests
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Seiden A.M. Postviral olfactory loss. Otolaryngol. Clin. North Am. 2004;37(6):1159–1166. doi: 10.1016/j.otc.2004.06.007. dezembro de. [DOI] [PubMed] [Google Scholar]
- 2.Paderno A., Schreiber A., Grammatica A., Raffetti E., Tomasoni M., Gualtieri T. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int. Forum Allergy Rhinol. 2020;10(8):955–962. doi: 10.1002/alr.22610. agosto de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.-Y., Lepiller Q., Gendrin V. Features of anosmia in COVID-19. Med. Mal. Infect. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. agosto de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meini S., Suardi L.R., Busoni M., Roberts A.T., Fortini A. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur. Arch. Otorhinolaryngol. 2020 Jun 4:1–5. doi: 10.1007/s00405-020-06102-8. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chary E., Carsuzaa F., Trijolet J.-P., Capitaine A.-L., Roncato-Saberan M., Fouet K. Prevalence and recovery from olfactory and gustatory dysfunctions in Covid-19 infection: a prospective Multicenter study. Am. J. Rhinol. Allergy. 2020 Sep;34(5):686–693. doi: 10.1177/1945892420930954. 12 de junho de. (1945892420930954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackman A.H., Doty R.L. Utility of a three-item smell identification test in detecting olfactory dysfunction. Laryngoscope. 2005;115(12):2209–2212. doi: 10.1097/01.mlg.0000183194.17484.bb. dezembro de. [DOI] [PubMed] [Google Scholar]
- 7.Doty R.L., Yousem D.M., Pham L.T., Kreshak A.A., Geckle R., Lee W.W. Olfactory dysfunction in patients with head trauma. Arch. Neurol. 1997;54(9):1131–1140. doi: 10.1001/archneur.1997.00550210061014. setembro de. [DOI] [PubMed] [Google Scholar]
- 8.Croy I., Nordin S., Hummel T. Olfactory disorders and quality of life--an updated review. Chem. Senses. 2014;39(3):185–194. doi: 10.1093/chemse/bjt072. março de. [DOI] [PubMed] [Google Scholar]
- 9.Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020 May 20 doi: 10.1001/jama.2020.8391. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]